- 1The Comprehensive Cancer Centre of Drum Tower Hospital, Medical School of Nanjing University & Clinical Cancer Institute of Nanjing University, Nanjing, China
- 2Department of General Surgery, Drum Tower Hospital, Nanjing, China
- 3Department of Pathology, Drum Tower Hospital, Nanjing, China
- 4Department of Radiology, Drum Tower Hospital, Nanjing, China
- 5The Medical Department, 3D Medicines Inc., Shanghai, China
Programmed death 1(PD-1) blockade has shown promising efficacy in advanced gastric cancer. Here, we performed a retrospective analysis of three patients with locally advanced gastric cancer who received adjuvant PD-1 plus chemoradiotherapy as neoadjuvant treatment. Neoadjuvant sintilimab plus concurrent chemoradiotherapy had an acceptable side-effect profile. All three patients underwent surgical gastrectomy after a median of 3.9 months. A major pathological response occurred in two resected tumors and a pathologic complete response was observed in one patient. Our results suggest that PD-1 blockade combined with chemoradiotherapy is a promising strategy as a neoadjuvant therapy in patients with unresectable locally advanced gastric cancer.
Introduction
Gastric cancer is one of the most common malignant tumors in the digestive system. The incidence of gastric cancer is higher in Asian countries compared with other regions around the world (1). Surgery remains the best therapy for gastric cancer with resectable tumor. But for the potentially resectable and unresectable patients, preoperative chemoradiation is a recommended option (2). Neoadjuvant chemoradiotherapy may improve overall resectability rate and the complete surgical resection rate of the primary tumor. More specifically, neoadjuvant chemoradiotherapy may eliminate latent micrometastases and reduce the risk of post-surgical recurrence. Thus chemoradiotherapy is regarded as one of the standard treatment options for preoperative treatment of locally advanced gastric carcinoma (3).
Immunotherapy drugs like PD-1/L1 inhibitors have shown promising efficacy in advanced gastric cancer as monotherapy or combined with chemotherapy (4, 5). Recently, some studies have reported that patients with solid cancer exhibited promising response rate to neoadjuvant immune checkpoint blockade. A phase I study has shown promising efficacy of neoadjuvant nivolumab in resectable non-small cell lung cancer with major pathological response rate of 45% (6). In addition, neoadjuvant ipilimumab and nivolumab treatment has also shown to be effective in high-risk resectable melanoma with pathologic complete response rate of 45% (7). Though it has been well established in some solid cancer, the neoadjuvant immunotherapy is a newer approach in Asian gastric cancer patients. Importantly, it has been reported that synergistic effects can be observed when anti-PD-1/L1 antibodies are used in combination with chemotherapy and/or radiotherapy (8). Here, we report the case series of three patients with locally advanced gastric cancer who exhibited a major pathological response to neoadjuvant PD-1 blockade combined with chemoradiotherapy.
Materials and Methods
Immunohistochemistry (IHC) Staining
Programmed death ligand-1 (PD-L1) staining using Ventana PD-L1 (SP263) kit (Roche, USA) was performed on a BenchMark XT system (Roche, USA), and the specimen was then counterstained with hematoxylin and coverslipped, according to the manufacturer’s instructions.
Multiplexed IHC (mIHC) Staining
mIHC was used to identify the immune cell subsets in the tumor immune microenvironment. mIHC staining was performed using PANO7-plex IHC kit (Panovue, Beijing, China), according to the manufacturer’s instructions. Different antibodies were used to identify the immune cells, including anti-CD8 (CST70306, USA), anti-CD56 (CST3576, USA), anti-CD68 (BX50031, China), anti-HLA-DR (ab92511, USA), anti-panCK (CST4545, USA), and anti-S100 (ab52642, USA). S100 staining was used to define the invasive margin and tumor parenchyma. The invasive margin is defined as the region centered on the border separating the normal part from the malignant part, with an extent of 1 mm. The tumor parenchyma corresponds to all the tissue inside the invasive margin. The Mantra System (Akoya, USA) was used to scan the stained slides and build image. The inForm software (Akoya, USA) was used for image analysis.
Case Presentation
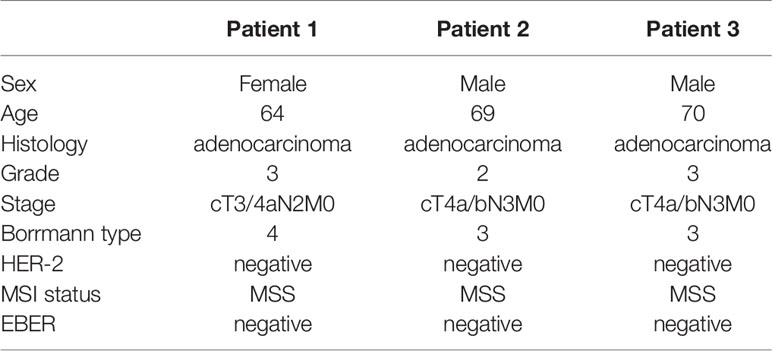
We identified three patients with locally advanced gastric cancer in our hospital from July, 2018 to February, 2019, treated with neoadjuvant anti-PD-1 therapy plus chemoradiotherapy. All of the three patients were gastric adenocarcinoma, MSS status, HER2 negative, and EBV negative. The TNM stage for the three patients was cT3/4aN2M0 with hepatogastric lymph node metastasis (Bulky N2) (Borrmann type 4), cT4a/bN3M0 (Borrmann type 3), cT4a/bN3M0 (Borrmann type 3), respectively. The baseline characteristics for the three patients are summarized in Table 1.
The three patients were treated with the same therapy. The selected therapeutic regimen consisted of one cycle of PD-1 blockade (sintilimab, 200 mg, on day 1), S-1 (40 mg/m2, twice daily, day 1 to 14), and Nab-PTX (100–120 mg/m2, on day 1 and 8), one cycle of sintilimab (200 mg, every 3 weeks) and weekly Nab-PTX (80–100 mg/m2), in combination with concurrent radiotherapy (RT) (45 Gy in 25 fractions), and another cycle of sintilimab, S-1, and Nab-PTX at the same dose used in the first cycle. After treatment above all, surgery was decided by Multiple Disciplinary Team (MDT). Another three cycles of sintilimab, S-1, and Nab-PTX was used after surgery. The timeline of the clinical treatment was shown in Figure 1A (Figure 1A). The three patients suffered from neutropenia with grade from 2 to 3. Patient 2 suffered from grade 2 rash and patient 3 suffered from grade 1 anorexia and grade 1 non-immunotherapy related pneumonitis. No deaths or discontinuation due to adverse events occurred. So neoadjuvant sintilimab plus concurrent chemoradiotherapy presented an acceptable side-effect profile.
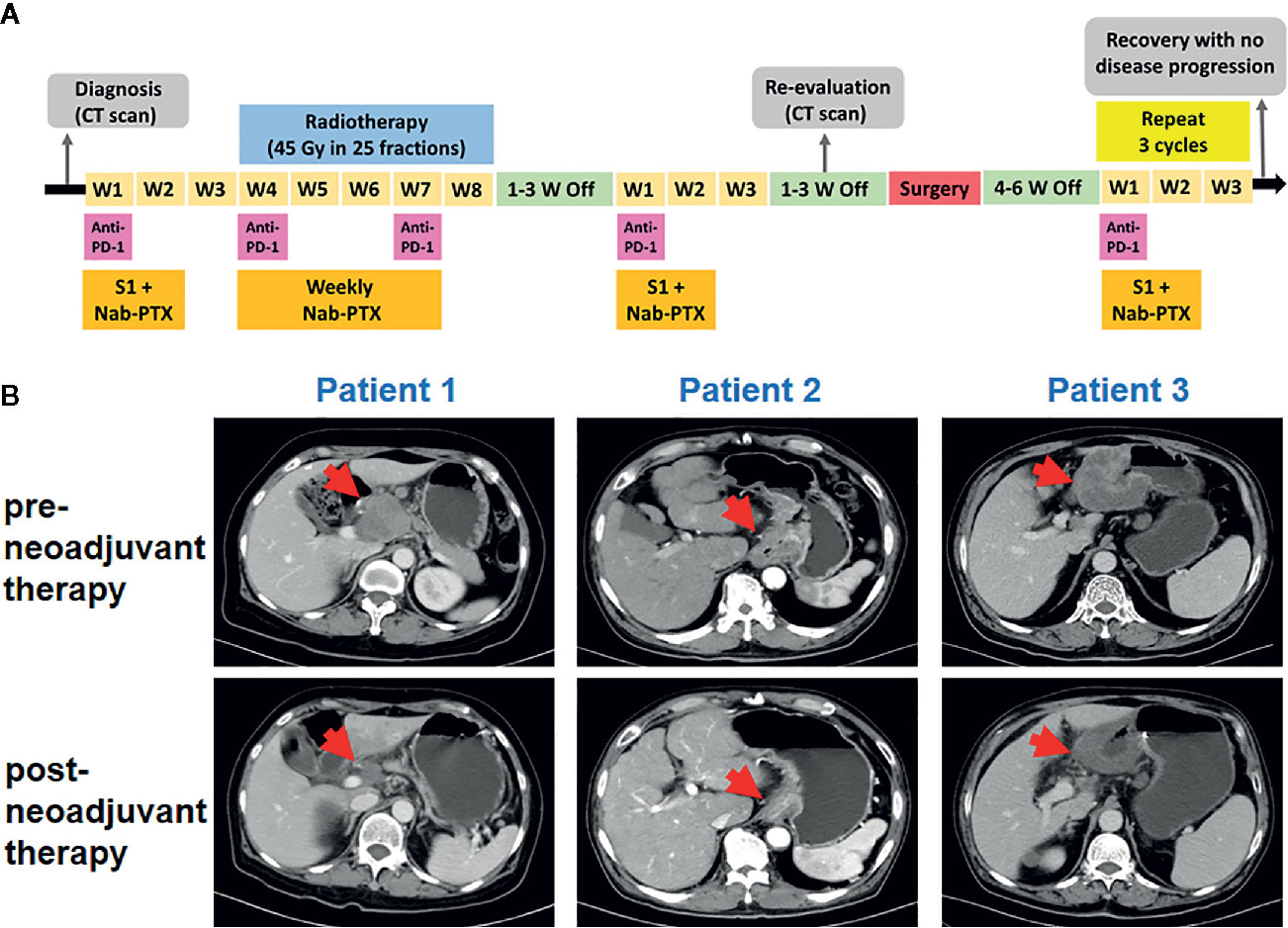
Figure 1 Tumor responses after neoadjuvant therapy. (A) Timeline of disease status and corresponding treatment regimens. W, week. (B) Tumor lesion of pre- and post-neoadjuvant therapy for three patients.
Pre- and post-neoadjuvant therapy, the computed tomography (CT) was performed in these three patients (Figure 1B). For patient 1, the CT had indicated significant reduction of the primary tumor and lymph nodes. For patient 2, tumor and metastatic lymph node volumes were clearly reduced upon CT examination. For patient 3, efficacy evaluation using CT revealed a partial response. All the three patients received surgical D2 gastrectomy. Post-operative pathology showed that a major pathological response occurred in two resected tumors (patient 1 and patient 3) and a pathologic complete response was observed in patient 2. The final TNM stage was ypT3N0M0, ypT0N0M0, and ypT3N2M0, respectively.
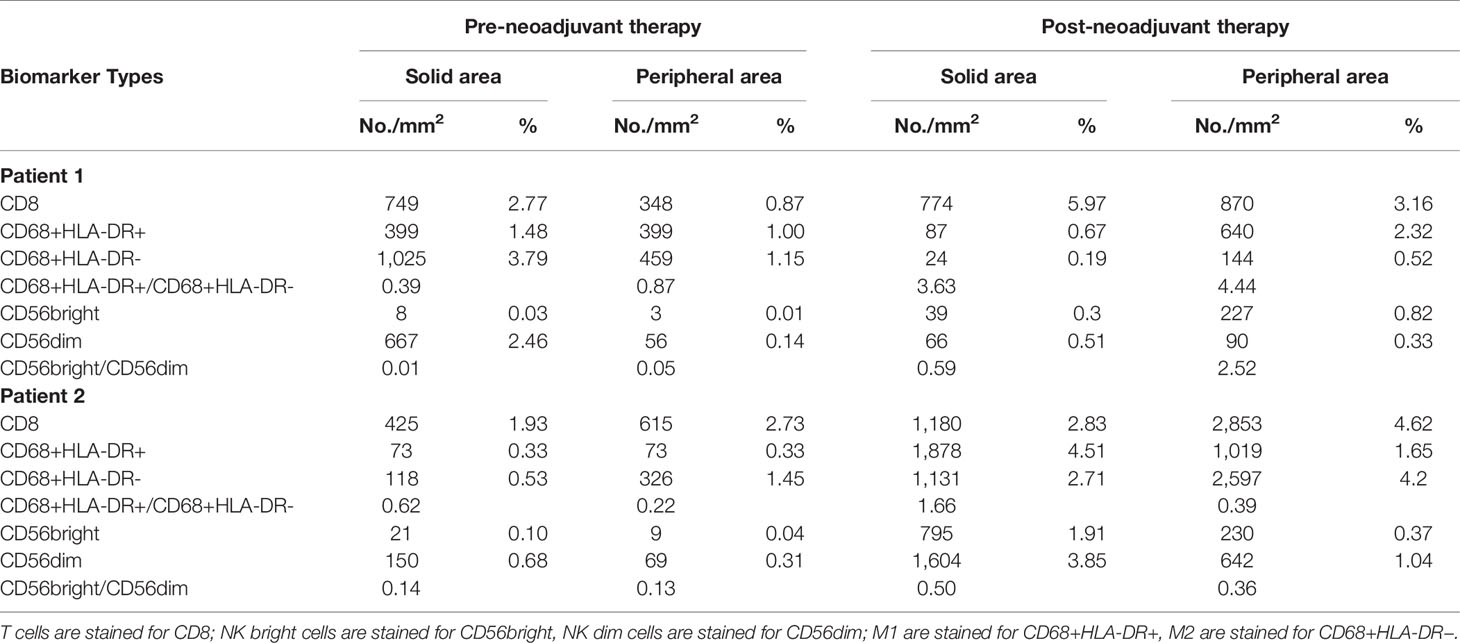
Pre- and post-neoadjuvant therapy immune microenvironment biomarkers were assessed in two patients (patient 1 and patient 2) with adequate tumor tissues (biopsy samples and surgical samples) (Figure 2). PD-L1 expression on immune cells (ICs), CD8+ T cells, NK cells, and tumor-associated macrophages (TAMs) were analyzed. For patient 1, pre- and post-neoadjuvant therapy, PD-L1 was expressed on 20% and 10% of ICs. For patient 2, pre- and post-neoadjuvant therapy, PD-L1 was Neoadjuvant therapy of gastric cancer expressed on 40% and 80% of ICs. NK cells were identified using the CD56 marker, and were divided into two categories according to the intensity of membrane staining for the CD56 protein: CD56dim (weak staining) and CD56bright (strong staining). TAMs were identified by CD68 and HLA-DR, and were divided into two categories: type M1 (CD68+ and HLA-DR+) and type M2 (CD68+ and HLA-DR−). These distinctions were made according to the existence of HLA-DR membrane staining. Biomarker analyses are summarized in Table 2. For patient 1, biomarker analyses suggested that CD8+ T cells, the ratio of NK bright/NK dim cells and M1/M2 was increased post-neoadjuvant therapy (Table 2) both in center and the invasive margin of tumor. Similar results were observed for patient 2 that the increased population of CD8+ T cells, the increased ratio of NK bright/NK dim cells and M1/M2 post-neoadjuvant therapy (Table 2). Taken together, these results suggested that the combination of PD-1 blockade and chemoradiotherapy would increase the immune-cell infiltration and the increase of immune-cell infiltration might be associated with the response to PD-1 blockade plus chemoradiotherapy.
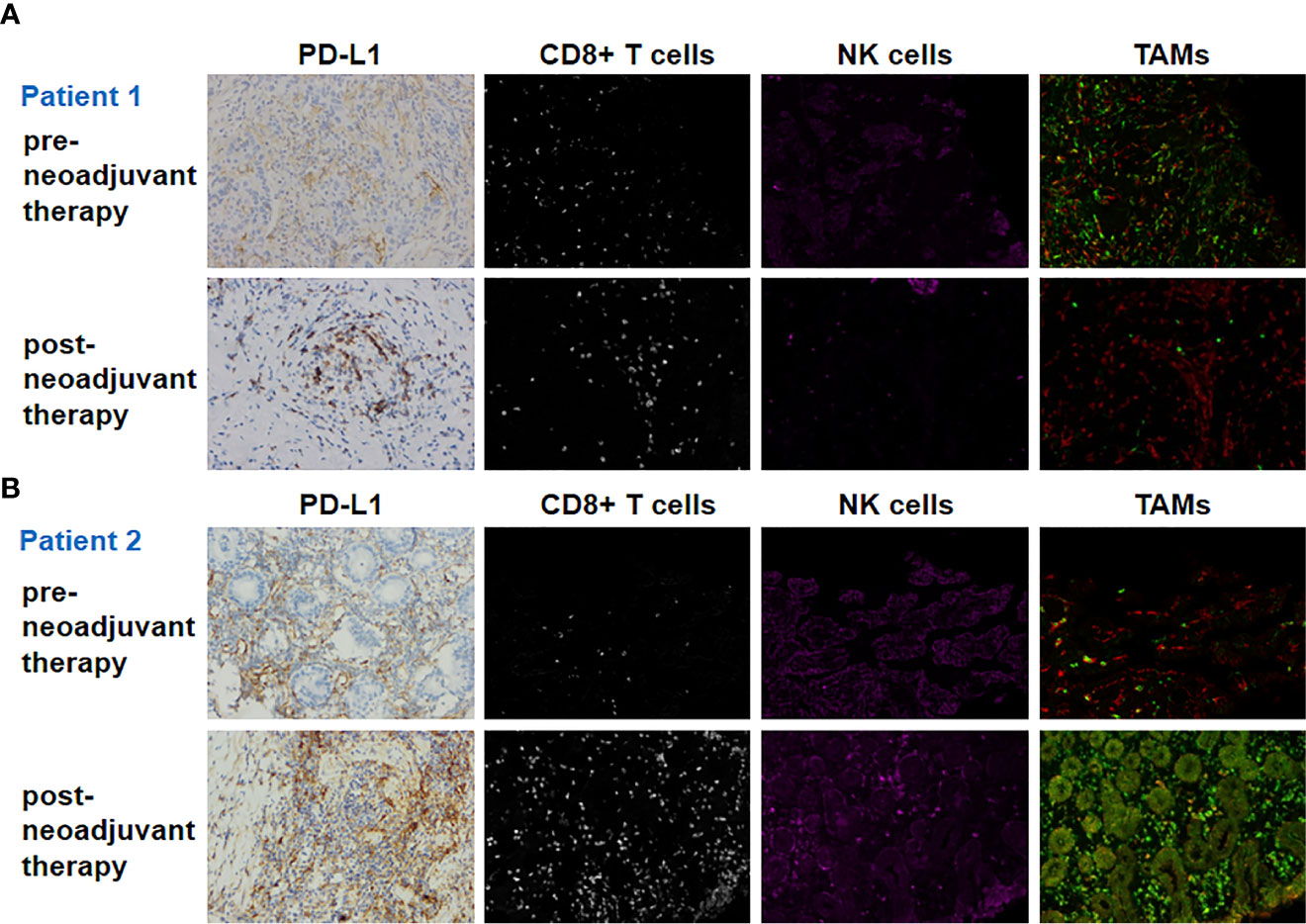
Figure 2 Biomarker findings of the tumor tissue samples. PD-L1, CD8+ T cells, NK cells, and TAMs (tumor-associated macrophages) of pre- and post-neoadjuvant therapy for patient 1 (A) and patient 2 (B). Original magnification ×200.
Discussion
In the present study, we demonstrated three patients with potentially unresectable locally advanced gastric cancer who received neoadjuvant PD-1 blockade plus concurrent chemoradiotherapy. Our results suggest neoadjuvant PD-1 blockade plus concurrent chemoradiotherapy may be efficacious and tolerable in potentially unresectable locally advanced gastric cancer. The increase of immune-cell infiltration may be associated with the response of PD-1 blockade plus concurrent chemoradiotherapy.
Anti-PD-1/PD-L1 therapies have been approved for advanced gastric cancer and further the Checkmate 649 study has shown that Nivolumab plus chemotherapy as first-line treatment for advanced gastric cancer can prolong the overall survival when compared with chemotherapy (5). Meanwhile, anti-PD-1/PD-L1 therapies have shown promising clinical benefit in non-metastasis stages of several cancers (9). The Checkmate 577 study showed that Nivolumab can be used as adjuvant therapy in resected esophageal or gastroesophageal junction cancer (GEJ) (10). For the unresectable non-small-cell lung cancer, the PACIFIC study showed that concurrent chemoradiotherapy followed by anti-PD-L1 drugs prolonged the overall survival when compared with placebo (11). One phase I/II study evaluated the efficacy of avelumab in combination with chemoradiation treatment in stage II/III resectable esophageal and GEJ cancer (12). The chemotherapy drugs and radiation kill tumor cells and promote the release of tumor antigens. This approach may result in a synergistic effect with immuno-checkpoint inhibitors in various therapies targeting malignancies (13). In the cases presented here, the clinical stages of patients were either cT3-4N2 or cT4N3. Regardless, one patient had a pCR and the remaining two patients had MPRs. Even though the final TNM stage for patient 3 was T3N2M0, only a little number of tumor cells was observed in primary tumor and lymph nodes (Supplemental Figures 1A, B). Besides, the follow-up had been lasting and until the case series were written, all the three patients were disease free. These results show the promising efficacy of neoadjuvant PD-1 blockade plus concurrent chemoradiotherapy in potentially unresectable locally advanced gastric cancer patients.
The tumor immune microenvironment could reflect the immune response, and changes in the numbers of CD8+ T cells, cancer-associated macrophages, and NK cells infiltrating in the tumor immune microenvironment correlate with clinical outcomes in various malignancies, including gastric cancer, melanoma, lung cancer, and others (14). And studies had also showed that PD-L1 expression and tumor-infiltrating lymphocytes were correlated with pCR in neoadjuvant therapy (15, 16). We found a significant elevation of CD8+ T cells and PD-L1 positivity of ICs after neoadjuvant therapy in patient 2, who achieved pCR after treatment. Several studies have suggested that the density of CD8+ T cells within tumor was higher in PD-1 inhibitor responders than non-responders. Moreover, the CD8+ T cell density in responders was increased during the treatment (17–19). In line with our findings, some studies have indicated that the pathologic and clinical responses of neoadjuvant PD-1 blockade therapy were associated with accumulation of exhausted CD8+ T cells (20, 21). Besides, we also observed the increase of M1/M2 macrophages ratio in patient 1 and 2. M1 macrophages were considered to be activated to participate in anti-tumor immune responses (22). And the ratio of M1/M2 macrophages in an ipilimumab response group have been shown to be significantly higher than that in a non-response group (23).
Our findings could provide a reference for the use of neoadjuvant PD-1 blockade plus concurrent chemoradiotherapy in patients with potentially unresectable locally advanced gastric cancer patients. However, there are some limitations such as the lack of control cases (neoadjuvant treatment with only chemoradiotherapy). In the future, prospective clinical studies with larger samples are required to validate the clinical activity of PD-1 blockade plus concurrent chemoradiotherapy in gastric cancer.
Ethics Statement
This case series was approved by the local ethical committee. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JW, WG, and BL conceived the experiments. JW performed most of the experiments. XL, QL, LL, SL, FL, YF, XF, YYA, CQ, and YYU helped with experiments. JW, WG, and BL analyzed the data and wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by grants from the National Major Projects for “Major New Drugs Innovation and Development”(No.2019ZX09301-150) and Fund for Distinguished Young Scholars of Jiangsu Province (BK20190001).
Conflict of Interest
Authors CQ and YYY were employed by the company 3D Medicines Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.554040/full#supplementary-material
Supplementary Figure 1 | Hematoxylin and eosin (H&E) staining of patient 3. (A) Primary gastric lesions; (B) Tumor cells in mucus pool.
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: gastric cancer (version 3.2020). Available at: https://www.nccn.org/professionals/physician_gls/default.aspx.
3. Petrillo A, Pompella L, Tirino G, Pappalardo A, Laterza MM, Caterino M, et al. Perioperative Treatment in Resectable Gastric Cancer: Current Perspectives and Future Directions. Cancers (Basel) (2019) 11:399. doi: 10.3390/cancers11030399
4. Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer (2020) 23:565–78. doi: 10.1007/s10120-020-01090-4
5. Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, et al. Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann Oncol (2020) 31(suppl_4):S1142–215. doi: 10.1016/annonc/annonc325
6. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med (2018) 378:1976–86. doi: 10.1056/NEJMoa1716078
7. Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med (2018) 24:1649–54. doi: 10.1038/s41591-018-0197-1
8. Kordbacheh T, Honeychurch J, Blackhall F, Faivre-Finn C, Illidge T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: building better translational research platforms. Ann Oncol (2018) 29:301–10. doi: 10.1093/annonc/mdx790
9. Vansteenkiste J, Wauters E, Reymen B, Ackermann CJ, Peters S, De Ruysscher D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann Oncol (2019) 30:1244–53. doi: 10.1093/annonc/mdz175
10. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Cutsem EV, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer following neoadjuvant chemoradiation therapy: first results of the CheckMate 577 study. Ann Oncol (2020) 31(suppl_4):S1142–215. doi: 10.1016/annonc/annonc325
11. Gray JE, Villegas AE, Daniel DB, Vicente D, Murakami S, Hui R, et al. Three-year overall survival update from the PACIFIC trial. J Clin Oncol (2019) 37(15_suppl):8526–6. doi: 10.1200/JCO.2019.37.15_suppl.8526
12. Uboha NV, Maloney JD, McCarthy D, Deming DA, LoConte NK, Matkowskyj K, et al. Safety of neoadjuvant chemoradiation (CRT) in combination with avelumab (A) in the treatment of resectable esophageal and gastroesophageal junction (E/GEJ) cancer. J Clin Oncol (2019) 37(15_suppl):4041–1. doi: 10.1200/JCO.2019.37.15_suppl.4041
13. Seliger B. Combinatorial Approaches With Checkpoint Inhibitors to Enhance Anti-tumor Immunity. Front Immunol (2019) 10:999. doi: 10.3389/fimmu.2019.00999
14. Zeng DQ, Li MY, Zhou R, Zhang JW, Sun HY, Shi M, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res (2019) 7:737–50. doi: 10.1158/2326-6066.CIR-18-0436
15. Fassan M, Cavallin F, Guzzardo V, Kotsafti A, Scarpa M, Cagol M, et al. PD-L1 expression, CD8+ and CD4+ lymphocyte rate are predictive of pathological complete response after neoadjuvant chemoradiotherapy for squamous cell cancer of the thoracic esophagus. Cancer Med (2019) 8:6036–48. doi: 10.1002/cam4.2359
16. Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res (2015) 3:326–32. doi: 10.1158/2326-6066.CIR-14-0133
17. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature (2014) 515:568–71. doi: 10.1038/nature13954
18. Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov (2016) 6:827–37. doi: 10.1158/2159-8290.CD-15-1545
19. Fumet JD, Richard C, Ledys F, Klopfenstein Q, Joubert P, Routy B, et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer (2018) 119:950–60. doi: 10.1038/s41416-018-0220-9
20. Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med (2018) 24:1655–61. doi: 10.1038/s41591-018-0198-0
21. Huang AC, Orlowski RJ, Xu XW, Mick R, George SM, Yan PK, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med (2019) 25:454–61. doi: 10.1038/s41591-019-0357-y
22. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217
Keywords: chemoradiotherapy, locally advanced gastric cancer, neoadjuvant, PD-1 inhibitor, immune microenvironment
Citation: Wei J, Lu X, Liu Q, Li L, Liu S, Liu F, Fu Y, Fan X, Yang Y, Qi C, Yu Y, Guan W and Liu B (2021) Case Report: Neoadjuvant PD-1 Blockade Plus Concurrent Chemoradiotherapy in Unresectable Locally Advanced Gastric Cancer Patients. Front. Oncol. 10:554040. doi: 10.3389/fonc.2020.554040
Received: 21 April 2020; Accepted: 07 December 2020;
Published: 05 February 2021.
Edited by:
Ignacio Melero, University of Navarra, SpainReviewed by:
Maria Alsina, Vall d’Hebron Institute of Oncology (VHIO), SpainMaria Esperanza Rodríguez-Ruiz, University of Navarra, Spain
Fernando Aranda, Instituto de Investigación Sanitaria de Navarra (IdiSNA), Spain
Copyright © 2021 Wei, Lu, Liu, Li, Liu, Liu, Fu, Fan, Yang, Qi, Yu, Guan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baorui Liu, YmFvcnVpbGl1QG5qdS5lZHUuY24=; Wenxian Guan, Z3Vhbi13eEAxNjMuY29t
 Jia Wei
Jia Wei Xiaofeng Lu2
Xiaofeng Lu2 Qin Liu
Qin Liu Yangyang Yu
Yangyang Yu Baorui Liu
Baorui Liu