- 1Department of Head and Neck Surgery, Fudan University Shanghai Cancer Center, Fudan University, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
Background: Recently, radioiodine refractory differentiated thyroid cancer (RR-DTC) has received increasing attention due to its poor prognosis. The roles of clinical, pathological, and molecular features in the development of RR-DTC remain controversial and require additional investigation. This study aimed to evaluate the association between these risk factors and the occurrence of RR-DTC.
Methods: We performed a systematic search for relevant literature following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) in PubMed, EMBASE, Medline, SCOPUS, and Web of Science up to the July 15, 2020. Observational studies that investigated the risk factors for RR-DTC were included. Fixed- or random-effects models were used to calculate pooled odds ratios (ORs) or mean differences (MD) with corresponding 95% confidence intervals.
Results: We included 13 eligible studies incorporating 1,431 cases, of which 603 were patients with RR-DTC. The pooled analysis indicated that four parameters significantly increased the risk of RR-DTC: extrathyroidal extension (ETE) (OR: 2.28, 95% CI: 1.43–3.64, I2 = 14%), BRAFV600E mutation (OR: 3.60, 95% CI: 1.74–7.46, I2 = 69%), TERT promoter mutation (OR: 9.84, 95% CI: 3.60–26.89, I2 = 61%) and high-risk histological subtype (OR: 1.94, 95% CI: 1.15–3.27, I2 = 15%), including tall cell variant papillary thyroid carcinoma (PTC), sclerosing diffuse PTC, hobnail variant PTC, follicular thyroid carcinoma (FTC) (including Hürthle cell), and poorly differentiated thyroid carcinoma (PDTC). However, there was no statistical significance regarding sex, age, tumor size, multifocality, or lateral lymph node metastasis. Subgroup and sensitivity analyses were conducted to further confirm the robustness of the results.
Conclusions: Histological subtype, ETE, BRAFV600E mutation, and TERT promoter mutation could be considered clinicopathological factors and biomarkers. They could assist in risk stratification, prognostic prediction, and individual therapy options for RR-DTC.
Introduction
In recent decades, thyroid cancer (TC) has emerged as a striking health issue, and the global incidence of TC is 6.7 per 100,000 (1). Papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) are collectively characterized as differentiated thyroid carcinoma (DTC) and account for more than 90% of all thyroid malignancies. Most DTC cases can be treated successfully by thyroidectomy, selective radioactive iodine (RAI) therapy, and thyroid stimulating hormone (TSH)-suppressive therapy and have a favorable prognosis. However, the incidences of local recurrence and distant metastases are ~30 and 10% (2), respectively. Among these patients, unfortunately, one third show initial or gradual loss of iodine uptake and even a decrease in sodium iodide symporter (NIS) expression in the plasma membrane, indicating a status of dedifferentiation known as RAI-refractory DTC (RR-DTC) (3). A long-term study showed that the 10- and 15-year survival rates of patients without any radioiodine uptake were much lower than those of patients with RAI uptake (10 vs. 56%, and 6 vs. 45%) (4).
The American Thyroid Association (ATA) Management Guidelines for Adult Patients with Thyroid Nodules and Thyroid Cancer has classified RR-DTC in four basic ways: (i) the malignant/metastatic tissue has no concentrated RAI, (ii) the tumor tissue has lost the ability to concentrate RAI after previous evidence of RAI-avid disease, (iii) RAI is concentrated in some lesions but not in others, and (iv) metastatic disease progresses despite significant concentration of RAI (5). There is an increasing demand to understand this kind of cancer better and to predict the response to RAI therapies earlier in the disease course. Clinicopathological characteristics as well as molecular features are meaningful indicators that could be utilized for further characterization and prognostication of this tumor. To provide a reliable reference for the prediction of RR-DTC, we conducted this meta-analysis.
Materials and Methods
The study was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria.
Search Strategy
The search for eligible studies was conducted in PubMed, EMBASE, Medline, SCOPUS, and Web of Science up to July 15, 2020, with English restriction. The following terms were used: “thyroid neoplasms” or “thyroid carcinoma” or “thyroid cancer” or “thyroid tumor,” “iodine radioisotopes” or 131I or radioiodine or “radioactive iodine,” refractory or negative or resistant* or fail* or resist, and predict* or factor or character* or feature or risk. The reference lists of relevant studies and review articles were hand-searched to identify any potential additional relevant articles. The literature search was conducted independently by two investigators, and any inconsistencies were resolved by consensus with a third investigator.
Selection Criteria
The inclusion criteria of the literature in this meta-analysis were as follows: (1) the original research; (2) observational studies designed to evaluate the association between clinical, pathological, or molecular factors and the development of RR-DTC, including retrospective and prospective studies; (3) patients with RR-DTC were well-defined, and the data of the control group were available; and (4) the data of variables reported could be used to calculate the log odds ratio (OR) or mean difference (MD) with 95% confidence interval (CI). Studies were excluded in cases of duplicated, unavailable, or incomplete data. Reviews, case reports, letters, editorials, and expert opinions were also excluded.
Data Extraction and Study Assessment
Two investigators (Y.L. and H.J.) extracted key data from the included articles in a standardized Excel sheet independently, and a third independent investigator (B.M.) checked the extracted data. For each article, data were extracted on the authors, year of publication, country, study type, recruitment period, sample size, demographics (age and sex), histological subtype, case number of patients in the RAI refractory (RAIR) group and the RAI-avid (RAIA) group, and RAIR classifications. Disagreements during data extraction were resolved through discussion among all authors. Study quality was assessed by two investigators (Y.L. and W.X.) using the Newcastle-Ottawa Scale (NOS). A third reviewer (X.W.) was available for mediation. The NOS assigns a maximum of 9 points based on three quality parameters, including selection, comparability, and outcome. A score ≥ 7 indicates good quality.
Statistics Analysis
Risk factors were included in the meta-analysis when they were reported in more than two studies. We considered only adjusted estimates to minimize the impact of confounding factors on pooled effect measures. Thus, for categorical variables, we entered the ratio measures of the (adjusted) effect as a log OR and the standard error (SE) of the log OR using the generic inverse-variance weighting method (6). The mean and standard deviation (SD) of continuous variables were also included in the study. All log ORs and MDs with 95% CIs were calculated. Generally, a fixed- or random-effects model was chosen depending on the heterogeneity, which was regarded as no significance between 25 and 50%, moderate degree between 50 and 75%, and high degree >75%. Different models can lead to quite different outcomes when the heterogeneity is considerable (I2 > 50%, according to the Cochrane Handbook), but this difference will be very slight with data of insignificant heterogeneity. Thus, we conservatively conducted random models for all data to make the results as reliable as possible. In this meta-analysis, subgroup analyses were conducted to explore the source of obvious heterogeneity, and a sensitivity analysis was performed to evaluate the reliability of the associations. A publication bias test was not conducted due to the small sample size. A p < 0.05 was considered statistically significant. All analyses were performed with R software version 3.6.2.
Results
Search Results and Characteristics of Patients
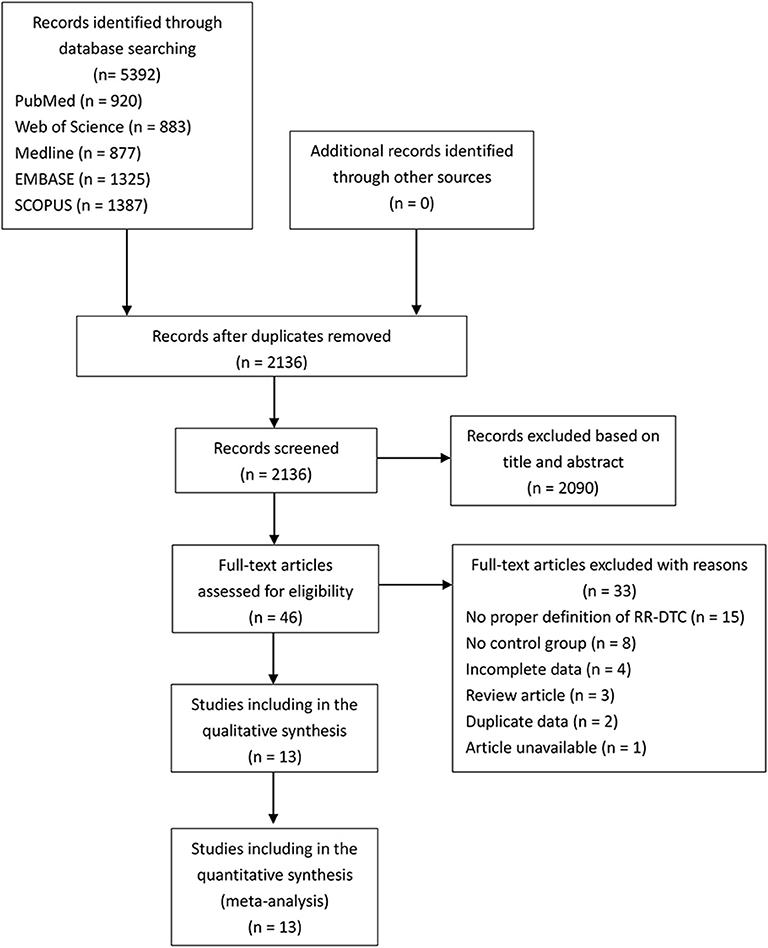
After duplicate studies were excluded, 2,136 articles from PubMed, EMBASE, Medline, SCOPUS, and Web of Science were screened. A total of 2,090 articles were inappropriate for our study according to the title and abstracts. Forty-six remaining studies were retrieved for assessment, and a flow chart showed the process of literature retrieval (Figure 1). Of 13 eligible articles, 12 were retrospective studies, and one study from China was prospective (7–19). Two appropriate reports were included, although they were conference abstracts. Among these 13 studies, five were conducted in China, two in France, two in Korea, two in the United States, and one each in Italy and Germany. There were 603 RR-DTCs and 828 controls involved in the present study. The basic characteristics and NOS results of the identified studies are shown in Table 1 and Supplementary Table 1.
Nine potential risk factors were analyzed to pool the log OR or MD with a 95% CI: age, sex, histological subtype, tumor size, multifocality, extrathyroidal extension (ETE), lateral lymph node metastasis (LLNM), TERT promoter (TERTp) mutation, and BRAFV600E mutation (Supplementary Tables 2, 3). In the meta-analysis, statistically significant clinical predictors were shown, as follows: histological subtype (p = 0.01, OR: 1.94, 95% CI: 1.15–3.27) (Figure 2A) and ETE (p < 0.01, OR: 2.28, 95% CI: 1.43–3.64) (Figure 2B). At the molecular level, significant effects were also found for both the TERTp mutation (p < 0.01, OR: 9.84, 95% CI: 3.60–26.89) and BRAFV600E mutation (p < 0.01, OR: 3.60, 95% CI: 1.74–7.46) (Figure 3). However, there was no statistical significance in sex (p = 0.95, OR: 1.01, 95% CI: 0.74–1.38) (Figure 2C), age (p = 0.24, MD: 1.48 years, 95% CI: −1.01 to 3.96) (Figure 2D), tumor size (p = 0.17, MD: 0.64 cm, 95% CI: −0.27 to 1.55) (Figure 2E), multifocality (p = 0.53, OR:1.24,95% CI: 0.63–2.42) (Figure 2F), or LLNM (p = 0.23, OR: 2.49, 95% CI: 0.56–11.01) (Figure 2G).
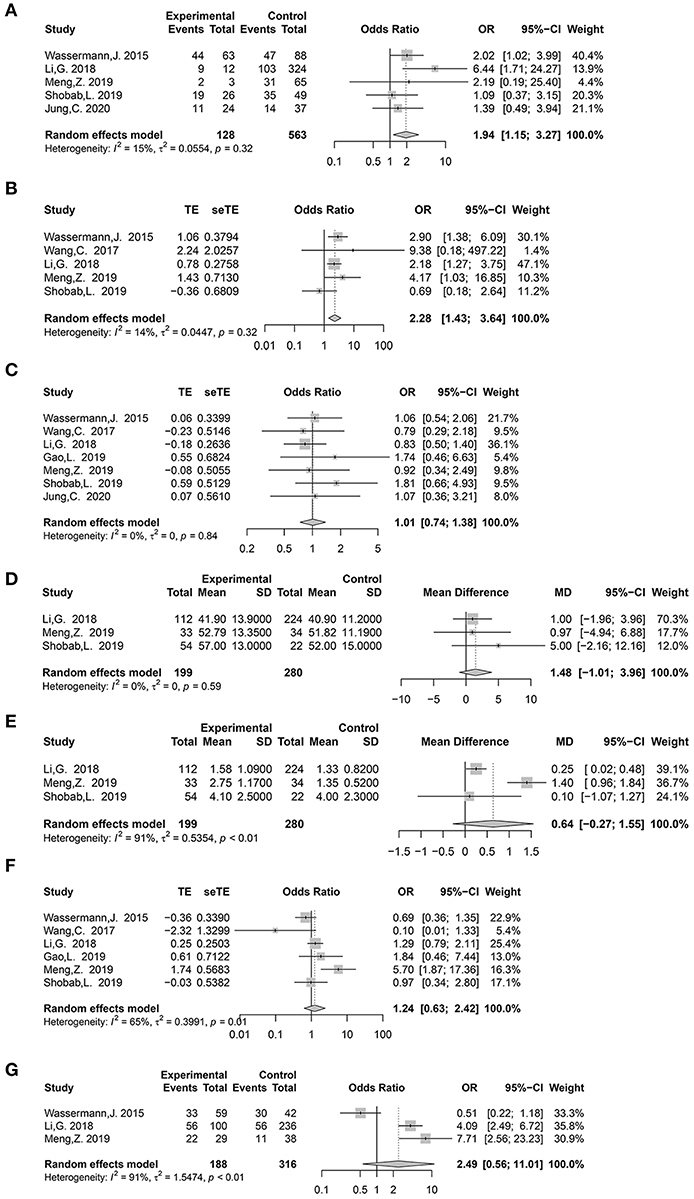
Figure 2. Forest plot detailing odds ratio and mean difference with 95% confidence intervals (CI) for the effect of histological subtype (A), extrathyroidal extension (B), gender (C), age (years) (D), tumor size (cm) (E), multifocality (F), and lateral lymph node metastasis (G).

Figure 3. Forest plot detailing the odds ratios with 95% confidence intervals for the effect of TERT promoter mutation (A) and BRAFV600E mutation (B).
Subgroup Analysis
Heterogeneities were obvious for the following five features: tumor size (I2 = 91%, p < 0.01), LLNM (I2 = 91%, p < 0.01), multifocality (I2 = 65%, p = 0.01), BRAFV600E mutation (I2 = 69%, p < 0.01), and TERTp mutation (I2 = 61%, p = 0.03) (Supplementary Tables 2, 3). Due to the limited number of studies included, we performed subgroup analyses for the latter three to explore whether heterogeneity might be caused by countries and recruitment periods (Supplementary Table 4 and Supplementary Figure 1).
Multifocality of Different Countries
When subanalyzing multifocality, Asian studies continued to demonstrate moderate heterogeneity (I2 = 70%, p = 0.02), whereas heterogeneity showed a marked decrease in Western countries (I2 = 0%, p = 0.60) (Figure 4A). Both Asian (p = 0.43) and Western countries (p = 0.35) demonstrated a statistically insignificant association between multifocality and RR-DTC.
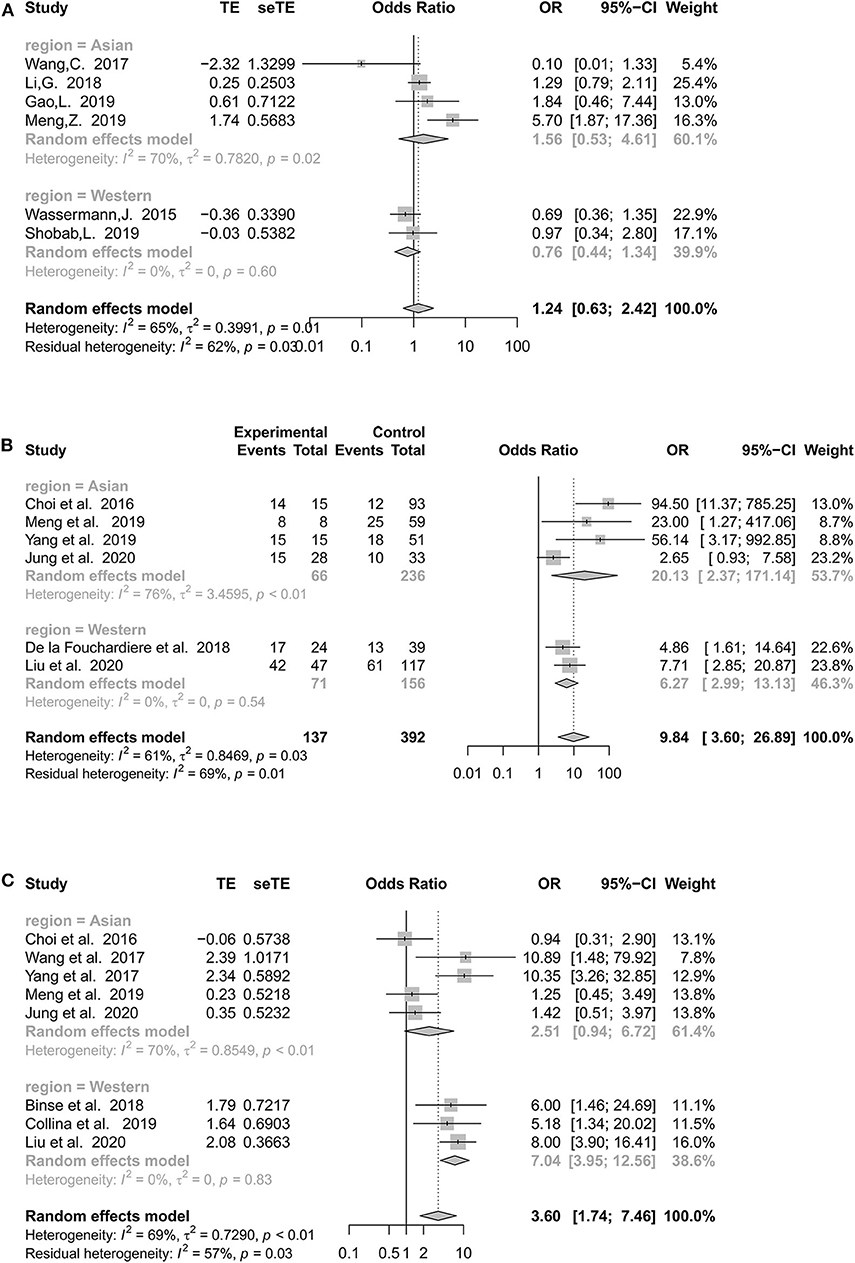
Figure 4. Forest plot detailing the odds ratios with 95% confidence intervals for the effect of multifocality (A), TERT promoter mutation (B), and BRAFV600E mutation (C) according to different regions.
TERTp Mutation of Different Countries
National classification did not decrease the heterogeneity of TERTp mutations in the Asian group (I2 = 76%, p < 0.01), and the pooled estimates remained positive (OR: 20.13, 95% CI: 2.37–171.14, p < 0.01). Interestingly, there was no significant heterogeneity in Western countries (Figure 4B).
BRAF Mutations in Different Countries
Asian populations continued to demonstrate considerable heterogeneity, with an I2 of 70%. Asian studies (OR: 2.51, 95% CI: 0.94–6.72; p = 0.07) failed to show a significant association between the BRAFV600E mutation and RR-DTC (Figure 4C). However, the heterogeneity of Western studies was 0% (OR: 7.04, 95% CI: 3.95–12.56, p < 0.01).
Genetic Factors of Different Recruitment Periods of Study
As shown in Table 1, the recruitment period differs in each study. We divided the data into two parts, before and after 2011, based on the median year of the inclusion time of each study. It showed that there was no obvious improvement in the heterogeneities of both TERTp and BRAFV600E mutations (Supplementary Figure 1).
Cumulative Meta-Analysis of Genetic Factors
To determine whether the predictive ability of TERTp and BRAFV600E mutation changes over time, we performed a cumulative meta-analysis depending on the main recruitment periods. The OR of the BRAFV600E mutation obviously declined over time, while there was no clear tendency in the TERTp mutation (Figure 5).
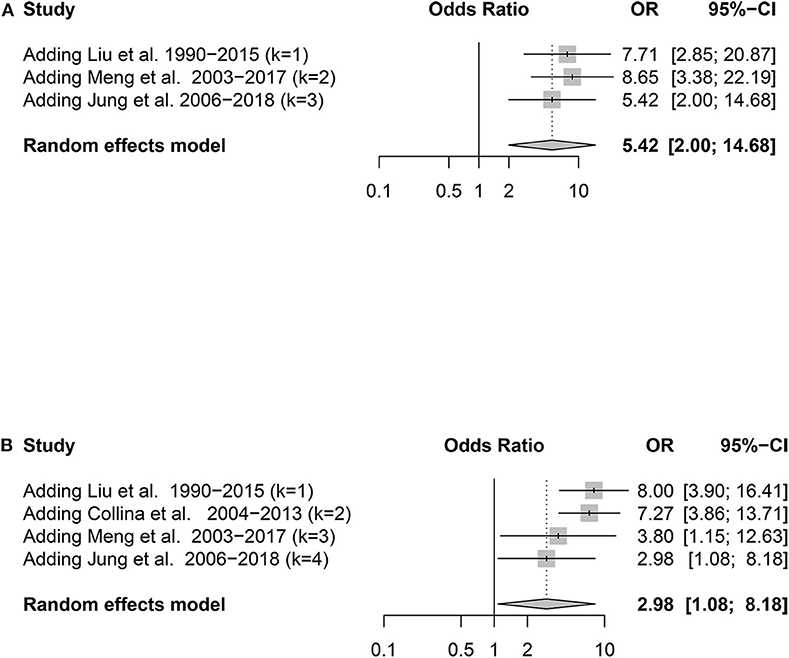
Figure 5. Forest plot detailing the odds ratios with 95% confidence intervals for the effect of TERT promoter mutation (A) and BRAFV600E mutation (B) in the cumulative meta-analysis.
Sensitivity Analysis
We performed a leave-one-out sensitivity analysis to evaluate the robustness of pooled ORs and MDs for all nine factors (Supplementary Figure 2). The effect size of age, sex, histological subtype, ETE, and BRAFV600E mutation revealed no significant change, indicating the stability of these risk factors.
The heterogeneity of tumor size was high (I2 = 91%), but there was no heterogeneity after removing the study (I2 = 0%) (11). The pooled OR was recalculated, and it was a statistically significant result (before: MD 0.64 cm, 95% CI: −0.27 to 1.55, p = 0.17; after MD 0.24 cm, 95% CI: 0.02–0.47, p = 0.03). For multifocality, we found that one study (11) could be the reason for the high heterogeneity, because the result became stable after excluding it (before: I2 = 65%; after: I2 = 34%). However, the p-value was still statistically insignificant despite the fact that a fixed-effect model was used to replace the former random-effects model, which indicated that multifocality was not a proper predictor of the occurrence of RR-DTC. During the sensitivity analysis of LLNM, it was found that the deletion of one study (18) turned the high heterogeneity (I2 = 91%) into no heterogeneity (I2 = 5%) with a statistically significant outcome (before: OR 2.49, 95% CI: 0.56–11.01, p = 0.23; after: OR 4.61, 95% CI: 2.84–7.48, p < 0.01).
At the molecular level, for TERTp mutation, when we removed one study (9), the pooled log OR of RR-DTC decreased from 9.84 (95% CI: 3.60–26.89, p < 0.01) to 6.13 (95% CI: 2.78–13.47, p < 0.01), and the statistical heterogeneity declined (before: I2 = 61%; after: I2 = 35%), which suggested that it could be the source of heterogeneity. However, there were few changes in the statistical heterogeneity after removing the literature sequentially in the sensitivity analysis of the BRAFV600E mutation, indicating its robust outcome.
Discussion
Recently, RR-DTC has become a tricky problem as a result of the increasing number of patients with PTC around the world. The present study of 11 observational studies synthesized and explored potential risk factors for RR-DTC. We found four risk factors, as follows: histological subtype, ETE, TERTp mutation, and BRAFV600E mutation.
Generally, grades of malignancy were related to age and sex in DTCs, wherein younger and female patients have a better prognosis. Some have reported that RAI avidity has a significant inverse correlation with age (12, 13, 20, 21). Males with RR-DTC tend to have poorer survival than females (4). However, our results showed that demographics, including age and sex, were not statistically significant. A possible explanation is that most of the literature included was age- and sex-matched. Nevertheless, to our experience, young age may be a protective factor, but further investigations of the association between demographics and RR-DTC are required. In addition, publication bias could not be ignored when making conclusions.
Histology has been known as an important predictor of prognosis in PTC. In patients with RR-DTC, some studies illustrated that FTC, Hürthle cell, and poorly differentiated thyroid carcinoma (PDTC) had a higher possibility of being refractory to radioiodine treatment (22–25). It was also found that there was a considerable amount of histologic plasticity between the primary lesions and metastases, indicating that tumor cells became less differentiated during the progression of 131I treatment (26). In the present study, classic and follicular variant PTCs were less aggressive histological subtypes, which were classified as the low-risk histological group, while the others were categorized as the high-risk histological group: tall cell variant PTC, sclerosing diffuse PTC, hobnail variant PTC, FTC (including Hürthle cell), and PDTC. The meta-analysis further confirmed that histological subtype was a predictor of RR-DTC (OR: 1.94, 95% CI: 1.15–3.27, p = 0.01) with low heterogeneity (I2 = 15%, p = 0.32). Sensitivity analysis also assessed the stability of the association.
Generally, a large tumor size is a useful feature for indicating the worse prognosis of PTC (27, 28), but it remains unknown in RR-DTC. Here, we drew the opposite conclusion. Three studies including 100 RR-DTCs and 115 controls were analyzed in this part: two from China (11, 17) and one from America (12). In the sensitivity analysis, deletion of one study (11) changed the high heterogeneity into no heterogeneity. Nevertheless, regardless of whether sensitivity analysis was performed, the MD between patients with RR-DTC and controls was minor (before sensitivity analysis: 0.64 cm; after sensitivity analysis: 0.24 cm). Thus, it has relatively low clinical significance and value. Of course, it is necessary to investigate and further prove this idea.
There is no doubt that ETE correlates with poor survival of PTCs (29–31), whereas the value of ETE in RR-DTC is unclear. The present study illustrated that ETE had a positive relationship with the development of RR-DTC (p < 0.01, OR: 2.28, 95% CI: 1.43–3.64), and the sensitivity analysis confirmed the robustness of the results. However, ETE is associated with other factors, such as histological subtype, the location of the primary lesion, and the timing of diagnosis. For example, the ETE probability of tumors is higher in the isthmus than in the thyroid gland lobe due to its anatomical position, while the incidence of RR-DTC might be similar on the assumed premise of the same clinical stage and the same histology. Thus, ETE could be a risk factor, but it needs to be combined with other factors when making clinical decisions and strategies, and further studies are urgently needed.
Both multifocality and LLNM had no statistical association with the occurrence of radioiodine resistance in the overall study. However, further analysis suggested a positive relationship between LLNM and RR-DTC (OR 4.61, 95% CI: 2.84–7.48, p < 0.01, I2 = 5%) by removing one study (18). This might be explained by the fact that lymph nodes were not evaluated by a pathologist (Nx) in more than one third of patients in both the refractory DTC and the control groups in one study (18). Therefore, the great difference would be reconciled if this part of the data were complete. Although the subgroup analysis for LLNM was not conducted due to the similar study number limitation as tumor size, the outcome was relatively reliable because the literature remaining (11, 17) contained many patients, specifically 403 patients with DTC, of whom 145 were diagnosed with RR-DTC. LLNM is a predictor of the prognosis of DTC (32–34), which reflects the aggressiveness of tumors similar to ETE. Unfortunately, there are few studies relevant to LLNM and RR-DTC at the moment, and only a tendency was shown here. More studies related to metastatic lymph nodes are required to prove this concept.
Molecular analysis provides useful insight into the role of predicting the occurrence of RR-DTC, and BRAFV600E/TERTp mutations are associated with greater aggressiveness of TC. Liu et al. (35) reported that the TERTp mutation was a malignant molecular marker for follicular cell-derived thyroid carcinoma. Possible mechanisms behind the association between genetic mutations and RR-DTC have been found. First, there are frequent mutations in C228T and C250T, two prevalent hot positions, which may induce hyperactivity of TERT and lead to over proliferation and carcinogenesis (36, 37). Second, one hallmark of dedifferentiation of DTC is impairment of NIS function (38). Tavares reported a genetic background in which BRAFV600E, TERTp, and NRAS inversely correlated with NIS expression (39). Currently, the BRAFV600E mutation has been found to repress NIS expression in two ways: the TGFβ-Smad3–PAX8 pathway and histone deacetylation of NIS (40–42). The meta-analysis indicated that positive BRAFV600E (p < 0.01, OR: 3.60, 95% CI: 1.74–7.46) and TERTp (p < 0.01, OR: 9.84, 95% CI: 3.60–26.89) mutations were risk factors for RR-DTC. However, according to the results of the cumulative meta-analysis, we found an obvious decreasing tendency of the prediction effect of the BRAFV600E mutation, which could be used to explain the considerable heterogeneity of the BRAFV600E mutation. Over the past few decades, an increased incidence of BRAFV600E mutations has been reported in PTCs, especially classic papillary PTCs (43–45). In the meantime, the incidence of RR-DTC remains stable relatively. Thus, the RAIR predictive ability of BRAFV600E mutations may decline because of the attenuation effect conducted by the increasing amount of PTCs with BRAFV600E mutations. The possible reason is the great advances in genetic testing technology with higher sensitivity and specificity currently so that we could detect the BRAFV600E mutations we could not before. Additionally, the predictive role of BRAF should be further considered in patients with papillary thyroid microcarcinoma (PTMC), defined as a tumor of 1 cm or less in size. Based on the available information, the 2015 ATA suggested that BRAF had a limited role in increasing the recurrence risk of patients with PTMC (46). Unfortunately, we lacked PTMC data to perform a subgroup analysis of PTMC in our study. A possible reason why there were no data could be the good prognosis and the quite low recurrence rate of PTMC, 2–6% locoregional recurrence, and 1–2% distant recurrence (47, 48). Thus, it is too rare for a patient with PTMC to develop RR-DTC. Hence, the BRAFV600E would be a predictor of RR-DTC for patients with PTC except PTMC. Interestingly, according to our subgroup and sensitivity analyses of TERTp mutation, a Korean study (9) was found to increase the heterogeneity and the pooled OR considerably (before: OR 9.84, p < 0.01, 95% CI: 3.60–26.89, I2 = 61%; after: OR 6.13, p < 0.01, 95% CI: 2.78–13.47, I2 = 35%). The possible explanation could be that the control group was the RAIA group without distant metastasis, within whom the TERTp mutation would be much lower. It could be improved if the control group had a similar ratio of local recurrence and distant metastasis to the RAIR group.
Moreover, when further considering the coexistence of TERT/BRAF mutations, the predictive power seems to be better. Vuong reported that concurrent TERT/BRAF mutations were associated with increased tumor aggressiveness than were PTCs with BRAFV600E or TERTp mutation alone, and TERTp mutation was more high risk than BRAFV600E in terms of tumor aggressiveness (49). It was also reported that distant metastases showed enrichment in TERTp mutations and a decrease in BRAF mutations in comparison with paired primary tumors (50, 51). In addition, Yang et al. (8) indicated a greater proportion of TERTp mutations in the RAIR group than in the BRAF group. Nevertheless, it remains unclear whether TERTp mutation dominates prognosis prediction or whether there are any other better predictive genetic factors, such as RAS. In summary, both the BRAFV600E mutation and TERTp mutation were meaningful predictors for RR-DTC, and a joint evaluation was required. Once the coexistence of TERT/BRAF mutations is confirmed, high attention should be paid to RAIR patients.
Despite our efforts, several potential limitations should be noted in the meta-analysis. First, the small number of eligible studies restricted the analytical process. The majority of studies included were retrospective. Second, the definition and classifications of RR-DTC varied slightly between the included studies. However, the current classifications that we could find were not necessarily sufficient evidence for diagnosing radioiodine refractory disease, although they were clinically useful (52). Third, consideration of confounding factors varied across studies, and certain valuable factors were not consistently reported, such as race, TNM stage, number of RAI treatments, and cumulative dose of RAI. Hence, the subgroup analysis was limited, and we cannot restore heterogeneous sources more authentically and meticulously. Finally, our meta-analysis was based on study-level data but not individual participant data. Individual participant-level meta-analysis could provide more reliable risk estimates than the study-level meta-analysis.
Conclusion
This meta-analysis indicated that high-risk histological subtypes, ETE, TERTp mutation, and BRAFV600E mutation are related to the occurrence of iodine resistance in PTCs. High-risk histological subtypes include tall cell variant PTC, sclerosing diffuse PTC, hobnail variant PTC, FTC (including Hürthle cell), and PDTC. In contrast, tumor diameter and multifocality were not predictors according to our results. Meanwhile, other factors, including age, sex, and LLNM, may be useful and valuable predictors, but their values in RR-DTC need to be further investigated.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
YW: conceptualization, supervision, and project administration. YL: methodology. YL, HJ, WX, XW, and BM: acquisition, analysis, curation, and validation of data. YL, HJ, WX, XW, BM, and TL: writing—original draft preparation, review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Science and Technology Commission of Shanghai Municipality (19411966600 to YW) and the Shanghai Anticancer Association (SACA-AX106 to YW and SACA-CY19B01 to BM).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.549882/full#supplementary-material
Supplementary Figure 1. Forest plot detailing TERT promoter mutation (A) and BRAFV600E mutation (B) according to different recruitment periods (before 2011 and after 2011).
Supplementary Figure 2. Forest plot detailing the leave-one-out sensitivity analysis for the effect of sex (A), age (years) (B), tumor size (cm) (C), multifocality (D), histological subtype (E), extrathyroidal extension (F), lateral lymph node metastasis (G), BRAFV600E mutation (H), and TERT promoter mutation (I).
Supplementary Table 1. Quality of eligible studies (Newcastle-Ottawa Scale).
Supplementary Table 2. Analysis of the categorical variables for patients with RR-DTC.
Supplementary Table 3. Analysis of the continuous variables for patients with RR-DTC.
Supplementary Table 4. Analysis of factors of RR-DTC patients in different regions.
References
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. (2019) 144:1941–53. doi: 10.1002/ijc.31937
2. Liu FH, Kuo S-F, Hsueh C, Chao T-C, Lin J-D. Postoperative recurrence of papillary thyroid carcinoma with lymph node metastasis. J Surg Oncol. (2015) 112:149–54. doi: 10.1002/jso.23967
3. Schlumberger M, Brose M, Elisei R, Leboulleux S, Luster M, Pitoia F, et al. Definition and management of radioactive iodine-refractory differentiated thyroid cancer. Lancet Diabetes Endocrinol. (2014) 2:356–8. doi: 10.1016/S2213-8587(13)70215-8
4. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. (2006) 91:2892–9. doi: 10.1210/jc.2005-2838
5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
6. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (eds). Cochrane Handbook for Systematic Reviews of Interventions, 2nd Edn. Chichester: John Wiley & Sons (2019).
7. Binse I, Pokladek J, Weber MM, Theurer S, Jentzen W, Nagarajah J, et al. BRAF mutation analysis for initial risk stratification in high risk papillary thyroid carcinoma. Eur J Nucl Med Mol. (2018) 45(Suppl. 1):S551. Available online at: https://posterng.netkey.at/eanm/viewing/index.php?module=viewing_poster&task=&pi=3174
8. Yang X, Li J, Li X, Liang Z, Gao W, Liang J, et al. TERT promoter mutation predicts radioiodine-refractory character in distant metastatic differentiated thyroid cancer. J Nucl Med. (2017) 58:258–65. doi: 10.2967/jnumed.116.180240
9. Choi SE, Pyo JY, Kim JS, Shin E, Hong SW. Molecular and histopathologic characteristics of radioiodine-refractory papillary thyroid cancer. Lab Invest. (2016) 1:147A−8. Available online at: https://ir.ymlib.yonsei.ac.kr/handle/22282913/148991
10. Gao L, Lin Y, Jiang Y, Li H, Gao Q, Xi X, et al. Ultrasound characteristics of cervical lesions in patients with radioiodine refractory differentiated thyroid cancer: a strobe-compliant article. Medicine. (2019) 98:e17876. doi: 10.1097/MD.0000000000017876
11. Meng Z, Matsuse M, Saenko V, Yamashita S, Ren P, Zheng X, et al. TERT promoter mutation in primary papillary thyroid carcinoma lesions predicts absent or lower 131 i uptake in metastases. IUBMB Life. (2019) 71:1030–40. doi: 10.1002/iub.2056
12. Shobab L, Gomes-Lima C, Zeymo A, Feldman R, Jonklaas J, Wartofsky L, et al. Clinical, pathological, and molecular profiling of radioactive iodine refractory differentiated thyroid cancer. Thyroid. (2019) 29:1262–8. doi: 10.1089/thy.2019.0075
13. Wang C, Zhang X, Li H, Li X, Lin Y. Quantitative thyroglobulin response to radioactive iodine treatment in predicting radioactive iodine-refractory thyroid cancer with pulmonary metastasis. PLoS One. (2017) 12:e0179664. doi: 10.1371/journal.pone.0179664
14. Collina F, La Sala L, Liotti F, Prevete N, La Mantia E, Chiofalo MG, et al. AXL is a novel predictive factor and therapeutic target for radioactive iodine refractory thyroid cancer. Cancers. (2019) 11:785. doi: 10.3390/cancers11060785
15. Liu J, Liu R, Shen X, Zhu G, Li B, Xing M. The genetic duet of BRAF V600E and TERT promoter mutations robustly predicts loss of radioiodine avidity in recurrent papillary thyroid cancer. J Nuclear Med. (2020) 61:177–82. doi: 10.2967/jnumed.119.227652
16. de la Fouchardiere C, Decaussin-Petrucci M, Berthiller J, Descotes F, Lopez J, Lifante JC, et al. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur J Cancer. (2018) 92:40–7. doi: 10.1016/j.ejca.2017.12.027
17. Li G, Lei J, Song L, Jiang K, Wei T, Li Z, et al. Radioiodine refractoriness score: a multivariable prediction model for postoperative radioiodine-refractory differentiated thyroid carcinomas. Cancer Med. (2018) 7:5448–56. doi: 10.1002/cam4.1794
18. Wassermann J, Bernier MO, Spano JP, Lepoutre-Lussey C, Buffet C, Simon JM, et al. Outcomes and prognostic factors in radioiodine refractory differentiated thyroid carcinomas. Oncologist. (2016) 21:50–8. doi: 10.1634/theoncologist.2015-0107
19. Jung CK, Jung SH, Jeon S, Jeong YM, Kim Y, Lee S, et al. Risk stratification using a novel genetic classifier including PLEKHS1 promoter mutations for differentiated thyroid cancer with distant metastasis. Thyroid. (2020) doi: 10.1089/thy.2019.0459. [Epub ahead of print].
20. Kikumori T, Iwano S. Relationship between age and radioactive iodine uptake of recurrent lesion of differentiated thyroid carcinoma. Thyroid. (2015) 1:A232.
21. Larwanou MM, Houda S, El Ouahabi H, Alaoui NI. Differentiated metastatic thyroid cancer in 70 cases. Med Nucl. (2019) 44:12–7. doi: 10.1016/j.mednuc.2019.08.002
22. Kim HJ, Lee JI, Kim NK, Min Y-K, Kim SW, Chung JH. Prognostic implications of radioiodine avidity and serum thyroglobulin in differentiated thyroid carcinoma with distant metastasis. World J Surg. (2013) 37:2845–52. doi: 10.1007/s00268-013-2213-4
23. Besic N, Vidergar-Kralj B, Frkovic-Grazio S, Movrin-Stanovnik T, Auersperg M. The role of radioactive iodine in the treatment of hürthle cell carcinoma of the thyroid. Thyroid. (2003) 13:577–84. doi: 10.1089/105072503322238845
24. Ghossein RA, Hiltzik DH, Carlson DL, Patel S, Shaha A, Shah JP, et al. Prognostic factors of recurrence in encapsulated Hurthle cell carcinoma of the thyroid gland : a clinicopathologic study of 50 cases. Cancer. (2006) 106:1669–76. doi: 10.1002/cncr.21825
25. Bonichon F, Schvartz C, Toubeau M, Soubeyran I, Godbert Y, Carrat X, et al. Hurthle cell thyroid carcinoma: a multicentric retrospective study of 130 patients. Thyroid. (2012) 1:A100.
26. Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM. Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer. (2008) 113:48–56. doi: 10.1002/cncr.23515
27. Tam S, Amit M, Boonsripitayanon M, Busaidy NL, Cabanillas ME, Waguespack SG, et al. Effect of tumor size and minimal extrathyroidal extension in patients with differentiated thyroid cancer. Thyroid. (2018) 28:982–90. doi: 10.1089/thy.2017.0513
28. Angell TE, Maurer R, Wang Z, Kim MI, Alexander CA, Barletta JA, et al. A cohort analysis of clinical and ultrasound variables predicting cancer risk in 20,001 consecutive thyroid nodules. J Clin Endocrinol Metab. (2019) 104:5665–72. doi: 10.1210/jc.2019-00664
29. Youngwirth LM, Adam MA, Scheri RP, Roman SA, Sosa JA. Extrathyroidal extension is associated with compromised survival in patients with thyroid cancer. Thyroid. (2017) 27:626–31. doi: 10.1089/thy.2016.0132
30. Jin BJ, Kim MK, Ji YB, Song CM, Park JH, Tae K. Characteristics and significance of minimal and maximal extrathyroidal extension in papillary thyroid carcinoma. Oral Oncol. (2015) 51:759–63. doi: 10.1016/j.oraloncology.2015.05.010
31. Shaha AR. Extrathyroidal extension-what does it mean. Oral Oncol. (2017) 68:50–2. doi: 10.1016/j.oraloncology.2017.03.008
32. Hirsch D, Levy S, Tsvetov G, Gorstein A, Slutzky-Shraga I, Akirov A, et al. Long term outcomes and prognostic factors in patients with differentiated thyroid cancer and distant metastases. Endocr Pract. (2017) 23:1193–200. doi: 10.4158/EP171924.OR
33. Liu Z, Chen S, Huang Y, Hu D, Zeng W, Wang M, et al. Synergic effects of histology subtype, tumor size, and lymph node metastasis on distant metastasis in differentiated thyroid cancer. Ann Transl Med. (2019) 7:533. doi: 10.21037/atm.2019.09.137
34. Dong W, Horiuchi K, Tokumitsu H, Sakamoto A, Noguchi E, Ueda Y, et al. Time-varying pattern of mortality and recurrence from papillary thyroid cancer: lessons from a long-term follow-up. Thyroid. (2019) 29:802–8. doi: 10.1089/thy.2018.0128
35. Liu X, Bishop J, Shan Y, Pai S, Liu D, Murugan AK, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. (2013) 20:603–10. doi: 10.1530/ERC-13-0210
36. Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. (2013) 339:959–61. doi: 10.1126/science.1230062
37. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. (2013) 339:957–9. doi: 10.1126/science.1229259
38. Paladino S, Melillo RM. Editorial: novel mechanism of radioactive iodine refractivity in thyroid cancer. J Natl Cancer Inst. (2017) 109. doi: 10.1093/jnci/djx106
39. Tavares C, Coelho MJ, Eloy C, Melo M, da Rocha AG, Pestana A, et al. NIS expression in thyroid tumors, relation with prognosis clinicopathological and molecular features. Endocrine Connect. (2018) 7:78–90. doi: 10.1530/EC-17-0302
40. Riesco-Eizaguirre G, Rodríguez I, De la Vieja A, Costamagna E, Carrasco N, Nistal M, et al. The BRAFV600E oncogene induces transforming growth factor beta secretion leading to sodium iodide symporter repression and increased malignancy in thyroid cancer. Cancer Res. (2009) 69:8317–25. doi: 10.1158/0008-5472.CAN-09-1248
41. Costamagna E. The functional interaction between the paired domain transcription factor Pax8 and Smad3 is involved in transforming growth factor- repression of the sodium/iodide symporter gene. J Biol Chem. (2003) 279:3439–46. doi: 10.1074/jbc.M307138200
42. Zhang Z, Liu D, Murugan AK, Liu Z, Xing M. Histone deacetylation of NIS promoter underlies BRAF V600E-promoted NIS silencing in thyroid cancer. Endocr Relat Cancer. (2014) 21:161–73. doi: 10.1530/ERC-13-0399
43. Romei C, Fugazzola L, Puxeddu E, Frasca F, Viola D, Muzza M, et al. Modifications in the papillary thyroid cancer gene profile over the last 15 years. J Clin Endocrinol Metab. (2012) 97:E1758–65. doi: 10.1210/jc.2012-1269
44. Vuong HG, Altibi AM, Abdelhamid AH, Ngoc PU, Quan VD, Tantawi MY, et al. The changing characteristics and molecular profiles of papillary thyroid carcinoma over time: a systematic review. Oncotarget. (2017) 8:10637–49. doi: 10.18632/oncotarget.12885
45. Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA Jr, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. (2014) 99:E276–85. doi: 10.1210/jc.2013-2503
46. Luster M, Aktolun C, Amendoeira I, Barczynski M, Bible KC, Duntas LH, et al. European perspective on 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: proceedings of an interactive international symposium. Thyroid. (2019) 29:7–26. doi: 10.1089/thy.2017.0129
47. Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. (2007) 13:498–512. doi: 10.4158/EP.13.5.498
48. Hay I. Management of patients with low-risk papillary thyroid carcinoma. Endocr Pract. (2007) 13:521–33. doi: 10.4158/EP.13.5.521
49. Vuong HG, Altibi AMA, Duong UNP, Hassell L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin Endocrinol. (2017) 87:411–7. doi: 10.1111/cen.13413
50. Melo M, Gaspar da Rocha A, Batista R, Vinagre J, Martins MJ, Costa G, et al. TERT, BRAF, and NRAS in primary thyroid cancer and metastatic disease. J Clin Endocrinol Metab. (2017) 102:1898–907. doi: 10.1210/jc.2016-2785
51. Vuong HG, Altibi AM, Duong UN, Ngo HT, Pham TQ, Tran HM, et al. Role of molecular markers to predict distant metastasis in papillary thyroid carcinoma: promising value of TERT promoter mutations and insignificant role of BRAF mutations-a meta-analysis. Tumour Boil. (2017) 39:1010428317713913. doi: 10.1177/1010428317713913
Keywords: thyroid cancer, radioactive iodine refractory (RAIR), poorly differentiated thyroid cancer, risk factors, meta-analysis
Citation: Luo Y, Jiang H, Xu W, Wang X, Ma B, Liao T and Wang Y (2020) Clinical, Pathological, and Molecular Characteristics Correlating to the Occurrence of Radioiodine Refractory Differentiated Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 10:549882. doi: 10.3389/fonc.2020.549882
Received: 07 April 2020; Accepted: 21 August 2020;
Published: 30 September 2020.
Edited by:
Paula Soares, Universidade do Porto, PortugalReviewed by:
Huy Gia Vuong, University of Oklahoma Health Sciences Center, United StatesRengyun Liu, The First Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2020 Luo, Jiang, Xu, Wang, Ma, Liao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Wang, bmVjazEzMEBob3RtYWlsLmNvbQ==
 Yi Luo
Yi Luo Hongyi Jiang1,2
Hongyi Jiang1,2