
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 21 October 2020
Sec. Women's Cancer
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.556718
This article is part of the Research TopicQuality of Life in Breast Cancer Patients and SurvivorsView all 14 articles
 Marco Invernizzi1*†
Marco Invernizzi1*† Alessandro de Sire1,2†
Alessandro de Sire1,2† Lorenzo Lippi1
Lorenzo Lippi1 Konstantinos Venetis3,4
Konstantinos Venetis3,4 Elham Sajjadi3
Elham Sajjadi3 Francesca Gimigliano5
Francesca Gimigliano5 Alessandra Gennari6
Alessandra Gennari6 Carmen Criscitiello7
Carmen Criscitiello7 Carlo Cisari1,8
Carlo Cisari1,8 Nicola Fusco3,9
Nicola Fusco3,9Breast cancer fatigue (BCF) is a complex and multidimensional condition characterized by a persistent sense of physical and/or mental stiffness, resulting in a substantial impairment of health-related quality of life in breast cancer survivors. Aim of this prospective cohort study was to evaluate the feasibility and the effectiveness of a 4-week rehabilitation protocol on BCF, muscle mass, strength, physical performance, and quality of life in breast cancer (BC) survivors. We recruited adult BC women with a diagnosis of BCF, according to the International Classification of Diseases 10 criteria, referred to the Outpatient Service for Oncological Rehabilitation of a University Hospital. All participants performed a specific physical exercise rehabilitative protocol consisting of 60-min sessions repeated 2 times/week for 4 weeks. All outcomes were evaluated at the baseline (T0), at the end of the 4-week rehabilitation treatment (T1), and at 2 months follow up (T2). The primary outcome measure was the Brief Fatigue Inventory (BFI); secondary outcomes included: Fat-Free Mass and Fat Mass, assessed by Bioelectrical Impedance Analysis (BIA); Hand Grip Strength Test (HGS); Short Physical Performance Battery (SPPB); 10-meter walking test (10 MWT); 6-min walking test (6 MWT); European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ–C30). Thirty-six women (mean age: 55.17 ± 7.76 years) were enrolled in the study. Significant reduction of BCF was observed both after the 4-week rehabilitation treatment (T1) (BFI: 5.4 ± 1.6 vs. 4.2 ± 1.7; p = 0.004) and at the follow-up visit (T2) (BFI: 5.4 ± 1.6 vs. 4.4 ± 1.6; p = 0.004). Moreover, significant differences (p < 0.001) HGS, SPPB, 10 MWT, 6 MWT, and EORTC QLQ-C30 were found at T1, while at T2 all the outcome measures were significantly different (p < 0.05) from the baseline. The rehabilitation protocol seemed to be feasible, safe, and effective in reducing BCF, improving muscle mass and function, and improving HRQoL in a cohort of BC survivors. The results of this study could improve awareness of this underestimated disease, suggesting the definition of a specific therapeutic exercise protocol to reduce BCF.
Breast cancer is the most common cancer in women and one of the leading causes of cancer-related death worldwide (1). Owing to the advances in the clinical management of this tumor, the number of long-term survivors has progressively increased during the past four decades (1). In this scenario, health-related quality of life has become more and more important in the overall patients' outcome evaluation (2–6).
Cancer-related fatigue, also known as cancer fatigue, is a highly prevalent long-term side effect among breast cancer survivors (7, 8). This complex and multidimensional condition is clinically characterized by a persistent sense of physical, emotional, and/or cognitive stiffness, resulting in a substantial impairment of health-related quality of life (7, 9). The etiology of breast cancer fatigue (BCF) is poorly understood and probably related to mitochondrial dysfunction, inflammation, and increased reactive oxygen species production (8, 10, 11). However, the wide subjectivity of BCF hinders further research to explain its pathogenesis. Several risk factors have been identified so far, including low socioeconomic status, sleep disturbance, emotional stress, anxiety, physical inactivity, high body mass index (BMI), radical surgery, chemotherapy, and radiotherapy (8, 9). According to the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) guidelines, specific screening programs for BCF should be performed (7, 12). In this respect, the gold standard for the evaluation of BCF is self-reporting using scales, questionnaires, and/or inventories (13). Regrettably, the great heterogeneity in these diagnostic methods, coupled with the lack of widely adopted guidelines, represents a major limitation in the clinical management of BCF (13, 14).
Several types of interventions have been proposed to treat or reduce BCF, including counseling, psycho-education, physical and mind-body activity, massage therapy, acupuncture, music therapy, supplements (e.g., ginseng, vitamin D, psychostimulants), and physical exercise (13). Among these, the supervised physical exercise is supported by the strongest evidence of safety and effectiveness in reducing BCF (15–19). Nevertheless, the optimal exercise interventions scheme (i.e., type, combination, frequency, intensity, and duration) to reduce BCF remains controversial. The aim of this study was to evaluate the feasibility and effectiveness of a 4-week rehabilitation protocol on BCF reduction.
This prospective cohort study involved a consecutive series of breast cancer survivors suffering from BCF. All patients referred to the Outpatient Service for the Oncological Rehabilitation of the Physical Medicine and Rehabilitation Unit of University Hospital “Maggiore della Carità” in Novara, Italy over a 24-month period, from January 2018 to December 2019. The inclusion criteria were the following: (1) diagnosis of invasive breast cancer (2) surgery performed at least 12 months earlier; (3) diagnosis of cancer fatigue according to the International Classification of Diseases Tenth Revision (ICD-10) criteria. The exclusion criteria were the following: (1) anemia, defined as hemoglobin <9 g/dl; (2) severe thrombocytopenia, defined as platelets <100,000/mm3; (3) history of bleeding; (4) hypothyroidism without replacement therapy; (5) persistent insomnia; (6) central nervous system primary and/or metastatic tumors. Inclusion and exclusion criteria are summarized in Table 1. The study protocol was approved by the local Institutional Review Board and was compliant with the ethical guidelines of the responsible governmental agency. At the enrollment, all the participants were asked to carefully read and sign an informed written consent. The investigators provided to protect the privacy and the study procedures according to the Declaration of Helsinki.
All participants were subjected to a specific physical exercise rehabilitative protocol consisting of 10 min of warm-up, 40 min of aerobic exercise (e.g., walking, cycling, rowing) and strength training (e.g., light weightlifting), and 10 min of cool-down. Each session was repeated 2 times/week with at least 2 days of rest for 4 weeks, under the supervision of an experienced physical therapist. The study flow chart is shown in Figure 1. At the end of the rehabilitation treatment, a booklet encompassing the pictures and instructions of the previously performed exercises was provided to the patients. To maintain the benefits obtained during the hospital treatment, all patients were trained and strongly encouraged to continue the exercises at home. In the case of BCF evolution and/or worsening of general clinical conditions, the rehabilitation treatment was stopped.
At the baseline (T0), demographic and anthropometric characteristics, cancer location and staging, as well as pharmacologic history, have been assessed. All outcomes were also evaluated at the end of the 4-week rehabilitation treatment (T1), and at 2 months follow-up (T2).
The primary outcome measure was the Brief Fatigue Inventory (BFI), a multidimensional self-report scale that assesses the effects of fatigue on health-related quality of life originally reported by Mendoza et al. (20, 21). This survey is composed of nine questions scored on a 0–10 point scale. The BFI is presented as two parts. Specifically, the first three questions rate the current, usual, and worst levels of fatigue over the last 24 h, while the remaining six questions are related to the impact of fatigue on activity, mood, walking, work, relationships, and enjoyment of life. A total BFI score is then calculated by the mean of the nine scores, where scores 1–3 indicate slight fatigue, scores 4–6 moderate fatigue, and scores 7–10 severe fatigue.
The secondary outcomes were the following. (1) Body composition in terms of fat-free mass (FFM) and fat mass (FM) by bioelectrical impedance analysis (BIA). For this study, the BIA101 Anniversary (Akern Srl, Pontassieve, Florence, Italy) was used. BIA evaluations were performed with patients in a supine position, with the upper and lower limbs abducted by about 30 and 45 degrees, respectively. The electrodes were placed on hands and feet at a minimum distance of 5 cm and connected to the cable with the red insulated tweezers (distal) and black (proximal). FFM and FM were determined according to the equation elaborated by Kyle et al. (22). (2) Handgrip strength test (HGS), using the Jamar® hydraulic hand dynamometer (Sammons Preston, Rolyon, Bolingbrook, IL, USA) to assess the isometric grip strength of the hand, according to the American College of Sports Medicine recommendations (23). This measure strongly correlates with global muscle strength (24). Briefly, the test was conducted with the participant seated on a chair, the shoulder adducted and neutral for rotation, with the elbow flexed at 90°, the forearm neutral for prono-supination and wrist extension between 0 and 30° with 0–15 degrees of ulnar deviation. The test was repeated three times to obtain the mean. (3) Short physical performance battery (SPPB), a composite scale ranging from 0 to 12, assessing walking speed, standing balance, and sit-to-stand performance (25, 26). (4) Ten-meter walking test (10MWT) to assess walking speed (27). (5) 6-min walking test (6 MWT) for the integrated response of cardiopulmonary and musculoskeletal systems (28). (6) European Organization for Research and Treatment of Cancer 30-item quality of life questionnaire (EORTC QLQ–C30), a unidimensional scale that assesses the severity of symptoms related to cancer and its treatment, consisting of functional scales (i.e., physical, role, cognitive, emotional, and social functioning), a global quality of life scale, symptom scales (i.e., fatigue, nausea and vomiting, and pain), and global health (i.e., appetite loss, diarrhea, dyspnea, constipation, insomnia, financial impact) (29). Furthermore, at T1, both the enrolled patients and the physical therapist expressed their satisfaction regarding this treatment, which was assessed using the Global perceived effect (GPE) Scale, ranging from 1 (best satisfaction) to 7 (unsatisfaction) (30).
Statistical analyses were performed using GraphPad Prism®, version 7.00 (GraphPad Software, La Jolla California USA). Due to the low numerosity of the sample, we assumed a non-gaussian distribution of the considered variables, as previously described (31). Differences between single variables at different time-points were assessed by the two-way Friedman Analysis of Variance (ANOVA) for repeated measure and Dunn's post hoc test. A type I error level of 0.05 was chosen. A p-value lower than 0.05 was considered statistically significant.
A total of 102 women with BCF were assessed. Among them, 48 (47%) did not meet the eligibility criteria and 18 (18%) refused to sign the informed consent. Taken together, 36 patients were enrolled in the study. The study flowchart is depicted in Figure 1.
The mean age at diagnosis of the 36 patients included in this study was 55.17 ± 7.76 years. Most of the women were of normal weight or borderline overweight (BMI = 25.15 ± 5.52 kg/m2). The rate of smokers was similar to that of the general women population (n = 8, 22.2%). All of them underwent breast surgery, with equal distribution between conservative and radical surgery (n = 19, 52.7%, and n = 17, 47.3%, respectively). The en bloc axillary dissection was performed in 16 (44.4%) patients, while 21 (58.3%) were subjected to radiotherapy, either in the supraclavicular fossa or in the chest wall. Breast cancer related lymphedema was present in 12 (33.3) patients. The baseline characteristics along with the therapeutic information are listed in Table 2.
We observed a statistically significant reduction of the BCF score after the 4-week rehabilitation treatment (T1) compared to T0 (4.2 ± 1.7 vs. 5.4 ± 1.6; p = 0.004). Despite the small sample size, the significance was substantially maintained at the follow-up visit (T2) (4.4 ± 1.6; p = 0.004), as showed in Figure 2 and Table 3. However, no statistical significance was observed between the T1 and theT2 stage. Furthermore, we found significant differences at T1 in terms of HGS (20.1 ± 5.8 vs. 22.5 ± 5.2: p < 0.001), SPPB (9.3 ± 2.0 vs. 11.3 ± 1.2; p < 0.001), 10 MWT (1.5 ± 0.3 vs. 1.8 ± 0.3; p < 0.001), 6 MWT (464.5 ± 62.9 vs. 554.1 ± 71.6; p < 0.001), EORTC QLQ-C30 Functional score (69.2 ± 14.9 vs. 76.9 ± 15.7; p < 0.001), EORTC QLQ-C30 Symptoms score (29.2 ± 14.9 vs. 21.2 ± 16.0: p < 0.001), and EORTC QLQ-C30 Global Health score (40.7 ± 12.5 vs. 67.6 ± 14.8; p < 0.001). At 2 months (T2), all the outcome measures significantly differ from the baseline (p < 0.05), including FFM (43.2 ± 6.4 vs. 45.5 ± 6.6; p < 0.001) and FM (24.0 ± 10.6 vs. 21.7 ± 10.0; p < 0.001), as showed by Table 3. Moreover, the GPE score measured at T1 was 2.20 considering patients' perspective and 2.40 considering physical therapists' perspective.
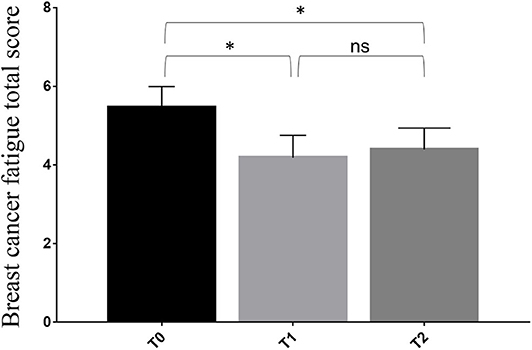
Figure 2. Differences in primary outcome measure from the baseline (T0) to the end of 4-week rehabilitation treatment (T1) and the follow-up assessment at 3 months from the baseline (T2). * p < 0.05; ns, non significant.
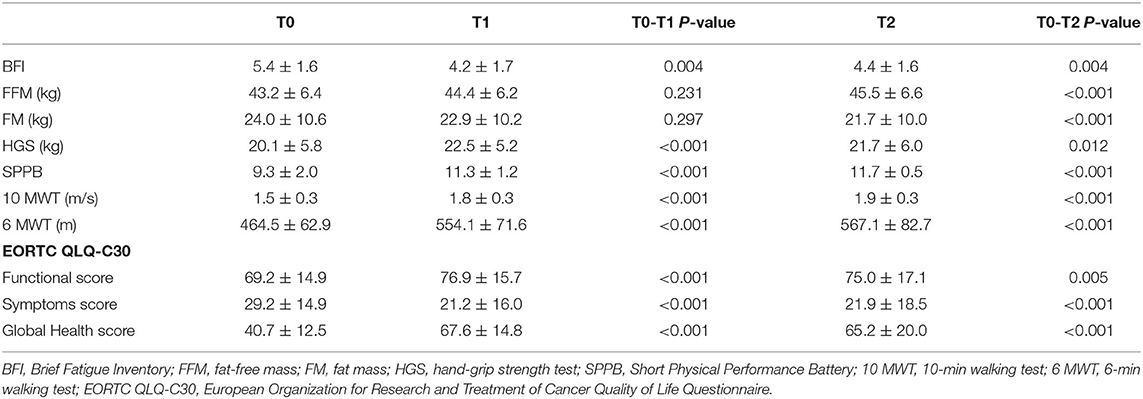
Table 3. Differences in outcome measures from baseline (T0) to the end of 4-week rehabilitation treatment (T1) and the follow-up assessment at 3 months from the baseline (T2).
Several exercise programs have been proposed to reduce BCF (17, 32–36). However, the choice of the most appropriate intervention to offer remains troubled in real-life clinical practice. Here, we subjected an exploratory cohort of breast cancer survivors suffering from BCF to a physical exercise rehabilitative protocol consisting of 10 min of warm-up, 40 min of aerobic exercise and strength training, and 10 min of cool-down, twice a week for 4 weeks. Taken together, a significant decrease of BCF was observed at the end of the program and was maintained at the follow-up visits.
To date, there is still little evidence about the multifactorial mechanisms underpinning BCF pathogenesis. Historically, the loss of muscle mass, metabolism disorders and ATP production impairment have been viewed as founder events (37). In our study, we noticed a significant improvement in the FFM and a significant reduction of the FM at a 2-months follow-up but not at T1, suggesting that muscle mass modifications need more time to manifest compared to the relatively fast improvement of all the functional outcomes assessed. These results highlight that rehabilitative physical exercise counters the main mechanisms underpinning BCF and might be considered as an effective and reliable treatment option. This notion, however, should be considered in the context of the small sample size investigated in the present work.
A recent randomized controlled trial investigated the effects of a specific training program to modulate systemic inflammation (38). After this intervention, serum levels of TNF-α, IL-6, and IL-10 were significantly lower in the intervention group, confirming the anti-inflammatory properties of physical exercise previously demonstrated in several pathological conditions (39–42) and suggesting a possible mechanism through which it intervenes in countering BCF clinical manifestations. Of note, given the lack of adverse events in our study group, we confirm the excellent safety profile of this physical exercise intervention. This approach proved to be feasible, considering the high treatment adherence (i.e., no dropouts) and the high GPE scores obtained by both patients and physical therapists.
The skeletal muscle system has been recently hypothesized to have a key role in fatigue pathogenesis (43, 44). Furthermore, there are multiple examples in literature on the direct mitochondria damage, inducing a dysfunction characterized by an increased intracellular oxidative stress and low energy supply (8, 45–48). Noteworthy, exercise training could remodel the mitochondrial network, influencing the mitochondria intrinsic plasticity through different mechanisms and modulating their shape in response to fission and fusion events (45, 47, 49–51). Furthermore, it has been recently proved that physical exercise is able to improve mitochondrial function and dynamics in fragile patients (51). Similarly, an endurance exercise protocol could have a key role in the prevention of muscle wasting by stimulating mitochondrial dynamics. Taken together, all these findings, coupled with our preliminary observations, could suggest that exercise therapy might have a crucial impact not only in the clinical and therapeutic management of BCF, but also interfering directly in its pathogenesis.
This study has several limitations. First, the relatively small sample size of women with BCF included in the study could have limited the clinical impact of our conclusions. It should be noted, however, that our pilot prospective study provides for the first time in literature evidence on the possible clinical application of a specific physical exercise rehabilitative treatment in this setting. Further prospective studies embracing larger cohorts of patients are warranted to define the implications of our observations. Second, due to the study design, we did not collect any data on bone mineral density, falls, and fracture rate. Indeed, a high prevalence (80.6%) of patients treated with aromatase inhibitor therapy, a well-known risk factor for osteoporosis (52), has been recruited in the present study. Considering the beneficial effects of physical exercise on bone mineral density in premenopausal and postmenopausal women (53), the improvement of all functional parameters that we observed after a 1-month protocol might constitute the basis for a possible role in contrasting osteoporosis, reducing the risk of falling and consequently the risk of fragility fractures. On the other hand, given that these women are at high risk of osteoporosis, it is mandatory to underline the role of an adequate therapeutic exercise to prevent fractures and all the disabling consequences. Third, the lack of a control group limits the translational relevance of our hypothesis. However, this study should be considered a proof-of-principle that rehabilitation interventions can be safety and effectively performed in breast cancer survivors. Lastly, we did not provide any data on long-term outcomes because all of the patients enrolled in this prospective study are still followed up by our multidisciplinary team.
Despite these limitations, we provide preliminary and previously unavailable evidence on the feasibility, reliability, and safety of a 1-month specific physical exercise rehabilitative protocol in reducing BCF, improving muscle mass, muscular-skeletal function, and health-related quality of life in breast cancer survivors. Our results advocate the need to define tailored physical exercise interventions that could be performed in common clinical practice as a first-line rehabilitative treatment to reduce BCF.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Comitato Etico Interaziendale Novara. The patients/participants provided their written informed consent to participate in this study.
MI and CCi: study concept and design. MI, CCi, and NF: supervision. MI and AS: manuscript writing (first draft). AS, LL, KV, FG, and AG: bibliography. MI: iconography. KV, ES, CCr, and NF: critical revision. All authors contributed to the article and approved the submitted version.
This research was partially funded by the Italian Ministry of Health with Ricerca Corrente funds.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Sabrina Pasqua, MD, Galliano Balloni, Alessia Marelli, and Stefano Rossi for their support.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. O'Higgins CM, Brady B, O'Connor B, Walsh D, Reilly RB. The pathophysiology of cancer-related fatigue: current controversies. Support Care Cancer. (2018) 26:3353–64. doi: 10.1007/s00520-018-4318-7
3. Michelotti A, Invernizzi M, Lopez G, Lorenzini D, Nesa F, De Sire A, et al. Tackling the diversity of breast cancer related lymphedema: perspectives on diagnosis, risk assessment, and clinical management. Breast. (2019) 44:15–23. doi: 10.1016/j.breast.2018.12.009
4. Triberti S, Savioni L, Sebri V, Pravettoni G. eHealth for improving quality of life in breast cancer patients: A systematic review. Cancer Treat Rev. (2019) 74:1–14. doi: 10.1016/j.ctrv.2019.01.003
5. de Sire A, Losco L, Cisari C, Gennari A, Boldorini R, Fusco N, et al. Axillary web syndrome in women after breast cancer surgery referred to an Oncological Rehabilitation Unit: which are the main risk factors? A retrospective case-control study. Eur Rev Med Pharmacol Sci. (2020) 24:8028–35. doi: 10.26355/eurrev_202008_22486
6. Nardin S, Mora E, Varughese FM, D'Avanzo F, Vachanaram AR, Rossi V, et al. Breast cancer survivorship, quality of life, and late toxicities. Front Oncol. (2020) 10:864. doi: 10.3389/fonc.2020.00864
7. Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical Oncology Clinical Practice Guideline Adaptation. J Clin Oncol. (2014) 32:1840–50. doi: 10.1200/JCO.2013.53.4495
8. Yang S, Chu S, Gao Y, Ai Q, Liu Y, Li X, et al. A narrative review of cancer-related fatigue (CRF) and its possible pathogenesis. Cells. (2019) 8:738. doi: 10.3390/cells8070738
9. Thong MSY, van Noorden CJF, Steindorf K, Arndt V. Cancer-related fatigue: causes and current treatment options. Curr Treat Options Oncol. (2020) 21:17. doi: 10.1007/s11864-020-0707-5
10. Gilliam LA, Moylan JS, Callahan LA, Sumandea MP, Reid MB. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve. (2011) 43:94–102. doi: 10.1002/mus.21809
11. Dirks-Naylor AJ, Tran NTK, Yang S, Mabolo R, Kouzi SA. The effects of acute doxorubicin treatment on proteome lysine acetylation status and apical caspases in skeletal muscle of fasted animals. J Cachexia Sarcopenia Muscle. (2013) 4:239–43. doi: 10.1007/s13539-013-0104-z
12. Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, et al. Cancer-related fatigue, version 2.2015. J Natl Compr Cancer Netw. (2015) 13:1012–39. doi: 10.6004/jnccn.2015.0122
13. Joly F, Lange M, Dos Santos M, Vaz-Luis I, Di Meglio A. Long-term fatigue and cognitive disorders in breast cancer survivors. Cancers. (2019) 11:1896. doi: 10.3390/cancers11121896
14. Wirtz P, Baumann FT. Physical activity, exercise and breast cancer - what is the evidence for rehabilitation, aftercare, and survival? A review. Breast Care. (2018) 13:93–101. doi: 10.1159/000488717
15. Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. (2012) 11:Cd006145. doi: 10.1002/14651858.CD006145.pub3
16. Meneses-Echavez JF, Gonzalez-Jimenez E, Ramirez-Velez R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. (2015) 15:77. doi: 10.1186/s12885-015-1069-4
17. Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, et al. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. (2016) 50:339–45. doi: 10.1136/bjsports-2015-094927
18. Juvet LK, Thune I, Elvsaas IKO, Fors EA, Lundgren S, Bertheussen G, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast. (2017) 33:166–77. doi: 10.1016/j.breast.2017.04.003
19. Wang R, Nakshatri H. Systemic actions of breast cancer facilitate functional limitations. Cancers. (2020) 12:194. doi: 10.3390/cancers12010194
20. Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. (1999) 85:1186–96. doi: 10.1002/(SICI)1097-0142(19990301)85:5<1186::AID-CNCR24>3.0.CO;2-N
21. Toh C, Li M, Finlay V, Jackson T, Burrows S, Wood FM, et al. The brief fatigue inventory is reliable and valid for the burn patient cohort. Burns. (2015) 41:990–7. doi: 10.1016/j.burns.2014.11.014
22. Kyle UG, Genton L, Karsegard L, Slosman DO, Pichard C. Single prediction equation for bioelectrical impedance analysis in adults aged 20–94 years. Nutrition. (2001) 17:248–53. doi: 10.1016/S0899-9007(00)00553-0
23. American College of Sports Medicine, Pescatello LS, Riebe D, Thompson PD. ACSM's Guidelines for Exercise Testing and Prescription. Philadelphia, PA: Wolters Kluwer Health (2014).
24. Porto JM, Nakaishi APM, Cangussu-Oliveira LM, Freire Junior RC, Spilla SB, Abreu DCC. Relationship between grip strength and global muscle strength in community-dwelling older people. Arch Gerontol Geriatr. (2019) 82:273–8. doi: 10.1016/j.archger.2019.03.005
25. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. (1994) 49:M85–94. doi: 10.1093/geronj/49.2.M85
26. Treacy D, Hassett L. The short physical performance battery. J Physiother. (2018) 64:61. doi: 10.1016/j.jphys.2017.04.002
27. Amatachaya S, Kwanmongkolthong M, Thongjumroon A, Boonpew N, Amatachaya P, Saensook W, et al. Influence of timing protocols and distance covered on the outcomes of the 10-meter walk test. Physiother Theory Pract. (2019) 1–6. doi: 10.1080/09593985.2019.1570577
28. Pera MC, Luigetti M, Pane M, Coratti G, Forcina N, Fanelli L, et al. 6MWT can identify type 3 SMA patients with neuromuscular junction dysfunction. Neuromuscul Disord. (2017) 27:879–82. doi: 10.1016/j.nmd.2017.07.007
29. Kasper B. The EORTC QLQ-C30 summary score as a prognostic factor for survival of patients with cancer: a commentary. Oncologist. (2020) 25:e610–1. doi: 10.1634/theoncologist.2019-0749
30. Meisingset I, Stensdotter AK, Woodhouse A, Vasseljen O. Predictors for global perceived effect after physiotherapy in patients with neck pain: an observational study. Physiotherapy. (2018) 104:400–7. doi: 10.1016/j.physio.2017.01.007
31. Invernizzi M, Corti C, Lopez G, Michelotti A, Despini L, Gambini D, et al. Lymphovascular invasion and extranodal tumour extension are risk indicators of breast cancer related lymphoedema: an observational retrospective study with long-term follow-up. BMC Cancer. (2018) 18:935. doi: 10.1186/s12885-018-4851-2
32. Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. (2005) 293:2479–86. doi: 10.1001/jama.293.20.2479
33. Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. (2010) 42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112
34. Brown JC, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. (2011) 20:123–33. doi: 10.1158/1055-9965.EPI-10-0988
35. Mangone M, Bernetti A, Agostini F, Paoloni M, De Cicco FA, Capobianco SV, et al. Changes in spine alignment and postural balance after breast cancer surgery: a rehabilitative point of view. Biores Open Access. (2019) 8:121–8. doi: 10.1089/biores.2018.0045
36. Paolucci T, Bernetti A, Paoloni M, Capobianco SV, Bai AV, Lai C, et al. Therapeutic alliance in a single versus group rehabilitative setting after breast cancer surgery: psychological profile and performance rehabilitation. Biores Open Access. (2019) 8:101–10. doi: 10.1089/biores.2019.0011
37. Morrow GR, Andrews PL, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Support Care Cancer. (2002) 10:389–98. doi: 10.1007/s005200100293
38. Alizadeh AM, Isanejad A, Sadighi S, Mardani M, Kalaghchi B, Hassan ZM. High-intensity interval training can modulate the systemic inflammation and HSP70 in the breast cancer: a randomized control trial. J Cancer Res Clin Oncol. (2019) 145:2583–93. doi: 10.1007/s00432-019-02996-y
39. Nimmo MA, Leggate M, Viana JL, King JA. The effect of physical activity on mediators of inflammation. Diabetes Obes Metab. (2013) 15 (Suppl. 3):51–60. doi: 10.1111/dom.12156
40. Khosravi N, Stoner L, Farajivafa V, Hanson ED. Exercise training, circulating cytokine levels and immune function in cancer survivors: a meta-analysis. Brain Behav Immun. (2019) 81:92–104. doi: 10.1016/j.bbi.2019.08.187
41. de Sire A, de Sire R, Petito V, Masi L, Cisari C, Gasbarrini A, et al. Gut–joint axis: the role of physical exercise on gut microbiota modulation in older people with osteoarthritis. Nutrients. (2020) 12:574. doi: 10.3390/nu12020574
42. Szymura J, Kubica J, Wiecek M, Pera J. The immunomodulary effects of systematic exercise in older adults and people with Parkinson's Disease. J Clin Med. (2020) 9:184. doi: 10.3390/jcm9010184
43. Invernizzi M, de Sire A, Carda S, Venetis K, Renò F, Cisari C, et al. Bone muscle crosstalk in spinal cord injuries: pathophysiology and implications for patients' quality of life. Curr Osteoporos Rep. (2020) 18:422–31. doi: 10.1007/s11914-020-00601-7
44. Invernizzi M, de Sire A, Renò F, Cisari C, Runza L, Baricich A, et al. Spinal cord injury as a model of bone-muscle interactions: therapeutic implications from in vitro and in vivo studies. Front Endocrinol. (2020) 11:204. doi: 10.3389/fendo.2020.00204
45. White JP, Puppa MJ, Sato S, Gao S, Price RL, Baynes JW, et al. IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+ mouse. Skelet Muscle. (2012) 2:14. doi: 10.1186/2044-5040-2-14
46. Argiles JM, Lopez-Soriano FJ, Busquets S. Muscle wasting in cancer: the role of mitochondria. Curr Opin Clin Nutr Metab Care. (2015) 18:221–5. doi: 10.1097/MCO.0000000000000164
47. Invernizzi M, Rizzi M, Carda S, Cisari C, Molinari C, Reno F. Mini invasive skeletal muscle biopsy technique with a tri-axial end cut needle. Eur Rev Med Pharmacol Sci. (2015) 19:2446–51.
48. Penna F, Ballaro R, Costelli P. The redox balance: a target for interventions against muscle wasting in cancer cachexia? Antioxid Redox Signal. (2020) 33:542–58. doi: 10.1089/ars.2020.8041
49. Invernizzi M, Carda S, Cisari C. Possible synergism of physical exercise and ghrelin-agonists in patients with cachexia associated with chronic heart failure. Aging Clin Exp Res. (2014) 26:341–51. doi: 10.1007/s40520-013-0186-7
50. Idorn M, Thor Straten P. Exercise and cancer: from “healthy” to “therapeutic”? Cancer Immunol Immunother. (2017) 66:667–71. doi: 10.1007/s00262-017-1985-z
51. Casuso RA, Huertas JR. The emerging role of skeletal muscle mitochondrial dynamics in exercise and ageing. Ageing Res Rev. (2020) 58:101025. doi: 10.1016/j.arr.2020.101025
52. Grizzi G, Ghidini M, Botticelli A, Tomasello G, Ghidini A, Grossi F, et al. Strategies for increasing the effectiveness of aromatase inhibitors in locally advanced breast cancer: an evidence-based review on current options. Cancer Manag Res Vol. (2020) 12:675–86. doi: 10.2147/CMAR.S202965
Keywords: breast cancer, quality of life, rehabilitation, fatigue, muscle strength, muscle performance, precision medicine
Citation: Invernizzi M, de Sire A, Lippi L, Venetis K, Sajjadi E, Gimigliano F, Gennari A, Criscitiello C, Cisari C and Fusco N (2020) Impact of Rehabilitation on Breast Cancer Related Fatigue: A Pilot Study. Front. Oncol. 10:556718. doi: 10.3389/fonc.2020.556718
Received: 28 April 2020; Accepted: 17 September 2020;
Published: 21 October 2020.
Edited by:
San-Gang Wu, First Affiliated Hospital of Xiamen University, ChinaReviewed by:
Deniz Can Guven, Hacettepe University, TurkeyCopyright © 2020 Invernizzi, de Sire, Lippi, Venetis, Sajjadi, Gimigliano, Gennari, Criscitiello, Cisari and Fusco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Invernizzi, bWFyY28uaW52ZXJuaXp6aUBtZWQudW5pdXBvLml0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.