- Department of Dermatology, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan
Recent clinical trials have demonstrated the efficacy of immune checkpoint inhibitors (ICIs) for treating melanoma. However, these previous studies comprised mainly Caucasian populations, in which cutaneous melanoma (CM) is the major clinical type. In contrast, Asian populations have a distinct profile of melanoma and show much higher frequencies of acral lentiginous melanoma (ALM) and mucosal melanoma (MCM). Compared with CM, ALM and MCM show poorer response to ICIs, but the mechanisms have not been fully understood. To evaluate the immune status in each melanoma subtype, we examined the number of total tumor-infiltrating lymphocytes (TILs), CD4+ TILs, CD8+ TILs, and tumor-infiltrating FoxP3+ regulatory T cells (Tregs) to evaluate the immune status in each melanoma subtype using data from 137 patients with melanoma. Total TIL numbers in ALM and MCM were significantly lower than that in CM. CD4+ TIL number in MCM was also lower than CM although CD4+ TIL number in ALM was comparable with CM. In contrast, CD8+ TIL numbers in both ALM and MCM were significantly lower than that in CM. Although number of tumor-infiltrating Tregs was comparable among the 3 subtypes, the proportion of tumor-infiltrating Tregs in CD4+ T cells in MCM was significantly higher than in CM and ALM. Multivariate regression analysis revealed that ALM and MCM were significantly associated with a lower total TIL number, but only MCM was significantly associated with a lower CD4+ TIL number. Multivariate regression analysis also revealed that both ALM and MCM were significantly associated with a lower CD8+ TIL number. Our results suggest that both ALM and MCM are independent factors of lower total TIL number, which may be associated with poorer responses to ICIs in ALM and MCM.
Introduction
Malignant melanoma is an aggressive malignant tumor with high mortality (1). However, recent clinical trials have demonstrated the efficacy of immune checkpoint inhibitors (ICIs) for treating malignant melanoma. Both anti-programmed death-1 (PD-1) monoclonal antibodies (nivolumab and pembrolizumab) and anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) monoclonal antibodies (ipilimumab) have been reported to prolong the overall survival in advanced melanoma patients (1–3). In addition, combination therapy of nivolumab plus ipilimumab showed better overall survival than ipilimumab alone (4). However, these previous studies comprised mainly Caucasian populations, in which cutaneous melanoma (CM) is the major clinical type of melanoma (5). In contrast, Asian populations reveal a distinct profile of melanoma from that of Caucasians with much higher frequencies of acral lentiginous melanoma (ALM) and mucosal melanoma (MCM). We previously showed that, compared with CM, ALM and MCM have a poorer response to ICIs (6, 7). However, the mechanisms underlying this poor response in ALM an MCM have not been fully elucidated.
PD-1 is an inhibitory receptor expressed mainly by activated T cells. CTLA-4 suppresses T cell activation through competing with CD28 in binding to CD80/86. Both anti-PD-1 antibodies and anti-CTLA-4 antibodies exert anti-tumor effects through T cell activation. Immune cells, including T cells, that infiltrate tumors may induce tumor regression following ICI treatment, and consistently, the number of tumor-infiltrating lymphocytes (TILs) have been reported to predict the tumor response to ICIs (8). Therefore, we speculate that TIL number may be lower in ALM and MCM than in CM. In this study, we examined number of total TILs, CD4+ TILs, CD8+ TILs, and tumor-infiltrating FoxP3+ regulatory T cells (Tregs) of the primary tumors for each melanoma subtype to evaluate the immune status.
Material and Methods
Patients
Data of patients with melanoma, who were referred to our department and whose primary tumors were resected at the University of Tsukuba Hospital from January 2004 to November 2019, were retrospectively collected. Diagnosis was confirmed using the pathological findings. Clinical factors including age, sex, melanoma subtype, tumor thickness, presence or absence of ulceration of primary tumors were collected. This study was approved by the institutional ethics committee of the University of Tsukuba Hospital. All the protocols in this study were performed in accordance with the Declaration of Helsinki.
Immunohistochemical Studies
The specimens were fixed in formalin and embedded in paraffin. They were sliced into 3-µm thicknesses, mounted on glass slides, deparaffinized in xylene, and rehydrated before antigen retrieval by boiling in citrate buffer (0.01M citrate containing 0.5% Tween 20, pH 6.0). The sections were incubated in 10% bovine serum albumin in PBS containing 0.01% Tween 20 at room temperature for 1 hour and with optimized concentrations for 60 min at room temperature. The primary antibodies used in this experiment were antibodies for human CD4 (M7310, 1:250 dilution; Dako), CD8 (M7103, 1:250 dilution; Dako) and FoxP3 (ab20034, 1:200 dilution; Abcam) overnight at 4°C, followed by biotinylated anti-rat IgG antibody (1:500; Vector Laboratories) and VECTASTATIN ABC reagent (Vector Laboratories) at room temperature for 60 and 30 min, respectively. Finally, the sections were stained with DAB Peroxidase Substrate Kit (Vector Laboratories) before imaging. The number of positive cells was counted in a ×400 microscopic field (high power fields: HPF). Six areas were counted in each case, and the numbers were averaged. Total TIL number was calculated as the total number of CD4+ TILs plus CD8+ TILs. In addition to TIL number, we also evaluated the numbers of total T cells, CD4+ T cells, CD8+ T cells, and FoxP+ regulatory T cells (Tregs) in peritumoral skin/mucosa and distant normal skin/mucosa as controls. The peritumoral skin/mucosa and normal skin/mucosa were defined as areas at a distance of 0.3–2 mm and more than 4 mm from the lesions, respectively.
Statistical Analysis
Kruskal-Wallis statistic, Mann-Whitney U test, and Chi square were used to compare clinical factors among melanoma subtypes. Univariate regression analyses were used to reveal the association of numbers of total TILs, CD4+ TILs or CD8+ TILs with clinical factors. For the factors shown to be significant in the univariate analysis, multivariate analyses in a stepwise procedure were performed to determine the independent factors associated with number of total TILs, CD4+ TILs or CD8+ TILs. In the procedure, a 0.15 significance level for entering and eliminating explanatory variables was used. Throughout the analyses, P-values of <0.05 were considered as statically significant. The statistical tests were 2-sided and carried out using Stat Flex version 6.0 (Artec, Osaka, Japan) and Prim version 6 (Graph Pad software, California, USA).
Results
Patient Background
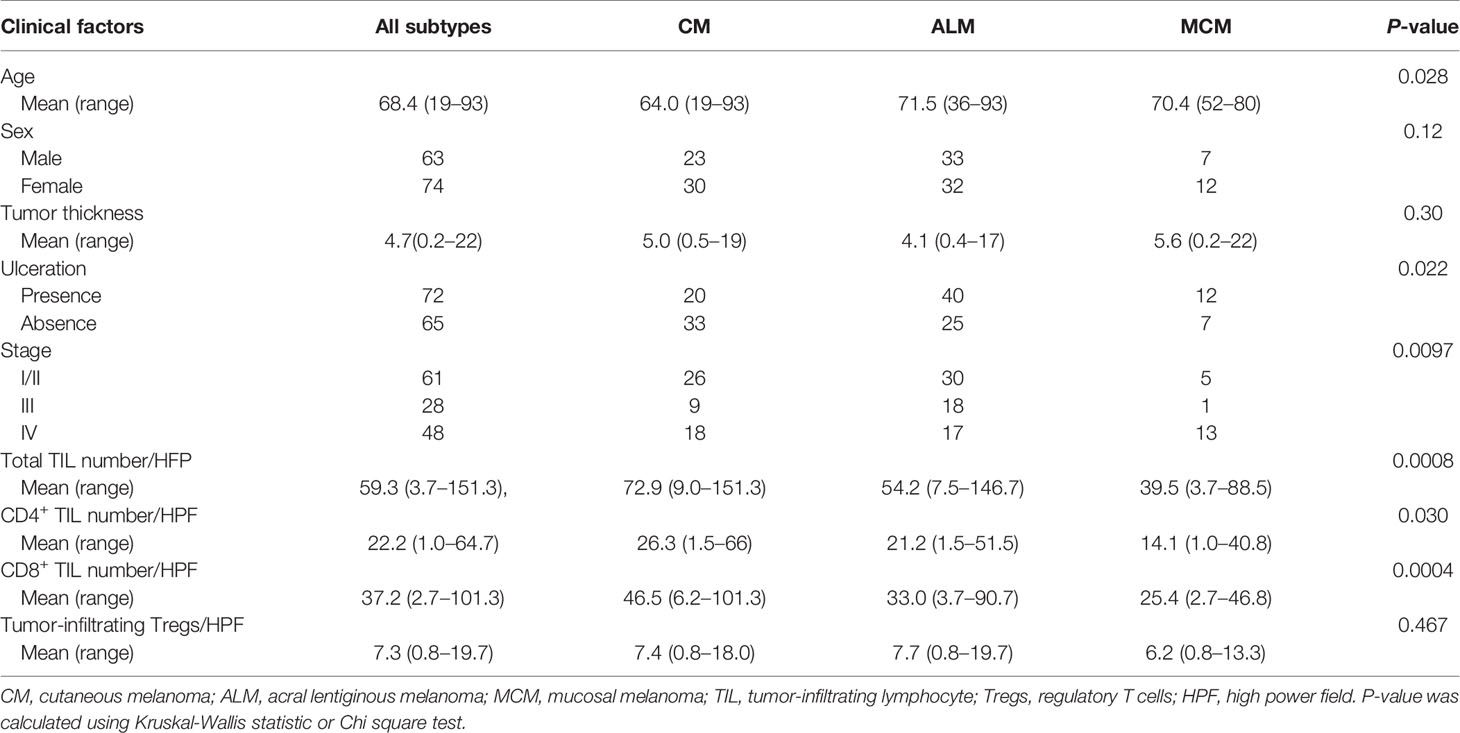
We collected data for 137 patients with melanoma (Table 1). The mean age was 68.4 (range: 19 to 93) and 63 patients (46.0%) were male. The most common melanoma type was ALM (65 patients, 47.4%), followed by CM (53 patients, 38.7%), and MCM (19 patients, 13.9%). Among the 19 MCM patients, 17 (89.4%) were referred to our department after the 2014 introduction of ICIs to Japan. Twenty-four patients (36.9%) with ungual melanoma were included in the ALM category. Primary tumor locations in the patients with MCM included: genital lesions (5 patients), conjunctiva lesions (5 patients), nasal cavity (4 patients), oral cavity (2 patients), anorectal lesion (2 patients), and esophageal lesion (1 patient). The mean tumor thickness was 4.7mm (range: 0.2 to 22) and ulceration was present in 72 patients (52.6%). There were 61 patients at stage I/II, 28 at stage III, and 48 at stage IV. The mean number of total TILs, CD4+ TILs, CD8+ TILs and tumor-infiltrating Tregs were 59.3/HPF (range: 3.7 to 151.3), 22.2/HPF (range: 1.0 to 64.7), 37.2/HPF (range: 2.7 to 101.3) and 7.3/HPF (range: 0.8 to 19.7), respectively. The mean number of total T cells, CD4+ T cells, CD8+ T cells and Tregs in peritumoral skin/mucosa were 22.1/HPF (range: 10.3 to 45.5), 11.1/HPF (range: 1.3 to 28.7), 11.9/HPF (range: 3.2 to 30.2) and 4.0/HPF (range: 0.3 to 10.8), respectively. The mean number of total T cells, CD4+ T cells, CD8+ T cells and Tregs in distant normal skin/mucosa were 9.9/HPF (range: 2.0 to 54.2), 5.5/HPF (range: 0.8 to 23.1), 4.4/HPF (range: 0.8 to 26.2) and 1.0/HPF (range: 0.2 to 6.7), respectively. The occurrence of distant normal skin/mucosa in 4 patients (1 with ALM patient and 3 with MCM) was not included in the resected specimens, and was excluded for the analysis.
Comparison of Clinical Factors Among Melanoma Subtypes
We compared the clinical factors among the melanoma subtypes and found that 2 factors, sex and tumor thickness, were comparable among the subtypes (Table 1). In contrast, patients with ALM were significantly older than those with CM (Table 1, Figure 1A). Frequency of ulceration in ALM was significantly higher than CM, and MCM also tended to show higher frequency than CM (Table 1, Figure 1B). The proportion of patients at stage I/II in CM and at stage III in ALM were significantly higher than those in MCM (Table 1, Figure 1C). In contrast, the proportions of patients at stage IV in CM and ALM were significantly lower than in MCM (Table 1, Figure 1C). Total TIL numbers in ALM and MCM were significantly lower than in CM (Figure 2A). CD4+ TIL number in MCM was also lower than in CM, although CD4+ TIL number in ALM was comparable with CM (Figure 2B, Supplemental Figures S1A–C). In contrast, CD8+ TIL numbers in both ALM and MCM were significantly lower than in CM (Figure 2C, Supplemental Figures S1D–F). Although the number of tumor-infiltrating Tregs was comparable among the 3 subtypes, the proportion of tumor-infiltrating Tregs in CD4+ T cells in MCM was significantly higher than in CM and ALM (Figures 2D, E, Supplemental Figure S1G–I). In peritumoral skin/mucosa, both number of Tregs and proportion of Tregs in CD4+ T cells were significantly higher in MCM than CM and ALM, although the numbers of total T cells, CD4+ T cells, and CD8+ T cells were comparable among the 3 subtypes (Figures 2F–J, Supplemental Figures S2A–C). In the normal skin/mucosa, the number of total T cells, CD4+ T cells, CD8+ T cells, Tregs, and proportion of Tregs in CD4+ T cells in MCM were all significantly higher than in CM and ALM (Figures 2K-O, Supplemental Figures S2D–F).
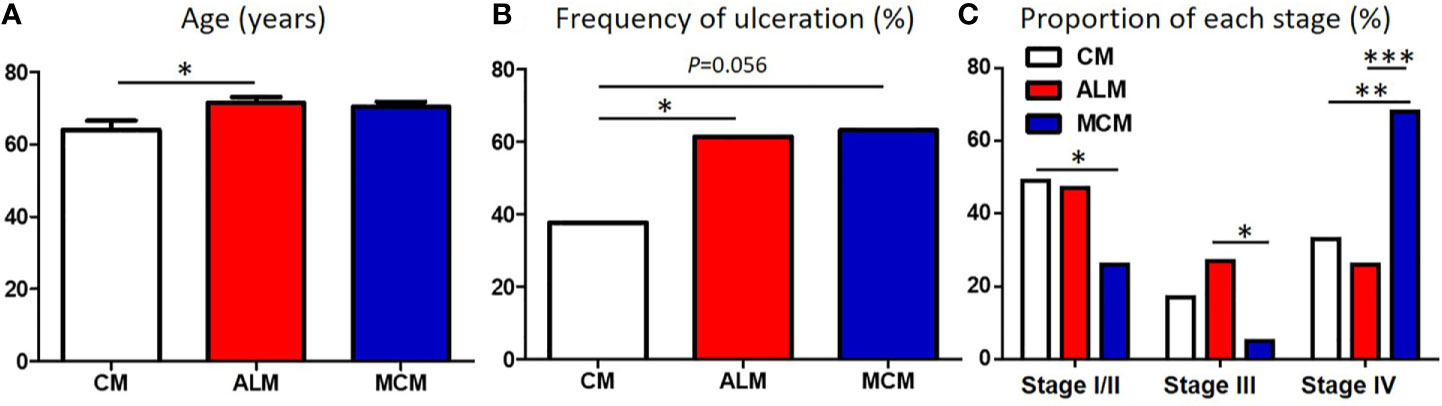
Figure 1 Comparison of patients’ background between each melanoma subtype. (A–C) Age (A), frequency of ulceration (B) and proportion of each stage (C) in patients with cutaneous melanoma (CM), acral lentiginous melanoma (ALM) and mucosal melanoma (MCM) were shown. P-values was calculated using Mann-Whitney U test (A) and Chi square test (B, C). *P<0.05, **<0.01, ***P<0.001.
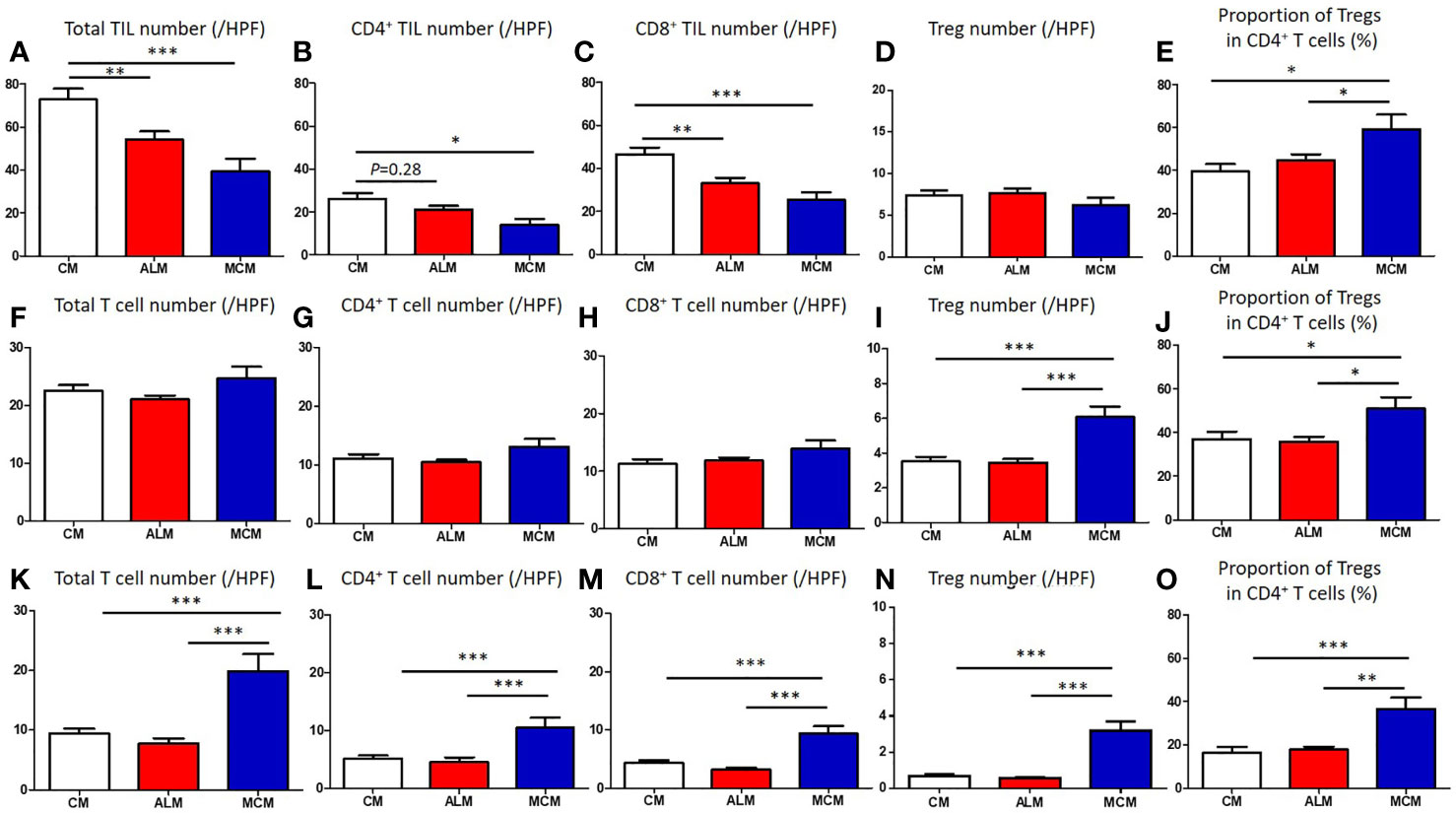
Figure 2 Comparison of T cells in tumor, peritumoral skin/mucosa and distant normal skin/mucosa of each melanoma subtype. (A–O) Numbers of total T cells, CD4+ T cells, CD8+ T cells, regulatory T cells (Tregs) and proportion of Tregs in CD4+ T cells in tumor (A–E), peritumoral skin/mucosa (F–J) and distant normal skin/mucosa (K–O) in patients with cutaneous melanoma (CM), acral lentiginous melanoma (ALM) and mucosal melanoma (MCM) were shown. P-values was calculated using Mann-Whitney U test. *P<0.05, **<0.01, ***P<0.001.
Associations of TIL Number With Clinical Factors
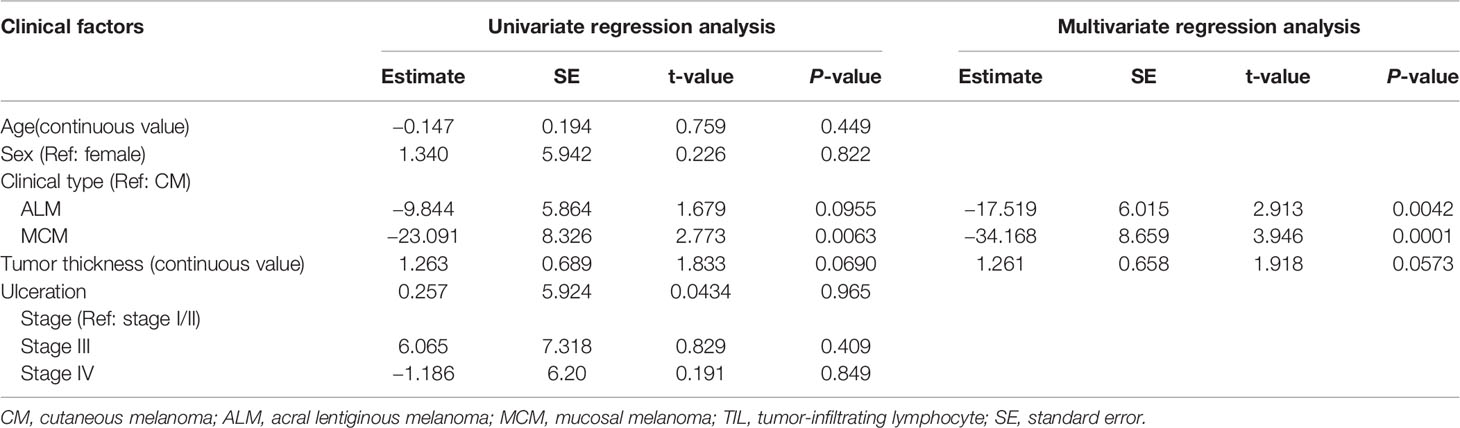
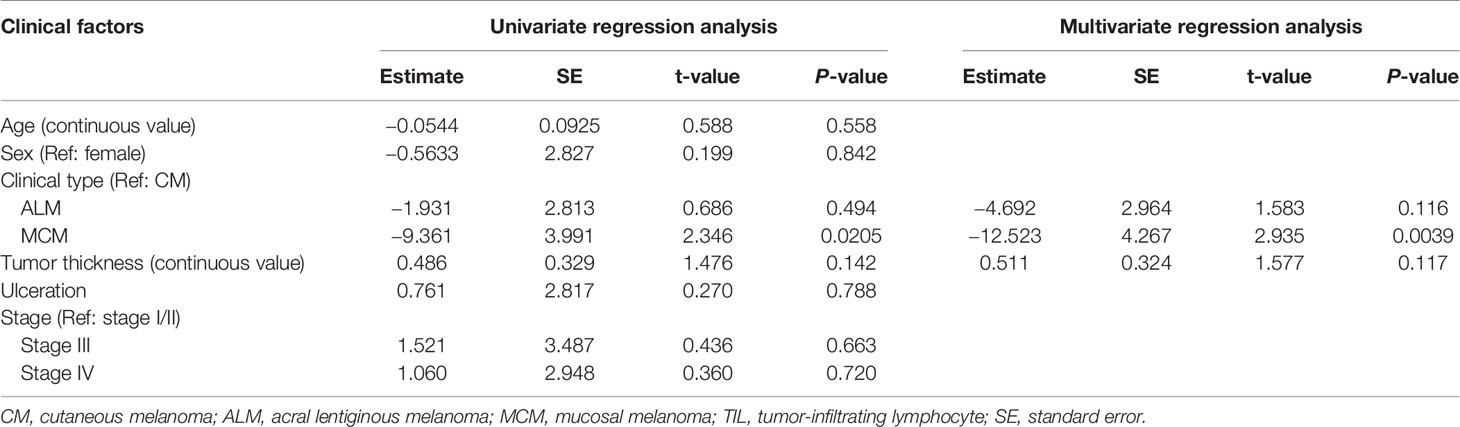
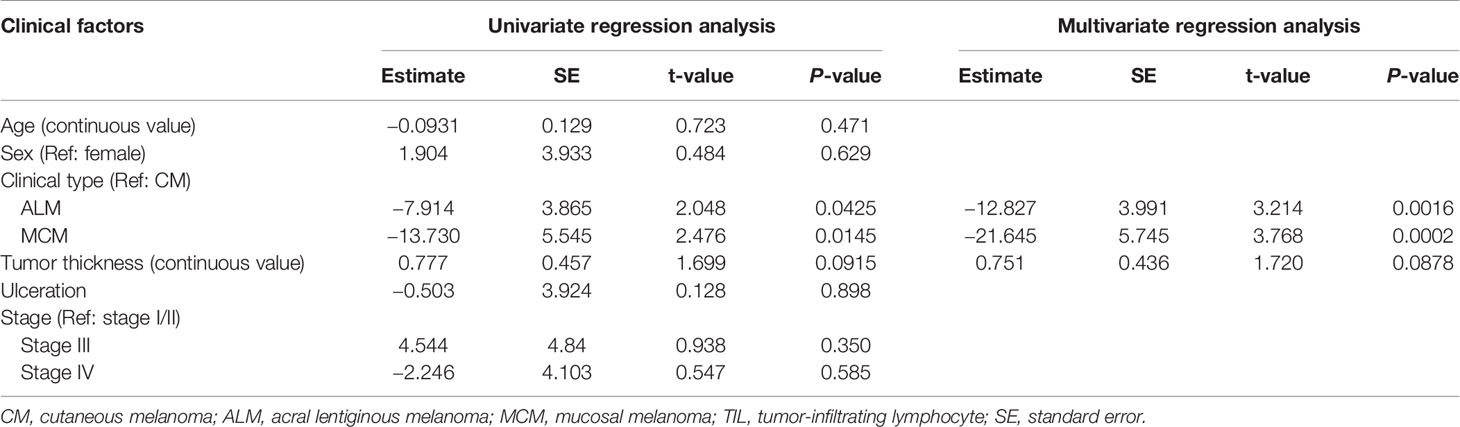
Univariate regression analysis showed that MCM was significantly associated with lower total TIL number, and that ALM and tumor thickness showed a tendency toward lower and higher numbers of total TILs, respectively (Table 2). Multivariate analysis revealed that ALM and MCM were significantly associated with lower total TIL number (Table 2), suggesting that both ALM and MCM were independent factors of lower total TIL number. As for CD4+ TILs, univariate regression analysis showed that only MCM was significantly associated with lower CD4+ TIL number, and the significance was retained in multivariate analysis (Table 3). In contrast, univariate regression analysis showed that both ALM and MCM were significantly associated with lower numbers of CD8+ TILs, and tumor thickness showed a tendency towards higher numbers of CD8+ TILs (Table 4). The significance of both ALM and MCM was also retained in multivariate analysis (Table 4).
Discussion
Previously, Wada et al. demonstrated that melanoma-specific survival in patients with ALM was comparable to those with CM when treated with dacarbazine-based chemotherapies before introduction of ICIs. They proposed that ALM does not differ in its biological behavior from CM (9). In contrast, it has been reported that ALM and MCM were less susceptible to ICI treatment than CM (6, 10). In addition, Maeda et al. demonstrated that overall survival and progression survival in patients with ALM and MCM tended to be shorter than those with CM when treated with nivolumab monotherapy, suggesting that the immune response to ALM and MCM may be poorer than CM (10). However, only a few reports have shown the comparison of basic immune status between each melanoma subtype (11).
Our study revealed that total TIL number in ALM and MCM were both significantly lower than in CM, and multivariate regression analysis showed ALM and MCM to be independent factors of lower total TIL number. The exact mechanism underlying the lower number of TILs in ALM and MCM remains unknown. However, given that ALM and MCM have been reported to show lower mutation burden than CM, the low mutation burden, which results in poor generation of tumor neo-antigen, may be involved in the poor proliferation of tumor-specific T cells in ALM and MCM (12).
Recently, Castaneda et al. demonstrated that the number of TILs in ALM was lower than that in CM, which is consistent with the findings in our study. However, we discovered that, in ALM, while the number of CD8+ TIL was significantly lower than in CM the number of CD4+ TILs was not, suggesting that lower number of TILs in ALM may be a result of the lower numbers of CD8+ TILs. Previous studies reported that there are differences in the molecules responsible for migration, retention, and maintenance between CD4+ T cells and CD8+ T cells (13–15). Therefore, the distinct expression patterns of these molecules associated with migration, retention, and maintenance in ALM and CM may have led to the finding that the number of CD8+ TILs, but not CD4+ TILs, in ALM was significantly lower than in CM, although the exact mechanism remains unknown.
Both CD4+ T cells and CD8+ T cells play crucial roles in the immune response to tumors. However, CD4+ T cells, including Tregs, may also exhibit significant immunosuppressive function for tumors (16, 17). In contrast, CD8+ TILs have been shown to predict favorable prognoses in many types of cancer (18, 19). Additionally, it has been reported that a high ratio of total TIL number to CD4+ TIL number predicted better survival in patients with bladder cancer (20). Moreover, anti-PD-1 antibody was totally dependent on CD8+ T cells to exert anti-tumor immunity in the mouse colon cancer model (21). Therefore, we speculate that poor infiltration of CD8+ T cells, but not CD4+ T cells, may play an important role in limiting the response of ALM to ICI treatment.
In MCM, the numbers of both CD4+ TILs and CD8+ TILs were significantly lower than those in CM. The digestive system manifests immune tolerance to a large variety of antigens, including dietary proteins and commensal bacteria (22). This mechanism within the digestive system includes enriched immune suppressive cells such as Tregs and inhibitory cytokines such as TGF-β and IL-10 (22). In addition, conjunctiva and genital tracts have been reported to show immune tolerance, which may also involve Tregs (23–25). Indeed, in our study, both the number of Tregs and proportion of Tregs in CD4+ T cells were significantly higher in MCM than in CM and ALM in both the peritumoral skin/mucosa and distant normal skin/mucosa. In addition, the proportion of tumor-infiltrating Tregs in CD4+ T cells in MCM was also significantly higher than in CM and ALM. Therefore, basic immune tolerance associated with mucosal site may also contribute the low number of both CD4+ TILs and CD8+ TILs in MCM observed in our study.
In conclusion, our study demonstrated that the number of TILs in ALM and MCM were significantly lower than CM, which may be associated with poorer responses to ICIs in ALM and MCM. Therefore, in addition to ICIs, novel therapies to increase the number of TILs are required for enhancing the tumor immune response in ALM and MCM. Our study was limited by the relatively low number of participants, especially MCM patients. In our study, only 2 of 19 patients with MCM (10.6%) were referred to our department before the introduction of ICIs. In addition, the proportion of patients at stage IV in MCM was significantly higher than in CM and ALM. Therefore, a substantial number of MCM patients who were not indicated for the recently-developed systemic therapies including ICIs may have been treated in other departments or other institutions, resulting in the low number of MCM patients in our study. Therefore, further large-scale studies with a larger number of MCM patients are needed to confirm our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
YN mainly conducted the research, analyzed the data, and wrote the manuscript. ZZ, KO, and RT supported the immunohistochemical studies. YI, NO, RW, and YF provided the helpful suggestions for the research. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Yuriko Hirota (Department of Dermatology, Faculty of Medicine, University of Tsukuba) for the technical support. We also thank Dr. Thomas D. Mayers of the University of Tsukuba Medical English Communication Center for English editing of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.524700/full#supplementary-material
Supplementary Figure 1 | Immunohistochemical studies for CD4, CD8 and FoxP3 in tumor of each melanoma subtype. (A–I) Representative images of immunohistologic analyses of CD4 in the tumor of cutaneous melanoma (CM) (A), acral lentiginous melanoma (ALM) (B), and mucosal melanoma (MCM) (C), CD8 in the tumor of CM (D), ALM (E), and MCM (F), and FoxP3 in the tumor of CM (G), ALM (H), and MCM (I) (×200, respectively). Scale bar, 50 μm.
Supplementary Figure 2 | Immunohistochemical studies for FoxP3 in peritumoral skin/mucosa and distant normal skin/mucosa of each melanoma subtype. Representative images of immunohistologic analyses of FoxP3 in the peritumoral skin/mucosa of cutaneous melanoma (CM) (A), acral lentiginous melanoma (ALM) (B), and mucosal melanoma (MCM) (C), and in the distant normal skin/mucosa of CM (D), ALM (E), and MCM (F) (×200, respectively). Scale bar, 50 μm.
References
1. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med (2015) 372:320–30. doi: 10.1056/NEJMoa1412082
2. Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med (2011) 364:2517–26. doi: 10.1056/NEJMoa1104621
3. Hamid O, Puzanov I, Dummer R, Schachter J, Daud A, Schadendorf D, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer (2017) 86:37–45. doi: 10.1016/j.ejca.2017.07.022
4. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2017) 377:1345–56. doi: 10.1056/NEJMoa1709684
5. Wang Y, Zhao Y, Ma S. Racial differences in six major subtypes of melanoma: descriptive epidemiology. BMC Cancer (2016) 16:691. doi: 10.1186/s12885-016-2747-6
6. Nakamura Y, Tanaka R, Maruyama H, Ishitsuka Y, Okiyama N, Watanabe R, et al. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn J Clin Oncol (2019) 49:431–7. doi: 10.1093/jjco/hyy201
7. Nakamura Y, Fujisawa Y, Tanaka R, Maruyama H, Ishitsuka Y, Okiyama N, et al. Use of immune checkpoint inhibitors prolonged overall survival in a Japanese population of advanced malignant melanoma patients: Retrospective single institutional study. J Dermatol (2018) 45:1337–9. doi: 10.1111/1346-8138.14637
8. Kristensen LK, Frohlich C, Christensen C, Melander MC, Poulsen TT, Galler GR, et al. CD4(+) and CD8a(+) PET imaging predicts response to novel PD-1 checkpoint inhibitor: studies of Sym021 in syngeneic mouse cancer models. Theranostics (2019) 9:8221–38. doi: 10.7150/thno.37513
9. Wada M, Ito T, Tsuji G, Nakahara T, Hagihara A, Furue M, et al. Acral lentiginous melanoma versus other melanoma: A single-center analysis in Japan. J Dermatol (2017) 44:932–8. doi: 10.1111/1346-8138.13834
10. Maeda T, Yoshino K, Nagai K, Oaku S, Kato M, Hiura A, et al. Efficacy of nivolumab monotherapy against acral lentiginous melanoma and mucosal melanoma in Asian patients. Br J Dermatol (2019) 180:1230–1. doi: 10.1111/bjd.17434
11. Castaneda CA, Torres-Cabala C, Castillo M, Villegas V, Casavilca S, Cano L, et al. Tumor infiltrating lymphocytes in acral lentiginous melanoma: a study of a large cohort of cases from Latin America. Clin Transl Oncol (2017) 19:1478–88. doi: 10.1007/s12094-017-1685-3
12. Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature (2017) 545:175–80. doi: 10.1038/nature22071
13. Iijima N, Iwasaki A. Tissue instruction for migration and retention of TRM cells. Trends Immunol (2015) 36:556–64. doi: 10.1016/j.it.2015.07.002
14. Gehad A, Al-Banna NA, Vaci M, Issekutz AC, Mohan K, Latta M, et al. Differing requirements for CCR4, E-selectin, and α4β1 for the migration of memory CD4 and activated T cells to dermal inflammation. J Immunol (2012) 189:337–46. doi: 10.4049/jimmunol.1102315
15. Manes TD, Pober JS. Significant Differences in Antigen-Induced Transendothelial Migration of Human CD8 and CD4 T Effector Memory Cells. Arterioscler Thromb Vasc Biol (2016) 36:1910–8. doi: 10.1161/atvbaha.116.308039
16. Liu Z, McMichael EL, Shayan G, Li J, Chen K, Srivastava R, et al. Novel Effector Phenotype of Tim-3(+) Regulatory T Cells Leads to Enhanced Suppressive Function in Head and Neck Cancer Patients. Clin Cancer Res (2018) 24:4529–38. doi: 10.1158/1078-0432.Ccr-17-1350
17. Ikemoto T, Shimada M, Ishikawa D, Kawashita Y, Teraoku H, Yoshikawa M, et al. Peripheral Tr1 and Foxp3(+) Treg as Markers of Recurrent Malignancies in Patients with Hepato-Biliary Pancreatic Cancers. Anticancer Res (2017) 37:5541–52. doi: 10.21873/anticanres.11986
18. Goode EL, Block MS, Kalli KR, Vierkant RA, Chen W, Fogarty ZC, et al. Dose-Response Association of CD8+ Tumor-Infiltrating Lymphocytes and Survival Time in High-Grade Serous Ovarian Cancer. JAMA Oncol (2017) 3:e173290. doi: 10.1001/jamaoncol.2017.3290
19. Wang H, Li Z, Dong B, Sun W, Yang X, Liu R, et al. Prognostic significance of PD-L1 expression and CD8+ T cell infiltration in pulmonary neuroendocrine tumors. Diagn Pathol (2018) 13:30. doi: 10.1186/s13000-018-0712-1
20. Shi MJ, Meng XY, Wu QJ, Zhou XH. High CD3D/CD4 ratio predicts better survival in muscle-invasive bladder cancer. Cancer Manag Res (2019) 11:2987–95. doi: 10.2147/cmar.S191105
21. Gao CE, Zhang M, Song Q, Dong J. PD-1 inhibitors dependent CD8(+) T cells inhibit mouse colon cancer cell metastasis. Onco Targets Ther (2019) 12:6961–71. doi: 10.2147/ott.S202941
22. Wang X, Sherman A, Liao G, Leong KW, Daniell H, Terhorst C, et al. Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Delivery Rev (2013) 65:759–73. doi: 10.1016/j.addr.2012.10.013
23. Galletti JG, Guzman M, Giordano MN. Mucosal immune tolerance at the ocular surface in health and disease. Immunology (2017) 150:397–407. doi: 10.1111/imm.12716
24. Xu L, Dong B, Wang H, Zeng Z, Liu W, Chen N, et al. Progesterone suppresses Th17 cell responses, and enhances the development of regulatory T cells, through thymic stromal lymphopoietin-dependent mechanisms in experimental gonococcal genital tract infection. Microbes Infect (2013) 15:796–805. doi: 10.1016/j.micinf.2013.06.012
Keywords: cutaneous, melanoma, acral lentiginous melanoma, mucosal melanoma, lymphocytes, checkpoint inhibitors
Citation: Nakamura Y, Zhenjie Z, Oya K, Tanaka R, Ishitsuka Y, Okiyama N, Watanabe R and Fujisawa Y (2020) Poor Lymphocyte Infiltration to Primary Tumors in Acral Lentiginous Melanoma and Mucosal Melanoma Compared to Cutaneous Melanoma. Front. Oncol. 10:524700. doi: 10.3389/fonc.2020.524700
Received: 12 June 2020; Accepted: 17 November 2020;
Published: 17 December 2020.
Edited by:
Wen-Qing Li, Peking University Cancer Hospital, ChinaReviewed by:
Susanna Dolci, University of Rome Tor Vergata, ItalyIoana Cosgarea, Newcastle University, United Kingdom
Copyright © 2020 Nakamura, Zhenjie, Oya, Tanaka, Ishitsuka, Okiyama, Watanabe and Fujisawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshiyuki Nakamura, eW5ha2FtdXJhLXR1a0B1bWluLmFjLmpw
 Yoshiyuki Nakamura
Yoshiyuki Nakamura Zhu Zhenjie
Zhu Zhenjie Ryota Tanaka
Ryota Tanaka Yosuke Ishitsuka
Yosuke Ishitsuka Rei Watanabe
Rei Watanabe Yasuhiro Fujisawa
Yasuhiro Fujisawa