- 1Department of Radiation Oncology, University of Mississippi Medical Center, Jackson, MS, United States
- 2Department of Health Sciences, University of Mississippi Medical Center, Jackson, MS, United States
- 3Department of Data Science, University of Mississippi Medical Center, Jackson, MS, United States
- 4Department of Radiology, University of Mississippi Medical Center, Jackson, MS, United States
Background: Inconsistent findings have been reported in the literature regarding racial differences in survival outcomes between African American and white patients with metastatic prostate cancer (mPCa). The current study utilized a national database to determine whether racial differences exist among the target population to address this inconsistency.
Methods: This study retrospectively reviewed prostate cancer (PCa) patient data (N = 1,319,225) from the National Cancer Database (NCDB). The data were divided into three groupings based on the metastatic status: (1) no metastasis (N = 318,291), (2) bone metastasis (N = 29,639), and (3) metastases to locations other than bone, such as brain, liver, or lung (N = 952). Survival probabilities of African American and white PCa patients with bone metastasis were examined through parametric proportional hazards Weibull models and Bayesian survival analysis. These results were compared to patients with no metastasis or other types of metastases.
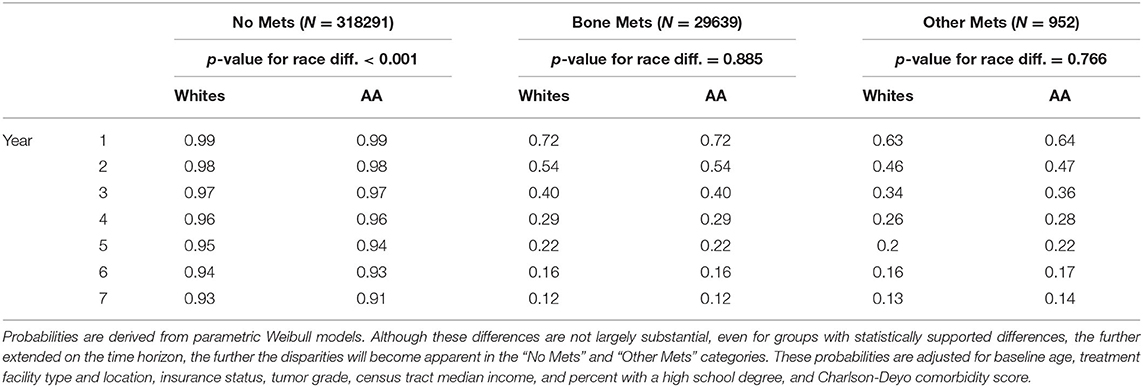
Results: No statistically supported racial disparities were observed for African American and white men with bone metastasis (p = 0.885). Similarly, there were no racial disparities in survival for those men suffering from other metastases (liver, lung, or brain). However, racial disparities in survival were observed among the two racial groups with non-metastatic PCa (p < 0.001) or when metastasis status was not taken into account (p < 0.001). The Bayesian analysis corroborates the finding.
Conclusion: This research supports our previous findings and shows that there are no racial differences in survival outcomes between African American and white patients with mPCa. In contrast, racial disparities in the survival outcome continue to exist among non-metastatic PCa patients. Further research is warranted to explain this difference.
Introduction
Prostate cancer (PCa) was the most frequently diagnosed cancer in over 100 countries in 2018, and the fifth leading cause of cancer-related deaths in men, according to Global Cancer Statistics 2018 (1). In the United States, PCa accounts for 20% of new cancer diagnoses in men (2). Race is a well-studied risk factor for PCa (3–7). It is widely recognized that when compared to white men, African American men are more likely to develop PCa, tend to be diagnosed at a younger age, exhibit higher prostate-specific antigen (PSA) levels, and demonstrate more advanced or metastatic disease at diagnosis (8–12). Besides, African American men are four times more likely to develop metastatic PCa (mPCa) than white men and more than twice as likely to die from PCa (13–19). It is of vital importance to continue research on racial disparity of PCa to better understand the disease and improve the survival outcomes of African American men.
The existing literature has consistently reported that racial survival differences exist between African American and white patients with localized PCa (20–24). However, inconsistent findings have been observed regarding the survival outcomes of African Americans and whites with mPCa. Some studies reported that African American men had poorer survival outcomes than white men (25–27); while other studies detected no differences (6, 28–33); and still others found that African American men might have better overall survival than white men (34, 35).
To address the inconsistency in the literature and to further investigate mPCa, we conducted a pilot study that examined African American and white PCa patients with bone metastasis at an academic medical center (36). The results revealed that when treatments were equal, no differences in survival outcomes were observed between African American and white men (36). Based on the pilot study, we hypothesized that mPCa might have different behavior from non-metastatic PCa in different races. Even though our initial study included 12-year retrospective data, the sample size was relatively small. Therefore, the current study utilized the National Cancer Database (NCDB), which contained nationwide cancer data collected from over 1,500 facilities and provided a sample size that was larger than the majority of existing studies. We aimed to evaluate any potential racial differences in the survival outcomes of African American and white patients with mPCa.
Methods
The National Cancer Database (NCDB) is a nationwide, hospital-based registry that consists of data from patients who received care at cancer centers accredited by the American College of Surgeons Commission on Cancer (CoC). It is estimated to capture ~70% of all patients newly diagnosed with cancer in the United States (37). The CoC's NCDB and the accredited facilities participating in the NCDB are the sources of the de-identified data used in this study. However, the CoC has not verified and are not responsible for the statistical validity or conclusions derived by the authors of this study. Because the NCDB contains de-identified patient data, this study did not meet our institutional review board's (IRB) criteria for human subject's research, and thus approval and review from IRB was not indicated. The use of de-identified data in this study complied with the terms specified in the NCDB Participant User File Data Use Agreement. Individual patients, hospitals, and healthcare providers were not identified.
The prostate cancer-specific dataset provided by NCDB was used to assess our primary research objectives. Of the available 1,380,357 men in the original data, 61,132 were excluded for non-white/African American race, leaving 1,319,225 in the initial analysis dataset. Of these, 348,882 had metastasis data available. Patients were divided into three metastases groupings: (1) no metastasis (N = 318,291) (2) bone metastasis, including bone and other metastases (N = 29,639), and (3) metastases to locations other than bone, such as brain, liver, or lung metastasis (N = 952). Full data were used in analyses where metastasis status was not considered.
Statistical Analysis
Means with standard deviations and counts with percentages were compiled for baseline comparisons between African Americans and whites within each metastasis category and in the overall cohort. All henceforth described models were adjusted in the respective regression models for baseline age, treatment facility type and location, insurance status, tumor grade, census tract median income, percent with a high school degree, and Charlson-Deyo comorbidity score.
Adjusted failure time curves were constructed from parametric proportional hazards models using Weibull distributions to model time-to-death first disregarding metastasis status and then stratified by metastasis category. From these curves, we estimated the yearly survival probabilities.
In order to incorporate as much prior information as possible, especially the wealth of studies that have shown African Americans have lower long-term survival than whites, Bayesian survival analysis was performed. Similar pooled and stratified Weibull models were constructed, as described above, with the exception being that now we place a highly informative Cauchy (log (2), 0.5) prior on the log-hazard for the African Americans main effect with all other parameters having non-informative priors. This model, particularly the log (2) location parameter, allows us to use the previously published information that African American men have twice the hazard of death compared to whites. This fully informative prior was compared to a non-informative prior. From our primary model, posterior histograms of estimated hazard ratios were plotted. Posterior means, medians, and 95% credible intervals were further compiled. All Bayesian posterior checks were performed to assure proper model convergence. All analyses were completed with Stata v15.1 (StataCorp, College Station, TX).
Results
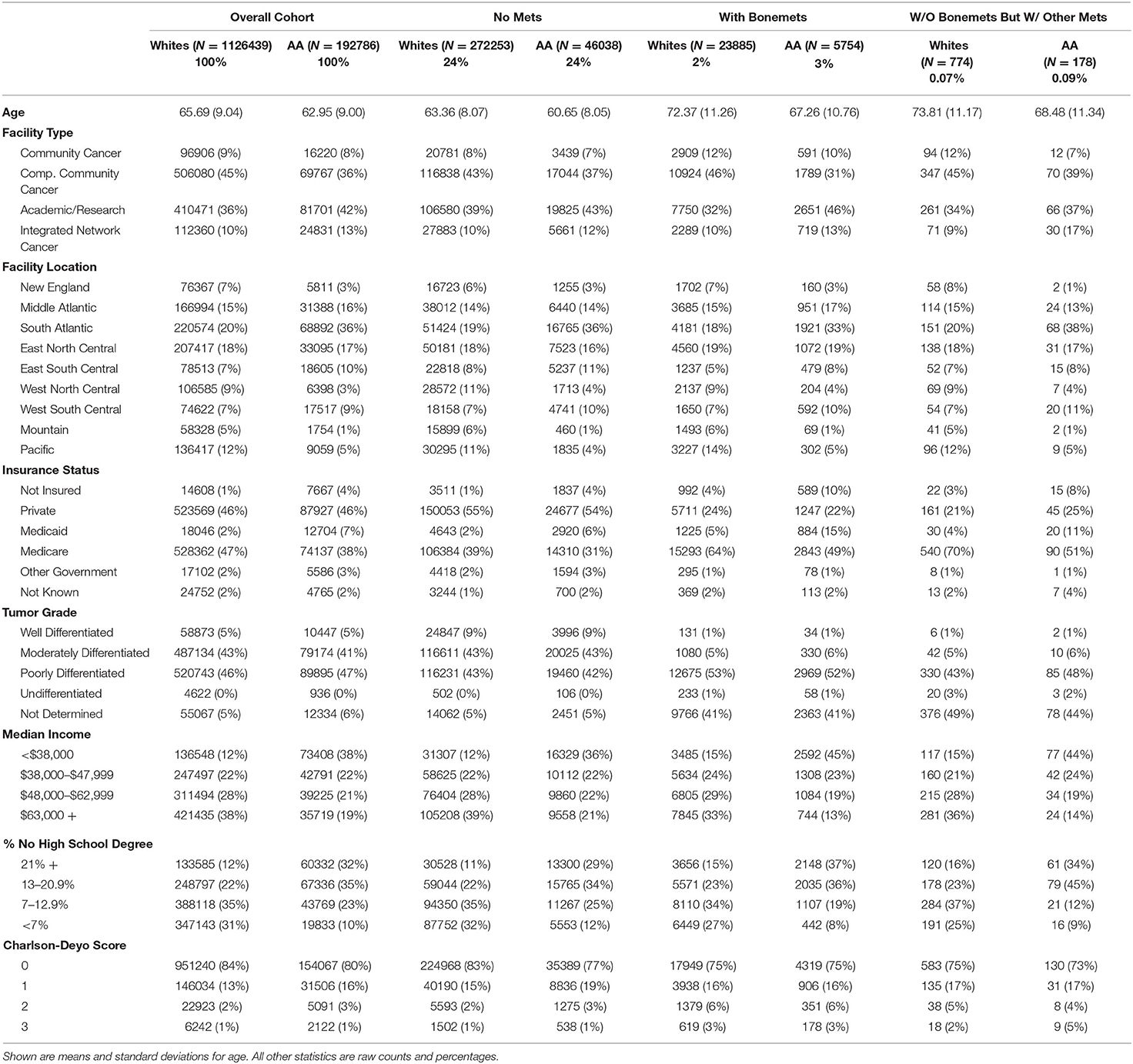
As shown in Table 1, the overall cohort had a mean age of 65.3 and a median follow-up time of 5.3 years. Those who were not missing metastasis data had a mean age of 65.1 and a median follow-up time of 3.4 years. Those with metastasis were slightly older in both whites and African Americans. The majority (>75%) of primary treatments occurred at comprehensive community cancer programs or academic/research programs. Most were either privately insured or on Medicare. Tumor grades were most likely to be moderately differentiated or poorly differentiated, except in the bone metastasis category, where a high percentage of tumor grades were not determined. African Americans tended to live in areas with lower incomes, and a higher percentage of residents without a high school diploma compared to whites. Charlson-Deyo scores were roughly evenly distributed across races.
Adjusted racial differences were observed when not taking metastasis status into account (p < 0.001). Similar differences were seen for those men having no metastasis (p < 0.001). These translated to 5 and 7-year survival probabilities of 0.95 vs. 0.94 and 0.93 vs. 0.91 (whites vs. African Americans), respectively. No statistically supported racial disparities were observed for those men with bone metastasis (p = 0.885), which translates to a five-year survival probability of 0.22 and seven-year survival probability of 0.12, regardless of race. Similar results were observed for those men suffering from other metastases. These results can be seen in Table 2 as well as Figure 1.
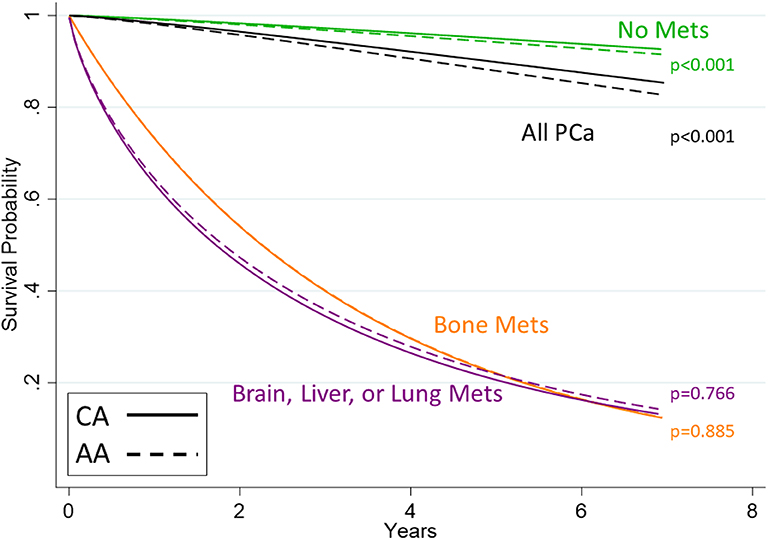
Figure 1. Survival curves for men with prostate cancer stratified by metastasis category. Shown are curves estimated from Weibull models. Whites are shown with solid lines and African Americans with dashed lines. The black line shows all PCa diagnoses, regardless of metastases status. Of note is that the lines representing racial distinctions are indistinguishable for the bone metastasis group (orange) since the African American effect contributes virtually nothing to the hazard of death (p = 0.885) nor for metastasis other than bone (purple, p = 0.766). This null effect was not observed in the group with no metastasis (p < 0.001, green lines).
Our Bayesian analysis corroborates the same information as the frequentist analysis presented in the preceding paragraph. As shown in Figure 2, even with priors assuming double the hazard of death for African Americans vs. whites, the data pulled the posterior hazard ratios more closely to 1. There was still a modest, but meaningful effect for those men without metastasis [HR = 1.17; 95% Cred. Int. (1.11–1.22)] as well as when we disregard metastasis status [HR = 1.20; 95% Cred. Int. (1.19–1.21)]. Once again, this effect disappeared for men with bone metastasis [HR = 0.99; 95% Cred. Int. (0.95–1.04)] and for men with metastasis other than bone [HR = 1.00; 95% Cred. Int. (0.79–1.24)].
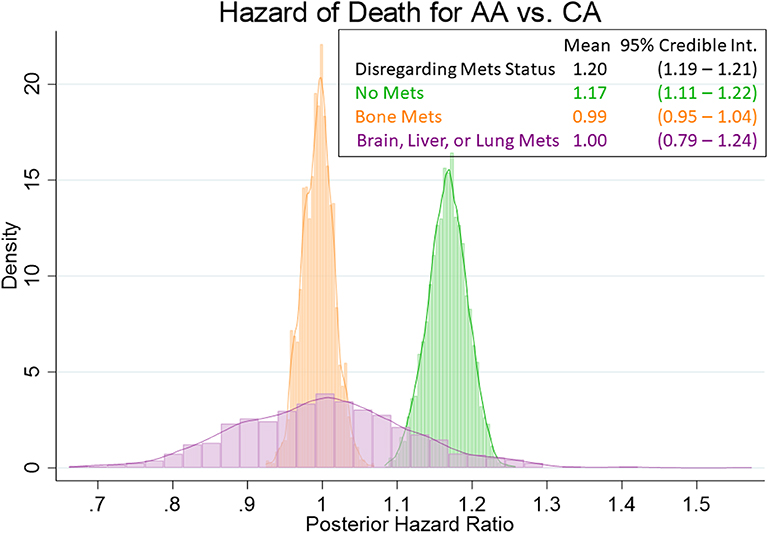
Figure 2. Posterior distributions of hazard ratios for the African American main effect. Posterior hazard ratios come from Bayesian Weibull models with informative Cauchy (log (2), 0.5) priors on the African American main effect. The histograms (coupled with analogous estimates) show that there is a demonstrable (if not modest) racial effect on the hazard of death for those men without metastasis (HR = 1.17), as well as with the overall cohort (HR = 1.20). However, the racial disparities are virtually nonexistent for those mean with bone metastasis (HR = 0.99) or with brain, liver, or lung metastasis (HR = 1.00).
Discussion
Our study found that there is no disparity in survival for African American and white patients with metastatic prostate cancer, even though a disparity exists for patients with non-metastatic prostate cancer. This study is the biggest study revealing an absence of a racial disparity in the survival outcome for metastatic prostate cancer. Our results are in agreement with studies that analyzed patients with metastatic prostate cancer—either androgen-sensitive metastatic prostate cancer (mPCa) or Castrate Resistant Prostate Cancer (mCRPC). However, there are some conflicting data in the available literature as well.
In the case of androgen-sensitive mPCa, Sassani et al. retrospectively reviewed 681 metastatic prostate cancer patients who received ADT as monotherapy in the Kaiser Permanente Southern California Cancer Registry between January 2003 and December 2006 (38, 39). They found that among these patients, African American men had a 34% lower risk of biochemical failure compared with white men (HR = 0.66, p = 0.03) (38, 39). McLeod et al. conducted an exploratory analysis of patient data from a double-blind, randomized, multicenter trial which evaluated the use of combined androgen blockade in 813 patients with mPCa and found that there were no significant differences in the survival outcomes among African Americans and whites (33). Gordy et al. recently reported in a retrospective study from our intuition an absence of a racial disparity in the treatment outcomes for metastatic prostate cancer patients (36). Thompson et al. analyzed patients enrolled into Southwest Oncology Group Study 8,894 randomized phase III trial that compared orchiectomy with or without flutamide in men with metastatic prostate cancer to determine if ethnicity was an independent predictor of survival (26, 40). Upon analysis of the data from 288 African American and 975 white patients in the trial, they found African American men had a worse survival (HR = 1.23, p = 0.018), after adjusting for confounding variables (26).
Regarding mCRPC, the controversy continues as to whether a racial disparity in outcome exists. Thatai et al. retrospectively reviewed 145 patients with androgen-independent prostate cancer enrolled in clinical trials at Wayne State University between 1991 and 2001. Even though there was no difference by race in the distribution of patient and disease characteristics, the race was found to be the only significant factor predicting time to PSA progression (4.6 months in whites vs. 2.3 months in African Americans, p = 0.02) (31). However, there was no difference in overall survival by race (31). However, when Halabi et al. retrospectively reviewed the data of men with Metastatic Castrate Resistant Prostate Cancer (mCRPC) enrolled in 9 phase III trials treated with Docetaxel containing regimens (34, 41), they found that African American patients were younger, and had worse performance status, higher testosterone, and prostate-specific antigen, and lower hemoglobin than whites. Despite these differences, both races had an equivalent median survival of 21 months. Multivariate analysis revealed that African American men had a 19% decreased risk of death compared to white men (HR = 0.81, 0.72 to 0.91, p < 0.001) (34, 41). In a different study by Halabi et al. analyzed patients with mCRPC enrolled in 8 Cancer and Leukemia Group B (CALGB) trials and reported that there is no significant difference in survival based on race (42). In a retrospective analysis of 1,902 mCRPC patients treated with Sipuleucel-T, African American men were found to have a significantly better median overall survival compared to white men (35.2 vs. 29.9 months, CI: 0.68–0.97, p = 0.03) (43).
McNamara et al. conducted a retrospective evaluation of chemotherapy-naïve mCRPC patients treated with Abiraterone or enzalutamide from a Veterans Health Administration (VHA) database that included 2,123 white and 787 African American mCRPC patients (35). Even though African American men had higher numbers of comorbidities, they still had better OS compared to white men (HR = 0.826; 95%CI [0.732-0.933]) (35). “Abi Race” (NCT01940276) is a prospective, multicenter study of African American and white patients with mCRPC treated with abiraterone acetate and prednisone, evaluating racial disparities in the outcomes (44). When George et al. reported the initial results of this prospective study, they found the primary endpoint radiographic progression-free survival (rPFS) was equal among the races (16.8 months) (44). Interestingly, African American men had more significant and more durable PSA progression-free survival, which was their secondary endpoint (28). Matzkin et al., Fowler et al. and Bergan et al. have all reported the absence of racial disparities in the treatment outcomes for mCRPC patients (29, 32, 45). These results should be analyzed acknowledging the disparities in the clinical trial participation among the races.
Our study agrees with the general literature regarding the presence of racial disparities in the outcome for localized prostate cancer (no metastasis group). There is evidence to suggest that African American men are disproportionately affected by prostate cancer (46, 47), and it has been reported in other countries other than the United States as well (48). There is some evidence to suggest that African American men have a genetically different cancer (16, 21, 31, 49, 50). However, other authors argue that there are no established genetic differences in PCa among African Americans and whites and that the disparities are due to differences in access to care (51). African American men tend to have poor access to screening for prostate cancer (52), and African American men tend to have a lower use of follow-through care even after having an elevated PSA identified during screening (53). Some authors have concluded that disparities continue to exist between African Americans and whites regarding treatments of localized PCa (54, 55), including African American men are less likely to receive definitive treatments (54, 56–58); less likely to undergo prostatectomy or pelvic node dissection during prostatectomy and are more likely to have complications during the operation and in the post-operative period (55, 59, 60).
There is some recent research suggesting that access to care is the causative factor leading to the racial disparities in the outcome. These authors argue that no racial disparities exist in the outcome if there is equal access to care, based on studies of patients who received care through Veterans Affairs hospital system or in a clinical trial, where similar treatments are guaranteed (6, 61). Krimphove et al. reported that when access to care, treatment, and cancer characteristics are accounted for, African American men were found to have even better OS as compared to white men (62).
Our study presents a difference in racial disparities in the outcome between metastatic and non-metastatic prostate cancer patients, which is hard to explain. One possible explanation is the treatment modality. Metastatic patients are treated with systemic therapy only, and that was mainly Androgen Deprivation Treatment (ADT) until recently. There is some evidence to suggest that African American men have higher testosterone and androgen receptor expression compared to white men (21, 63, 64). These might be contributing to a better response to ADT, which in turn might nullify other socio-economic factors contributing to the disparities in the outcome. Another possible explanation is the wide availability and relatively inexpensive nature of ADT, leading to equal opportunities for treatment regardless of race and socioeconomic status. Localized PCa is a very heterogeneous disease with multiple significantly different treatment options, which might lead to disparities in the outcome (65). In contrast, mPCa is a relatively homogeneous disease with only a few treatment options and thus have less chance of racial disparity in the treatment outcomes. Further research is warranted to determine why there is an absence of racial disparities in the mPCa and its implications.
The primary limitation of our study is the retrospective nature of the NCDB database which accounts for patients receiving care only in Commission on Cancer (CoC) accredited facilities. This is a hospital-based registry and there is possibility that we might be missing more number of patients, unlike in a population-based registry (37). We did not evaluate the treatment outcomes based on the treatment options. The cause of death or cancer-specific survival was not available due to the nature of the NCDB database (37).
Conclusions
Current NCDB review aimed to validate our previously published results of an absence of racial disparities in the survival outcome among PCa patients with bone metastases. This research supports our previous finding and reveals that there are no racial differences in survival outcomes between African American and white patients with metastatic prostate cancer (bone or other metastases) in a national database. In contrast, racial disparities in the outcome continue to exist among non-metastatic PCa patients. Further Research is warranted find the cause of this difference.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
TV and XG are the lead authors who share first authorship. SL participated in data analysis, table/figure creation, and data interpretation. AA contributed to manuscript writing and review. DG contributed to manuscript writing and review. SV and VV are the senior authors who came up with the original idea for this research and guided the research and writing process. All authors contributed to the article and approved the submitted version.
Funding
SL is partially supported by the Mississippi Center for Clinical and Translational Research and Mississippi Center of Excellence in Perinatal Research COBRE funded by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers 5U54GM115428 and P20GM121334.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
3. Kheirandish P, Chinegwundoh F. Ethnic differences in prostate cancer. Br J Cancer. (2011) 105:481–5. doi: 10.1038/bjc.2011.273
4. Rebbeck TR. Prostate cancer genetics: variation by race, ethnicity, and geography. Sem Rad Oncol. (2017) 27:3–10. doi: 10.1016/j.semradonc.2016.08.002
5. Williams VL, Awasthi S, Fink AK, Pow-Sang JM, Park JY, Gerke T, et al. African-American men and prostate cancer-specific mortality: a competing risk analysis of a large institutional cohort, 1989-2015. Cancer Med. (2018) 7:2160–71. doi: 10.1002/cam4.1451
6. Dess RT, Hartman HE, Mahal BA, Soni PD, Jackson WC, Cooperberg MR, et al. Association of black race with prostate cancer–specific and other-cause mortalityassociation of black race with prostate cancer–specific and other-cause mortalityassociation of black race with prostate cancer–specific and other-cause mortality. JAMA Oncol. (2019) 5:975–83. doi: 10.1001/jamaoncol.2019.0826
7. Hanson HA, Martin C, O'Neil B, Leiser CL, Mayer EN, Smith KR, et al. The relative importance of race compared to healthcare and social factors in predicting prostate cancer mortality: a random forest approach. J Urol. (2019) 202:1209–16. doi: 10.1097/ju.0000000000000416
8. Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. (2005) 97:1407–27. doi: 10.1093/jnci/dji289
9. Aus G, Abbou CC, Bolla M, Heidenreich A, Schmid HP, van Poppel H, et al. EAU guidelines on prostate cancer. Eur Urol. (2005) 48:546–51. doi: 10.1016/j.eururo.2005.06.001
10. Powell IJ, Banerjee M, Sakr W, Grignon D, Wood Jr. DP, Novallo M, et al. Should African-American men be tested for prostate carcinoma at an earlier age than white men? Cancer. (1999) 85:472–7. doi: 10.1002/(SICI)1097-0142(19990115)85:2<472::AID-CNCR27>3.0.CO;2-0
11. Powell IJ, Heilbrun LK, Sakr W, Grignon D, Montie J, Novallo M, et al. The predictive value of race as a clinical prognostic factor among patients with clinically localized prostate cancer: A multivariate analysis of positive surgical margins. Urology. (1997) 49:726–31. doi: 10.1016/S0090-4295(96)00618-8
12. Powell IJ, Vigneau FD, Bock CH, Ruterbusch J, Heilbrun LK. Reducing prostate cancer racial disparity: evidence for aggressive early prostate cancer PSA testing of African American men. Cancer Epidemiol Biomed Prev. (2014) 23:1505–11. doi: 10.1158/1055-9965.Epi-13-1328
13. Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in White American men, and influences racial progression and mortality disparity. J Urol. (2010) 183:1792–7. doi: 10.1016/j.juro.2010.01.015
14. Kumar S, Singh R, Malik S, Manne U, Mishra M. Prostate cancer health disparities: an immuno-biological perspective. Cancer Lett. (2018) 414:153–65. doi: 10.1016/j.canlet.2017.11.011
15. Shenoy D, Packianathan S, Chen AM, Vijayakumar S. Do African-American men need separate prostate cancer screening guidelines? BMC Urol. (2016) 16:19. doi: 10.1186/s12894-016-0137-7
16. Shiina M, Hashimoto Y, Kato T, Yamamura S, Tanaka Y, Majid S, et al. Differential expression of miR-34b and androgen receptor pathway regulate prostate cancer aggressiveness between African-Americans and Caucasians. Oncotarget. (2017) 8:8356–68. doi: 10.18632/oncotarget.14198
17. American Cancer Society. Prostate Statistics. Atlanta, GA: American Cancer Society. (2018). Available online at: https://cancerstatisticscenter.cancer.org/?_ga=2.128503500.1947676907.1545064325-1467894297.1545064325#!/cancer-site/Prostate (Accessed 12/17/2018 2018)
18. American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society. (2018). Available online at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf (Accessed 12/17/2018).
19. Hawkins LD. QuickStats: age-adjusted death rates* from prostate cancer, (†) by race/ ethnicity - national vital statistics system, United States, 1999-2017. MMWMorb Mort Weekly Rep. (2019) 68:531. doi: 10.15585/mmwr.mm6823a4
20. Xin H. Racial disparity in localized prostate cancer mortality. J Natl Med Assoc. (2017) 109:86–92. doi: 10.1016/j.jnma.2017.01.007
21. Gaston KE, Kim D, Singh S, Ford OH, 3rd, Mohler JL. Racial differences in androgen receptor protein expression in men with clinically localized prostate cancer. J Urol. (2003) 170:990–3. doi: 10.1097/01.ju.0000079761.56154.e5
22. Godley PA, Schenck AP, Amamoo MA, Schoenbach VJ, Peacock S, Manning M, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst. (2003) 95:1702–10. doi: 10.1093/jnci/djg094
23. Schapira MM, McAuliffe TL, Nattinger AB. Treatment of localized prostate cancer in African-American compared with Caucasian men. Less use of aggressive therapy for comparable disease. Med Care. (1995) 33:1079–88.
24. Steele CB, Li J, Huang B, Weir HK. Prostate cancer survival in the United States by race and stage (2001-2009): Findings from the CONCORD-2 study. Cancer. (2017) 123 (Suppl 24):5160–77. doi: 10.1002/cncr.31026
25. Smith ZL, Eggener SE, Murphy AB. African-American prostate cancer disparities. Curr Urol Rep. (2017) 18:81. doi: 10.1007/s11934-017-0724-5
26. Thompson IM, Tangen CM, Tolcher A, Crawford ED, Eisenberger M, Moinpour CM. Association of African-American ethnic background with survival in men with metastatic prostate cancer. J National Cancer Inst. (2001) 93:219–25. doi: 10.1093/jnci/93.3.219
27. Hoffman RM, Gilliland FD, Eley JW, Harlan LC, Stephenson RA, Stanford JL, et al. Racial and ethnic differences in advanced-stage prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. (2001) 93:388–95. doi: 10.1093/jnci/93.5.388
28. Alexander M, Zhu K, Cullen J, Byrne C, Brown D, Shao S, et al. Race and overall survival in men diagnosed with prostate cancer in the Department of Defense Military Health System, 1990-2010. Cancer Causes Cont. (2019) 30:627–35. doi: 10.1007/s10552-019-01163-5
29. Bergan RC, Walls RG, Figg WD, Dawson NA, Headlee D, Tompkins A, et al. Similar clinical outcomes in African-American and non-African-American males treated with suramin for metastatic prostate cancer. J Natl Med Assoc. (1997) 89:622–8.
30. Spratt DE, Chen YW, Mahal BA, Osborne JR, Zhao SG, Morgan TM, et al. Individual patient data analysis of randomized clinical trials: impact of black race on castration-resistant prostate cancer outcomes. Eur Urol Focus. (2016) 2:532–9. doi: 10.1016/j.euf.2016.03.010
31. Thatai LC, Banerjee M, Lai Z, Vaishampayan U. Racial disparity in clinical course and outcome of metastatic androgen-independent prostate cancer. Urology. (2004) 64:738–43. doi: 10.1016/j.urology.2004.05.024
32. Fowler JE, Bigler SA, Renfroe DL, Dabagia MD. Prostate specific antigen in black and white men after hormonal therapies for prostate cancer. J Urol. (1997) 158:150–4. doi: 10.1097/00005392-199707000-00047
33. McLeod DG, Schellhammer PF, Vogelzang NJ, Soloway MS, Sharifi R, Block NL, et al. Exploratory analysis on the effect of race on clinical outcome in patients with advanced prostate cancer receiving bicalutamide or flutamide, each in combination with LHRH analogues. The Casodex Combination Study Group. Prostate. (1999) 40:218–24. doi: 10.1002/(sici)1097-0045(19990901)40:4<218::aid-pros2>3.0.co;2-6
34. Halabi S, Dutta S, Tangen CM, Rosenthal M, Petrylak DP, Thompson IM, et al., et al. Overall survival of black and white men with metastatic castration-resistant prostate cancer treated with docetaxel. J Clin Oncol. (2019) 37:403–10. doi: 10.1200/jco.18.01279
35. McNamara MA, George DJ, Ramaswamy K, Lechpammer S, Mardekian J, Schultz NM, et al. Overall survival by race in chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC) patients treated with abiraterone acetate or enzalutamide. J Clin Oncol. (2019) 37(7_suppl):212. doi: 10.1200/JCO.2019.37.7_suppl.212
36. Gordy DP, Gordy XZ, Lirette S, Vijayakumar S, Vijayakumar V. Bone metastatic prostate cancer among African Americans: the survival equivalence parodox. Biomed Sci Instrum. (2019) 55:109–15.
37. Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, et al. Using the national cancer database for outcomes research: a review. JAMA Oncol. (2017) 3:1722–8. doi: 10.1001/jamaoncol.2016.6905
38. Sassani P, Blumberg Jeremy M, Cheetham Craig T, Niu F, Jacobsen Steven J, Williams Stephen G, et al. African American men have lower rate of treatment failure on primary LHRH agonist therpay for prostate cancer. J Urol. (2009) 181:62–3. doi: 10.1016/S0022-5347(09)60185-3
39. Sassani P, Blumberg JM, Cheetham TC, Niu F, Williams SG, Chien GW. Black men have lower rates than white men of biochemical failure with primary androgen-deprivation therapy. Perm J. (2011) 15:4–8. doi: 10.7812/tpp/11-096
40. Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. (1998) 339:1036–42. doi: 10.1056/NEJM199810083391504
41. Duke University Medical Center. Survival Benefit for African-Americans With Advanced Prostate Cancer. Durham, NC: Duke University Medical Center (2018). Available online at: https://medicalxpress.com/news/2018-06-survival-benefit-african-americans-advanced-prostate.html
42. Halabi S, Small EJ, Vogelzang NJ, Barrier Jr RC, George SL, Gilligan TD. Impact of race on survival in men with metastatic hormone-refractory prostate cancer. Urology. (2004) 64:212–7.
43. Sartor AO, Armstrong AJ, Ahaghotu C, McLeod DG, Cooperberg MR, Penson DF, et al. Overall survival (OS) of African-American (AA) and Caucasian (CAU) men who received sipuleucel-T for metastatic castration-resistant prostate cancer (mCRPC): Final PROCEED analysis. Am Soc Clin Oncol. (2019) 64:212–7. doi: 10.1016/j.urology.2004.04.014
44. George DJ, Heath EI, Sartor AO, Sonpavde G, Berry WR, Healy P, et al. Abi Race: a prospective, multicenter study of black (B) and white (W) patients (pts) with metastatic castrate resistant prostate cancer (mCRPC) treated with abiraterone acetate and prednisone (AAP). Am Soc Clin Oncol. (2018) 37:5035–5. doi: 10.1200/JCO
45. Matzkin H, Rangel MC, Soloway MS. Relapse on endocrine treatment in patients with stage D2 prostate cancer. Does second-line hormonal therapy affect survival? Urology. (1993) 41:144–8. doi: 10.1016/0090-4295(93)90167-9
46. Burks DA, Littleton RH. The epidemiology of prostate cancer in black men. Henry Ford Hosp Med J. (1992) 40:89–92.
47. McGinley KF, Tay KJ, Moul JW. Prostate cancer in men of African origin. Nat Rev Urol. (2016) 13:99–107. doi: 10.1038/nrurol.2015.298
48. Reddy S, Shapiro M, Morton R, Jr, Brawley OW. Prostate cancer in black and white Americans. Cancer Metastasis Rev. (2003) 22:83–6.
49. Powell IJ, Dyson G, Land S, Ruterbusch J, Bock CH, Lenk S, et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiol Biomarkers Prev. (2013) 22:891–7. doi: 10.1158/1055-9965.epi-12-1238
50. Farrell J, Petrovics G, McLeod DG, Srivastava S. Genetic and molecular differences in prostate carcinogenesis between African American and Caucasian American men. Int J Mol Sci. (2013) 14:15510–31. doi: 10.3390/ijms140815510
51. Freedland SJ, Isaacs WB. Explaining racial differences in prostate cancer in the United States: sociology or biology? Prostate. (2005) 62:243–52. doi: 10.1002/pros.20052
52. Mahal BA, Chen YW, Muralidhar V, Mahal AR, Choueiri TK, Hoffman KE, et al. Racial disparities in prostate cancer outcome among prostate-specific antigen screening eligible populations in the United States. Ann Oncol. (2017) 28:1098–104. doi: 10.1093/annonc/mdx041
53. Barocas DA, Grubb R, Black A, Penson DF, Fowke JH, Andriole G, et al. Association between race and follow-up diagnostic care after a positive prostate cancer screening test in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer. (2013) 119:2223–9. doi: 10.1002/cncr.28042
54. Underwood W, De Monner S, Ubel P, Fagerlin A, Sanda MG, Wei JT. Racial/ethnic disparities in the treatment of localized/regional prostate cancer. J Urol. (2004) 171:1504–7.
55. Hayn MH, Orom H, Shavers VL, Sanda MG, Glasgow M, Mohler JL, et al. Racial/ethnic differences in receipt of pelvic lymph node dissection among men with localized/regional prostate cancer. Cancer. (2011) 117:4651–8. doi: 10.1002/cncr.26103
56. Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. (2011) 71:985–97. doi: 10.1002/pros.21314
57. Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. (2002) 94:334–57. doi: 10.1093/jnci/94.5.334
58. Friedlander DF, Trinh QD, Krasnova A, Lipsitz SR, Sun M, Nguyen PL, et al. Racial disparity in delivering definitive therapy for intermediate/high-risk localized prostate cancer: the impact of facility features and socioeconomic characteristics. Eur Urol. (2017) 71:985–97. doi: 10.1016/j.eururo.2017.07.023
59. Schmid M, Meyer CP, Reznor G, Choueiri TK, Hanske J, Sammon JD, et al. Racial differences in the surgical care of medicare beneficiaries with localized prostate cancer. JAMA Oncol. (2016) 2:85–93. doi: 10.1001/jamaoncol.2015.3384
60. Brawley OW. The meaning of race in prostate cancer treatment. JAMA Oncol. (2016) 2:93–4. doi: 10.1001/jamaoncol.2015.3615
61. Graham-Steed T, Uchio E, Wells CK, Aslan M, Ko J, Concato J. ‘Race' and prostate cancer mortality in equal-access healthcare systems. Am J Med. (2013) 126:1084–8. doi: 10.1016/j.amjmed.2013.08.012
62. Krimphove MJ, Cole AP, Fletcher SA, Harmouch SS, Berg S, Lipsitz SR, et al. Evaluation of the contribution of demographics, access to health care, treatment, and tumor characteristics to racial differences in survival of advanced prostate cancer. Prost Cancer Prost Dis. (2019) 22:125.
63. Hu H, Odedina FT, Reams RR, Lissaker CT, Xu X. Racial differences in age-related variations of testosterone levels among US males: potential implications for prostate cancer and personalized medication. J Racial Ethn Health Disparities. (2015) 2:69–76. doi: 10.1007/s40615-014-0049-8
64. Ross R, Bernstein L, Judd H, Hanisch R, Pike M, Henderson B. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst. (1986) 76:45–8.
Keywords: prostate cancer, metastasic, racial and ethnic differences, African American (AA) men, white men, survival outcomes
Citation: Vengaloor Thomas T, Gordy XZ, Lirette ST, Albert AA, Gordy DP, Vijayakumar S and Vijayakumar V (2020) Lack of Racial Survival Differences in Metastatic Prostate Cancer in National Cancer Data Base (NCDB): A Different Finding Compared to Non-metastatic Disease. Front. Oncol. 10:533070. doi: 10.3389/fonc.2020.533070
Received: 06 February 2020; Accepted: 17 August 2020;
Published: 18 September 2020.
Edited by:
Cora N. Sternberg, San Camillo-Forlanini Hospital, ItalyReviewed by:
Fred Saad, University of Montreal Hospital Centre (CRCHUM), CanadaPaul Denis Leger, National Cancer Institute, National Institutes of Health (NIH), United States
Copyright © 2020 Vengaloor Thomas, Gordy, Lirette, Albert, Gordy, Vijayakumar and Vijayakumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Srinivasan Vijayakumar, c3ZpamF5YWt1bWFyQHVtYy5lZHU=; Vani Vijayakumar, dnZpamF5YWt1bWFyQHVtYy5lZHU=
†These authors have contributed equally to this work and share first authorship
 Toms Vengaloor Thomas
Toms Vengaloor Thomas Xiaoshan Z. Gordy
Xiaoshan Z. Gordy Seth T. Lirette3
Seth T. Lirette3 Srinivasan Vijayakumar
Srinivasan Vijayakumar Vani Vijayakumar
Vani Vijayakumar