- 1Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Clinical Medicine, West China Hospital, Sichuan University, Chengdu, China
- 3Laboratory of Clinical Nuclear Medicine, Department of Nuclear Medicine, West China Hospital, Sichuan University, Chengdu, China
- 4State Key Laboratory of Biotherapy, Department of Pathology and Laboratory of Pathology, West China Hospital, Sichuan University, Chengdu, China
Purpose: To evaluate the accuracy of 68Ga-PSMA positron emission tomography/computerized tomography (PET/CT) for preoperative lymph node staging using histopathological results of pelvic lymph node dissection (PLND) as reference standard in patients with intermediate/high risk of prostate cancer.
Material and Methods: A systematic search of PubMed, Embase, and the Cochrane Library was completed up to May 2020. We included studies investigating accuracy of 68Ga-PSMA PET/CT in primary lymph node staging before radical prostatectomy and PLND. The pooled sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic odds ratio (DOR), and the summary receiver operating characteristic (SROC) curve with an area under the curve (AUC) were synthesized.
Results: Eleven studies comprising 904 patients were identified. Based on per-patient analysis, the pooled sensitivity and specificity reached 0.63 (95% CI: 0.46–0.78) and 0.93 (95% CI: 0.88–0.96), respectively, with the DOR of 22 (95% CI: 10–47). An overall accuracy was revealed by the SROC curve with AUC of 0.91 (95% CI: 0.88–0.93). Using the lymph node as unit, the pooled sensitivity and specificity were 0.70 (95% CI: 0.49–0.85) and 0.99 (95% CI: 0.96–1.00), respectively. And the DOR reached 167 (95% CI: 40–695) with an AUC of 0.96 (95% CI: 0.94–0.98). The pooled PPV and NPV all reached above 0.8 on basis of per-patient or per-node analysis.
Conclusions: 68Ga-PSMA PET/CT represented as a promising test for preoperative lymph node staging and patients without lymph node metastatic status can rarely be misdiagnosed. However, its sensitivity ought to be improved before forgoing PLND.
Introduction
Prostate cancer (PCa) is regarded as the most commonly diagnosed cancer in men in western countries (1). Radical prostatectomy (RP) remains one widely used curative treatment for patients with localized PCa (2). However, nearly 15% of patients, especially those of intermediate and high risk, harbor lymph node invasion (LVI) during RP with extended pelvic lymph node dissection (PLND) (3). Accurate staging of lymph nodes is important which helps determine the optimal multimodal treatment to achieve better cancer control for patients with metastatic nodes (4).
Currently, PLND remains the gold standard for lymph node staging which provides important information for prognosis (5). However, the frequent complications (lymphocele/lymphedema/venous thromboembolism), high cost, as well as lack of evidence to improve oncological outcomes limited the routine application of this invasive procedure in clinical practice (6, 7). Multiple preoperative nomogram tools have been identified to figure out optimal candidates for PLND, however, lacking an ideal cut-off value because of various patient selections (8–10). In addition, such models were originated from general populations which may not be perfect for one specific individual (11). More specific and non-invasive imaging modalities including computed tomography (CT) and magnetic resonance imaging (MRI) have been assessed for lymph node staging but without satisfactory accuracy (12, 13).
Prostate-specific membrane antigen (PSMA) is overexpressed on the surface of most PCa cells (14). And 68Ga-PSMA ligand positron emission tomography/computerized tomography (PET/CT) has been reported to be superior to conventional modalities in the detection of metastatic PCa, especially in the identification of recurrent PCa after primary treatment failure (15, 16). However, the diagnostic role of 68Ga-PSMA PET/CT in primary lymph node staging before RP has yet to be fully revealed. We conducted this meta-analysis and tried to evaluate the accuracy of 68Ga-PSMA PET/CT for preoperative lymph node staging using histopathological results of dissected lymph nodes as reference standard in patients with intermediate/high risk of PCa.
Materials and Methods
Search Strategy
A systematic review was performed in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines (17). PubMed, Embase, and the Cochrane Library were searched up to May 2020. The search strategy was applied to identify all trials by using medical subject headings in combination with keywords of “Positron-Emission Tomography,” “PSMA,” “Gallium,” and “prostate cancer.” We limited studies to human. We also manually screened the references of included studies for additional citations.
Selection Criteria
Two authors evaluated all potential articles independently. The inclusion criteria were as follows: (1) studies investigating the accuracy of 68Ga-PSMA PET/CT in primary lymph node staging; (2) patients underwent preoperative 68Ga-PSMA PET/CT imaging test before RP with PLND; (3) the patients recruited in the studies did not receive other treatment (hormone treatment, pelvic radiation, or chemotherapy) for PCa previously and had no previous/concurrent other malignancies; (4) relevant data in terms of 68Ga-PSMA PET/CT and histopathological results (positive/negative) for PLND can be extracted. We excluded studies which evaluated the utility of the 68Ga-PSMA PET/CT in the secondary staging for patients (detecting recurrent PCa after primary treatment failure). We also excluded studies of case reports or series (<10 patients), conference abstracts, and reviews.
Data Extraction
We extracted basic characteristics including study design, recruiting place and time, and study inclusion criteria. Participant details included age and prostate specific antigen (PSA). PET/CT test details (CT technique, uptake time, definition of positive imaging test) and details of reference standard were also summarized. Outcome data in terms of 68Ga-PSMA PET/CT and pathological results (positive/negative) for PLND on the basis of patient unit (per-patient) or node unit (per-node) were both extracted into 2 × 2 contingency tables.
Quality Assessment
We set 68Ga-PSMA PET/CT imaging prior to RP and PLND as the index test. And the histopathological status of the lymph nodes after PLND was defined as the reference standard. The Quality Assessment Tool for Diagnosis Accuracy Studies (QUADAS-2) was used to evaluate the quality of each trial, which included four domains: patient selection, index test, reference standard, and participant flow and timing (18). We judged a study to have “low risk of bias” if it was evaluated as “low” on all four domains. A study might be evaluated as a high risk of bias if more than one domain (including one) was judged “high” or “unclear.” Any discrepancies were resolved by a third reviewer.
Statistical Analysis
The pooled sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the summary receiver operating characteristic (SROC) curve with an area under the curve (AUC) of 68Ga-PSMA PET/CT test were synthesized. Positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were also pooled to better evaluate diagnostic role of the index test. The results were based on a per-patient analysis (setting one patient as unit) and a per-node analysis (setting one node as unit). More specifically, per-patient analysis (or per-node analysis) was calculated by the proportions of patients (or lymph nodes) with positive/negative histological results who (or which) were correctly identified by PSMA PET/CT. Heterogeneity was valued with the Chi-square and the Higgins-Thompson I2 method, and publication bias was determined by Deeks' funnel plot (19, 20). Sensitivity analysis was performed to exclude heterogeneous studies. We performed all the statistical analyses using the STATA, version 12.0 (Stata Corporation, College Station, TX).
Results
Literature Search and Description of the Included Studies
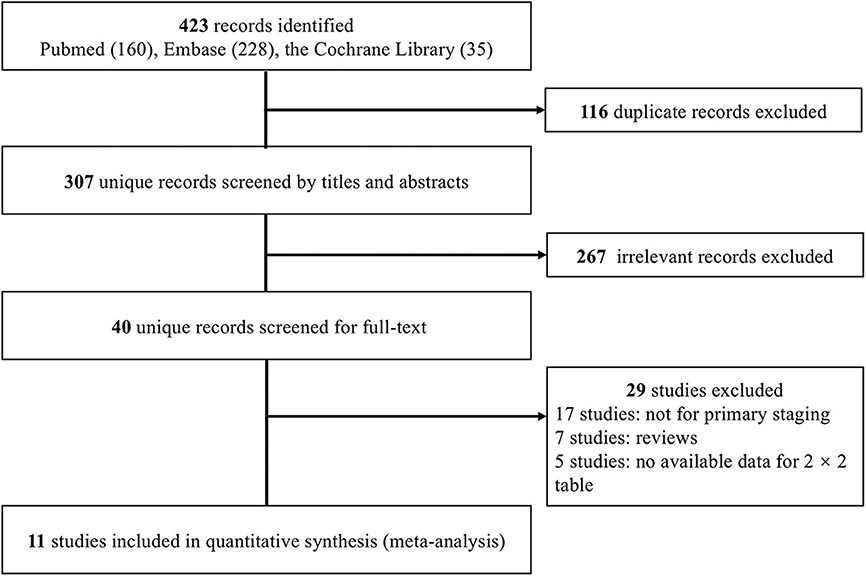
We initially identified 423 records. Following the removal of duplicates, 307 records were screened by titles and abstracts, and 40 studies were deemed relevant for full-text assessment. Ultimately, 11 studies (21–31) fulfilled the inclusion criteria and were included in the meta-analysis (Figure 1).
Overall, 11 studies comprising 904 patients who underwent 68Ga-PSMA PET/CT before RP with PLND were included. All studies recruited patients with confirmed PCa of intermediate/high risk, and three studies (25, 26, 31) additionally applied nomogram tools (9, 32) to identify patients of high risk of lymph node metastases. All studies set 68Ga-PSMA PET/CT imaging as an index test and defined a positive test with increased 68Ga-PSMA uptake above background. Of note, one study combined PET information with MRI in a proportion of recruited participants (28). And two studies (24, 25) quantified maximum standardized uptake value (SUVmax) for positive lymph nodes. The histopathological results from extended PLND (ePLND) were regarded as reference standard in five studies (22, 23, 25, 26, 30), and those from PLND were used as the reference standard in the other six studies. Patients in the studies harbored ages mostly ranging from 60 to 70, while PSA values varied in different studies. Basic information was summarized in Table 1.
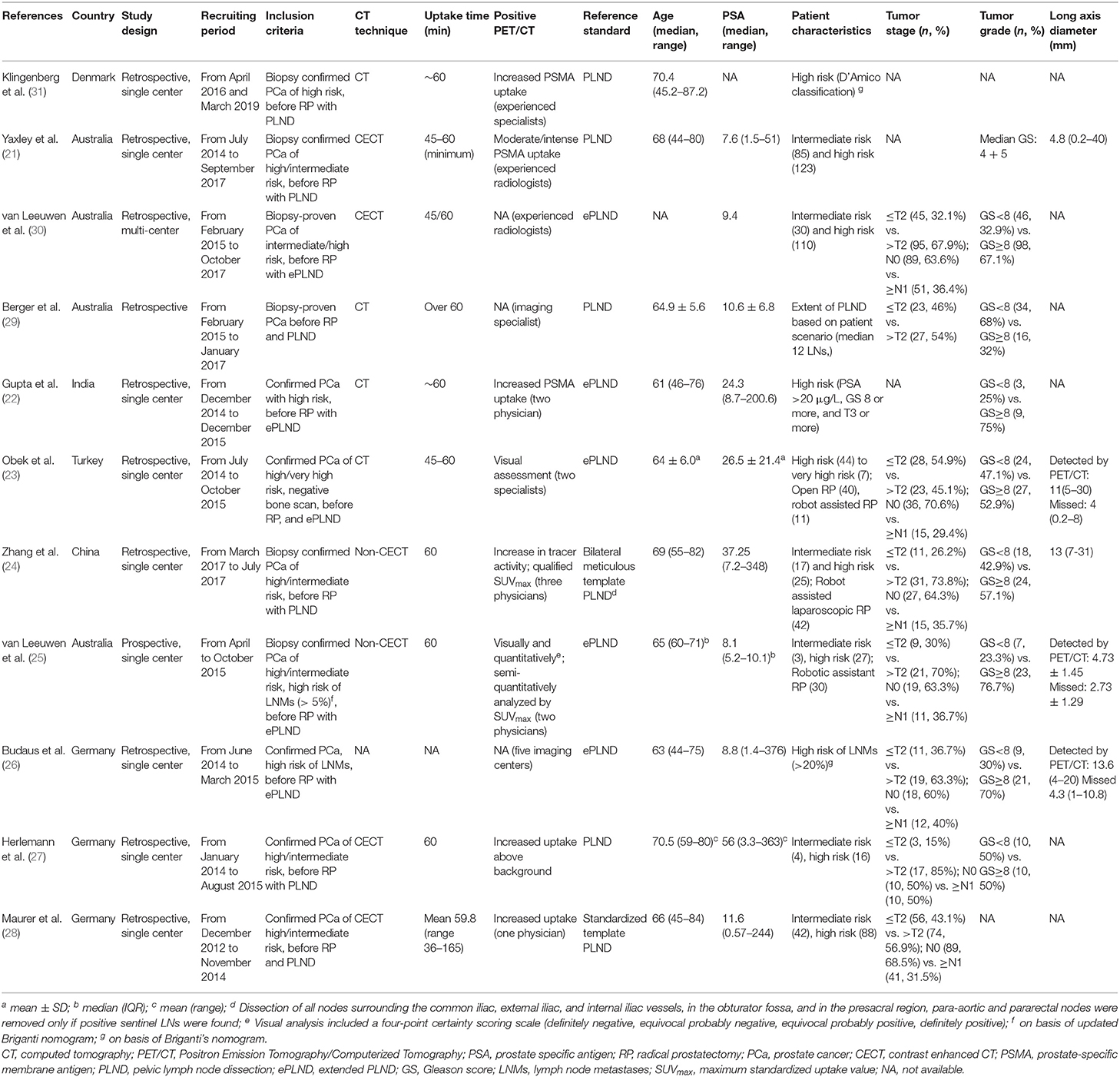
Table 1. Characteristics of included studies aiming at investigating role of 68Ga-PSMA PET/CT for primary lymph node staging in patients with PCa.
Diagnostic Accuracy of 68Ga-PSMA PET/CT for Preoperative Lymph Node Staging: Per-Patient Analysis
For per-patient analysis, 11 studies (21–31) with 904 patients were assessed (Table 2). Of the patients, 16.3% (147/904) and 23.7% (214/904) were diagnosed as positive by 68Ga-PSMA PET/CT test and histology of PLND, respectively. The sensitivity and specificity for PET/CT in primary staging ranged from 0.31 to 1.00 and from 0.67 to 1.00, reaching a pooled sensitivity of 0.63 (95% CI: 0.46–0.78), specificity of 0.93 (95% CI: 0.88–0.96), PPV of 0.79 (95% CI: 0.66–0.88), and NPV of 0.84 (95% CI: 0.79–0.89; Table 2). The PLR was 8.7 (95% CI: 5.2–14.5), the NLR was 0.39 (95% CI: 0.25–0.62), and the DOR was 22 (95% CI: 10–47). An overall accuracy was revealed by the SROC curve with AUC of 0.91 (95% CI: 0.88–0.93; Figure 2).
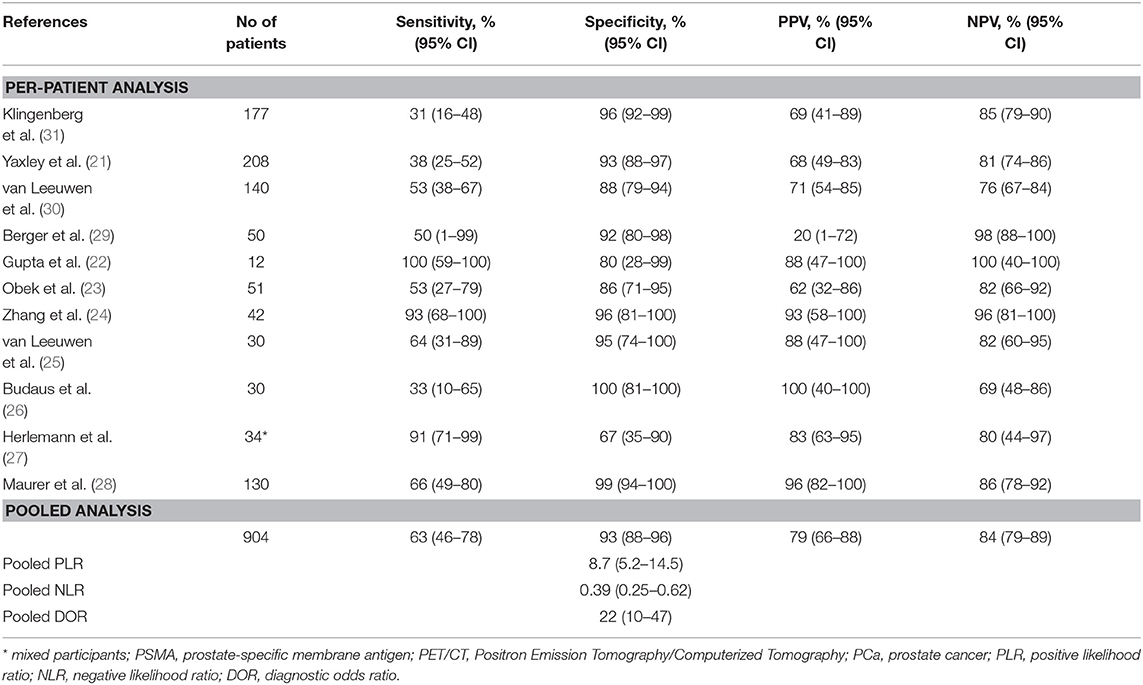
Table 2. Summary of studies for 68Ga-PSMA PET/CT in primary lymph node staging for PCa patients based on per-patient analysis.
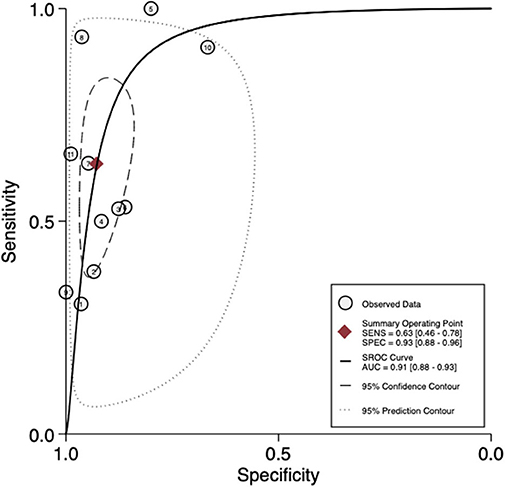
Figure 2. SROC curves for the diagnostic accuracy of 68Ga-PSMA PET/CT on a per-patient analysis. SROC curves: summary receiver operating characteristic curves.
Diagnostic Accuracy of 68Ga-PSMA PET/CT for Preoperative Lymph Node Staging: Per-Node Analysis
For per-node analysis, seven studies (21, 22, 24–28) comprising 5,773 lymph nodes were dissected by performing PLND (Table 3). Of the lymph nodes, 6.2% (356/5,773) and 8.4% (483/5,773) were diagnosed as positive by 68Ga-PET/CT and histology of PLND, respectively. The sensitivity and specificity for PET/CT in primary staging ranged from 0.24 to 0.96 and from 0.82 to 1.00, reaching a pooled sensitivity of 0.70 (95% CI: 0.49–0.85), specificity of 0.99 (95% CI: 0.96–1.00), PPV of 0.85 (95% CI: 0.69–0.94), and NPV of 0.97 (0.93–0.98; Table 3). The PLR was 50.7 (95% CI: 15.9–162.1), the NLR was 0.30 (95% CI: 0.16–0.56), and the DOR was 167 (95% CI: 40–695). An overall accuracy was revealed by the SROC curve with AUC of 0.96 (95% CI: 0.94–0.98; Figure 3).
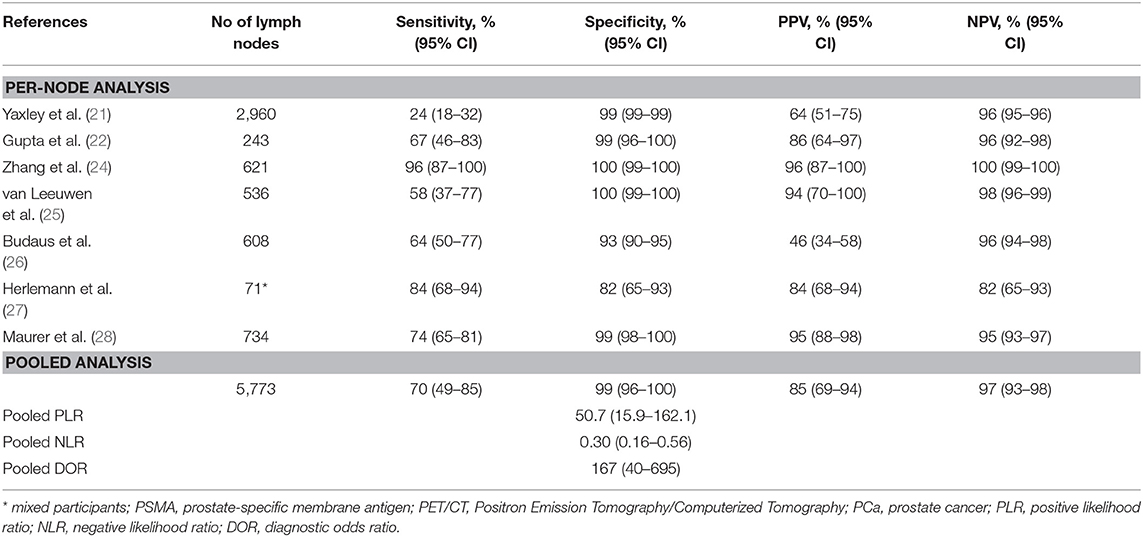
Table 3. Summary of studies for 68Ga-PSMA PET/CT in primary lymph node staging for PCa patients based on per-node analysis.
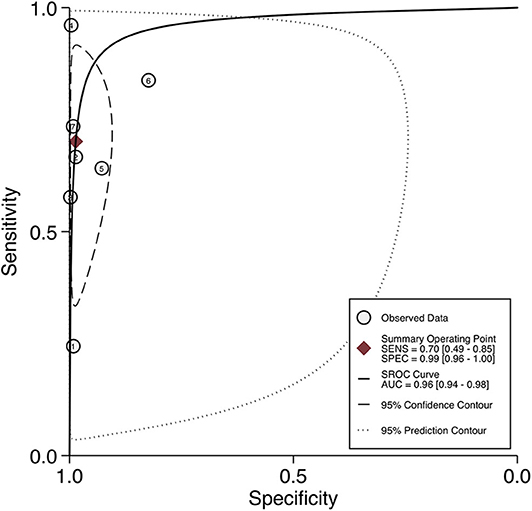
Figure 3. SROC curves for the diagnostic accuracy of 68Ga-PSMA PET/CT on a per-node analysis. SROC curves: summary receiver operating characteristic curves.
Quality Assessment of the Included Studies
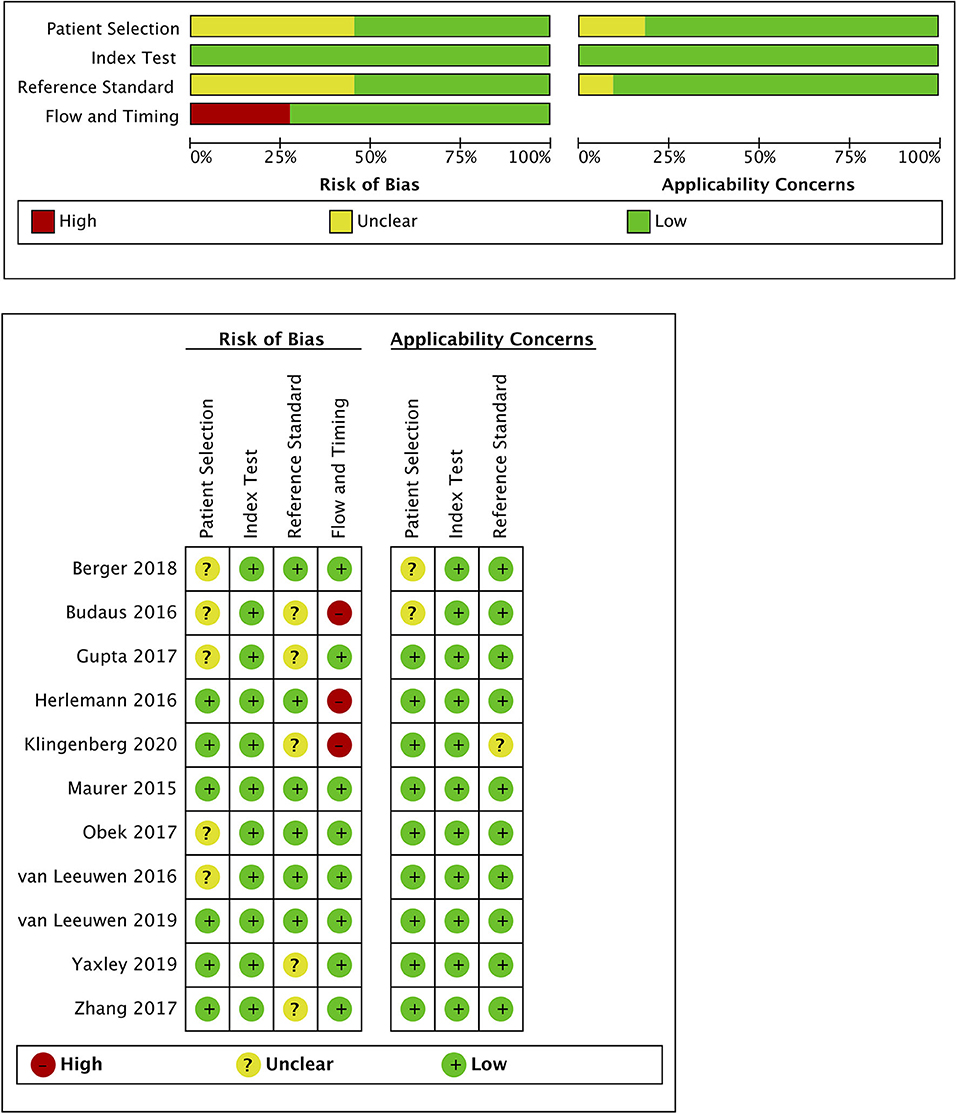
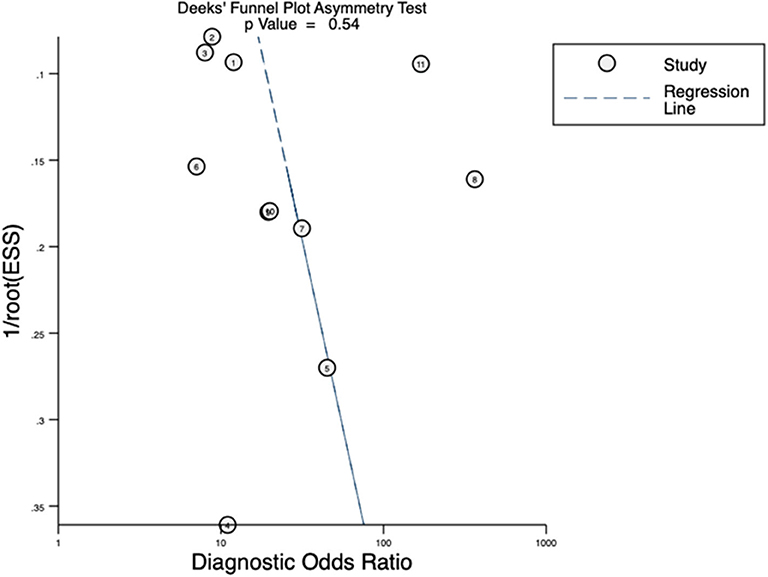
Quality assessment of individual studies was summarized in Figure 4. In the patient selection domain, three studies (22, 26, 29) were rated as unclear risk for no specific information (consecutive/random) on patient recruitment. As to the reference standard, four studies (21, 22, 24, 26) were rated as unclear risk because we could not assess whether pathologists were blinded to PET/CT results. In the flow and timing, three studies were considered to be at high risk. In Budaus's study, they initially identified 58 patients; however, their final analyses were restricted to the homogenous cohort with 30 patients (26). And in Herlemann's study, the authors included 14 patients receiving secondary PLND after primary treatment failure, and their final analysis was based on the mixed group (27). And Klingenberg et al. only recruited those patients (177/691) who received PLND for final analysis (31). No publication bias was identified by Deek's funnel plot asymmetry test (p = 0.54; Figure 5).
Discussion
Our study revealed that patients without lymph node metastatic status can rarely be misdiagnosed by PSMA PET/CT. However, the sensitivity of <70% may be not strong enough to forgo additional lymph node staging by PLND.
It is generally accepted that PLND provides important information for staging and prognosis for intermediate/high risk patients of PCa (5). Recently, Abdollah et al. have identified specific categories of pN1 patients (using the PLND information) who would benefit from adjuvant therapy after RP (33, 34). However, PLND itself lacked an definitely therapeutic effect based on current literature (7). Moreover, PLND is associated with worse intraoperative and perioperative outcomes in terms of longer operating time, more blood loss, as well as increased lymphocele/lymphedema rates and venous thromboembolism rates leading to longer hospital stay (6). All these factors raised concern for its routine use in clinical practice. Multiple preoperative nomogram tools have been identified to figure out optimal candidates benefitting from such expensive and invasive procedures (8, 9). However, strict cut-off values of these predictive tools which were originated from various patient selections may not be suitable and optimal for one specific individual, and may put a non-negligible proportion of patients (nearly 12%) under risk of LNI without adjuvant therapy (11).
During the last decades, some more specific as well as non-invasive staging tools for individuals have been investigated. And the conventional imaging procedures used for assessing nodal staging first came to CT and MRI. Hovels et al. performed a meta-analysis on the basis of 24 studies in 2008 to reveal their roles in primary staging using histological evaluation as the gold standard (12). Their results indicated the pooled sensitivity of 0.42 (95% CI 0.26–0.56)/0.39 (95% CI 0.22–0.56) and pooled specificity of 0.82 (95% CI 0.8–0.83)/0.82 (95% CI 0.79–0.83) for CT and MRI, respectively. No significant difference was observed between these two tests. However, both tests were far too intensive in their ability to detect nodal malignancy which can be attributed to using a size criterion of >8 millimeters (morphology) as malignancy (35). In fact, nearly 45% of metastatic lymph nodes are <4 millimeters in diameter which is below the spatial resolution of CT/MRI (36). Moreovenlargement in non-metastatic lymph nodes can mimic a malignant lesion, such as inflammation status or in elderly patients (37). While combining the morphological imaging and functional imaging together, Evangelista et al. performed a systematic review in 2013 and evaluated the diagnostic abilities of 18F-choline/11C-choline PET/CT for assessing the involvement of lymph nodes before RP in PCa patients (13). Their meta-analysis included 10 studies with 441 patients. Though with the pooled specificity of 0.95 (95% CI 0.92–0.97), the relatively unsatisfactory sensitivity (0.49, 95% CI 0.40–0.58) limited their routine utility regarding lymph node involvement detection.
To our knowledge, the first meta-analysis investigating the diagnostic accuracy in primary staging of PCa for 68Ga-PSMA PET/CT was conducted by von Eyben et al. in 2016 (38). They included four available studies revealing a pooled sensitivity of 0.61 (95% CI 0.47–0.72) and specificity of 0.97 (95% CI 0.85–0.99) in patient-based analysis. And the pooled sensitivity and specificity in node-based analysis were 0.70 (95% CI 0.53–0.83) and 0.84 (95% CI 0.24–0.99), respectively. Thereafter, Corfield et al. performed a critical review, recruiting nearly the same study cohorts, and summarized the role of 68Ga-PSMA PET/CT for primary staging of high-risk PCa (39). Their result revealed PSMA PET/CT appeared to outperform traditional imaging modalities but without performing quantitative synthesis. Perera's group also have tried to synthesize the role of 68Ga-PSMA PET in patients of advanced PCa; however, they mainly focused on assessing predictors of positive tests for patients with biochemical recurrence (40). And further updated work reported the predictive ability of PSMA-PET/CT imaging for primary staging. Of note, failure of including currently all available evidence may cause potential bias (41, 42).
To provide more concise and updated evidence on the role of 68Ga-PSMA PET/CT for predicting lymph node metastases before definitive treatment, we conducted a thorough literature research and included currently all available studies. Using histological status of resected lymph nodes as the reference standard, our study found the pooled specificity can reach above 90% both in per-patient analysis and in per-node analysis, which indicated that patients without lymph node metastatic status can rarely be misdiagnosed by 68Ga-PSMA PET/CT. The relatively low misdiagnosis rate can be further supported with the superior PPV and NPV (above 80%). However, PSMA PET/CT failed to reach high diagnostic evidence criteria defined by Jaeschke et al. (PLR of 5–10 and NLR of 0.1–0.2) (43), mainly due to its relatively low sensitivity. With a sensitivity of <70%, if completely on the basis of 68Ga-PSMA PET/CT test, more than 30 out of 100 truly positive patients with metastatic lymph nodes would be missed and thereafter be under additional risk of progression without adjuvant therapy. However, these values were superior to those for traditional imaging approaches. The staging performance of 68Ga-PET imaging co-registered with MRI was currently less investigated. Grubmuller et al. prospectively recruited 80 patients who received preoperative PSMA-PET/mpMRI followed by RP and ePLND and reported a sensitivity of 68.8% and a specificity of 100% for the prediction of lymph node metastasis on patient base (44). Thalgott's retrospective study recruiting 73 patients of high-risk prostate cancer has reported comparable results (sensitivity: 60%; specificity 100%) (45).
Our study has several limitations. Firstly, the majority data was derived from retrospective, single-institutional studies with small sample sizes. However, through a careful and thorough literature search, our study provided the most up-to-date evidence on basis of per-patient and per-node analysis. Secondly, there was considerable heterogeneity in our pooled analysis. We assumed some confounders may have impact on the different diagnostic accuracy across included cohorts. For example, the sensitivity and specificity in Zhang's study (24) were obviously higher than those in Yaxley's study (21), which can be partly owing to different PSA levels between their cohorts (median 37.25 vs. 7.7 ng/ml). Higher diagnostic accuracy was observed in Herlemann's study which included a portion of patients receiving salvage PLND (27). Varied diameters of lymph nodes caused by different inclusion PLND criteria (e.g., using nomogram tool or not) may also contribute to the heterogeneity (24–26). Moreover, the application of PLND or ePLND, the different sample size (22), study quality, and experiences of radiologists/urologists and pathologists may also have impacts. Nevertheless, they are regarded as common pitfalls for real-world clinical practice. Thirdly, as a review, we cannot correlate the positive lymph nodes with the preoperative PSA level and the Gleason score. In combination with such parameters may improve sensitivity and specificity of PSMA PET/CT. We also cannot evaluate the role of SUVmax value due to limited data available. Finally, issues about the additional cost, clinical feasibility, and potential benefit should also be considered and balanced for individuals in clinical practice, however, which are out of the scope of our study.
In conclusion, we synthesized currently available evidence to evaluate the role of 68Ga-PSMA PET/CT for preoperative lymph nodes staging in patients with intermediate/high risk PCa. Our study found patients without lymph node metastatic status can rarely be misdiagnosed by PSMA PET/CT. However, the relatively low sensitivity of <70%, though superior to that for traditional imaging approaches, is not strong enough to forgo lymph node staging by PLND. Future studies should focus on the evaluation of lymph node involvement in a stricter patient selection, such as those with a very high risk of lymph node dissemination or those specific patients involved in controversial status of lymph node metastasis based on current preoperative risk estimation nomograms.
Take-Home Message
1. 68Ga-PSMA PET/CT can provide more accurate staging information of lymph nodes (Sen 0.63, Spe 0.93) for intermediate/high risk PCa compared with traditional imaging approaches (CT: Sen 0.42, Spe 0.39; MRI: Sen 0.39, Spe 0.82; 18F-choline/11C-choline PET/CT: Sen 0.49, Spe 0.95).
2. Patients without lymph node metastatic status can rarely be misdiagnosed by 68Ga-PSMA PET/CT (Spe: 0.93, 95% CI 0.88–0.96). However, the pooled sensitivity (0.63, 95% CI 0.46–0.78) indicated more than 30 out of 100 truly positive patients with metastatic lymph nodes would be missed and thereafter be under additional risk of progression without adjuvant therapy.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Author Contributions
XT, CZ, and ZL: protocol development, data collection or management, data analysis, and manuscript drafting. GS and XW: data management, manuscript review, and revision. LN: data management, manuscript review, and revision. TC: data collection and management. HX and YB: data collection or management and data analysis. LY and QW: study concept and design, manuscript review, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National key research and development program of China (Grant No. SQ2017YFSF090096), Programs from Science and Technology Department of Sichuan Province (Grant No. 2017HH0063), and Young Investigator Award of Sichuan University 2017.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The poster's abstract was published in Paper Abstracts in the World Journal of urology, hyperlink with: https://link.springer.com/article/10.1007/s00345-019-02955-9.
Abbreviations
PET/CT, positron emission tomography/computerized tomography; PCa, prostate cancer; PLND, pelvic lymph node dissection; RP, radical prostatectomy; PPV, positive predictive value; NPV, negative predictive value; SROC, summary receiver operating characteristic; AUC, area under the curve; LVI, lymph node invasion; CT, computed tomography; MRI, magnetic resonance imaging; PSMA, prostate-specific membrane antigen; PRISMA, Preferred Reporting Items for Systematic Review and Meta-analysis; PSA, prostate specific antigen; QUADAS, the Quality Assessment Tool for Diagnosis Accuracy Studies; PLR, positive likelihood ratio; NLR, negative likelihood ratio; DOR, diagnostic odds ratio; ePLND, extended pelvic lymph node dissection.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Cary KC, Punnen S, Odisho AY, Litwin MS, Saigal CS, Cooperberg MR. Nationally representative trends and geographic variation in treatment of localized prostate cancer: the Urologic Diseases in America project. Prostate Cancer Prostatic Dis. (2015) 18:149–54. doi: 10.1038/pcan.2015.3
3. Abdollah F, Suardi N, Gallina A, Bianchi M, Tutolo M, Passoni N, et al. Extended pelvic lymph node dissection in prostate cancer: a 20-year audit in a single center. Ann Oncol. (2013) 24:1459–66. doi: 10.1093/annonc/mdt120
4. Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N, et al. Predicting survival of patients with node-positive prostate cancer following multimodal treatment. Eur Urol. (2014) 65:554–62. doi: 10.1016/j.eururo.2013.09.025
5. Mottet N, van den Bergh RCN, Briers E, Bourke L, Cornford P, De Santis M, et al. EAU - ESTRO - ESUR - SIOG guidelines on prostate cancer 2018. In: European Association of Urology Guidelines. 2018 Edition. Vol presented at the EAU Annual Congress Copenhagen 2018. Arnhem: EAU Guidelines Office (2018). Available online at: http://uroweb.org/guidelines/compilations-of-all-guidelines/
6. Briganti A, Chun FK, Salonia A, Suardi N, Gallina A, Da Pozzo LF, et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol. (2006) 50:1006–13. doi: 10.1016/j.eururo.2006.08.015
7. Fossati N, Willemse PM, Van den Broeck T, van den Bergh RCN, Yuan CY, Briers E, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol. (2017) 72:84–109. doi: 10.1016/j.eururo.2016.12.003
8. Gandaglia G, Fossati N, Zaffuto E, Bandini M, Dell'Oglio P, Bravi CA, et al. Development and internal validation of a novel model to identify the candidates for extended pelvic lymph node dissection in prostate cancer. Eur Urol. (2017) 72:632–40. doi: 10.1016/j.eururo.2017.03.049
9. Briganti A, Larcher A, Abdollah F, Capitanio U, Gallina A, Suardi N, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. (2012) 61:480–7. doi: 10.1016/j.eururo.2011.10.044
10. Cagiannos I, Karakiewicz P, Eastham JA, Ohori M, Rabbani F, Gerigk C, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. (2003) 170:1798–803. doi: 10.1097/01.ju.0000091805.98960.13
11. Leyh-Bannurah SR, Budaus L, Pompe R, Zaffuto E, Briganti A, Abdollah F, et al. North American population-based validation of the national comprehensive cancer network practice guideline recommendation of pelvic lymphadenectomy in contemporary prostate cancer. Prostate. (2017) 77:542–8. doi: 10.1002/pros.23292
12. Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. (2008) 63:387–95. doi: 10.1016/j.crad.2007.05.022
13. Evangelista L, Guttilla A, Zattoni F, Muzzio PC, Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol. (2013) 63:1040–8. doi: 10.1016/j.eururo.2012.09.039
14. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. (1997) 3:81–5.
15. Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. (2017) 44:1258–68. doi: 10.1007/s00259-017-3711-7
16. Kimura S, Abufaraj M, Janisch F, Iwata T, Parizi MK, Foerster B, et al. Performance of [68Ga] Ga-PSMA 11 PET for detecting prostate cancer in the lymph nodes before salvage lymph node dissection: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. (2019) 23:1–10. doi: 10.1038/s41391-019-0156-z
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
18. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
20. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J F Clin Epidemiol. (2005) 58:882–93. doi: 10.1016/j.jclinepi.2005.01.016
21. Yaxley JW, Raveenthiran S, Nouhaud FX, Samartunga H, Yaxley AJ, Coughlin G, et al. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with 68Ga-PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology: can preoperative 68Ga-PSMA positron emission tomography/computerized tomography replace pelvic lymph node dissection for prostate cancer staging? J Urol. (2019) 201:815–20. doi: 10.1097/JU.0000000000000053
22. Gupta M, Choudhury PS, Hazarika D, Rawal S. A comparative study of 68gallium-prostate specific membrane antigen positron emission tomography-computed tomography and magnetic resonance imaging for lymph node staging in high risk prostate cancer patients: an initial experience. World J Nucl Med. (2017) 16:186–91. doi: 10.4103/1450-1147.207272
23. Obek C, Doganca T, Demirci E, Ocak M, Kural AR, Yildirim A, et al. The accuracy of 68Ga-PSMA PET/CT in primary lymph node staging in high-risk prostate cancer. Eur J Nucl Med Mol Imaging. (2017) 44:1806–12. doi: 10.1007/s00259-017-3752-y
24. Zhang Q, Zang S, Zhang C, Fu Y, Lv X, Zhang Q, et al. Comparison of 68Ga-PSMA-11 PET-CT with mpMRI for preoperative lymph node staging in patients with intermediate to high-risk prostate cancer. J Transl Med. (2017) 15:230. doi: 10.1186/s12967-017-1333-2
25. van Leeuwen PJ, Emmett L, Ho B, Delprado W, Ting F, Nguyen Q, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Inter. (2017) 119:209–15. doi: 10.1111/bju.13540
26. Budaus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of 68Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. (2016) 69:393–6. doi: 10.1016/j.eururo.2015.06.010
27. Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. (2016) 70:553–7. doi: 10.1016/j.eururo.2015.12.051
28. Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of 68gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. (2016) 195:1436–43. doi: 10.1016/j.juro.2015.12.025
29. Berger I, Annabattula C, Lewis J, Shetty DV, Kam J, Maclean F, et al. 68Ga-PSMA PET/CT vs. mpMRI for locoregional prostate cancer staging: correlation with final histopathology. Prostate Cancer Prostatic Dis. (2018) 21:204–11. doi: 10.1038/s41391-018-0048-7
30. van Leeuwen PJ, Donswijk M, Nandurkar R, Stricker P, Ho B, Heijmink S, et al. Gallium-68-prostate-specific membrane antigen (68 Ga-PSMA) positron emission tomography (PET)/computed tomography (CT) predicts complete biochemical response from radical prostatectomy and lymph node dissection in intermediate- and high-risk prostate cancer. BJU Inter. (2019) 124:62–8. doi: 10.1111/bju.14506
31. Klingenberg S, Jochumsen MR, Ulhøi BP, Fredsøe J, Sørensen KD, Borre M, et al. 68Ga-PSMA PET/CT for primary NM staging of high-risk prostate cancer. J Nucl Med. (2020). doi: 10.2967/jnumed.120.245605. [Epub ahead of print].
32. Briganti A, Chun FK, Salonia A, Zanni G, Scattoni V, Valiquette L, et al. Validation of a nomogram predicting the probability of lymph node invasion among patients undergoing radical prostatectomy and an extended pelvic lymphadenectomy. Eur Urol. (2006) 49:1019–26; discussion 1026–17. doi: 10.1016/j.eururo.2006.01.043
33. Abdollah F, Karnes RJ, Suardi N, Cozzarini C, Gandaglia G, Fossati N, et al. Impact of adjuvant radiotherapy on survival of patients with node-positive prostate cancer. J Clin Oncol. (2014) 32:3939–47. doi: 10.1200/JCO.2013.54.7893
34. Abdollah F, Dalela D, Sood A, Keeley J, Alanee S, Briganti A, et al. Impact of adjuvant radiotherapy in node-positive prostate cancer patients: the importance of patient selection. Eur Urol. (2018) 74:253–6. doi: 10.1016/j.eururo.2018.04.017
35. McSherry SA, Levy F, Schiebler ML, Keefe B, Dent GA, Mohler JL. Preoperative prediction of pathological tumor volume and stage in clinically localized prostate cancer: comparison of digital rectal examination, transrectal ultrasonography and magnetic resonance imaging. J Urol. (1991) 146:85–9. doi: 10.1016/S0022-5347(17)37720-0
36. Davis GL. Sensitivity of frozen section examination of pelvic lymph nodes for metastatic prostate carcinoma. Cancer. (1995) 76:661–8. doi: 10.1002/1097-0142(19950815)76:4<661::AID-CNCR2820760419>3.0.CO;2-S
37. Osborne BM, Butler JJ. Clinical implications of nodal reactive follicular hyperplasia in the elderly patient with enlarged lymph nodes. Mod Pathol. (1991) 4:24–30.
38. von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Euro Urol Focus. (2018) 4:686–93. doi: 10.1016/j.euf.2016.11.002
39. Corfield J, Perera M, Bolton D, Lawrentschuk N. 68Ga-prostate specific membrane antigen (PSMA) positron emission tomography (PET) for primary staging of high-risk prostate cancer: a systematic review. World J Urol. (2018) 36:519–27. doi: 10.1007/s00345-018-2182-1
40. Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. (2016) 70:926–37. doi: 10.1016/j.eururo.2016.06.021
41. Perera M, Papa N, Roberts M, Williams M, Udovicich C, Vela I, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. (2019) 77:403–17. doi: 10.1016/j.eururo.2019.01.049
42. Wu H, Xu T, Wang X, Yu YB, Fan ZY, Li DX, et al. Diagnostic performance of 68gallium labelled prostate-specific membrane antigen positron emission tomography/computed tomography and magnetic resonance imaging for staging the prostate cancer with intermediate or high risk prior to radical prostatectomy: a systematic review and meta-analysis. World J Men's Health. (2020) 38:208–19. doi: 10.5534/wjmh.180124
43. Jaeschke R, Guyatt GH, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test B. What are the results and will they help me in caring for my patients? The evidence-based medicine working group. JAMA. (1994) 271:703–7. doi: 10.1001/jama.271.9.703
44. Grubmüller B, Baltzer P, Hartenbach S, D'Andrea D, Helbich TH, Haug AR, et al. PSMA ligand PET/MRI for primary prostate cancer: staging performance and clinical impact. Clin Cancer Res. (2018) 24:6300–7. doi: 10.1158/1078-0432.CCR-18-0768
Keywords: lymph node dissection, meta-analysis, positron emission tomography, PET/CT, prostate cancer, prostate-specific membrane antigen
Citation: Tu X, Zhang C, Liu Z, Shen G, Wu X, Nie L, Chang T, Xu H, Bao Y, Yang L and Wei Q (2020) The Role of 68Ga-PSMA Positron Emission Tomography/Computerized Tomography for Preoperative Lymph Node Staging in Intermediate/High Risk Patients With Prostate Cancer: A Diagnostic Meta-Analysis. Front. Oncol. 10:1365. doi: 10.3389/fonc.2020.01365
Received: 21 April 2020; Accepted: 29 June 2020;
Published: 18 August 2020.
Edited by:
Rosa M. Nadal, National Heart, Lung, and Blood Institute (NHLBI), United StatesReviewed by:
Benjamin Maughan, University of Utah, United StatesMaria Picchio, San Raffaele Hospital (IRCCS), Italy
Copyright © 2020 Tu, Zhang, Liu, Shen, Wu, Nie, Chang, Xu, Bao, Yang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wei, d2VpcWlhbmcxNjMxNjNAMTYzLmNvbQ==; Lu Yang, d3ljbGVmbHVlQDE2My5jb20=
†These authors have contributed equally to this work
 Xiang Tu1†
Xiang Tu1† Ling Nie
Ling Nie Lu Yang
Lu Yang Qiang Wei
Qiang Wei