- 1State Key Laboratory of Oncology in South China, Department of Medical Oncology, Sun Yat-sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 2State Key Laboratory of Oncology in South China, Department of Molecular Diagnostics, Sun Yat-sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 3State Key Laboratory of Oncology in South China, Department of Experimental Research, Sun Yat-sen University Cancer Center, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
Background: BRAFV600E mutation is associated with poor prognosis of colorectal cancer (CRC) patients, but the comparison of clinic-pathologic features between V600E and non-V600E mutation was not well-known in CRC patients. The aim of this study is to evaluate the clinical and pathological features, prognostic value of BRAF mutations in CRC.
Methods: We conducted a retrospective study to characterize the clinical and pathological features and survival of patients with BRAF mutated CRC. Patients were classified according to BRAF status as BRAFV600E mutation and non-V600E mutations. Difference of characteristics and survival between the two groups was analyzed.
Results: There was no significant difference in gender, family history, location of primary tumor, metastatic sites between patients with BRAF-V600E mutation and non-V600E mutations. Patients with V600E mutation were younger than those with non-V600E mutations (p = 0.002). Patients with BRAFV600E mutation showed a poorer outcome than those with non-V600E mutations (23.1 vs. 49.9 months, respectively, p = 0.0024). Lack of CDX2 expression was associated with worse prognosis (mOS: 9.4 m vs. not reached, respectively, p = 0.016). Status of V600E mutation did not affect the mPFS and ORR of first-line or second-line treatment.
Conclusion: BRAFV600E mutation defines a distinct subgroup of CRC with worse prognosis. Lack of CDX2 expression is associated with poor OS. Status of V600E mutation did not affect the mPFS of first-line or second-line treatment.
Introduction
Colorectal cancer (CRC) is the third most prevalent malignancy worldwide (1). CRC is widely recognized as a molecularly heterogeneous disease, resulted from accumulation of genetic and/or epigenetic changes involving several pathways, such as microsatellite instability (MSI), chromosomal instability (CIN), RAS-RAF-MEK-ERK-MAPK pathway. Among them, mutations in RAS and BRAF (v-raf murine sarcoma viral oncogene homolog B) genes are most widely used in clinical decision making (2). BRAF, a proto-oncogene, plays an important role in cell differentiation, proliferation and survival through MAPK pathway (3). Therefore, its aberrant activation is critical for tumorigenesis in many types of malignancies, such as melanoma, hairy cell leukemia, papillary thyroid carcinoma, non-small cell lung cancer (NSCLC) as well as CRC (4–9). In CRC, the incidence of BRAF mutation is about 3–10% (4, 10–12). The most common BRAF mutation is due to a CTGCAG change in the nucleotide 1,799 of exon 15 (T1799A), which leads to an amino acid substitution from valine to glutamate at codon 600 (p.V600E). This mutation is known as BRAFV600E mutation, which accounts for 56–90% of BRAF mutations (13–16). Many studies have demonstrated the negative prognostic value of BRAF V600E mutation on metastatic CRC patients (4, 8). However, in our clinical practice, we found that not all the BRAFV600E patients had poor prognosis. Moreover, non-V600E BRAF mutations are less common in CRC, and their clinical and pathological features, prognostic and predictive value were less discussed.
Since the behavior of BRAFV600E mutated mCRC is aggressive, the PFS of traditional chemotherapy is poor and only 60% of patients can receive second-line treatment. Hence, intensive combination of targeted therapy and chemotherapy may be effective. FOLFOXIRI plus bevacizumab regimen has demonstrated an improved PFS and OS (17). So, it is recommended during first-line treatment for BRAFV600E mutated mCRC. During second-line treatment, combined approach with several targeted inhibitors against different key components of MAPK pathway has showed promising results, with a median progression free survival (PFS) of 7.7 months by vemurafenib, irinotecan, and cetuximab (18, 19), or 8.0 months by encorafenib, binimetinib, and cetuximab (20). To our knowledge, there are no studies about effectiveness of chemotherapy for Chinese CRC patients with BRAF mutation. In this study, we evaluated these mutations and tried to provide new insights of Chinese BRAF mutations CRC patients.
Methods
Clinical Data
In this study, we retrospectively review CRC patients with BRAF mutation who were diagnosed between April 2013 to January 2020 at Sun Yat-sen University Cancer Center (Guangzhou, China). All the patients were diagnosed as CRC by hematoxylin and eosin (HE) staining and histologically analysis. Clinic records, including gender, age, primary tumor location, TNM stage at diagnosis, metastatic sites, family history, MSI/MMR status, date of diagnosis and date of last contact, were collected by our medical record system.
Ethics and Consent Statement
The studies involving human participants were reviewed and approved by ethics committee of Sun Yat-sen University Cancer Center. The patients provided written informed consent to participate in this study.
DNA Extraction, and NGS Library Preparation and Sequencing
DNA from the tumor tissues and their paired normal tissues or peripheral blood cells were extracted using the QIAamp DNA FFPE Tissue kit (Qiagen, Hilden, Germany) according to the protocols recommended by the manufacturer as previously described (21). DNA concentration was measured using the Qubit dsDNA HS Assay kit on a Qubit Fluorometer 3.0 (Life Technologies, Carlsbad, CA, USA). Gene mutations of samples collected before February 2019 were tested by the OncoCarta Panel version 1.0 (Sequenom Inc., San Diego, CA, USA) which covered a total of 238 possible mutations in 19 common oncogenes as previously described (12). OncoScreen Panel (Burning Rock Biotech Ltd, Guangdong, China) was used for detection of 295 key genes since February 2019. The threshold of input DNA quantity was 200 ng for samples to proceed to library preparation, as previously described (22, 23). Fragments between 200 and 400 bp were purified by AGEcout AMPure beads (Beckman Coulter, Pasadena, USA). Hybridization, hybrid selection and PCR amplification were then performed according to the commercial protocol, and the indexed samples were sequenced on an Illumina NextSeq500 sequencer with pair-end reads (Illumina, Inc., San Diego, USA). A minimal median unique sequencing depth of 500X was necessary and sufficient to assess low frequency mutations for each tumor sample.
Statistical Methods
The patients' clinicopathological features were summarized with descriptive statistics. Categorical variables were compared using Chi square test, and comparisons of continuous variables were performed using Student's t-test. Five-year cause specific survival (CSS) was calculated from the date of diagnosis to the date of cancer-specific death. Survival among different variables was compared using Kaplan-Meier estimates and the log-rank test. Statistical analysis was carried out by the IBM SPSS Statistics 22.0.0 package software (SPSS Inc) and the Intercooled Stata 13.0 (Stata Corporation, College Station, TX). All the P-values were two-sided, and statistical significance was set at P < 0.05.
Results
Patients Characteristics
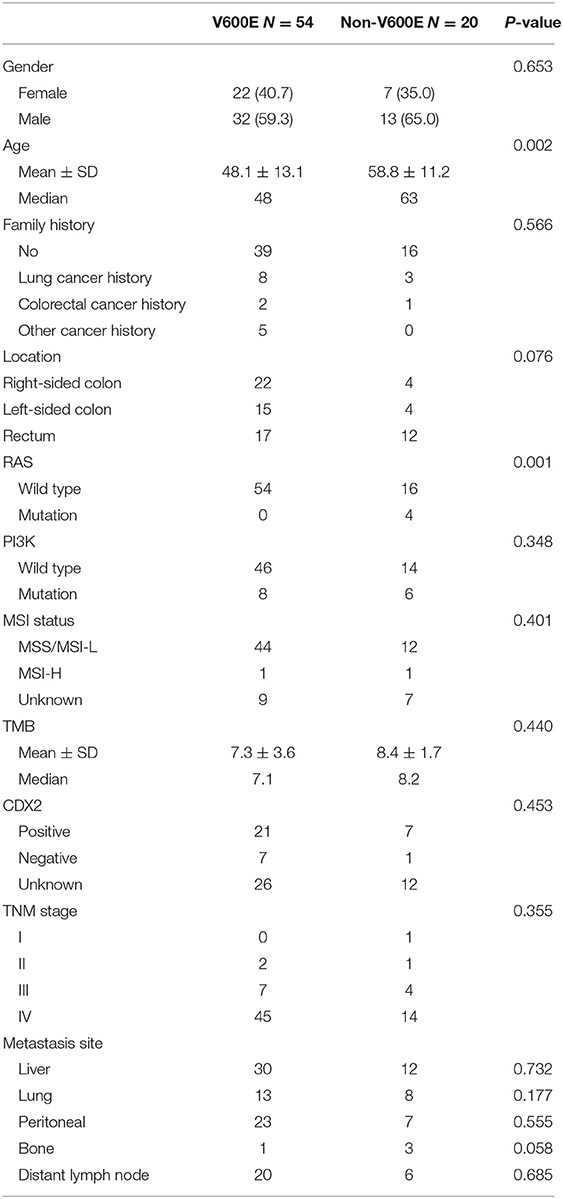
From April 2013 to January 2020, 74 Chinese CRC patients with BRAF mutations were investigated in Sun Yat-sen University Cancer Center. Fifty four (73.0%) were BRAFV600E mutated. Most patients were diagnosed at advanced stage (59/74, 79.7% at stage IV). There were 26 (35.1%) right-sided (cecum to transverse colon) and 19 (25.7%) left-sided (splenic flexure to sigmoid colon) cases, and the rest were in rectum (29/74, 39.2%). Patients with V600E mutation were much younger than those with non-V600E mutations (48.1 vs. 58.8 years old, p = 0.002). The most common sites of non V600E mutations are codon 469, 464, and 594. There was no significant difference in gender, family history, location of primary tumor, metastatic sites, CDX2 status, MSI status or TMB level between V600E and non-V600E groups. Though RAS and BRAF genes were thought to be mutually exclusive, 4 cases with RAS and BRAF co-mutations were found in our study. All of them were non-V600E mutated. The clinical and pathological features are showed in Table 1.
Eight patients with negative CDX2 expression were found in our study. The median age was 47.3 (30–69) years. Most of them (6/8, 75%) were male. Seven (87.5%) of them were BRAFV600E mutated. In terms of primary tumor location, there were 3 cases on each side of colon, and the rest 2 cases were located in the rectum. No remarkable difference of age, gender, location, differentiation, metastatic site was found in patients with negative CDX2 compared to those with positive CDX2 expression.
Treatment
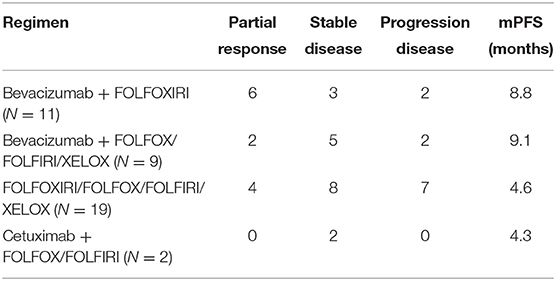
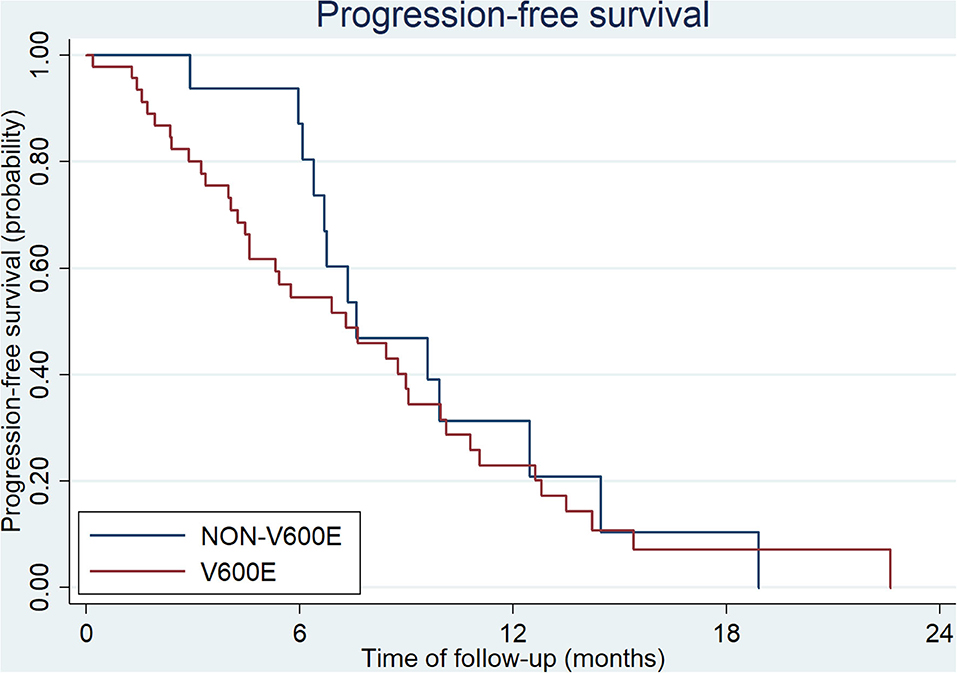
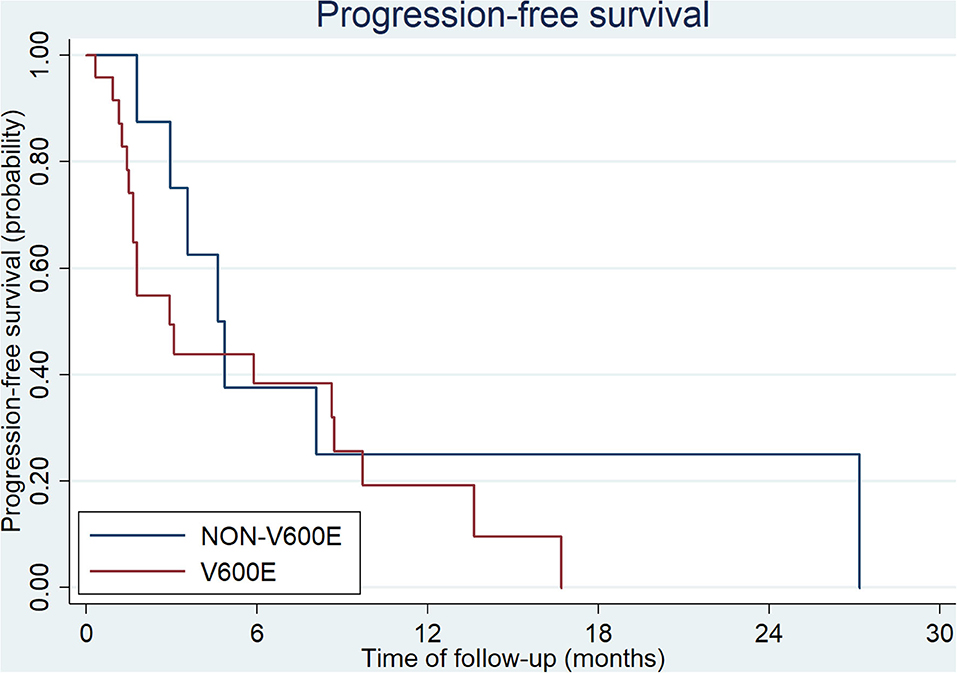
All patients at stage I, II, and III (9/54 in V600E group and 6/20 in non-V600E group, respectively), received radical surgery. Sixty five patients received first-line therapy and 58 of them were evaluable. Forty eight patients with BRAFV600E mutation received first-line treatment, and the regimen mostly used was bevacizumab plus two or three-drug chemotherapy (Table 2). Besides, 2 patients received local therapies for primary tumor and metastatic sites in the condition of disease controlled after systemic treatment. Among the 20 cases with BRAFnon−V600E mutation, 17 patients received first-line treatment, including 10 cases with chemotherapy alone, 5 with chemotherapy plus cetuximab, and 2 with chemotherapy plus bevacizumab. Two patients received local therapies for liver/lung metastasis. Median progression free survival (mPFS) and objective response rate (ORR) of patients with BRAFV600E mutation was 7.3 months (95%CI: 4.6–9.1 months) and 30.1%, while patients with non-V600E mutations had an mPFS of 7.6 months (95%CI: 6.4–12.5 months) and an ORR of 37.5%. PFS and ORR during first-line treatment was not affected by status of V600E mutation (p = 0.51 and 0.64, respectively, Figure 1). PFS of different regimens for BRAFV600E mutated patients is showed in Table 2. It seems that regimens with Bevacizumab + chemotherapy had a better PFS than chemotherapy alone or chemotherapy plus cetuximab, but the statistical significance was not reached.
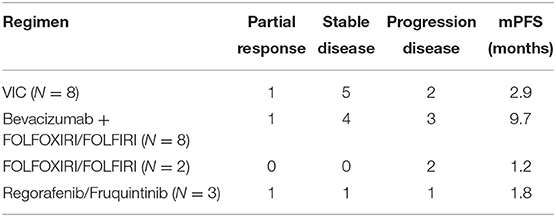
Thirty three patients received second-line therapy. the regimens mostly used for patients with BRAFV600E mutation were VIC (vemurafenib, irinotecan and cetuximab) and bevacizumab plus chemotherapy (Table 3). Among patients with BRAFnon−V600E mutation, only 8 patients (8/17, 47%) received second-line treatment. Four of them received chemotherapy alone, 3 received bevacizumab plus chemotherapy, and 1 patient received cetuximab plus chemotherapy. mPFS for patients with BRAF V600E and non-V600E mutations was 2.9 months (95%CI: 1.7–8.7 months) and 4.6 months (95%CI: 1.8- months), respectively (p = 0.30, Figure 2). The ORR of BRAF V600E and non-V600E mutations was 14.3 and 12.5%, respectively, p = 0.90. The effect of different regimens for patients with BRAFV600E mutation is presented in Table 3. Bevacizumab + chemotherapy seemed to have an improved PFS (9.7 months) compared to other regimens, though it was not statistically different.
Among the 8 patients with loss of CDX2 expression, 6 patients received first-line treatment. Two of them were treated with bevacizumab plus chemotherapy, and the other 4 patients used chemotherapy alone. The ORR was 16.7% (1/6) and mPFS was 3.2 months.
Survival Analysis
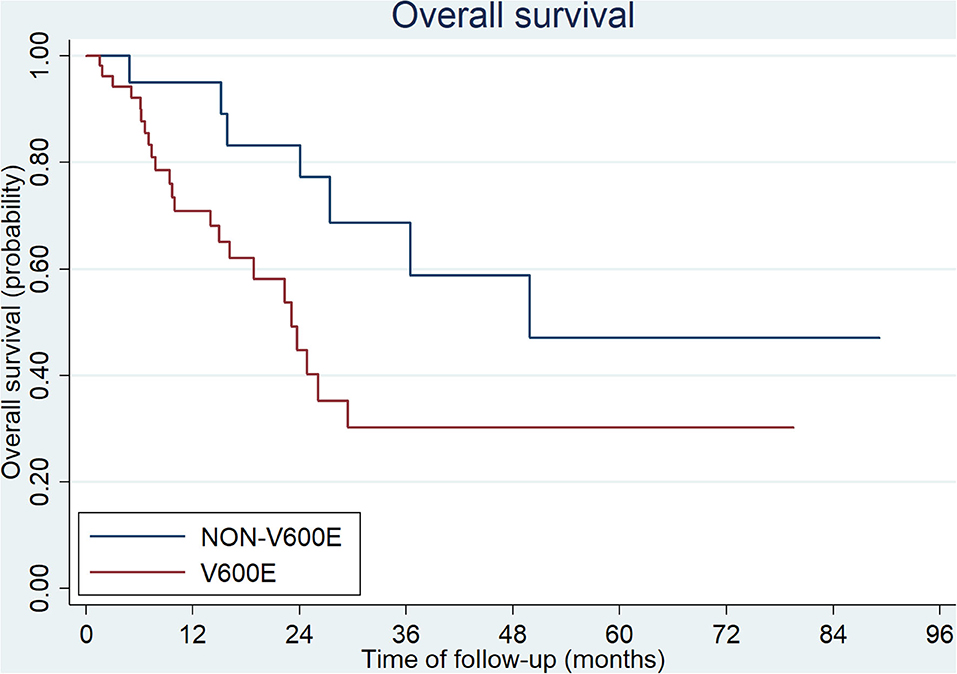
The median overall survival (OS) of all patients was 27.4 months in our study. Patients with BRAFV600E mutation showed a poorer outcome than those with non-V600E mutations (23.1 vs. 49.9 months, p = 0.0024, Figure 3). There were 15 patients diagnosed at early stage (stage I, II, and III; 9/54 with V600E mutation and 6/20 with non-V600E mutation). All of them received radical surgery and 10/15 received adjuvant chemotherapy. The median disease-free survival (DFS) was 15.3 months (3.0–63.9 months). No statistical difference was found between V600E/non-V600E patients (14.0 vs. 15.3 m, respectively, p = 0.257). However, Non-V600E mutant type at early stage showed better OS than V600E mutant type (not reached vs. 26.1 m, respectively, p = 0.05).
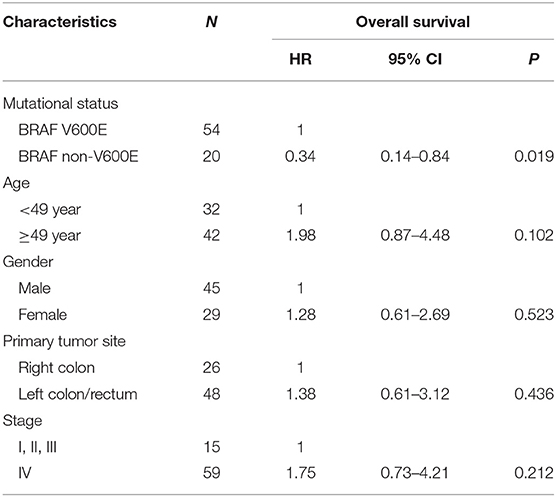
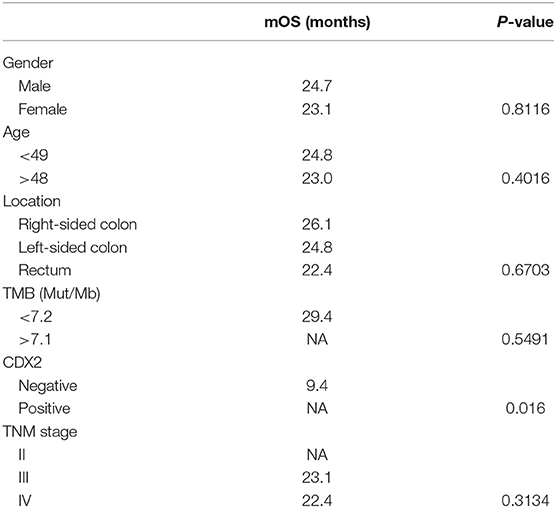
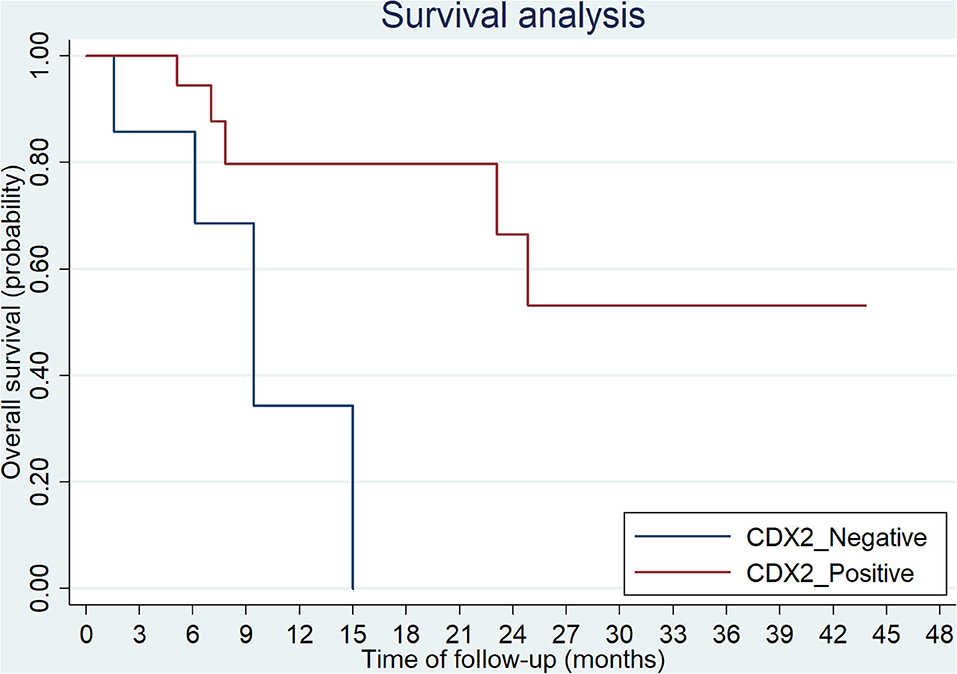
The overall survival after recurrence or metastasis was 18.9 months in BRAFV600E group and not reached in BRAFnon−V600E group (p = 0.051). Multivariate analysis was showed in Table 4 and BRAFV600E was an independent prognostic factor for survival. The univariate analysis showed that only CDX2 expression was related with prognosis of BRAFV600E mutation patients, while gender, age, tumor location, tumor mutational burden (TMB) level and TNM stage were not (Table 5). Patients with negative CDX2 expression have worse outcome compared to those with positive CDX2 (mOS: 9.4 months vs. not reached, p = 0.016, Figure 4).
There were 3 BRAFV600E patients who were alive for more than 40 months. One was a 66-year old male diagnosed as at stage II in 2013, who received radical surgery. The immunohistochemistry of primary tumor showed CDX2 positive and dMMR (MSH2 deficient). He got single lung metastasis after 5 years and received resection of the metastatic tumor and oral S1 as chemotherapy. He had multiple brain metastasis and received local radiotherapy in 2019. This patient was still in the follow-up. The other two patients were diagnosed at stage IV with pMMR and unknown CDX2 status. One got tumor located in rectum, with concurrent lung and peritoneum metastasis. The other one got left-sided colon cancer with peritoneum metastasis. Both patients received XELOX as first-line treatment and bevacizumab plus FOLFIRI regimen as second-line treatment.
Discussion
It has been reported that BRAF mutated CRC patients have specific clinical, pathological and molecular characteristics, compared to patients with wild-type BRAF (14). Clinically, BRAF mutated CRCs are more often seen in elderly women, located in right-sided colon, and accompany with peritoneal and/or distant lymph node metastasis (4, 24). Regarding to pathological features, BRAFV600E mutated CRCs are characterized by mucinous components, poor differentiation and highly aggressive behavior (14). In addition, BRAFV600E mutation is associated with MSI-H/dMMR status, and mutually exclusive with RAS mutations (25–27). However, few studies have described the clinical, pathological and molecular features of non-V600E mutations. We tried to summarize the similarities and differences between V600E and non-V600E mutations in our single institution in China. The frequency of non-V600E mutations was 27% (20/74) in our study, similar with the rates reported in other literatures (15). Patients with non-V600E mutations showed no difference in gender, family history, metastatic sites, CDX2 status or TMB level compared with patients with V600E mutation. Regarding primary tumor sidedness, some studies demonstrated that CRC with non-V600E mutations might be more often on left side, but others found no relation between sidedness and non-V600E mutations (15, 16, 28). In this study, we found most of non-V600E mutated CRC located on left colon and rectum, but due to the small sample size, the difference was not statistically significant. Besides, we found 4 cases (4/20, 20%) with concomitant presence of both BRAF non-V600E and RAS mutations, though BRAF mutation was thought to be mutually exclusive with RAS mutations. Jones et al. also reported that patients with non-V600E mutant CRC were more likely with concomitant RAS mutation (15). According to previous literatures, though some subtypes of BRAF mutants had impaired or no kinase activity, they might retain oncogenic function by co-expression with other mutations, such as RAS/EGFR mutations (29). In fact, some researchers have identified BRAF mutations as three classes according to their acting pattern and RAS dependency: class 1 (V600 mutations) is activated monomers when RAS activity is low; class 2 (codon 464, 469, 597 and 601) acts as a RAS-independent dimer; class 3 (codon 287, 459, 466, 467, 469, 581, 594, 595, and 596) acts as a dimer with impaired kinase activity, so the oncogenic potential is RAS-dependent (29, 30). This may explain the concomitant presence of BRAF non-V600E and RAS mutations in some cases.
It has been broadly demonstrated that BRAFV600E mutation is associated with poor prognosis of CRC patients regardless of stage (25, 31). According to our analysis, patients with non-V600E mutations had better OS than those with V600E mutation (p = 0.0239), especially for patients diagnosed at early stage. Shimada et al. reported V600E mutant type showed poorer OS than non-V600E mutant type after R0 resection (p = 0.038), which was consistent with our result. However, the prognostic value of non-V600E mutations is still controversial due to limited clinical data of this subgroup. Cremolini et al. found that some subtypes of non-V600E mutations (codon 594 and 596) might indicate a favorable outcome (28). More recently, Jones et al. reported a longer OS in patients with BRAF non-V600 mutations (60.7 months), which not only exceeded the OS of 11.4 months for patients with BRAFV600E mutation, but also the survival of 43.0 months for patients with wild-type BRAF gene (15). Besides, they explored if the kinase activity (which was discussed above) would influence the OS of BRAFnon−V600E mutant patients. It turned out there was no significant difference in OS for patients with activated vs. impaired kinase (p = 0.544) (15). Hence the non-V600E mutated CRC may be a totally different subtype of CRC regarding to prognostic value.
Though BRAFV600E mutation was associated with poorer survival in CRC, it has been observed that some patients with BRAFV600E mutation have a relatively poorer outcome than others. Loupakis et al. classified patients with BRAFV600E mutation as three different prognostic groups according to ECOG score, CA19-9 and LDH level, grade of tumor, status of metastasis (lung, liver and lymph nodes) (32). Prognosis of patients with BRAFV600E mutation could be related to MMR/MSI status, or some genetic events occurring in pathogenesis of CRC (29, 30, 33). Recently, it was reported that CDX2 might play a significant role in prognosis of CRC (34, 35). CDX2 is a transcription factor and a specific marker of differentiation of intestine, which could be used to identified tumors originating from intestine (36). Aasebo et al. reported that CDX2 expression, which accounts for 53% of patients with BRAF mutation in their study, was associated with much better prognosis (34). Our study also demonstrated that loss of CDX2 expression indicated worse survival in patients with BRAFV600E mutation (p = 0.016). Therefore, the loss of CDX2 expression may define a subgroup of poor prognosis in CRC patients, especially those with BRAFV600E mutation.
Though the prognostic value was widely discussed in many studies, the predictive role of BRAF mutation in CRC patients received chemotherapy or targeted therapy remains unclear. Some studies showed BRAFV600E mutated patients had worse PFS during first-line chemotherapy (10, 37); on the contrary, other studies reported that BRAF mutation was not associated with PFS of first-line treatment (8, 11, 38). The ambiguous results might depend on the small number of patients enrolled in the studies. Due to the aggressive behavior observed in BRAFV600E mutated CRC, intensive chemotherapy combined with targeted therapy was used in first-line treatment and proved to be effective (39). It has been reported that FOLFOXIRI plus bevacizumab showed an improved response rate and PFS compared to chemotherapy alone in BRAF mutated CRC (17). In our study, bevacizumab plus chemotherapy regimen had a better response rate and longer median PFS compared to chemotherapy alone in BRAFV600E mutated CRC patients, though the statistical significance was not reached. The response rate of bevacizumab plus FOLFOXIRI was 54.5%, which was an inspiring result considering the aggressiveness of BRAFV600E mutated CRC.
Regimens including specific inhibitors against BRAF mutation and other components of MAPK pathway were proved to be effective in second-line treatment. The phase II SWOG S1406 trial showed that combination of vemurafenib, irinotecan and cetuximab (the “VIC” regimen) for BRAFV600E mutant, RAS wild-type mCRC had an improved PFS compared with irinotecan plus cetuximab regimen (4.4 vs. 2.0 months) (18). Recently, the phase III BEACON trial proved an advantage of response rate and overall survival for combination of the BRAF inhibitor (encorafenib), MEK inhibitor (binimetinib) and cetuximab (20). Since MEK inhibitor was not available in China, we recorded only eight patients receiving the VIC regimen during second-line treatment. The ORR was 12.5% (1/8) and PFS was 2.9 months. The potential predictive value of different BRAF subtypes was less explored. Our studies showed that the subtypes of BRAF mutations had no significant impact on PFS during first-line or second-line treatment (p = 0.51 and 0.30, respectively).
There are some limitations of our study. First, it is a retrospective study and patients are from a single institution; hence selection bias inevitably exists. Most patients in this study were diagnosed at advanced stage, so the frequency of BRAF mutation in early staged CRC might be underestimated and its prognostic and predictive value is not clear. Second, given the rareness of BRAF mutation, especially non-V600E mutation, the sample size is too small to summarize the whole picture of CRC patients with BRAF mutation. Third, we were limited by lack of complete follow-up and treatment information for some patients.
Conclusion
In summary, the clinical and pathological features and outcomes of BRAF mutated CRC patients are heterogeneous. While BRAFV600E mutation is related with poor prognosis, non-V600E mutations define a subgroup of CRC patients with better outcome. Besides, some molecular basis like CDX2 status may affect the prognosis. So, it could be valuable to further classify BRAF mutated CRCs according to their molecular basis. The predictive value of BRAF mutation in CRC is still controversial; combination of different therapies may have better response compared to traditional chemotherapy. More efforts are needed to explore the molecular mechanism of BRAF mutation.
Data Availability Statement
The data from this study can be found at the following link: http://download.omicsbio.info/files/BRAF_mut/.
Ethics Statement
The studies involving human participants were reviewed and approved by ethics committee of Sun Yat-sen University Cancer Center. The patients provided written informed consent to participate in this study.
Author Contributions
M-ZQ, F-HW, and R-HX: study design. W-LG and M-ZQ: literature search and writing. W-LG, M-ZQ, L-QY, YJ, Z-QW, Y-HL, F-HW, and R-HX: data collecting. C-YH: gene mutations tested. W-LG, M-ZQ, and L-QY: data analysis, figure, and tables. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (Grant numbers. 82073377, 81772587); the third outstanding young talents training plan and Medical Scientist program of Sun Yat-sen University Cancer Center.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the patients and their families.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.563407/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Sepulveda AR, Hamilton SR, Allegra CJ, Greody W, Cushman-Vokoun AM, Funkhouser WK, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline summary from the American society for clinical pathology, college of American pathologists, association for molecular pathology, and American society of clinical oncology. J Oncol Pract. (2017) 13:333–7. doi: 10.1200/JOP.2017.022152
3. Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. (2015) 89:867–82. doi: 10.1007/s00204-015-1472-2
4. Chen D, Huang JF, Liu K, Zhang LQ, Yang Z, Chuai ZR, et al. BRAFV600E mutation and its association with clinicopathological features of colorectal cancer: a systematic review and meta-analysis. PLoS ONE. (2014) 9:e90607. doi: 10.1371/journal.pone.0090607
5. Chen D, Zhang LQ, Huang JF, Liu K, Chuai ZR, Yang Z, et al. BRAF mutations in patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS ONE. (2014) 9:e101354. doi: 10.1371/journal.pone.0101354
6. Kim TH, Park YJ, Lim JA, Ahn HY, Lee EK, Lee YJ, et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer. (2012) 118:1764–73. doi: 10.1002/cncr.26500
7. Sanchez JN, Wang T, Cohen MS. BRAF and MEK inhibitors: use and resistance in BRAF-mutated cancers. Drugs. (2018) 78:549–66. doi: 10.1007/s40265-018-0884-8
8. Seligmann JF, Fisher D, Smith CG, Richman SD, Elliott F, Brown S, et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: analysis from 2530 patients in randomised clinical trials. Ann Oncol. (2017) 28:562–8. doi: 10.1093/annonc/mdw645
9. Tiacci E, Trifonov V, Schiavoni G, Holmes A, Kern W, Martelli MP, et al. BRAF mutations in hairy-cell leukemia. N Engl J Med. (2011) 364:2305–15. doi: 10.1056/NEJMoa1014209
10. Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. (2009) 361:98–9. doi: 10.1056/NEJMc0904160
11. Tie J, Gibbs P, Lipton L, Christie M, Jorissen RN, Burgess AW, et al. Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAF(V600E) mutation. Int J Cancer. (2011) 128:2075–84. doi: 10.1002/ijc.25555
12. Ye ZL, Qiu MZ, Tang T, Wang F, Zhou YX, Lei MJ, et al. Gene mutation profiling in Chinese colorectal cancer patients and its association with clinicopathological characteristics and prognosis. Cancer Med. (2020) 9:745–56. doi: 10.1002/cam4.2727
13. Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. (2004) 116:855–67. doi: 10.1016/S0092-8674(04)00215-6
14. Fanelli GN, Dal Pozzo CA, Depetris I, Schirripa M, Brignola S, Biason P, et al. The heterogeneous clinical and pathological landscapes of metastatic Braf-mutated colorectal cancer. Cancer Cell Int. (2020) 20:30. doi: 10.1186/s12935-020-1117-2
15. Jones JC, Renfro LA, Al-Shamsi HO, Schrock AB, Rankin A, Zhang BY, et al. (Non-V600) BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J Clin Oncol. (2017). 35:2624–30. doi: 10.1200/JCO.2016.71.4394
16. Shinozaki E, Yoshino T, Yamazaki K, Muro K, Yamaguchi K, Nishina T, et al. Clinical significance of BRAF non-V600E mutations on the therapeutic effects of anti-EGFR monoclonal antibody treatment in patients with pretreated metastatic colorectal cancer: the biomarker research for anti-egfr monoclonal antibodies by comprehensive cancer genomics (BREAC) study. Br J Cancer. (2017) 117:1450–8. doi: 10.1038/bjc.2017.308
17. Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. (2014) 371:1609–18. doi: 10.1056/NEJMoa1403108
18. Kopetz S, McDonough SL, Morris VK, Lenz H-J, Magliocco AM, Atreya CE. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406) [abstract]. J Clin Oncol. (2017) 35:3505. doi: 10.1200/JCO.2017.35.15_suppl.3505
19. Hong DS, Morris VK, El Osta B, Sorokin AV, Janku F, Fu S, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. (2016) 6:1352–65. doi: 10.1158/2159-8290.CD-16-0050
20. van Cutsem E, Huijberts S, Grothey A, Yaeger R, Cuyle PJ, Elez E, et al. Binimetinib, encorafenib, and cetuximab triplet therapy for patients with BRAF V600E-mutant metastatic colorectal cancer: safety lead-in results from the phase III BEACON colorectal cancer study. J Clin Oncol. (2019) 37:1460–9. doi: 10.1200/JCO.18.02459
21. Qiu MZ, He CY, Lu SX, Guan WL, Wang F, Wang XJ, et al. Prospective observation: clinical utility of plasma Epstein-Barr virus DNA load in EBV-associated gastric carcinoma patients. Int J Cancer. (2020) 146:272–80. doi: 10.1002/ijc.32490
22. Zhang X, Shi Y, Song L, Shen C, Cai Q, Zhang Z, et al. Identification of mutations in patients with acquired pure red cell aplasia. Acta Biochim Biophys Sin. (2018) 50:685–92. doi: 10.1093/abbs/gmy052
23. Li M, Zhao BR, Liu SQ, An J, Deng PB, Han-Zhang H, et al. Mutational landscape and clonal diversity of pulmonary adenoid cystic carcinoma. Cancer Biol Ther. (2018) 19:898–903. doi: 10.1080/15384047.2018.1480296
24. Jang MH, Kim S, Hwang DY, Kim WY, Lim SD, Kim WS, et al. BRAF-mutated colorectal cancer exhibits distinct clinicopathological features from wild-type BRAF-expressing cancer independent of the microsatellite instability status. J Korean Med Sci. (2017) 32:38–46. doi: 10.3346/jkms.2017.32.1.38
25. Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. (2011) 117:4623–32. doi: 10.1002/cncr.26086
26. Lochhead P, Kuchiba A, Imamura Y, Liao X, Yamauchi M, Nishihara R, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. (2013) 105:1151–6. doi: 10.1093/jnci/djt173
27. Morkel M, Riemer P, Blaker H, Sers C. Similar but different: distinct roles for KRAS and BRAF oncogenes in colorectal cancer development and therapy resistance. Oncotarget. (2015) 6:20785–800. doi: 10.18632/oncotarget.4750
28. Cremolini C, Di Bartolomeo M, Amatu A, Antoniotti C, Moretto R, Berenato R, et al. BRAF codons 594 and 596 mutations identify a new molecular subtype of metastatic colorectal cancer at favorable prognosis. Ann Oncol. (2015) 26:2092–7. doi: 10.1093/annonc/mdv290
29. Yao Z, Yaeger R, Rodrik-Outmezguine VS, Tao A, Torres NM, Chang MT, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. (2017) 548:234–8. doi: 10.1038/nature23291
30. Yao Z, Torres Neilawattie M, Tao A, Gao Y, Luo L, Li Q, et al. BRAF mutants evade ERK-dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell. (2015) 28:370–83. doi: 10.1016/j.ccell.2015.08.001
31. Van Cutsem E, Köhne C-H, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. (2011) 29:2011–9. doi: 10.1200/JCO.2010.33.5091
32. Loupakis F, Intini R, Cremolini C, Orlandi A, Sartore-Bianchi A, Pietrantonio F, et al. A validated prognostic classifier for BRAF-mutated metastatic colorectal cancer: the ‘BRAF BeCool’ study. Eur J Cancer. (2019) 118:121–30. doi: 10.1016/j.ejca.2019.06.008
33. Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. (2005) 65:6063–9. doi: 10.1158/0008-5472.CAN-05-0404
34. Aasebo K, Dragomir A, Sundstrom M, Mezheyeuski A, Edqvist PH, Eide GE, et al. CDX2: a prognostic marker in metastatic colorectal cancer defining a better BRAF mutated and a worse KRAS mutated subgroup. Front Oncol. (2020) 10:8. doi: 10.3389/fonc.2020.00008
35. Loupakis F, Biason P, Prete AA, Cremolini C, Pietrantonio F, Pella N, et al. CK7 and consensus molecular subtypes as major prognosticators in (V600E)BRAF mutated metastatic colorectal cancer. Br J Cancer. (2019) 121:593–9. doi: 10.1038/s41416-019-0560-0
36. Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. (2003) 27:303–10. doi: 10.1097/00000478-200303000-00003
37. Souglakos J, Philips J, Wang R, Marwah S, Silver M, Tzardi M, et al. Prognostic and predictive value of common mutations for treatment response and survival in patients with metastatic colorectal cancer. Br J Cancer. (2009) 101:465–72. doi: 10.1038/sj.bjc.6605164
38. Kayhanian H, Goode E, Sclafani F, Ang JE, Gerlinger M, Gonzalez de Castro D, et al. Treatment and survival outcome of BRAF-mutated metastatic colorectal cancer: a retrospective matched case-control study. Clin Colorectal Cancer. (2018) 17:e69–76. doi: 10.1016/j.clcc.2017.10.006
39. Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo oncologico nord ovest. J Clin Oncol. (2007) 25:1670–6. doi: 10.1200/JCO.2006.09.0928
Keywords: BRAF, V600E, CDX2, colorectal cancer, prognosis
Citation: Guan W-L, Qiu M-Z, He C-Y, Yang L-Q, Jin Y, Wang Z-Q, Li Y-H, Xu R-H and Wang F-H (2020) Clinicopathologic Features and Prognosis of BRAF Mutated Colorectal Cancer Patients. Front. Oncol. 10:563407. doi: 10.3389/fonc.2020.563407
Received: 18 May 2020; Accepted: 16 September 2020;
Published: 23 November 2020.
Edited by:
Claudia Von Arx, Imperial College London, United KingdomReviewed by:
Feng Wei, Tianjin Medical University Cancer Institute and Hospital, ChinaMaxime Chénard-Poirier, Centre de Recherche du CHU de Québec, Canada
Copyright © 2020 Guan, Qiu, He, Yang, Jin, Wang, Li, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-Hua Wang, d2FuZ2ZoQHN5c3VjYy5vcmcuY24=
†These authors have contributed equally to this work
 Wen-Long Guan
Wen-Long Guan Miao-Zhen Qiu
Miao-Zhen Qiu Cai-Yun He
Cai-Yun He Li-Qiong Yang3
Li-Qiong Yang3 Yu-Hong Li
Yu-Hong Li Rui-Hua Xu
Rui-Hua Xu