- 1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2Department of Neurology, Medical School, University of Minnesota, Minneapolis, MN, United States
Gliomas account for more than half of all adult primary brain tumors. Epilepsy is the most common initial clinical presentation in gliomas. Glioma related epilepsy (GRE) is defined as symptomatic epileptic seizures secondary to gliomas, occurring in nearly 50% in high-grade glioma (HGG) patients and up to 90% in patients with low-grade glioma (LGG). Uncontrolled seizures, which have major impact on patients’ quality of life, are caused by multiple factors. Although the anti-seizure medications (ASMs), chemotherapy and radiation therapy are also beneficial for seizure treatment, the overall seizure control for GRE continue to be unsatisfactory. Due to the close relationship between GRE and glioma, surgical resection is often the treatment of choice not only for the tumor treatment, but also for the seizure control. Despite aggressive surgical treatment, there are about 30% of patients continue to have poor seizure control postoperatively. Furthermore, the diagnostic criteria for GRE is not well established. In this review, we propose an algorithm for the diagnosis and perioperative management for GRE.
Introduction
Epilepsy is among the most common clinical presentations of brain tumors, and seizures often present as the first clinical symptom. Glioma related epilepsy (GRE) is defined as symptomatic epileptic seizures secondary to gliomas. The incidences of epilepsy in patients with gliomas range from 40 to 90%, depending on the tumor types (1–5). The mechanisms of glioma-related epilepsy are thought to be multifactorial and share common pathways with glioma growth processes (6–8). The pathogenesis of GRE involves many factors including tumor histology, tumor location, microenvironment; specific genetic alterations such as isocitrate dehydrogenase 1 (IDH1) gene mutation, O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation; aberrant expression of potassium chloride cotransporter (KCC2), solute carrier family 7 member 11 (SLC7A11), glutamate, etc (8–11). In terms of postoperative seizure control, extent of resection, age, seizure type, seizure duration and Ki-67 expression have been identified as predictors (2, 12).
With the development of new imaging technology, more and more patients with glioma and GRE are diagnosed at an early stage. Nevertheless, the diagnostic criteria for GRE remain controversial, making it difficult to have a treatment guideline for GRE. Therefore, it is very important to have a standard diagnostic criteria for GRE.
Because tumor itself contributes significantly to seizure occurrence, most GRE patients could become seizure-free in the early stage after tumor resection. Gross total resection remains an independent positive predictor for postoperative seizure control (2, 12, 13). However, uncontrolled seizures could occur in 20–40% of patients post resection (2, 4, 11, 14). Recurrent seizures substantially impact quality of life, especially as newer therapies for gliomas have extended life expectancies. As a highly tumor associated disease, GRE is being treated on the second purpose of surgery followed by oncologic tumor control. Surgical treatment of gliomas has the potential to ameliorate seizures when combining with individual and normalized treatment of anti-seizure medications (ASMs) and radiochemotherapy. In this review, we propose an algorithm for the diagnosis and perioperative management for GRE, hoping to provide a better standard for improving seizure control in patients with gliomas.
Diagnosis of GRE
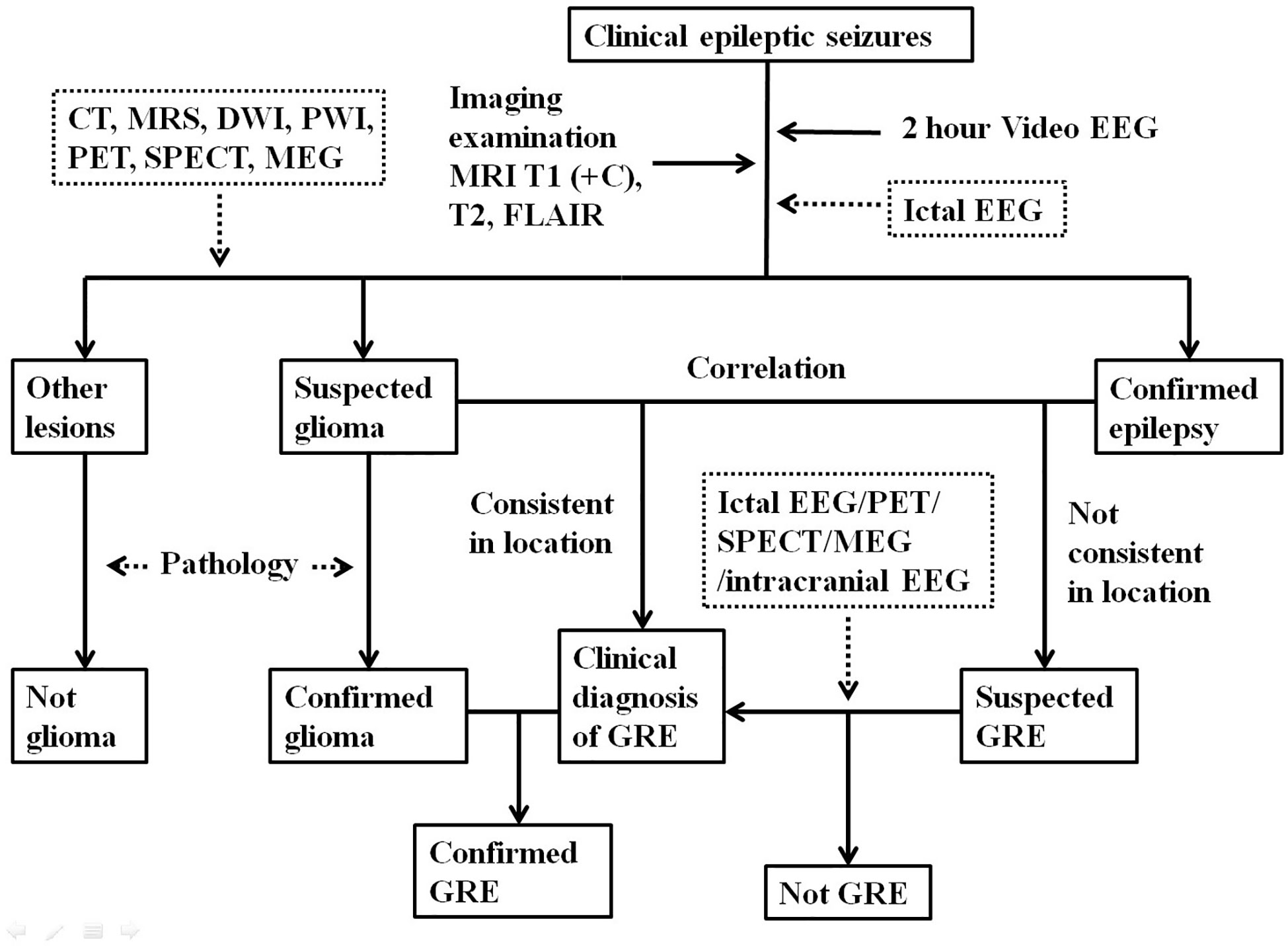
Unlike patients with idiopathic epilepsy, for patients with GRE, seizure risk is influenced by tumor types. It is important to have comprehensive history-taking and physical examination during the initial visit. Seizure characteristics and relevant clinical information should be documented in details (15). Seizure type should be classified according to the 2017 International League Against Epilepsy (ILAE) guidelines in order to guide management (16). Patients with suspected GRE should receive magnetic resonance imaging (MRI) and electroencephalogram (EEG) examinations. The diagnosis of GRE should be based on both the clinical information and imaging findings.
First of all, for the diagnosis of glioma, MRI with T2-weighted and contrast-enhanced T1-weighted imaging are essential. The other conventional sequences, such as diffusion-weighted imaging (DWI), perfusion-weighted imaging (PWI) and fluid-attenuated inversion recovery imaging (FLAIR) should also be included. In addition, computed tomography (CT), magnetic resonance spectroscopy (MRS) and positron emission tomography (PET) are also helpful for differential diagnosis. Pathological evaluation is the gold standard for the diagnosis of glioma. For the 2016 World Health Organization (WHO) classification (17), molecular pathological examination should be performed.
Secondly, for the diagnosis of epilepsy, a video EEG for more than 2 hours is recommended for patients with definite or possible seizure history (15). Ictal EEG is helpful for the differential diagnosis to rule out non-epileptic attacks in patients without typical clinical seizures or interictal epileptiform discharges (15, 18). PET, single-photon emission CT (SPECT), and magnetoencephalography (MEG) can also be used to localize the epileptogenic zone (19, 20).
To make the diagnosis of GRE, a clear correlation between glioma and epilepsy should be established. Although intracranial video EEG is rarely performed for patients with GRE due to significant cost and risks, this is a useful diagnostic tool for cases that show no clear causal relationship between the tumor and seizures. Intracranial video EEG monitoring is recommended for epilepsy patients whose epileptogenic zones are thought to be unrelated to the brain tumor (15). A preoperative diagnosis of GRE is generally considered a clinical diagnosis which mainly depends on clinical signs, EEG, imaging studies. A diagnosis of GRE can only be confirmed after pathological validation (Figure 1).
Preoperative ASMS Application for GRE
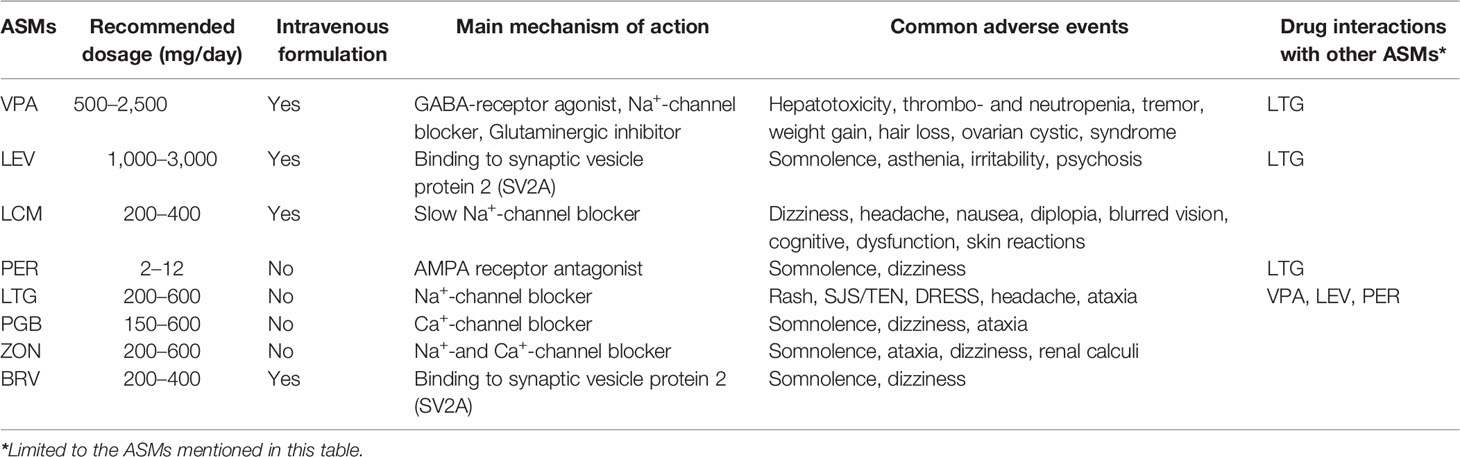
It is generally recommended to start ASMs medication when epilepsy is confirmed preoperatively because of great risk of seizure recurrence (21). The selection of ASMs should follow an individualized treatment plan based on age, sex, comorbidity, and concurrent medication therapy. One of the principles for the initial ASMs use in GRE patients is monotherapy with adequate drug dosage and duration. Given the fact that concomitant use of steroids and chemotherapy is common for postoperative treatment of glioma, ASMs with potential drug interactions as well as hepatic enzyme induction effects should be avoided (22, 23). At present time, evidence based medicine had suggested that the drug of choice for AED monotherapy for GRE are levetiracetam (LEV) and valproic acid (VPA) (24–26).
Recent studies on GRE treatment using LEV have suggested good efficacy and favorable safety profile (27, 28). With the advantages of improving the cognitive functioning and enhancing the chemotherapeutic efficacy of Temozolomide (TMZ), LEV is widely used for treating GRE as either monotherapy or add-on therapy (28, 29). VPA is a broad spectrum, well-tolerated AED that is widely used in epilepsy with brain tumors. As a histone deacetylase inhibitor, VPA may also inhibit glioma-genesis and prolong survival in patients with glioblastoma (GBM) (7, 30). If the first monotherapy AED has insufficient seizure control, switching to a different AED may be considered. Recently, there is a trend for polytherapy with combination of VPA with LEV if monotherapy with either one is ineffective (7, 25).
If neither LEV nor VPA or their combination is ineffective for seizure control, lacosamide (LCM), perampanel (PER), lamotrigine (LTG), pregabalin (PGB), zonisamide (ZON), and Brivaracetam (BRV) may be considered as add-on ASMs in GRE. LCM has a good efficacy and fewer side effects in GRE patients compared to other ASMs (31). LCM was also shown to have antineoplastic effect on glioma cells in vitro (32). PER, which functions as a noncompetitive AMPA receptor antagonist (33), was shown to inhibit GBM cell growth and glutamate release in vitro (34). Seizure-freedom periods could be achieved in drug-resistant epilepsy in patients with GRE when PER was used (35, 36). Because of their good tolerability and their synergistic activities with VPA, LTG, PGB, and ZON could also be considered as add-on agents for GRE (24, 29, 37). Brivaracetam is also a promising antiepileptic drug in this group of patients (38). We summarized the main characteristics of the antiepileptic drugs mentioned above in Table 1 (21, 24, 28, 39).
For patients without preoperative seizures, most studies and clinical guidelines advise against prophylactic ASMs use before surgery in patients with glioma (40, 41) unless they have the following risk factors: younger age, temporal lobe gliomas and cortex involving gliomas (2, 4, 42, 43).
Surgical Management of GRE
The goal of surgery in patients with GRE is not only tumor removal, but also seizure control, especially for LGG patients who usually have longer survival. Gross total resection is a known predictor of seizure freedom in glioma (2, 44). For gliomas with deep location or involving eloquent cortex, advanced intraoperative technologies, such as neuronavigation, awake surgery and “engraving surgery”, are recommended to maximize resection while preserving function. Electrocorticography (ECoG) in the intraoperative setting can help to identify epileptogenic zone to guide resection. ECoG has been shown of great value for LGG patients with preoperative refractory GRE (45). In addition, supratotal resection in noneloquent area can be helpful to optimize seizure control (6, 46).
Intraoperative direct electrical stimulation for functional cortical or subcortical mapping may induce seizures during awake craniotomy for gliomas. The incidence is approximately 8% (47). Patients with the following risk factors are more likely to experience intraoperative seizures: younger age, supplementary motor area involvement, preoperative seizure history, and IDH1 mutation (43, 47, 48). Prophylactic intravenous infusion of LEV or VPA half an hour before surgery can be used for these high-risk patients. The surgeons must stop stimulation immediately and irrigate the cortex with ice-cold Ringer’s solution or saline as soon as possible once intraoperative seizures occur (49). In case of status epilepticus, benzodiazepines or anesthesia enhancement should be initiated in a timely manner. It is worth mentioning that intraoperative electromyographic monitoring and ECoG could usually detect potential seizure onset early (43, 45).
Treatment of Early Postoperative Seizures
Early postoperative seizures (EPS) occur within the first week of surgery. Although clinical evidences have suggested no benefit for prophylactic ASMs during the perioperative period for GRE patients (40, 50), AED prophylaxis are still used routinely in clinical practice (51). One of the goals is to minimize the risk of EPS. Given the increased risk of EPS due to surgery, ASMs may be considered for 1 week after surgical resection whether or not patients had history of GRE. To evaluate the extent of tumor resection accurately, postoperative MRI with contrast-enhancement should be performed within 24–72 h after surgery.
In the case of EPS, intravenous or intramuscular injection of short acting ASMs should be administered as soon as possible to avoid status epilepticus, followed by performing electrocardiography and blood tests to exclude conditions caused by cardiac dysfunction, hypoglycemia, or electrolytes disturbances. Subsequently, CT or MRI must be performed to exclude intracranial hemorrhage, infarction and pneumocephalus. If the patient did not receive prophylactic ASMs perioperatively, AED monotherapy should be used (52). The blood concentrations of ASMs should be monitored regularly when more than two seizure episodes occur and add-on treatment with a second AED must be considered when seizures are not controlled with a single agent (43). A 2 h EEG monitoring is recommended to evaluate the interictal activities and to guide subsequent medication use (15).
Long-Term Postoperative Management of GRE
The majority of GRE can be managed effectively by either monotherapy or with combination of ASMs. The choice of ASMs should be individualized based on patients’ responses and comorbidities. Recent evidences have shown that antitumor treatment with radiotherapy and chemotherapy may lead to improved seizure control (6, 53). Therefore, the potential interaction with chemotherapy agents should be considered when ASMs is selected. Given the fact that there are few known harmful drug interactions of LEV and VPA with chemotherapeutic agents (22, 23), LEV and VPA are recommended for monotherapy in patients with GRE after surgery to improve seizure control and quality of life (25, 26, 29, 54).
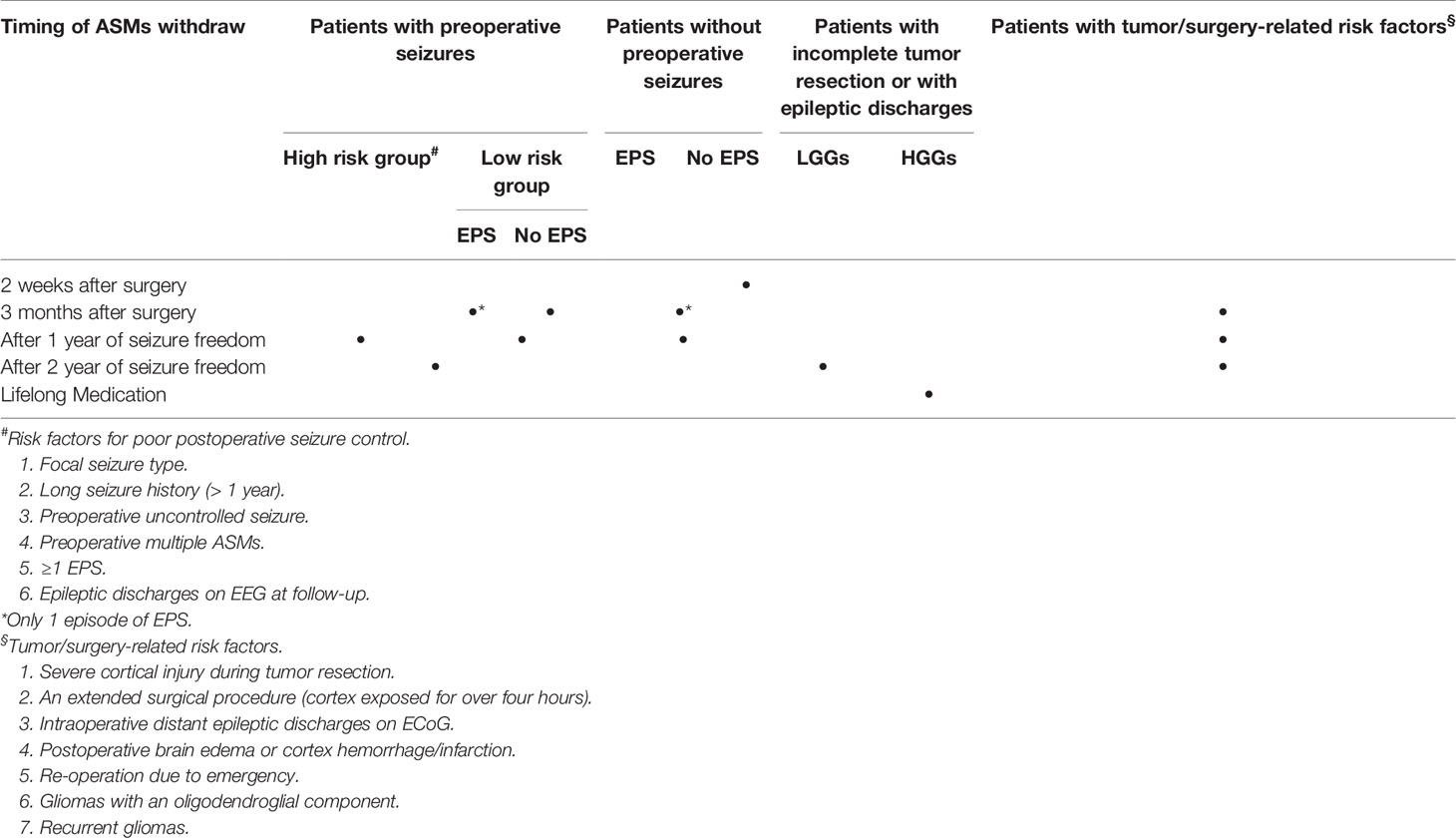
Some patients with glioma and GRE may achieve long-term seizure freedom after gross total resection and adjunct antitumor therapy, raising doubts about the necessity to continue ASMs. The withdrawal of ASMs remains a complex and controversial issue for glioma patients, as seizure risk is highly influenced by tumor types, stages and location, and tumor treatment. It remains very difficult to predict the precise risk of seizure recurrence in GRE patients. In general, it is recommended to avoid AED withdrawal in patients with high risk of seizure recurrence (55). This relapse risk depends on various factors including, but not limited to, patient age, seizure types, tumor location and grade, the extent of a surgical resection, tumor genetic features and types of antitumor treatment (2, 4, 34, 55). A recent study has suggested that AED withdrawal should only be considered in patients with a low risk of tumor progression, even the patients had experienced long-term seizure freedom (56). However, long-time use of AED may lead to significant side effects and should be only given to carefully selected patients. At present time, guidelines regarding the timing of ASMs withdrawal are urgently needed for GRE patients. Based on current evidence (2, 4, 42–44, 55–57), we propose the following recommendations in regarding the timing of ASMs withdrawal for patients with GRE (Table 2):
1. For patients without preoperative seizures and EPS, prophylactic ASMs should be withdrawal 2 weeks after surgery.
2. For patients with preoperative low-risk seizures (generalized seizure type, short-term seizure duration and drug controlled seizures) and without EPS, ASMs may be tapered off after 3 months postoperatively.
3. For patients with preoperative high-risk seizures (focal seizure type, long-term seizure duration and uncontrolled seizures), EPS or repeated postoperative seizures, ASMs withdrawal should be considered after a minimum of 1 year of seizure freedom.
4. For patients with incomplete tumor resection, intraoperative distant epileptic discharges on ECoG or epileptic discharges on EEG at follow-up, AED should be withdrawn no sooner than 2 years seizures freedom after the surgery.
5. For patients with the following tumor/surgery related risk factors, the postoperative duration of ASMs should be prolonged accordingly: (a) severe cortical injury during tumor resection; (b) an extended surgical procedure (cortex exposed for over four hours); (c) postoperative brain edema or cortex hemorrhage/infarction; (d) re-operation due to emergency; (e) gliomas with an oligodendroglial component; (f) recurrent gliomas.
6. For all patients with GBM or anaplastic glioma without complete tumor resection, AED withdrawal is not recommended.
Previous studies have shown that seizure recurrence following a prolonged seizure-free period could be suggestive of tumor progression, which would require prompt repeat imaging and re-evaluation of ASMs (11, 52). If patients suffer from postoperative seizures without evidence of tumor recurrence, adjustment of ASMs should be considered after adequate AED blood levels are confirmed. In cases of drug resistant GRE, epilepsy surgery or radiotherapy could be considered when quality of life is significantly impacted due to frequent seizures (15, 43, 53). Other factors, such as side effects from ASMs, financial and psychosocial aspects should also be considered when managing drug resistant GRE.
Conclusions
Diagnosis of GRE should be based on the coexistence and causal relationship of glioma and epilepsy. Seizure prognosis should be considered as important factor in addition to survival in managing GRE, especially for patients with LGG. Gross total resection of tumor is the most effective therapies for GRE. Intraoperative ECoG and postoperative EEG are useful for patients with perioperative refractory GRE. Standardized ASMs application and appropriate antitumor treatment are highly recommended for the management of GRE. Timing of postoperative ASMs withdrawal should be individualized based on perioperative risk factors.
Future Prospects
Accurate diagnosis of GRE is important from stand point of view of both neuro-oncology and epileptology. Accurate localization seizure onset zone and optimal tumor resection for gliomas may be achieved by incorporating tools used in epilepsy surgery and contemporary neuroimaging technologies. Large well-designed randomized controlled trials are needed to determine the usage of prophylaxis ASMs perioperatively in GRE. Possible survival benefits from ASMs therapy should be further investigated and confirmed. More genetic research is needed to further explore the underlying pathogenesis, and eventually to provide effective treatment for GRE.
Author Contributions
GY wrote the manuscript. ZS and TJ revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (No.81871013), Beijing Municipal Education Commission Science and Technology Plan General Project (No.1192050172), and Beijing Tiantan Hospital Young Scientist Program (YSP201705).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
WHO, World Health Organization; ILAE, International League Against Epilepsy; LGG, low-grade glioma; HGG, high-grade glioma; GBM, glioblastoma; GRE, glioma related epilepsy; ASMs, anti-seizure medications; EEG, electroencephalogram; ECoG, electrocorticography; EPS, early postoperative seizures; IDH1, isocitrate dehydrogenase 1; MGMT, O6-methylguanine-DNA methyltransferase; KCC2, potassium chloride cotransporter; SLC7A11, solute carrier family 7 member 11; MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; PWI, perfusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery imaging; MRS, magnetic resonance spectroscopy; CT, computed tomography; PET, positron emission tomography; SPECT, single-photon emission CT; MEG, magnetoencephalography; TMZ, temozolomide; LEV, levetiracetam; VPA, valproic acid; LCM, lacosamide; PER, perampanel; LTG, lamotrigine; PGB, pregabalin; ZON, zonisamide; BRV, brivaracetam.
References
1. Pallud J, Audureau E, Blonski M, Sanai N, Bauchet L, Fontaine D, et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain (2014) 137(Pt 2):449–62. doi: 10.1093/brain/awt345
2. You G, Sha ZY, Yan W, Zhang W, Wang YZ, Li SW, et al. Seizure characteristics and out comes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro Oncol (2012) 14(2):230–41. doi: 10.1093/neuonc/nor205
3. Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia (2013) 54(Suppl 9):12–7. doi: 10.1111/epi.12437
4. Yu Z, Zhang N, Hameed NUF, Qiu T, Zhuang D, Lu J, et al. The Analysis of Risk Factors and Survival Outcome for Chinese Patients with Epilepsy with High-Grade Glioma. World Neurosurg (2019) 125:e947–57. doi: 10.1016/j.wneu.2019.01.213
5. Yang P, Liang T, Zhang C, Cai J, Zhang W, Chen B, et al. Clinicopathological factors predictive of postoperative seizures in patients with gliomas. Seizure (2016) 35:93–9. doi: 10.1016/j.seizure.2015.12.013
6. Pallud J, McKhann GM. Diffuse Low-Grade Glioma-Related Epilepsy. Neurosurg Clin N Am (2019) 30(1):43–54. doi: 10.1016/j.nec.2018.09.001
7. Huberfeld G, Vecht CJ. Seizures and gliomas-towards a single therapeutic approach. Nat Rev Neurol (2016) 12(4):204–16. doi: 10.1038/nrneurol.2016.26
8. Robert SM, Buckingham SC, Campbell SL, Robel S, Holt KT, Ogunrinu-Babarinde T, et al. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci Transl Med (2015) 7(289):289ra286. doi: 10.1126/scitranslmed.aaa8103
9. Li Y, Shan X, Wu Z, Wang Y, Ling M, Fan X. IDH1 mutation is associated with a higher preoperative seizure incidence in low-grade glioma: A systematic review and meta-analysis. Seizure (2018) 55:76–82. doi: 10.1016/j.seizure.2018.01.011
10. Feyissa AM, Worrell GA, Tatum WO, Chaichana KL, Jentoft ME, Guerrero Cazares H, et al. Potential influence of IDH1 mutation and MGMT gene promoter methylation on glioma-related preoperative seizures and postoperative seizure control. Seizure (2019) 69:283–9. doi: 10.1016/j.seizure.2019.05.018
11. Samudra N, Zacharias T1, Plitt A, Lega B, Pan E. Seizures in glioma patients: An overview of incidence, etiology, and therapies. J Neurol Sci (2019) 404:80–5. doi: 10.1016/j.jns.2019.07.026
12. Yuan Y, Xiang W, Yanhui L, Ruofei L, Shuang L, Yingjun F, et al. Ki-67 overexpression in WHO grade II gliomas is associated with poor postoperative seizure control. Seizure (2013) 22(10):877–81. doi: 10.1016/j.seizure.2013.08.004
13. Yang K, Nath S, Koziarz A, Badhiwala JH, Ghayur H, Sourour M, et al. Biopsy versus subtotal versus gross total resection in patients with low-grade glioma: a systematic review and meta-analysis. World Neurosurg (2018) 120:e762–75. doi: 10.1016/j.wneu.2018.08.163
14. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol (2007) 6(5):421–30. doi: 10.1016/S1474-4422(07)70103-5
15. CAAE. Chinese guideline of diagnosis and treatment for epilepsy. Beijing: People’s Medical Publishing House (2015).
16. Fisher RS, Cross JH, D’Souza C, French JA, Haut SR, Higurashi N, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia (2017) 58(4):531–42. doi: 10.1111/epi.13671
17. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
18. Cavanna AE, Seri S. Neurophysiological investigations for the diagnosis of non-epileptic attack disorder in neuropsychiatry services: from safety standards to improved effectiveness. Acta Neuropsychiatr (2016) 28(4):185–94. doi: 10.1017/neu.2016.10
19. van Dellen E, Douw L, Hillebrand A, Ris-Hilgersom IH, Schoonheim MM, Baayen JC, et al. MEG network differences between low- and high-grade glioma related to epilepsy and cognition. PloS One (2012) 7(11):e50122. doi: 10.1371/journal.pone.0050122
20. Chandra PS, Vaghania G, Bal CS, Tripathi M, Kuruwale N, Arora A, et al. Role of concordance between ictal-subtracted SPECT and PET in predicting long-term outcomesafter epilepsy surgery. Epilepsy Res (2014) 108(10):1782–9. doi: 10.1016/j.eplepsyres.2014.09.024
21. Perucca E. Optimizing antiepileptic drug treatment in tumoral epilepsy. Epilepsia (2013) 54(Suppl. 9):97–104. doi: 10.1111/epi.12452
22. Iuchi T, Kuwabara K, Matsumoto M, Kawasaki K, Hasegawa Y, Sakaida T. Levetiracetam versus phenytoin for seizure prophylaxis during and early after craniotomy for brain tumours: a phase II prospective, randomised study. J Neurol Neurosurg Psychiatry (2015) 86(10):1158–62. doi: 10.1136/jnnp-2014-308584
23. Bähr O, Hermisson M, Rona S, Rieger J, Nussbaum S, Körtvelyessy P, et al. Intravenous and oral levetiracetam in patients with a suspected primary brain tumor and symptomatic seizures undergoing neurosurgery: the HELLO trial. Acta Neurochir (2012) 154(2):229–235 (discussion 235). doi: 10.1007/s00701-011-1144-9
24. Englot DJ, Chang EF, Vecht CJ. Epilepsy and brain tumors. Handb Clin Neurol (2016) 134:267–85. doi: 10.1016/B978-0-12-802997-8.00016-5
25. Kerkhof M, Dielemans J, van Breemen MS, Zwinkels H, Walchenbach R, Taphoorn MJ, et al. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol (2013) 15(7):961–7. doi: 10.1093/neuonc/not057
26. Rosati A, Buttolo L, Stefini R, Todeschini A, Cenzato M, Padovani A. Efficacy and safety of levetiracetam in patients with glioma: a clinical prospective study. Arch Neurol (2010) 67(3):343–6. doi: 10.1001/archneurol.2009.335
27. Nasr ZG, Paravattil B, Wilby KJ. Levetiracetam for seizure prevention in brain tumor patients: a systematic review. J Neurooncol (2016) 129(1):1–13. doi: 10.1007/s11060-016-2146-5
28. Cardona AF, Rojas L, Wills B, Bernal L, Ruiz-Patiño A, Arrieta O, et al. Efficacy and safety of Levetiracetam vs. other antiepileptic drugs in Hispanic patients with glioblastoma. J Neurooncol (2018) 136(2):363–71. doi: 10.1007/s11060-017-2660-0
29. Rossetti AO, Jeckelmann S, Novy J, Roth P, Weller M, Stupp R. Levetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors. A phase II randomized study. Neuro Oncol (2014) 16(4):584–8. doi: 10.1093/neuonc/not170
30. Redjal N, Reinshagen C, Le A, Walcott BP, McDonnell E, Dietrich J, et al. Valproic acid, compared to other antiepileptic drugs, is associated with improved overall and progression-free survival in glioblastoma but worse outcome in grade II/III gliomas treated with temozolomide. J Neurooncol (2016) 127(3):505–14. doi: 10.1007/s11060-016-2054-8
31. Maschio M, Zarabla A, Maialetti A, Fabi A, Vidiri A, Villani V, et al. Quality of life, mood and seizure control in patients with brain tumor related epilepsy treated with lacosamide as add-on therapy: A prospective explorative study with a historical control group. Epilepsy Behav (2017) 73:83–9. doi: 10.1016/j.yebeh.2017.05.031
32. Rizzo A, Donzelli S, Girgenti V, Sacconi A, Vasco C, Salmaggi A, et al. In vitro antineoplastic effects of brivaracetam and lacosamide on human glioma cells. J Exp Clin Cancer Res (2017) 36(1):76. doi: 10.1186/s13046-017-0546-9
33. Patsalos PN. The clinical pharmacology profile of the new antiepileptic drug perampanel: A novel non-competitive AMPA receptor antagonist. Epilepsia (2015) 56(1):12–27. doi: 10.1111/epi.12865
34. Lange F, Weßlau K, Porath K, Hörnschemeyer J, Bergner C, Krause BJ, et al. AMPA receptor antagonist perampanel affects glioblastoma cell growth and glutamate release in vitro. PloS One (2019) 14(2):e0211644. doi: 10.1371/journal.pone.0211644
35. Vecht C, Duran-Peña A, Houillier C, Durand T, Capelle L, Huberfeld G. Seizure response to perampanel in drug-resistant epilepsy with gliomas: early observations. J Neurooncol (2017) 133(3):603–7. doi: 10.1007/s11060-017-2473-1
36. Izumoto S, Miyauchi M, Tasaki T, Okuda T, Nakagawa N, Nakano N, et al. Seizures and Tumor Progression in Glioma Patients with Uncontrollable Epilepsy Treated with Perampanel. Anticancer Res (2018) 38(7):4361–6. doi: 10.21873/anticanres.12737
37. Novy J, Stupp R, Rossetti AO. Pregabalin in patients with primary brain tumors and seizures: a preliminary observation. Clin Neurol Neurosurg (2009) 111:171–3. doi: 10.1016/j.clineuro.2008.09.009
38. Maschio M, Maialetti A, Mocellini C, Domina E, Pauletto G, Costa C, et al. Effect of Brivaracetam on Efficacy and Tolerability in Patients With Brain Tumor-Related Epilepsy: A Retrospective Multicenter Study. Front Neurol (2020) 11:813. doi: 10.3389/fneur.2020.00813
39. Zaccara G, Perucca E. Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord (2014) 16(4):409–32. doi: 10.1684/epd.2014.0714
40. Wu AS, Trinh VT, Suki D, Graham S, Forman A, Weinberg JS, et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. J Neurosurg (2013) 118(4):873–83. doi: 10.3171/2012.12.JNS111970
41. Wang X, Zheng X, Hu S, Xing A, Wang Z, Song Y, et al. Efficacy of perioperative anticonvulsant prophylaxis in seizure-naïve glioma patients: A meta-analysis. Clin Neurol Neurosurg (2019) 186:105529. doi: 10.1016/j.clineuro.2019.105529
42. Zhang J, Yao L, Peng S, Fang Y, Tang R, Liu J. Correlation between glioma location and preoperative seizures: a systematic review and meta-analysis. Neurosurg Rev (2019) 42(3):603–18. doi: 10.1007/s10143-018-1014-5
43. Liang S, Fan X, Zhao M, Shan X, Li W, Ding P, et al. Clinical practice guidelines for the diagnosis and treatment of adult diffuse glioma-related epilepsy. Cancer Med (2019) 8(10):4527–35. doi: 10.1002/cam4.2362
44. Shan X, Fan X, Liu X, Zhao Z, Wang Y, Jiang T. Clinical characteristics associated with postoperative seizure control in adult low-grade gliomas: a systematic review and meta-analysis. Neuro Oncol (2018) 20(3):324–31. doi: 10.1093/neuonc/nox130
45. Yao PS, Zheng SF, Wang F, Kang DZ, Lin YX. Surgery guided with intraoperative electrocorticography in patients with low-grade glioma and refractory seizures. J Neurosurg (2018) 128(3):840–5. doi: 10.3171/2016.11.JNS161296
46. Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. Clin Article J Neurosurg (2011) 115(2):232–9. doi: 10.3171/2011.3.JNS101333
47. Yuan Y, Peizhi Z, Xiang W, Yanhui L, Ruofei L, Shu J, et al. Intraoperative seizures and seizures outcome in patients undergoing awake craniotomy. J Neurosurg Sci (2019) 63(3):301–7. doi: 10.23736/S0390-5616.16.03880-7
48. Gonen T, Grossman R, Sitt R, Nossek E, Yanaki R, Cagnano E, et al. Tumor location and IDH1 mutation may predict intraoperative seizures during awake craniotomy. J Neurosurg (2014) 121(5):1133–8. doi: 10.3171/2014.7.JNS132657
49. Boetto J, Bertram L, Moulinie G, Herbet G, Moritz-Gasser S, Duffau H. Low rate of intraoperative seizures during awake craniotomy in a prospective cohort with 374 supratentorial brain lesions: electrocorticography is not mandatory. World Neurosurg (2015) 84(6):1838–44. doi: 10.1016/j.wneu.2015.07.075
50. Dewan MC, White-Dzuro GA, Brinson PR, Zuckerman SL, Morone PJ, Thompson RC, et al. The Influence of Perioperative Seizure Prophylaxis on Seizure Rate and Hospital Quality Metrics Following Glioma Resection. Neurosurgery (2017) 80(4):563–70. doi: 10.1093/neuros/nyw106
51. Dewan MC, Thompson RC, Kalkanis SN, Barker FG2, Hadjipanayis CG. Prophylactic antiepileptic drug administration following brain tumor resection: results of a recent AANS/CNS Section on Tumors survey. J Neurosurg (2017) 126(6):1772–8. doi: 10.3171/2016.4.JNS16245
52. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist (2014) 19(7):751–9. doi: 10.1634/theoncologist.2014-0060
53. Koekkoek JA, Kerkhof M, Dirven L, Heimans JJ, Reijneveld JC, Taphoorn MJ. Seizure outcome after radiotherapy and chemotherapy in low-grade glioma patients: a systematic review. Neuro Oncol (2015) 17(7):924–34. doi: 10.1093/neuonc/nov032
54. Bénit CP, Vecht CJ. Seizures and cancer: drug interactions of anticonvulsants with chemotherapeutic agents, tyrosine kinase inhibitors and glucocorticoids. Neurooncol Pract (2016) 3(4):245–60. doi: 10.1093/nop/npv038
55. Koekkoek JK, Dirven L, Taphoorn MJ. The withdrawal of antiepileptic drugs in patients with low-grade and anaplastic glioma. Expert Rev Neurother (2017) 17(2):193–202. doi: 10.1080/14737175.2016.1219250
56. Kerkhof M, Koekkoek J, Vos MJ, van den Bent MJ, Taal W, Postma TJ, et al. Withdrawal of antiepileptic drugs in patients with low grade and anaplastic glioma after long-term seizure freedom: a prospective observational study. J Neurooncol (2019) 142(3):463–70. doi: 10.1007/s11060-019-03117-y
Keywords: diagnosis, epilepsy, glioma, seizure, surgery, treatment
Citation: You G, Sha Z and Jiang T (2021) Clinical Diagnosis and Perioperative Management of Glioma-Related Epilepsy. Front. Oncol. 10:550353. doi: 10.3389/fonc.2020.550353
Received: 09 April 2020; Accepted: 24 November 2020;
Published: 14 January 2021.
Edited by:
Letteria Minutoli, University of Messina, ItalyReviewed by:
James Tao, University of Chicago, United StatesJuan Manuel Sepulveda Sanchez, University Hospital October 12, Spain
Copyright © 2021 You, Sha and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Jiang, dGFvamlhbmcxOTY0QDE2My5jb20=; Zhiyi Sha, enlzaGFAdW1uLmVkdQ==
 Gan You
Gan You Zhiyi Sha
Zhiyi Sha Tao Jiang
Tao Jiang