- 1Department of Urology, Nanchong Central Hospital, The Second Clinical Medical College, North Sichuan Medical College, Nanchong, China
- 2Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Neurology, The Affiliated Hospital of Medical College, North Sichuan Medical College, Nanchong, China
- 4Department of Otolaryngology, Nanchong Central Hospital, The Second Clinical Medical College, North Sichuan Medical College, Nanchong, China
Background: The use of robot-assisted radical nephrectomy (RARN) for renal cell carcinoma (RCC) has increased in recent years, but the advantages of RARN over laparoscopic radical nephrectomy (LRN) remain controversial. This study aimed to compare the perioperative outcomes between RARN and LRN.
Methods: We systematically searched the EMBASE, PubMed, Web of Science, and CNKI databases to identify eligible comparative studies. The parameters were perioperative outcomes including operating time (OT), estimated blood loss (EBL), length of stay (LOS), conversion rate, and complications. Stata 15.0 software was used for the meta-analysis.
Results: Seven studies with 1,832 patients were included in the analysis. Among them, 532 underwent RARN and 840 underwent LRN for RCC. There were no significant differences in OT (weighted mean difference [WMD], 29.05; 95% confidence interval [CI], −0.31, 58.41; p = 0.05), EBL (WMD, −4.56; 95% CI, −29.79, 20.67; p = 0.72), LOS (WMD, −0.34; 95% CI, −0.68, 0.00; p = 0.05), conversion rate (WMD, 2.67; 95% CI, 0.68, 10.46; p = 0.05), transfusion rate (odds ratio [OR], 1.30; 95% CI, 0.74, 2.27; p = 0.36), intraoperative complications (OR, 1.13; 95% CI, 0.61, 2.12; p = 0.62), and postoperative complications (OR, 1.07; 95% CI, 0.68, 1.67; p = 0.62) between the two groups.
Conclusion: RARN was not superior to LRN in patients with RCC in terms of perioperative outcomes. Before establishing conclusive clinical recommendations, high-quality prospective large-scale randomized controlled trials with long-term follow-up are needed.
Introduction
Renal cell carcinoma (RCC) is the most common genitourinary malignancy, with estimated 403,262 new cases and 175,098 associated deaths worldwide in 2018 (1). Radical nephrectomy (RN) is the standard procedure for the management of large kidney tumors or tumors not suitable for nephron-sparing surgery (2, 3). In recent years, after the development of minimally invasive techniques, laparoscopic RN (LRN) has been considered an alternative to traditional open RN because it is associated with less trauma and fewer perioperative complications (4). However, laparoscopic technology has limited flexibility and operability, and its learning curve is steep (5).
In 2005, a new surgical procedure for RN was performed by Klingler et al. (6), known as robot-assisted radical nephrectomy (RARN). Since its introduction, RARN has been considered a safe and powerful procedure for nephrectomy by multiple institutions, and its use has increased significantly during the past decade (7). Robotic procedures have several significant advantages over laparoscopy, including higher definition displays, finer manipulations, and a greater range of motion (8).
Although the robotic procedure for RN has been increasingly adopted worldwide, its advantages in treating renal tumors are still controversial. Several studies have reported that RARN is associated with similar perioperative outcomes and higher hospital charges than the standard LRN (9–11). However, others have found that RARN is associated with lower surgical morbidity (12). This study aimed to perform a standard meta-analysis of the literature to compare the perioperative outcomes of RARN and LRN in patients with RCC.
Methods
Search Strategy
This meta-analysis was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) criteria (13) and was prospectively registered in the PROSPERO (CRD42020143279). We searched the EMBASE, PubMed, Web of Science, and CNKI databases for articles published between January 2005 and January 2020. The following search terms were used: “robot-assisted,” “robotic-assisted,” “robotic,” “robot,” “laparoscopic,” “laparoscopy,” “radical,” and “nephrectomy.” No language restriction was used. We also manually retrieved the reference list of eligible studies and reviewed conference records. Each included study was evaluated independently by two reviewers (J.L. and L.P.), and any differences were resolved by consensus.
Inclusion/Exclusion Criteria
The following inclusion criteria were used: (1) study type was randomized controlled trial, cohort study, or case-control study; (2) studies performed in adults diagnosed with RCC; (3) studies comparing RARN with LRN; (4) evaluation of at least one perioperative outcome such as operating time (OT), estimated blood loss (EBL), length of stay (LOS), conversion rate, transfusion rate, and complications.
The exclusion criteria were as follows: (1) reviews, letters to editors, case reports, and unpublished articles; (2) no comparison performed between RARN and LRN; (3) patients with benign kidney tumors; (4) studies with unavailable or unclear data.
Data Extraction
Data extraction was conducted independently by two authors (J.L. and L.P.), and disagreement was resolved through negotiation. The following data were extracted from eligible studies: first author, publication date, study type, surgical procedure, number of patients, age, body mass index, tumor size, follow-up time, and outcome measures (including OT, EBL, LOS, conversion rate, transfusion rate, and complications). If continuous variables in the article were expressed as median (interquartile range), we converted it to mean ± standard deviation (14).
Risk-of-Bias Assessments
Publication bias was evaluated using the Risk of Bias in Non-Randomized Studies-of Interventions (ROBINS-I) tool (15). This tool assesses seven domains: confounding bias, selection bias, bias in measurement classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported result.
Quality Assessment
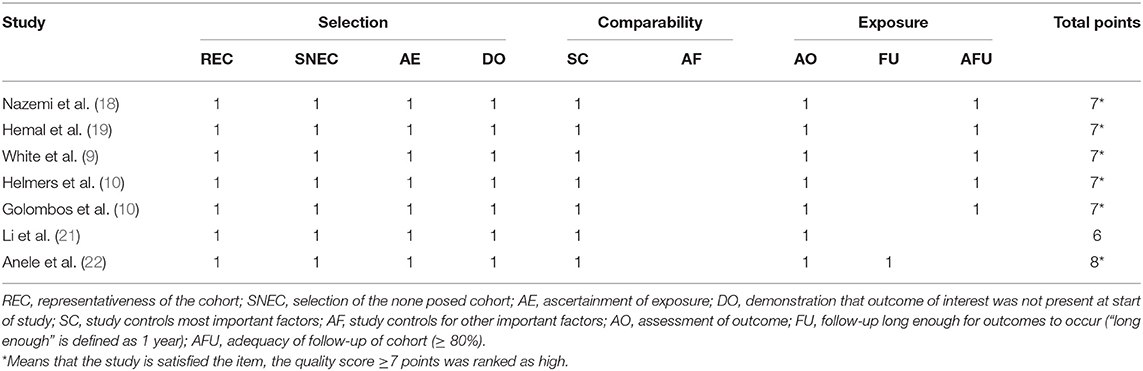
The quality of all included studies was estimated using the Newcastle–Ottawa scale (maximum score 9) (16). A score of ≥6 was considered high quality, whereas a score of ≤ 5 indicated low quality. Additionally, the level of evidence for each study was appraised according to the evidence evaluation criteria published by the Oxford Evidence-based Medicine Center (17). Two reviewers (J.L. and L.P.) performed quality assessment and level of evidence on the included studies, and differences were resolved through negotiation.
Statistical Analysis
The meta-analysis was performed using Stata v.15.0 software (Stata Corp, College Station, TX, USA). Continuous and dichotomous variables were pooled as weighted mean difference (WMD) and odds ratio (OR), respectively. All data were reported with 95% confidence intervals (CIs). The Z test was conducted to determine all merged effects, and statistical significance was defined as p < 0.05. Heterogeneity between studies was estimated using the χ2-test and inconsistency (I2) test; p < 0.10 or I2 > 50% indicated significant heterogeneity, and the random-effects model was applied; otherwise, a fixed-effects model was adopted. Sensitivity analysis was performed by omitting individual studies one by one for some outcomes such as OT, EBL, and LOS. Because fewer than 10 studies were included in this meta-analysis, no funnel plot was used to evaluate publication bias.
Results
Study Characteristics
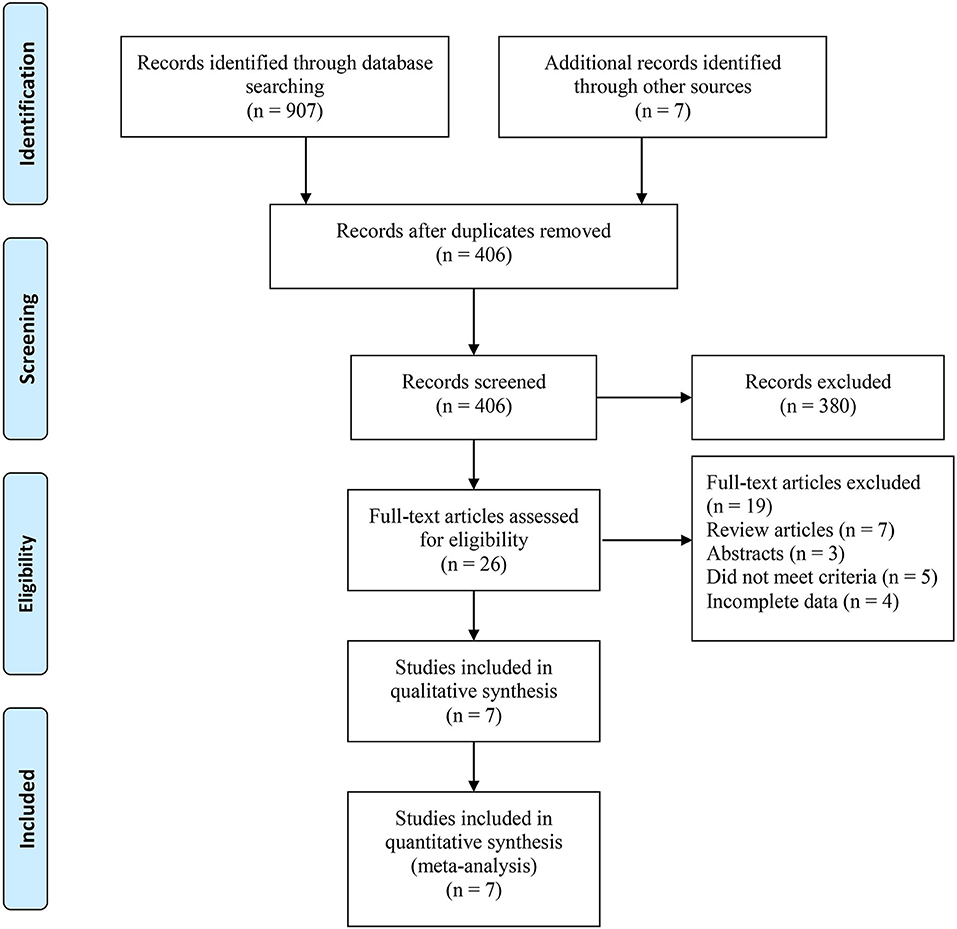
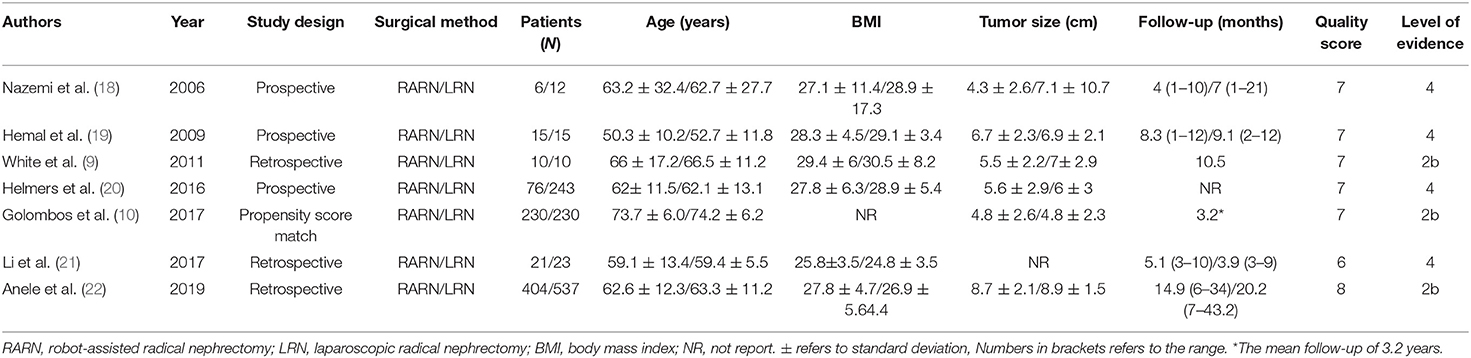
Based on the search strategy, we identified 914 related articles from the database. Among them, 406 studies remained after removing duplicates. After reading the title and abstract, 380 articles were excluded. Finally, seven full-text studies involving 1,832 patients (726 RARN vs. 1,070 LRN) were included in this meta-analysis (9, 10, 18–22) (Figure 1). Among the seven studies, there were three prospective (18–20) and four retrospective studies (9, 10, 21, 22). Only one study reported patient matching (10). The characteristics and level of evidence of all the included studies are presented in Table 1, and the quality evaluation of the included studies is presented in Table 2.
Demographic Variables
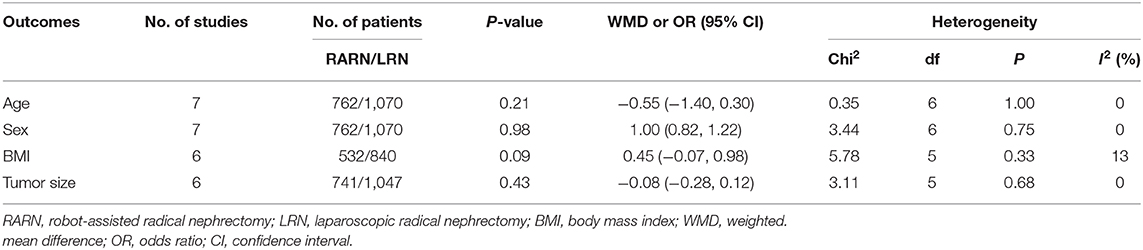
There were no significant differences between the two groups in terms of age (WMD, −0.55; 95% CI, −1.40, 0.30; p = 0.21), sex (male/total: OR, 1.00; 95% CI, 0.82, 1.22; p = 0.98), body mass index (WMD, 0.45; 95% CI, −0.07, 0.98; p = 0.09), and tumor size (WMD, −0.08; 95% CI, −0.28, 0.12; p = 0.43; Table 3).
Operating Time
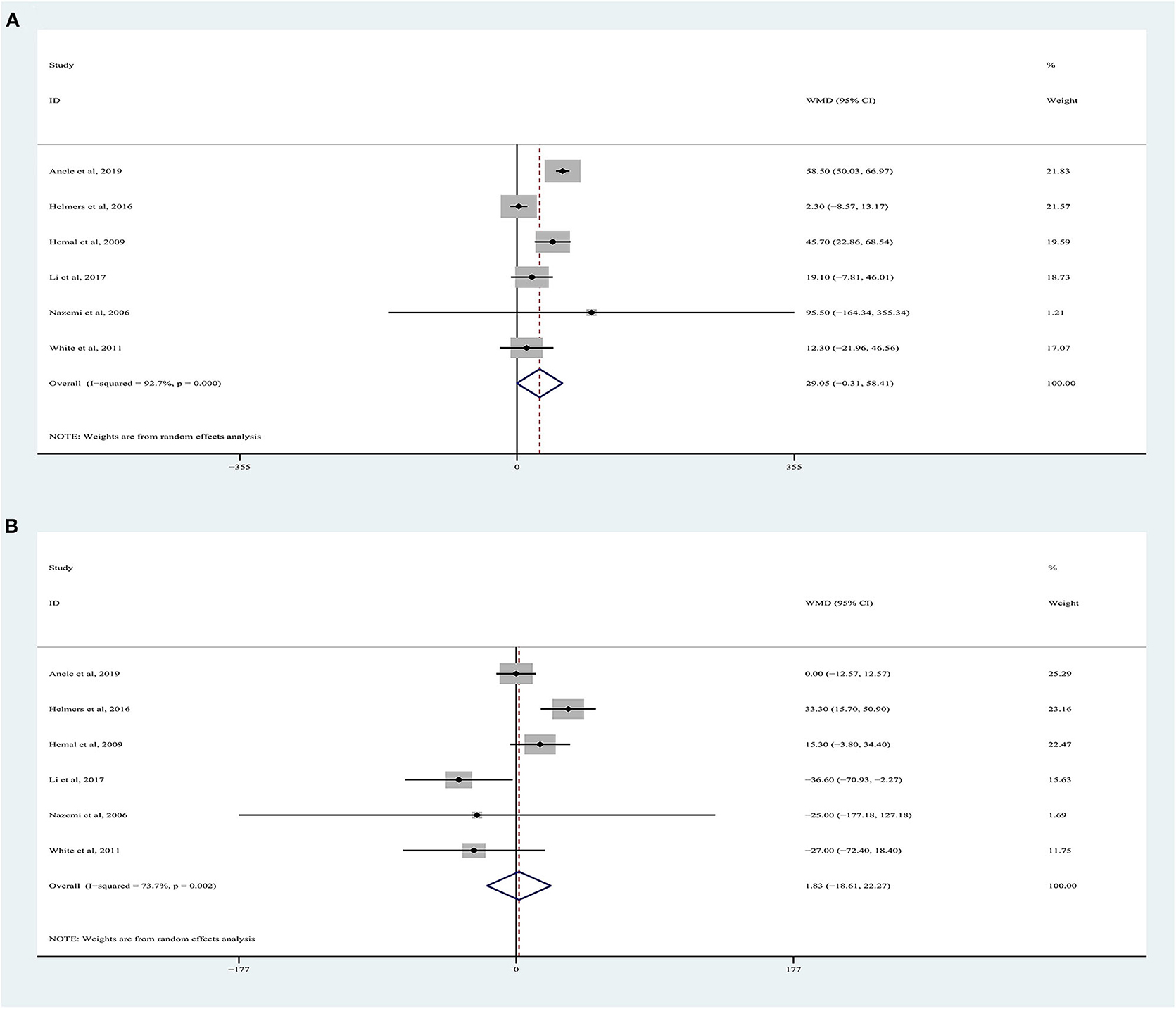
OT data were obtained from six studies (9, 18–22), totaling 1,372 patients (532 RARN vs. 840 LRN). The pooled analysis indicated no significant differences for OT between RARN and pure LRN (random-effects model: WMD, 29.05; 95% CI, −0.31, 58.41; p = 0.05; I2 = 93%), albeit at a greater heterogeneity (Figure 2A).
Estimated Blood Loss
Six articles were included in the meta-analysis (9, 18–22), including 1,372 patients. Among them, 532 underwent RARN, and 840 underwent LRN (Figure 2B). Because higher heterogeneity was present, a random-effects model was used (I2 = 74%). The pooled outcome supported that EBL in the RARN group was similar to that in the standard LRN group (WMD, 1.83, 95% CI, −18.61, 22.27; p = 0.86).
Length of Hospital Stay
LOS data were reported in seven studies involving 1,832 patients (Figure 3A) (9, 10, 18–22), of which 762 underwent RARN and 1,070 underwent LRN. No significant difference was observed between the two groups regarding LOS (random-effects model: LOS, −0.34; 95% CI, −0.68, 0.00; p = 0.05; I2 = 85%), despite greater heterogeneity.
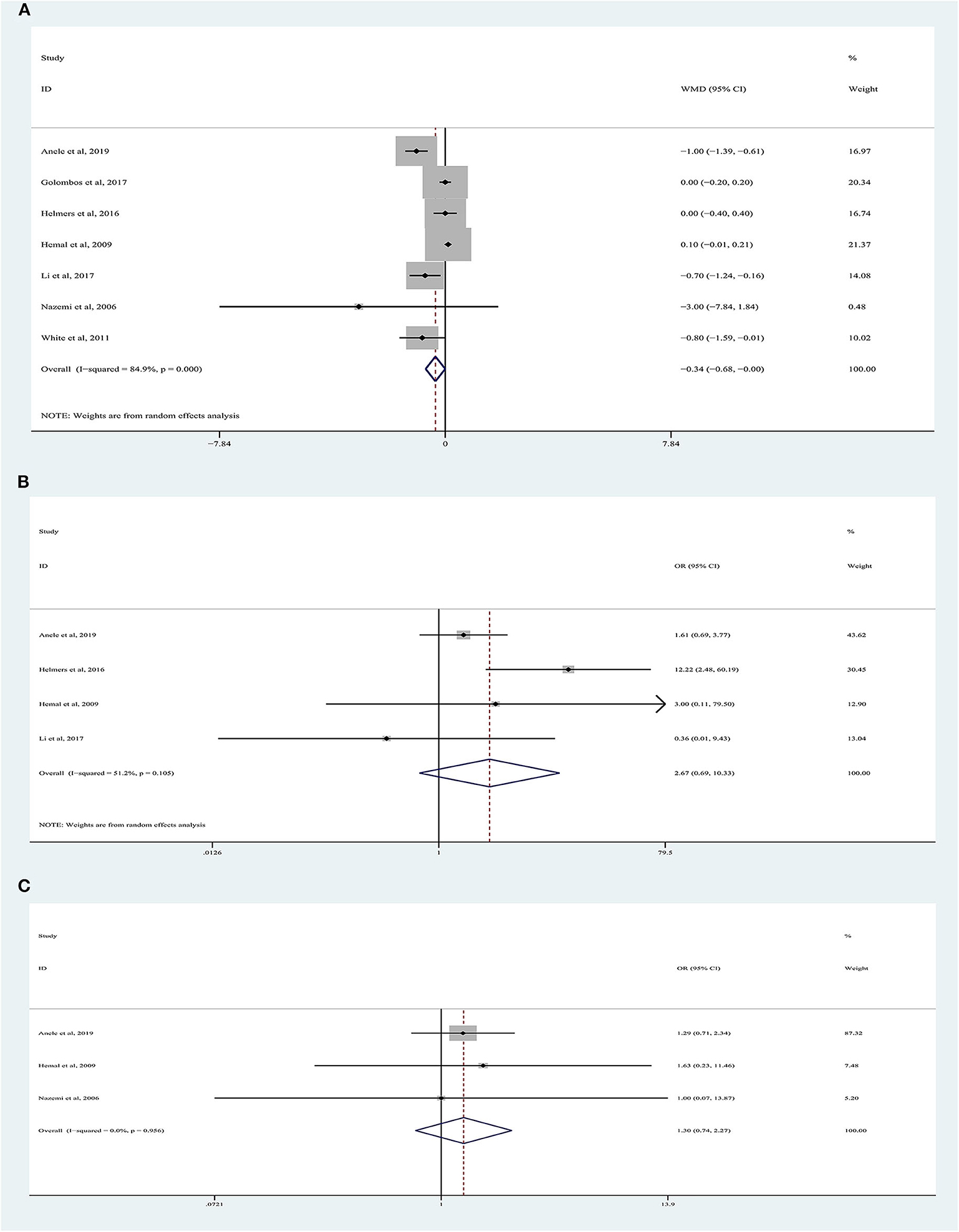
Figure 3. Forest plots of perioperative outcomes: (A) length of hospital stay, (B) transfusion rate, (C) conversion rate.
Conversion Rate
There were 1,334 patients analyzed in four studies (19–22). The conversion rate was reported in 3.88% (20/516) of patients who underwent RARN and in 1.60% (13/813) of patients who underwent LRN (Figure 3B). Meta-analysis demonstrated that RARN offers a comparable conversion rate to LRN (random-effects model: WMD, 2.67; 95% CI, 0.69, 10.33; p = 0.16; I2 = 51%).
Transfusion Rate
Three articles were analyzed (18, 19, 22). A total of 989 patients were included, of whom 425 underwent RARN and 564 underwent LRN (Figure 3C). The blood transfusion rate was similar between the two groups, and no heterogeneity was found (fixed-effects model: OR, 1.30; 95% CI, 0.74, 2.27; p = 0.36; I2 = 0%).
Complications
Forest plots of perioperative complications are illustrated in Figure 4. There were no significant differences in intraoperative complications (random-effects model: OR, 1.13; 95% CI, 0.61, 2.12; p = 0.62; I2 = 61%) and postoperative complications (fixed-effects model: OR, 1.07; 95% CI, 0.68, 1.67; p = 0.62; I2 = 0%) between the two approaches.
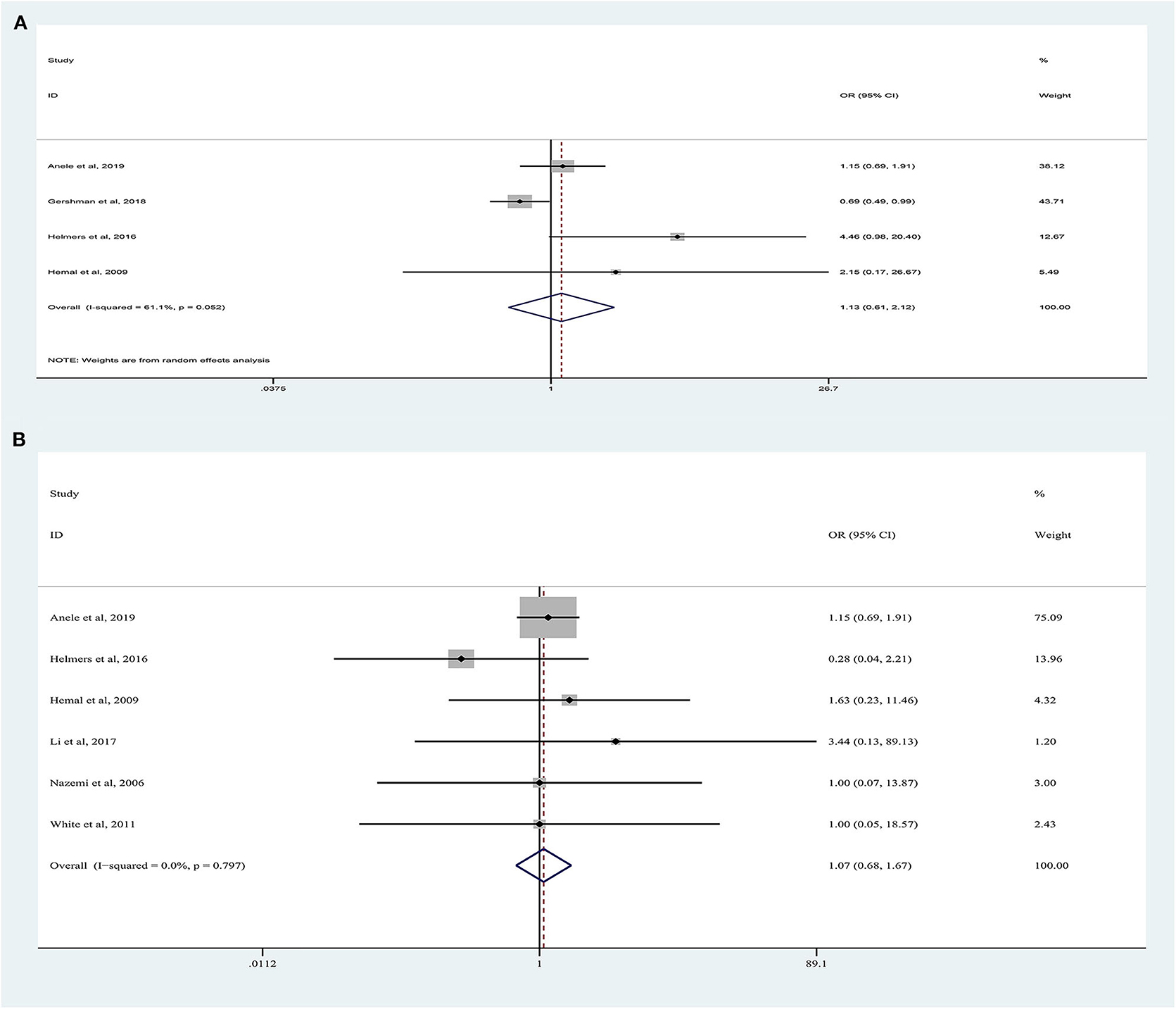
Figure 4. Forest plots of perioperative outcomes: (A) intraoperative complications, (B) postoperative complications.
Sensitivity Analysis and Publication Bias
Although the quality of the included studies was high (all scores were six or higher), some parameters were highly heterogeneous, such as OT, EBL, and LOS. A sensitivity analysis was performed on these parameters to improve the reliability of the analysis. Studies were removed one by one to recalculate the combined mean difference, and all the new pooled mean differences remained constant after deleting any study (Figure 5). The ROBINS-I tool was used to assess publication bias, and these results suggested that all comparative studies had a moderate risk of bias (9, 10, 18–22).
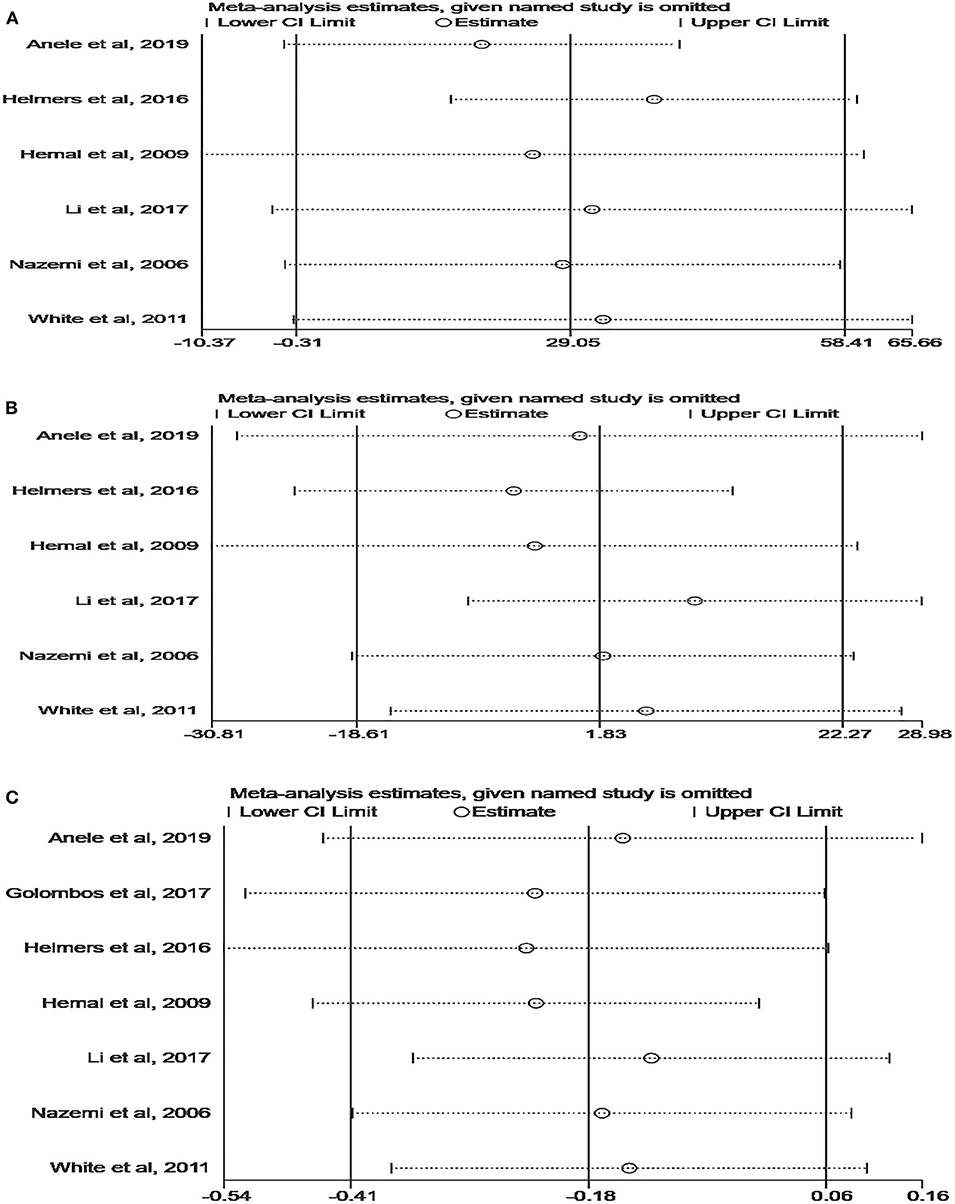
Figure 5. Sensitivity analysis of perioperative outcomes: (A) operating time, (B) estimated blood loss, (C) length of hospital stay.
Discussion
During the past decade, minimally invasive techniques have been used in the management of RCC (23). Both laparoscopic and robotic procedures are considered as alternatives to open surgery (7). In fact, the robotic procedure has higher definition displays and a shorter learning curve. Furthermore, RARN has a role in training for partial nephrectomy, in which robotic surgery offers some advantages compared to laparoscopy, such as finer manipulations and a greater range of motion, thus influencing the decision to continue a program of robotic surgery in which RN can be propaedeutic to partial nephrectomy.
Several studies have compared clinical parameters between RARN and standard LRN, but the advantage of these two technologies is still debatable (9, 12, 24). To the best of our knowledge, this is the first meta-analysis of reported comparative outcomes of RARN vs. LRN. The present study indicates that there were no significant differences between the two procedures in terms of OT, EBL, LOS, conversion rates, intraoperative complications, and postoperative complications. Therefore, RARN appears to be an effective and safe technique, although only the perioperative outcomes were compared.
In a retrospective cohort study conducted by Jeong et al. (11), the rate of prolonged OT (>4 h) in patients receiving RARN was higher than that for patients undergoing conventional LRN (11). Subsequently, using the multi-institutional renal masses database, Anele et al. (22) found that the duration of surgery for RARN was significantly longer than that for LRN, with a median OT increase of approximately 60 min (median = 185 and 126 min for RARN and LRN, respectively). Contrary to the results of both studies, our findings suggest that OT was comparable between RARN and LRN. Considering that greater heterogeneity was observed in the analysis (I2 = 93%), this result needs to be interpreted with caution.
In fact, there were different opinions on which method was superior in terms of OT. The duration of the operation may be related to the technical proficiency of the surgeon, and centers with less experience in robotic procedures may have longer OTs (25). Jaffe et al. (26) found that, after 180 cases, the OT of robotic surgery could be reduced from the initial 240 to 120 min. Similarly, Wolanski et al. (27) reported a steep improvement in operative duration as the robotic surgery experience increased. They concluded that the robot approach may have had significant advantages regarding console time compared to traditional laparoscopic surgery. As mentioned, differences in physician experience may lead to dramatic changes in surgical time, especially the time required for the suture part of the procedure. Moreover, different definitions of surgical time in the included studies may contribute to variations in results.
Our meta-analysis suggested that the EBL was similar between RARN and LRN (p = 0.86), which is consistent with prior studies (9, 19, 22). Helmers et al. (20) performed a retrospective study with 319 cases (243 RARN and 76 LRN). They described that the RARN group had a greater EBL than the LRN group (median = 100 vs. 50 mL, p < 0.05). However, Li et al. (21) found that the mean EBL was significantly lower for RARN (53.8 mL) than for standard LRN (90.4 mL). They explained that this difference is due to the operator's ability to accurately separate renal arteries and veins in the high-definition field of view provided by the robot, which can better protect blood vessels and reduce blood loss during surgery.
Of note, all included comparative studies reported LOS, and the pooled results demonstrated that no significant difference was found in LOS between the two technologies. One prospective study, instead, reported the LOS in RARN was significantly shorter than that in LRN (4.4 vs. 5.1 days, p < 0.05) (21). Anele et al. (22) found RARN had a lower median LOS (3 days in the RARN group vs. 5 days in the LRN group, p < 0.001). In addition, the flexible operation of the robotic method may increase the surgeon's confidence in the quality of the anastomosis. This may shorten the retention time of the drainage tube, thereby reducing the LOS. Heterogeneity was also found in the analysis for this outcome (I2 = 85%); however, no change in heterogeneity was observed after deleting any study, and the merger results were still not significant, which undoubtedly increased the credibility of our study.
Another interesting finding was the similar conversion rate for the RARN and LRN groups, according to the present study. This is different from the data reported Helmers et al. (20). Their results indicated that RARN was associated with a higher conversion rate than traditional LRN (10.3 vs. 1.0%, p < 0.01). A higher conversion rate in RARN was caused by bleeding and dense adhesions, but the median tumor size of the converted RRN cases was 9 cm. A larger tumor makes the dissection of kidney blood vessels more difficult, increasing the risk of causing serious vascular damage (19). Furthermore, confounding factors that are difficult to analyze may also be important, such as kidney anatomical variation and surgeon technical proficiency. In terms of blood transfusion rate, there was no steep difference between RARN and LRN, which is highly compatible with the results of previous comparative studies (18, 19, 22). However, prospective randomized controlled trials are needed to confirm our findings.
Complications are an important parameter for estimating the safety of surgical techniques. Our study suggested that RARN had similar intraoperative and postoperative complications as those of LRN. However, using the Nationwide Inpatient Sample data from 2010 to 2013, Gershman et al. (12) found that RARN was associated with lower perioperative morbidity (20.4 vs. 27.2%, p < 0.001), and surgeons with extensive RARN experience can diminish or avoid collateral injuries during surgery. Given the potential confounding factors, including patient characteristics, tumor complexity, and hospital location, this issue requires further investigation.
Since the introduction of RARN, one of the major concerns has been the high cost, which was supposed to be the chief obstruction of the broad implementation of this technology. In a cost comparison analysis, Jeong et al. (11) reported that the average 90-day direct hospital costs for RARN were significantly higher than those for LRN (US $19,530 vs. $16,851, p = 0.004). Similarly, Gershman et al. (12) also found that RARN had higher total hospital charges (US $16,207 vs. $15,037, p < 0.001). Interestingly, in the single-institution study conducted by Helmers et al. (20), there was no significant difference in total inpatient costs between the procedures (median = US $14,913 vs. $16,265, p = 0.171). As discussed by Kates et al. (28), the shorter LOS of the RARN procedure may save hospital charges; however, additional prospective studies are needed to explain this issue. In addition, the cost variations may depend on the size, location, and profit status of the medical center (29).
Limited studies have reported direct comparisons between RARN and standard LRN regarding oncologic outcomes. Golombos et al. (10) reported no significant differences in overall and cancer-specific survival between RARN and LRN at midterm follow-up. According to a comparative study, the median follow-up for RARN and LRN was 15 and 20 months, respectively, and no difference was noted in overall survival between the two groups (22). However, RARN was associated with a higher positive margin rate and greater risk of recurrence or metastases than LRN (all p < 0.01). As described, the higher staging and grading of malignancies in the RARN group may contribute to this result. Chopra et al. (30) reported results of RARN in patients with RCC with inferior vena cava tumor thrombus. The authors reported that no patient died during a median follow-up of 16 months (range = 12–39 months). Thus, RARN appears to have acceptable short-term oncologic outcomes, including locally advanced/progressed RCC, and in the case of renal cancer with inferior vena cava tumor thrombosis (7, 30, 31).
Our study is of clinical value as the first meta-analysis to directly compare the perioperative outcomes of RARN and LRN in patients with RCC. However, there are some limitations to the present analysis. First, most of the included studies had a retrospective design, and the sample size of some studies was small. Therefore, the level of evidence in this study was low. Second, some outcomes were heterogeneous, including OT, EBL, and LOS. Sensitivity analysis explained partial heterogeneity, but subgroup analysis was not available to further analyze other confounding factors with limited data. Considering these, our findings should be interpreted with caution. Third, many studies reported a shorter follow-up period, and only two studies had a median follow-up of more than 1 year. As a result, the oncologic outcomes of the two technologies cannot be assessed. Finally, a cost analysis was not evaluated because of lack of data; indeed, cost is the main factor restricting the implementation of robotics.
In summary, the study demonstrated that RARN was not superior to LRN in patients with RCC, in terms of perioperative outcomes. Before establishing final clinical recommendations, high-quality prospective large-scale randomized controlled trials with long-term follow-up are needed.
Data Availability Statement
All datasets generated for this study are included in the article/ supplementary material.
Author Contributions
YL and QW: conception and design. JL, LP, DC, and BC: acquisition of data and critical revision of the manuscript for important intellectual content. JL, LP, and HG: analysis and interpretation of data. JL and LP: drafting of the manuscript. YL: supervision. All authors: read and agree to the published version of the manuscript.
Funding
This article was funded by the special project of Nanchong City School Cooperation (NSMC20170457) and by the Technology Department Project of Sichuan Science (2020YFS0320).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms Mengqi Chen for providing continuous encouragement to JL to pursue his career in medicine.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Ljungberg B, Albiges L, Abu-Ghanem Y, Bensalah K, Dabestani S, Fernandez-Pello S, et al. European Association of Urology Guidelines on renal cell carcinoma: the 2019 update. Eur Urol. (2019) 75:799–810. doi: 10.1016/j.eururo.2019.02.011
3. Motzer RJ, Jonasch E, Michaelson MD, Nandagopal L, Gore JL, George S, et al. NCCN guidelines insights: kidney cancer, Version 2.2020. J Natl Compr Canc Netw. (2019) 17:1278–85. doi: 10.6004/jnccn.2019.0054
4. Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal mass and localized renal cancer: AUA guideline. J Urol. (2017) 198:520–9. doi: 10.1016/j.juro.2017.04.100
5. Barbash GI, Glied SA. New technology and health care costs–the case of robot-assisted surgery. N Engl J Med. (2010) 363:701–4. doi: 10.1056/NEJMp1006602
6. Klingler DW, Hemstreet GP, Balaji KC. Feasibility of robotic radical nephrectomy–initial results of single-institution pilot study. Urology. (2005) 65:1086–9. doi: 10.1016/j.urology.2004.12.020
7. Asimakopoulos AD, Miano R, Annino F, Micali S, Spera E, Iorio B, et al. Robotic radical nephrectomy for renal cell carcinoma: a systematic review. BMC Urol. (2014) 14:75. doi: 10.1186/1471-2490-14-75
8. Caputo PA, Ko O, Patel R, Stein R. Robotic-assisted laparoscopic nephrectomy. J Surg Oncol. (2015) 112:723–7. doi: 10.1002/jso.24033
9. White MA, Autorino R, Spana G, Laydner H, Hillyer SP, Khanna R, et al. Robotic laparoendoscopic single-site radical nephrectomy: surgical technique and comparative outcomes. Eur Urol. (2011) 59:815–22. doi: 10.1016/j.eururo.2011.02.020
10. Golombos DM, Chughtai B, Trinh QD, Mao J, Te A, O'Malley P, et al. Adoption of technology and its impact on nephrectomy outcomes, a U.S. population-based analysis (2008-2012). J Endourol. (2017) 31:91–9. doi: 10.1089/end.2016.0643
11. Jeong IG, Khandwala YS, Kim JH, Han DH, Li S, Wang Y, et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA. (2017) 318:1561–8. doi: 10.1001/jama.2017.14586
12. Gershman B, Bukavina L, Chen Z, Konety B, Schumache F, Li L, et al. The association of robot-assisted versus pure laparoscopic radical nephrectomy with perioperative outcomes and hospital costs. Eur Urol Focus. (2020) 6:305–12. doi: 10.1016/j.euf.2018.10.004
13. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
14. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
15. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
16. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
17. Brozek JL, Akl EA, Compalati E, Kreis J, Terracciano L, Fiocchi A, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines part 3 of 3. The GRADE approach to developing recommendations. Allergy. (2011) 66:588–95. doi: 10.1111/j.1398-9995.2010.02530.x
18. Nazemi T, Galich A, Sterrett S, Klingler D, Smith L, Balaji KC. Radical nephrectomy performed by open, laparoscopy with or without hand-assistance or robotic methods by the same surgeon produces comparable perioperative results. Int Braz J Urol. (2006) 32:15–22. doi: 10.1590/S1677-55382006000100003
19. Hemal AK, Kumar A. A prospective comparison of laparoscopic and robotic radical nephrectomy for T1-2N0M0 renal cell carcinoma. World J Urol. (2009) 27:89–94. doi: 10.1007/s00345-008-0321-9
20. Helmers MR, Ball MW, Gorin MA, Pierorazio PM, Allaf ME. Robotic versus laparoscopic radical nephrectomy: comparative analysis and cost considerations. Can J Urol. (2016) 23:8435–40.
21. Li F, Zhang G, Song Y, Li B, Sun L. Clinical effect of Da Vinci robot-assisted laparoscopic radical nephrectomy versus retroperitoneal laparoscopic radical nephrectomy inthetreatment of renal carcinoma: a comparative analysis. Med J Qilu. (2017) 32:394–6. doi: 10.13362/j.qlyx.201704006
22. Anele UA, Marchioni M, Yang B, Simone G, Uzzo RG, Lau C, et al. Robotic versus laparoscopic radical nephrectomy: a large multi-institutional analysis (ROSULA Collaborative Group). World J Urol. (2019) 37:2439–50. doi: 10.1007/s00345-019-02657-2
23. Bragayrac LA, Abbotoy D, Attwood K, Darwiche F, Hoffmeyer J, Kauffman EC, et al. Outcomes of minimal invasive vs open radical nephrectomy for the treatment of locally advanced renal-cell carcinoma. J Endourol. (2016) 30:871–6. doi: 10.1089/end.2016.0082
24. Yang DY, Monn MF, Bahler CD, Sundaram CP. Does robotic assistance confer an economic benefit during laparoscopic radical nephrectomy? J Urol. (2014) 192:671–6. doi: 10.1016/j.juro.2014.04.018
25. Light A, Karthikeyan S, Maruthan S, Elhage O, Danuser H, Dasgupta P. Peri-operative outcomes and complications after laparoscopic vs robot-assisted dismembered pyeloplasty: a systematic review and meta-analysis. BJU Int. (2018) 122:181–94. doi: 10.1111/bju.14170
26. Jaffe J, Castellucci S, Cathelineau X, Harmon J, Rozet F, Barret E, et al. Robot-assisted laparoscopic prostatectomy: a single-institutions learning curve. Urology. (2009) 73:127–33. doi: 10.1016/j.urology.2008.08.482
27. Wolanski P, Chabert C, Jones L, Mullavey T, Walsh S, Gianduzzo T. Preliminary results of robot-assisted laparoscopic radical prostatectomy (RALP) after fellowship training and experience in laparoscopic radical prostatectomy (LRP). BJU Int. (2012) 110(Suppl. 4):64–70. doi: 10.1111/j.1464-410X.2012.11479.x
28. Kates M, Ball MW, Patel HD, Gorin MA, Pierorazio PM, Allaf ME. The financial impact of robotic technology for partial and radical nephrectomy. J Endourol. (2015) 29:317–22. doi: 10.1089/end.2014.0559
29. Bai G, Anderson GF. Extreme markup: the fifty US hospitals with the highest charge-to-cost ratios. Health Aff. (2015) 34:922–8. doi: 10.1377/hlthaff.2014.1414
30. Chopra S, Simone G, Metcalfe C, de Castro Abreu AL, Nabhani J, Ferriero M, et al. Robot-assisted Level II-III inferior vena cava tumor thrombectomy: step-by-step technique and 1-year outcomes. Eur Urol. (2017) 72:267–74. doi: 10.1016/j.eururo.2016.08.066
Keywords: renal cell carcinoma, radical nephrectomy, robotic, laparoscopic, meta-analysis
Citation: Li J, Peng L, Cao D, Cheng B, Gou H, Li Y and Wei Q (2020) Comparison of Perioperative Outcomes of Robot-Assisted vs. Laparoscopic Radical Nephrectomy: A Systematic Review and Meta-Analysis. Front. Oncol. 10:551052. doi: 10.3389/fonc.2020.551052
Received: 15 April 2020; Accepted: 12 August 2020;
Published: 18 September 2020.
Edited by:
Zongbing You, Tulane University, United StatesReviewed by:
Alfonso Recordare, Ospedale dell'Angelo, ItalyLei Li, Xi'an Jiaotong University, China
Copyright © 2020 Li, Peng, Cao, Cheng, Gou, Li and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunxiang Li, bGl5dW54aWFuZzM2OUAxMjYuY29t; Qiang Wei, d2VpcWlhbmc5MzNAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jinze Li1†
Jinze Li1† Yunxiang Li
Yunxiang Li