- 1Department of Radiation Oncology, Peking University Third Hospital, Beijing, China
- 2Department of Radiation Oncology, Bayannur Hospital, Bayannur, China
Background: Management of locally recurrent rectal cancer (LRRC) after surgery or external beam radiotherapy (EBRT) remains a clinical challenge, given the limited treatment options and unsatisfactory outcomes. This study aimed to assess long-term outcomes of computed tomography (CT)-guided radioactive 125I seed implantation in patients with LRRC and associated prognostic factors.
Methods: A total of 101 patients with LRRC treated with CT-guided 125I seed implantation from October 2003 to April 2019 were retrospectively studied. Treatment procedures involved preoperative planning design, 125I seed implantation, and postoperative dose evaluation. We evaluated the therapeutic efficacy, adverse effects, local control (LC) time, and overall survival (OS) time.
Results: All the patients had previously undergone surgery or EBRT. The median age of patients was 59 (range, 31–81) years old. The median follow-up time was 20.5 (range, 0.89–125.8) months. The median LC and OS time were 10 (95% confidence interval (CI): 8.5–11.5) and 20.8 (95% CI: 18.7–22.9) months, respectively. The 1-, 2-, and 5-year LC rates were 44.2%, 20.7%, and 18.4%, respectively. The 1-, 2-, and 5-year OS rates were 73%, 31.4%, and 5%, respectively. Univariate analysis of LC suggested that when short-time tumor response achieved partial response (PR) or complete response (CR), or D90>129 Gy, or GTV ≤ 50 cm3, the LC significantly prolonged (P=0.044, 0.041, and <0.001, respectively). The multivariate analysis of LC indicated that the short-time tumor response was an independent factor influencing LC time (P<0.001). Besides, 8.9% (9/101) of the patients had adverse effects (≥grade 3): radiation-induced skin reaction (4/101), radiation-induced urinary reaction (1/101), fistula (2/101), and intestinal obstruction (2/101). The cumulative irradiation dose and the activity of a single seed were significantly correlated with adverse effects ≥grade 3 (P=0.047 and 0.035, respectively).
Conclusion: CT-guided 125I seed implantation is a safe and effective salvage treatment for LRRC patients who previously underwent EBRT or surgery. D90 and GTV significantly influenced prognosis of such patients.
Introduction
Locally recurrent rectal cancer (LRRC) refers to the recurrence, progression, or development of new sites within the pelvis after previous standard treatment for rectal cancer (1). Although preoperative chemoradiotherapy followed by total mesorectal excision (TME) significantly decreased the local recurrence rate, local recurrence has been reported in 5–11% of patients (2). Prognosis in LRRC patients is poor, with a median survival time of 10 months without treatment (3), and a reported 5-year survival rate of 10% (4). The majority of patients have severe symptoms such as pain, hematochezia, and fistula.
Surgery is an effective option and radical (R0) resection is an independent prognostic factor. Because the tumor typically shows extensive involvement in the pelvis, less than one-sixth of patients are eligible for R0 resection (5). The benefits of reirradiation include possible palliation by decreased steroid use, improvement in neurological symptoms, and extension of progression-free survival (PFS) and overall survival (OS) in some patients. Nevertheless, considering previous irradiation to the normal tissue, sufficient doses can hardly be delivered to the recurrent tumor in the pelvis (6). Furthermore, locally recurrent tumors are mostly located in the previously irradiated field, making it more challengeable for patients to undergo reirradiation (7). In addition, reirradiation with conventional radiation therapy confers a high rate of grade 3 adverse effects and late toxicities.
Nevertheless, 125I seed implantation can overcome the above-mentioned limitations. The dose of 125I seed is inversely proportional to the square of the distance, indicating that the dose is remarkably reduced surrounding the tumor. Interstitial implantation of 125I seeds delivers a high dose of radiation (140–180 Gy) to the tumor and spares surrounding normal tissues. In addition, 125I seed provides a slow continuous release of radiation that allows repair of sublethal damage and reoxygenation of hypoxic areas in the late-responding tissues. Therefore, radioactive 125I seed implantation might be a promising choice for the treatment of malignant tumors owing to its curative effect, minimal surgical trauma, and tolerable complications. The present study aimed to evaluate the efficacy and safety of computed tomography (CT)-guided 125I seed implantation for LRRC patients who underwent external beam radiotherapy (EBRT) or surgery, in addition to analysis of some prognostic factors.
Patients and Methods
Patients
This retrospective study collected the data of 101 patients with LRRC who were treated with CT-guided 125I seed implantation from October 2003 to April 2019. The study protocol was approved by the Ethics Committee of our hospital. All patients signed the written informed consent. The inclusion criteria were as follows: (1) patients with LRRC who were pathologically diagnosed; (2) extraluminal pelvic recurrence, without distal metastasis or with controllable oligometastasis; (3) tumor size < 7cm; (4) recurrence after surgery or EBRT, or refusal of surgery or EBRT; (5) life expectancy≥3 months. The patients’ median age was 59 (range, 31–81) years old. After tumor recurrence, all the patients received chemotherapy before seed implantation. And 17 (16.8%) patients received second-line or further chemotherapy. All the patients had received curative surgery or EBRT previously. Except for one case, 100 patients underwent surgery. Among all patients, 12 patients had no history of undergoing irradiation, 74 patients received one course of EBRT, and 14 patients received two courses of EBRT. The median cumulative dose in the pelvis was 50 (range, 30–130) Gy. All the patients had received chemotherapy previously. Demographic and clinical data of patients are listed in Table 1.
CT-Guided 125I Seed Implantation
Supine or prone position was chosen according to the tumor location. All the patients underwent contrast-enhanced CT scan with a slice thickness of 5 mm, one week before the implantation. CT data were transmitted to the brachytherapy treatment planning system (BTPS) (KLSIRPS-3D) which was provided by the Beijing University of Aeronautics and Astronautics and Beijing Astro Technology Co., Ltd. The radiation oncologists delineated the gross target volume (GTV) and organs at risk (OARs). Planned target volume (PTV) was defined as an extension of 5–10 mm from GTV. The optimal access for implantation (site, direction, and depth), prescription dose, number of seeds, single-seed activity, and seed distribution were designed.
Spinal anesthesia was induced in all patients. Under CT guidance, the needles were inserted into the planned site and arranged in parallel 5–10 mm apart. Then, the 125I seeds (6711_1985, Shanghai GMS Pharmaceutical Co., Ltd.) were implanted using a Mick seed implantation gun (Mick Radio-Nuclear Inc., Mount Vernon, NY, USA), by maintaining 1 cm between two seeds. After finishing seeds implantation, another CT scan was performed to verify the distribution of the seeds, and to calculate the dosimetric parameters (Figure 1). The postoperative parameters included: D90 (dose delivered to 90% of target volume), D100 (dose delivered to 100% of target volume), V100 (percentage of the target volume that was covered by 100% of the prescription dose), V150 (percentage of the target volume that was covered by 150% of the prescription dose), HI (homogeneity index), CI (conformal index), and EI (external index).

Figure 1 The first line presents the preoperative plan. The second line presents the intraoperative plan. The third line presents the postoperative plan.
Follow-Up
The patients were followed-up every 3 months by the radiation oncologists. The examinations included routine blood test, blood chemistry, tumor markers, magnetic resonance imaging (MRI) of pelvis, CT of the abdomen, and chest radiography. Positron emission tomography-CT (PET-CT) was employed when there were signs of metastasis. The local response was evaluated three months after 125I seed implantation by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (8). Complete response (CR) was defined as disappearance of all target lesions; partial response (PR) was defined as at least a 30% decrease in the diameters of the target lesion; progressive disease (PD) was defined as at least a 20% increase in the diameters of the target lesion; and stable disease (SD) was between PR and PD. The numeric rating scale (NRS) was used to assess the pain level. Adverse effects were evaluated according to the toxicity criteria of the Radiation Therapy Oncology Group (RTOG). Local control (LC) was defined as lack of tumor progression of the implanted volume.
Statistical Analysis
All statistical analyses were carried out by SPSS 18.0 software (IBM, Armonk, NY, USA). LC and OS rates were calculated by plotting Kaplan–Meier curves. The log-rank test was employed for univariate analysis, and Cox proportional hazards regression model was used for multivariate analysis. The Chi-square test and Fisher’s exact test were undertaken to analyze factors correlated with adverse effects. The two-tailed P<0.05 was considered statistically significant. The curves were plotted with GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA).
Results
Parameters of the Implantation
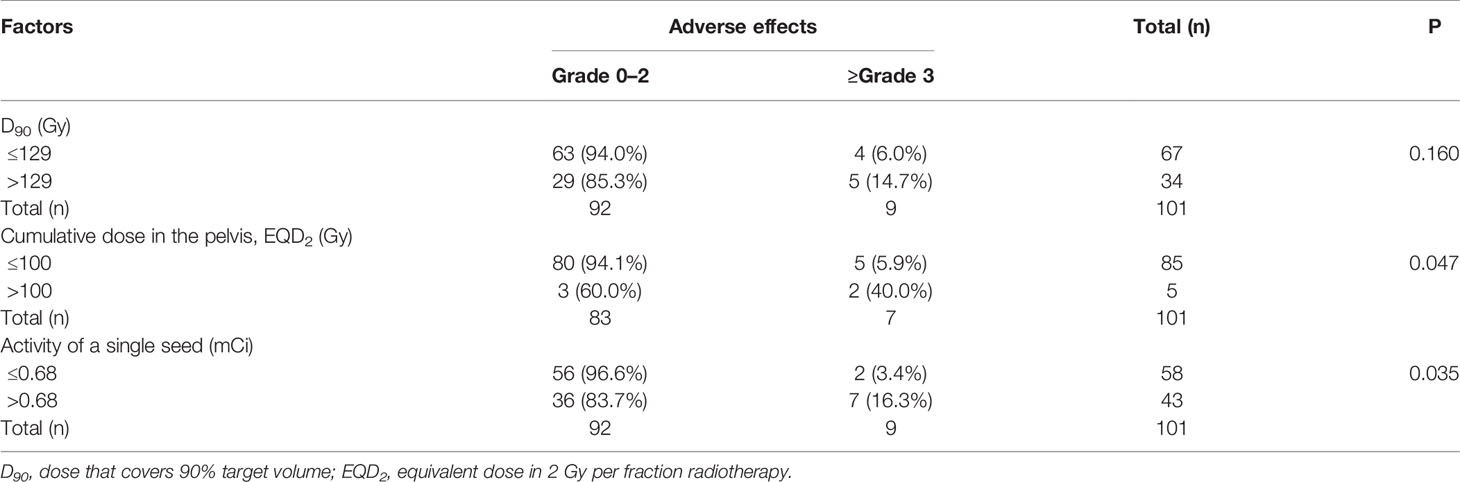
The volume of GTV was 6.5–234.8 (median, 66.9) cm3. The activity of a single radioactive seed was 0.4–0.8 (median, 0.66) mCi. The number of seeds was 6–137 (median, 70). The postoperative parameters included D90 (110.7 ± 33.7) Gy, D100 (46.8 ± 24.4) Gy, V100 (68.9 ± 36.4) %, V150 (56.8 ± 17.5) %, HI (0.34 ± 0.14), CI (0.91 ± 0.55), and EI (0.79 ± 1.6).
Efficacy and Adverse Effects
The follow-up time was 0.89–125.8 (median, 20.5) months. The local response included 23 cases of CR, 35 cases of PR, 33 cases of SD, and 10 cases of PD. The objective response rate (ORR) was 57.4% (58/101). Adverse effects occurred in 14 (13.9%) patients, including 21 cases. Besides, 12 (57.1%) of the cases showed grades 1–2 adverse effects, including neuropathy (n=1, 4.8%), radiation-induced skin reaction (n=4, 19%), and radiation-induced urinary reaction (n=7, 33.3%). Additionally, 9 (42.9%) cases had adverse effects with ≥grade 3, including radiation-induced urinary reaction (n=1, 4.8%), fistula (n=2, 9.5%), intestinal obstruction (n=2, 9.5%), and radiation-induced skin reaction (n=4, 19.1%). Concerning implantation-related complications, seed migration was observed in two patients during the follow-up, and one patient developed needle-tract implantation metastases. No correlation was found between D90 (D90 ≤ 129 Gy vs. D90>129 Gy) and adverse effects ≥grade 3 (P=0.160) (Table 2). The cumulative irradiation dose (≤100 Gy vs. >100 Gy) and the activity of a single seed (≤0.68 mCi vs. >0.68 mCi) were significantly correlated with adverse effects ≥grade 3 (P=0.047 and 0.035, respectively). The rates of adverse effects (grade ≥3) for cumulative dose ≤100 Gy and >100 Gy were 5.9% and 40%, respectively, and the rates of adverse effects (grade ≥3) for the activity of a single seed ≤0.68 mCi and >0.68 mCi were 3.4% and 16.3%, respectively.
Local Control
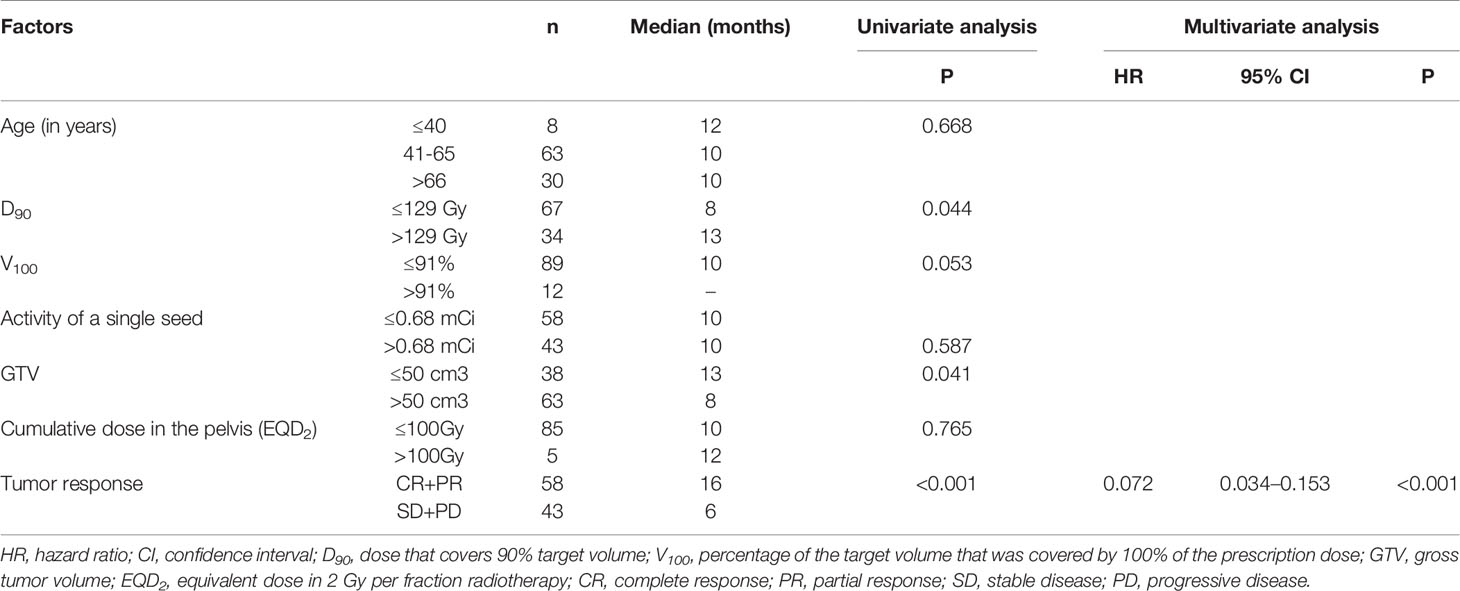
The median LC time was 10 (95% confidence interval (CI): 8.5–11.5) months. The 1-, 2-, and 5-year LC rates were 44.2%, 20.7%, and 18.4%, respectively. The univariate analysis of LC showed that D90 (≤129 Gy vs. >129 Gy), GTV (≤50 cm3 vs. >50 cm3), and short-time tumor response (CR+PR vs. SD+PD) significantly influenced LC time (P=0.044, 0.041, and <0.001, respectively) (Table 3). Besides, a prolonged trend was shown in LC when V100>91% (P=0.053) (Figure 2). The 1-year LC rate for V100 ≤ 91% and V100>91% was 42.1% and 62.5%, respectively. Multivariate analysis of these factors influencing LC time indicated that short-time tumor response was an independent factor of LC time (hazard ratio [HR] = 0.072; 95% CI=0.034–0.153; P<0.001). The LC of CR and PR was superior to that of SD and PD. The median LC time for (CR+PR) and (SD+PD) was 16.0 and 6.0 months, respectively.
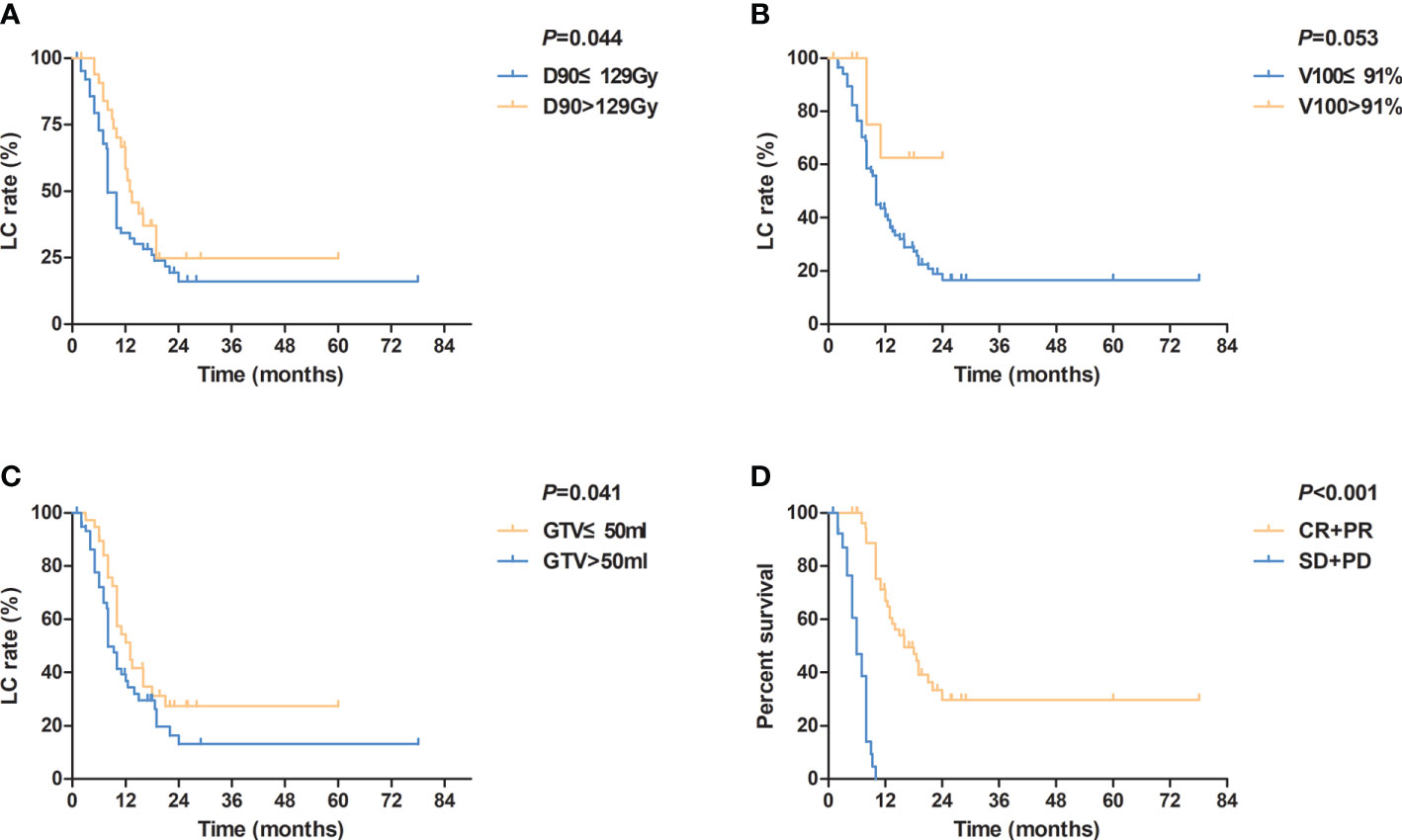
Figure 2 Kaplan-Meier curves for local control according to (A) different values of D90 (≤ 129Gy vs. >129Gy); (B) different values of V100 (≤ 91% vs. >91%); (C) different values of GTV (≤ 50 ml vs. >50 ml); (D) different tumor responses (CR+PR vs. SD+PD). D90, dose that covers 90% target volume; V100, percentage of the target volume that was covered by 100% of the prescription dose; GTV, gross tumor volume; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Overall Survival
The median OS time was 20.8 (95% CI: 18.7–22.9) months. The 1-, 2-, and 5-year OS rates were 73%, 31.4%, and 5%, respectively. In the current research, 93 patients died at the end of the follow-up. Of these, 20 patients died of local recurrence, 54 died of metastasis, seven died of non-tumor causes, and 12 died of unknown causes. For the univariate analysis, only the short-time tumor response was significantly correlated with OS time (P=0.017). The median OS time for (CR+PR) and (SD+PD) was 22.0 and 14.8 months, respectively.
Discussion
Numerous therapeutic modalities have been used for patients with LRRC, including surgery, EBRT, intraoperative radiotherapy (IORT), high-dose-rate (HDR) brachytherapy, chemotherapy, etc. Previously irradiated patients were found less sensitive to chemotherapy than those who did not receive pelvic radiotherapy (6, 9). Furthermore, chemotherapy alone is not effective for controlling pelvic recurrence. It was reported that the 5-year survival rate for R0 resection ranged from 43% to 60% (10). However, only a limited number of patients were eligible for R0 resection. LRRC typically presents with extensive involvement of the tumor in the pelvis, making it a great challenge to perform resection. Moreover, distorted anatomical structures and tissue fibrosis from previous irradiation increase the difficulty in surgery (6, 11). Besides, extensive resection is typically followed by high morbidity and mortality risks (10).
Several scholars have pointed out that re-irradiation is a reasonable option for patients with LRRC who have undergone EBRT previously. Besides, it could relieve symptoms to some extent (11–13). A systematic review reported 375 patients with LRRC who were reirradiated. Reirradiation was mostly administered using hyperfractionated or 1.8 Gy once-daily chemoradiotherapy. Median survival time was 39–60 months for resected patients and 12–16 months for palliative patients. The symptomatic relief rate was 82%–100% (12). Nevertheless, it is challenging to deliver a definitive dose to the tumor lesions considering history of irradiation to the normal pelvic tissue (6). Late toxicity rates were high. Recently, with the advances of cutting-edge technologies, a more precise radiotherapy technique, namely stereotactic ablative radiotherapy (SABR), was used to treat LRRC. Murray et al. (14) performed a systematic review regarding the use of SABR for the reirradiation of recurrent malignant disease within the pelvis, to guide the clinical implementation of this technique, and demonstrated that for previously irradiated patients with recurrent pelvic disease, SABR re-irradiation could be a feasible intervention for those who otherwise have limited options. Dagoglu et al. (15) reported the outcomes of a series of patients with pelvic recurrences from colorectal cancer reirradiated with SBRT. They employed Cyberknife Robotic Stereotactic Radiosurgery system with fiducial based real time tracking, and noted that one patient had small bowel perforation and required surgery (grade IV), two patients had symptomatic neuropathy (grade III), and one patient developed hydronephrosis from ureteric fibrosis requiring a stent (grade III).
IORT refers to the direct irradiation of the tumor surgically. A number of scholars have used IORT alone in the treatment of patients with history of undergoing irradiation. However, their results were not satisfactory. The LC and OS rates of IORT alone were significantly lower than those of IORT combined with EBRT (13, 16, 17). Besides, a significant increase was observed in the rate of complications related to the wound and neuropathy.
Several studies reported HDR brachytherapy for the management of LRRC. HDR intraluminal brachytherapy plays a great therapeutic role in the treatment of intraluminal tumor recurrence. And HDR interstitial brachytherapy has also shown an impressive therapeutic efficacy. Sakurai et al. (18) reported that LC was achieved in 7 of 18 patients with LRRC at a median follow-up time of 14.4 months. Morimoto et al. (19) studied 9 patients, and it was demonstrated that the 8-year OS, LC, and PFS rates were 56%, 44%, and 33%, respectively. Three patients had grade 3 adverse effects. Nevertheless, it should be noted that the tumor location of these patients could be reached by a needle applicator through the perineum. Lateral or presacral recurrence was contraindicated for HDR brachytherapy, which, however, could be managed with 125I seed implantation.
Recurrent tumor after EBRT or surgery is typically associated with a poor blood supply. Permanent implantation of 125I seeds has significant advantages in killing hypoxic tumor cells by consistently radiating low-dose rays. Furthermore, it also has the major advantages of delivering a high dose of irradiation to the tumor with a very sharp fall-off outside the implanted volume. For patients who are not eligible candidates for reirradiation, surgery or HDR interstitial brachytherapy, 125I seed implantation might be an alternative treatment option.
The results of the current research were comparable to those reported previously related to application of 125I seed implantation for LRRC. In our study, the median LC time was 10 months, and the median OS time was 20.8 months. The first report related to application of brachytherapy for LRRC included 30 patients (20). The seeds were implanted after radical or debulking surgical resection. The LC rate was 37.5% for gross residual disease and 66% for microscopic residual disease. The tumors in 64% (18/30) of patients were still under control at the last follow-up. No mortality was observed, and the morbidity rate was low. Martinez et al. (21) reported 29 patients with recurrent colorectal cancer in the pelvis or the paraaortic lymph nodes treated with intraoperative 125I seed implantation. The implanted volume received a median minimal peripheral dose of 140 Gy to total decay. The 1-, 2-, and 4-year LC rates were 38%, 17%, and 17%, (median, 11 months), respectively. The 1-, 2-, and 4-year OS rates were 70%, 35%, and 21%, (median, 18 months), respectively. Overall, 45% (13/29) of patients experienced 15 adverse events. Image-guided percutaneous 125I seed implantation which was minimally invasive has gradually become the mainstream treatment approach. In Wang et al.’s study (22), 15 patients with LRRC received 125I or 103Pd seed implantation under CT guidance. The median minimal peripheral dose was 150 Gy. The median follow-up, LC, and OS time were 8, 7, and 9 months, respectively. Only one patient had a grade 4 toxic event. Wang et al. (23) reported 20 patients with LRRC who were treated with CT-guided 125I seed implantation. The median peripheral dose was 120 Gy. CR or PR was achieved in 75% of patients. The median survival time was 18.8 months. The 1- and 2-year survival rates were 75% and 25%, respectively. Nevertheless, none of the above-mentioned studies analyzed optimal parameters and factors related to adverse effects. The optimal dosimetric parameters for 125I seed implantation are still elusive except for prostate cancer. In prostate cancer, the prognosis of patients with D90≥140 Gy was significantly greater than those with D90<140 Gy. The outcomes of patients with V100≥90% were also markedly superior than those with V100<90% (24–26). Similarly, for LRRC, the present study revealed that patients with D90>129 Gy achieved a notably longer LC time than those with D90 ≤ 129 Gy. The median LC time for D90>129 Gy and D90 ≤ 129 Gy was 8 and 13 months, respectively. Moreover, a trend of prolonged LC time was observed in patients with V100>91%. The 1-year LC rate for V100 ≤ 91% and V100>91% was 42.1% and 62.5%, respectively.
Regarding adverse effects, a meta-analysis of irradiation for LRRC showed that the rates of adverse effects (≥grade 3) for acute and late complications were 11.7% and 25.2%, respectively (12). Bhangu et al. (27) summarized surgical outcomes of 22 studies on LRRC and revealed that the overall rate of complications was 51%. In the current research, the overall rate of adverse effects was 13.9% (14/101), and 8.9% (9/101) of patients had ≥grade 3 adverse effects. The complication rates reported in our study were relatively lower than those reported in studies that used other treatment modalities. Cumulative irradiation dose (≤100 Gy vs. >100 Gy) and the activity of a single seed (≤0.68 mCi vs. >0.68 mCi) were found to be correlated with adverse effects (≥grade 3). We considered that the adverse effects in 2 patients with cumulative irradiation dose >100 Gy might be attributed to the late complications of previous high-dose irradiation. When low-activity seeds are used, the influence of a single seed on dosimetry is reduced, leading to a better dose homogeneity. The misplacement of a single seed would cause less damage to surrounding normal tissue. Sloboda et al. (28) reported that a range of 0.4–0.6 mCi per seed was optimal to cover the target volume and spare the urethra in prostate cancer. However, in the present study, the activity of a single seed had no effect on either LC or OS.
There are a number of limitations in this study. First, considering the short half-life of 125I seed, all the dosimetric parameters were postoperative parameters which were calculated immediately after 125I seed implantation, with assumption of complete dose delivery. According to the physical characteristic of 125I seed, 65% of prescription dose was delivered in 3 months and 90% was delivered in about 6 months. All the patients were still alive 3 months after 125I seed implantation except for one patient who died of pulmonary infection one month after the implantation. Moreover, the majority of patients were still alive 6 months after 125I seed implantation. Thus, it could be concluded that the postoperative dosimetry was nearly close to the delivered dosimetry. Nevertheless, there may still exist some minor errors that require further investigation. Second, the treatment modalities used for LRRC patients before 125I seed implantation were not consistent (e.g., some patients did not receive irradiation), which might influence patients’ sensitivity to 125I seed implantation and clinical outcomes. In addition, treatment modalities used for LRRC patients after 125I seed implantation were not consistent as well. A number of patients received postoperative chemotherapy, while others poorly tolerated, which might lead to the low efficiency of 125I seed implantation on OS. Third, it was sometimes difficult to indicate whether the adverse effects were caused by 125I seed implantation, tumor progression, or previous high-dose irradiation. In such cases, we attributed the adverse effects to 125I seed implantation, which might lead to an overestimation of the rates of adverse effects. Last but not the least, this was a single-center retrospective study with small sample size.
In conclusion, CT-guided 125I seed implantation is a safe, effective, and minimally invasive treatment for LRRC patients with mild adverse effects. This treatment does not require patients to have a high physical strength and is not limited by previous irradiation dose. Patients with LRRC after previous EBRT with limited treatment options are especially proper candidates for 125I seed implantation. Nevertheless, multicenter studies with a larger sample size and prospective design are needed to further investigate the effects of 125I seed implantation on LRRC patients.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Boards of Peking University Third Hospital (IRB00006761). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LW, HW, HS, and GD were responsible for the collection of the clinical data. LW and HW drafted the manuscript. JW was in charge of verifying the patients’ implantation plan and directing the writing. YJ, ZJ, FG, PJ, XL, YC, and JF were responsible for the supplement and refinement of the clinical data and collectively carried out the implantation plan. HS was responsible for the design and production of radioactive seed implantation plan. All authors read and approved the final manuscript. LW and HW contributed equally to the article and are co-first authors of the article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all our supporting colleagues in the Department of Radiation Oncology, Peking University Third Hospital.
References
1. Beyond TMEC. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg (2013) 100(8):1009–14. doi: 10.1002/bjs.9192
2. van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol (2011) 12:575–82. doi: 10.1016/S1470-2045(11)70097-3
3. Rasanen M, Carpelan-Holmstrom M, Mustonen H, Renkonen-Sinisalo L, Lepistö A. Pattern of rectal cancer recurrence after curative surgery. Int J Color Dis (2015) 30(6):775–85. doi: 10.1007/s00384-015-2182-1
4. Guyot F, Faivre J, Manfredi S, Meny B, Bonithon-Kopp C, Bouvier AM. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann Oncol (2005) 16:756–61. doi: 10.1093/annonc/mdi151
5. Westberg K, Palmer G, Hjern F, Nordenvall C, Johansson H, Holm T, et al. Population-based study of factors predicting treatment intention in patients with locally recurrent rectal cancer. Br J Surg (2017) 104:1866–73. doi: 10.1002/bjs.10645
6. Hildebrandt B, Wust P, Drager J, Lüdemann L, Sreenivasa G, Tullius SG, et al. Regional pelvic hyperthermia as an adjunct to chemotherapy (oxaliplatin, folinic acid, 5-fluorouracil) in pre-irradiated patients with locally recurrent rectal cancer: a pilot study. Int J Hyperthermia (2004) 20(4):359–69. doi: 10.1080/02656730310001645010
7. Yu T-K, Bhosale PR, Crane CH, Iyer RB, Skibber JM, Rodriguez-Bigas MA, et al. Patterns of locoregional recurrence after surgery and radiotherapy or chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys (2008) 71:1175–80. doi: 10.1016/j.ijrobp.2007.11.018
8. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
9. Alberda WJ, Haberkorn BC, Morshuis WG, Oudendijk JF, Nuyttens JJ, Burger JW, et al. Response to chemotherapy in patients with recurrent rectal cancer in previously irradiated area. Int J Colorectal Dis (2015) 30(8):1075–80. doi: 10.1007/s00384-015-2270-2
10. Hagemans JAW, van Rees JM, Alberda WJ, Rothbarth J, Nuyttens JJME, van Meerten E, et al. Locally recurrent rectal cancer; long-term outcome of curative surgical and non-surgical treatment of 447 consecutive patients in a tertiary referral centre. Eur J Surg Oncol (2020) 46(3):448–54. doi: 10.1016/j.ejso.2019.10.037
11. Lee J, Kim CY, Koom WS, Rim CH. Practical effectiveness of re-irradiation with or without surgery for locoregional recurrence of rectal cancer: A meta-analysis and systematic review. Radiother Oncol (2019) 140:10–9. doi: 10.1016/j.radonc.2019.05.021
12. Guren MG, Undseth C, Rekstad BL, Brændengen M, Dueland S, Spindler KL, et al. Reirradiation of locally recurrent rectal cancer: a systematic review. Radiother Oncol (2014) 113(2):151–7. doi: 10.1016/j.radonc.2014.11.021
13. Chung SY, Koom WS, Keum KC, Chang JS, Shin SJ, Ahn JB, et al. Treatment Outcomes of Re-irradiation in Locoregionally Recurrent Rectal Cancer and Clinical Significance of Proper Patient Selection. Front Oncol (2019) 9:529. doi: 10.3389/fonc.2019.00529
14. Murray LJ, Lilley J, Hawkins MA, Henry AM, Dickinson P, Sebag-Montefiore D. Pelvic re-irradiation using stereotactic ablative radiotherapy (SABR): A systematic review. Radiother Oncol (2017) 125(2):213–22. doi: 10.1016/j.radonc.2017.09.030
15. Dagoglu N, Mahadevan A, Nedea E, Poylin V, Nagle D. Stereotactic body radiotherapy (SBRT) reirradiation for pelvic recurrence from colorectal cancer. J Surg Oncol (2015) 111(4):478–82. doi: 10.1002/jso.23858
16. Susko M, Lee J, Salama J, Thomas S, Uronis H, Hsu D, et al. The Use of Re-irradiation in Locally Recurrent, Non-metastatic Rectal Cancer. Ann Surg Oncol (2016) 23(11):3609–15. doi: 10.1245/s10434-016-5250-z
17. Marzi S, Saracino B, Petrongari MG, Arcangeli S, Gomellini S, Arcangeli G, et al. Modeling ofalpha/beta for late rectal toxicity from a randomized phase II study: conventional versus hypofractionated scheme for localized prostate cancer. J Exp Clin Cancer Res (2009) 28:117. doi: 10.1186/1756-9966-28-117
18. Sakurai H, Mitsuhashi N, Harashima K, Muramatsu H, Ishikawa H, Kitamoto Y, et al. CT-fluoroscopy guided interstitial brachytherapy with image-based treatment planning for unresectable locally recurrent rectal carcinoma. Brachytherapy (2004) 3:222–30. doi: 10.1016/j.brachy.2004.09.007
19. Morimoto M, Isohashi F, Yoshioka Y, Suzuki O, Seo Y, Ogata T, et al. Salvage high-dose-rate interstitial brachytherapy for locally recurrent rectal cancer: long-term follow-up results. Int J Clin Oncol (2014) 19(2):312–8. doi: 10.1007/s10147-013-0567-0
20. Goes RN, Beart RW Jr, Simons AJ, Gunderson LL, Grado G, Streeter O. Use of brachytherapy in management of locally recurrent rectal cancer. Dis Colon Rectum (1997) 40:1177–9. doi: 10.1007/BF02055163
21. Martinez-Monge R, Naq S, Martin EW. 125Iodine brachytherapy for colorectal adenocarcinoma recurrent in the pelvis and paraortics. Int J Radiat Oncal Biol Phys (1998) 42:545–50. doi: 10.1016/S0360-3016(98)00269-7
22. Wang JJ, Yuan HS, Li JN, Jiang YL, Tian SQ, Yang RJ. CT-guided radioactive seed implantation for recurrent rectal carcinoma after multiple therapy. Med Oncol (2010) 27(2):421–9. doi: 10.1007/s12032-009-9227-7
23. Wang ZM, Lu J, Liu L, Liu T, Chen K, Liu F, et al. Clinical application of CT-guided 125I seed interstitial implantation for local recurrent rectal carcinoma. Radiat Oncol (2011) 6:138. doi: 10.1186/1748-717X-6-138
24. Keyes M, Morris WJ, Spadinger I, Araujo C, Cheung A, Chng N, et al. Radiation oncology and medical physicists quality assurance in British Columbia Cancer Agency Provincial Prostate Brachytherapy Program. Brachytherapy (2013) 12(4):343–55. doi: 10.1016/j.brachy.2012.03.006
25. Zapatero A, Garcia-Vicente F, Martin de Vidales C, Cruz Conde A, Ibáñez Y, Fernández I, et al. Long-term results after high-dose radiotherapy and adjuvant hormones in prostate cancer: how curable is high-risk disease? Int J Radiat Oncol Biol Phys (2011) 81(5):1279–85. doi: 10.1016/j.ijrobp.2010.07.1975
26. Stock RG, Stone NN, Tabert A, Iannuzzi C, DeWyngaert JK. A dose-response study for I-125 prostate implantation. Int J Radiat Oncol Biol Phys (1998) 41(1):101–8. doi: 10.1016/S0360-3016(98)00006-6
27. Bhangu A, Ali SM, Darzi A, Brown G, Tekkis P. Metaanalysis of survival based on resection margin status following surgery for recurrent rectal cancer. Color Dis (2012) 14(12):1457–66. doi: 10.1111/j.1463-1318.2012.03005.x
Keywords: locally recurrent rectal cancer, 125I seed implantation, dosimetry, prognosis, adverse effects
Citation: Wang H, Wang L, Jiang Y, Ji Z, Guo F, Jiang P, Li X, Chen Y, Sun H, Fan J, Du G and Wang J (2021) Long-Term Outcomes and Prognostic Analysis of Computed Tomography-Guided Radioactive 125I Seed Implantation for Locally Recurrent Rectal Cancer After External Beam Radiotherapy or Surgery. Front. Oncol. 10:540096. doi: 10.3389/fonc.2020.540096
Received: 03 March 2020; Accepted: 04 December 2020;
Published: 21 January 2021.
Edited by:
Daniel Michael Trifiletti, Mayo Clinic Florida, United StatesReviewed by:
Ananth Ravi, Independent Researcher, Toronto, CanadaToms Vengaloor Thomas, University of Mississippi Medical Center, United States
Copyright © 2021 Wang, Wang, Jiang, Ji, Guo, Jiang, Li, Chen, Sun, Fan, Du and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Wang, anVuamlld2FuZ19lZHVAc2luYS5jbg==
†These authors have contributed equally to this work and share first authorship
 Hao Wang1†
Hao Wang1† Lu Wang
Lu Wang Zhe Ji
Zhe Ji Ping Jiang
Ping Jiang