
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 July 2020
Sec. Cancer Imaging and Image-directed Interventions
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01238
 Longbo Zheng1†
Longbo Zheng1† Xunying Zhang1†
Xunying Zhang1† Jilin Hu1
Jilin Hu1 Yuan Gao1
Yuan Gao1 Xianxiang Zhang1
Xianxiang Zhang1 Maoshen Zhang1
Maoshen Zhang1 Shuai Li2
Shuai Li2 Xiaoming Zhou3
Xiaoming Zhou3 Tianye Niu4
Tianye Niu4 Yun Lu1,5*
Yun Lu1,5* Dongsheng Wang1*
Dongsheng Wang1*Background: The accurate prediction of the tumor infiltration depth in the gastric wall based on enhanced CT images of gastric cancer is crucial for screening gastric cancer diseases and formulating treatment plans. Convolutional neural networks perform well in image segmentation. In this study, a convolutional neural network was used to construct a framework for automatic tumor recognition based on enhanced CT images of gastric cancer for the identification of lesion areas and the analysis and prediction of T staging of gastric cancer.
Methods: Enhanced CT venous phase images of 225 patients with advanced gastric cancer from January 2017 to June 2018 were retrospectively collected. Ftable LabelImg software was used to identify the cancerous areas consistent with the postoperative pathological T stage. The training set images were enhanced to train the Faster RCNN detection model. Finally, the accuracy, specificity, recall rate, F1 index, ROC curve, and AUC were used to quantify the classification performance of T staging on this system.
Results: The AUC of the Faster RCNN operating system was 0.93, and the recognition accuracies for T2, T3, and T4 were 90, 93, and 95%, respectively. The time required to automatically recognize a single image was 0.2 s, while the interpretation time of an imaging expert was ~10 s.
Conclusion: In enhanced CT images of gastric cancer before treatment, the application of Faster RCNN to diagnosis the T stage of gastric cancer has high accuracy and feasibility.
Gastric cancer is currently ranked fifth in the global cancer incidence rate, and its mortality rate ranks third. Its high morbidity and mortality rates indicate a serious threat to human health (1). Tumor, node, and metastasis (TNM) stage and histological subtype is routinely used for risk stratification and treatment decision-making. For certain stage II and stage III, adjuvant chemotherapy is recommended as a standard preoperative treatment (2). Accurate preoperative T gastric cancer staging is critical for selecting treatment plans and predicting postoperative outcomes (T stage pattern of gastric cancer, see Figure 1). Therefore, early diagnosis and accurate staging before surgery are key to improving the accurate and prognosis. Because computed tomography (CT) is noninvasive, practical, convenient, and stable, it is a routine method used for the preoperative staging of gastric cancer (3). The application of abdominal enhanced CT greatly improves the accuracy of gastric cancer staging, and its accuracies of preoperative T staging and N staging are 62–75% and 75–80%, respectively (4–7). However, the final interpretation of a CT image still depends on the clinical experience and personal opinion of radiologists. Current research shows that texture analysis of CT images can be used to detect subtle differences that are unrecognizable by the human eye and that quantitative information regarding tumor heterogeneity can be obtained by analyzing the pixel intensity distribution and strength in images, thereby improving the diagnostic value of CT (8).
Artificial intelligence-mediated data processing has fast calculation speeds and high precision. Recently, convolutional neural networks (CNNs) have been increasingly used in clinical practice to evaluate medical images. This technique has been shown to have high diagnostic performance in diagnostic imaging, such as for coronary computed tomography angiography (CTA) (9) and X-ray detection in breast cancer (10). CNNs are the most mature algorithms among various deep learning models. This study was based on the powerful ability of CNNs to process and recognize images. We established a CNN-based clinical diagnostic system for progressive gastric cancer T staging utilizing preoperative abdominal enhanced CT images and verified and evaluated its accuracy. Artificial intelligence can be used to assist radiologists in the clinical T staging of gastric cancer based on preoperative abdominal CT. The process for establishing the system and the learning results are provided in this report.
Our research team retrospectively collected data from 225 cases of advanced gastric cancer treated at the Affiliated Hospital of Qingdao University, China, from January 2017 to June 2018 (Figure 2). The study was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University. All patients signed an informed consent form for the application of iodine contrast agent. The inclusion criteria were as follows: patients who underwent gastroscopy before surgery, were diagnosed with gastric cancer via pathological diagnosis and were not diagnosed with tumors in other areas; patients who underwent a preoperative upper abdominal enhanced CT examination in our hospital; and patients who underwent radical gastric cancer resection in our hospital, from which postoperative pathology led to a diagnosis of advanced gastric cancer.
We excluded patients if the primary tumor could not be identified on CT, or if patients had previously received adjuvant chemotherapy.
A total of 225 patients were enrolled in this study. Basic patient information is provided in Table 1. Upper abdominal enhanced CT is a routine auxiliary examination for patients with gastric cancer and is used to screen patients who are highly suspected of having gastric cancer. Venous phase-enhanced CT imaging of the upper abdomen is superior to arterial phase-enhanced CT imaging for the diagnosis of gastric tumor infiltration; therefore, this study chose venous phase-enhanced CT images of the upper abdomen (11). A total of 3,500 images of advanced gastric cancer were obtained, including 990 T2 images, 1,134 T3 images, and 1,376 T4 images. All patients in this study underwent an upper abdominal enhanced CT scan using a Philips Brilliance iCT scanner. The scanning parameters included a layer thickness of 1 mm, an interlayer distance of 1 mm, and a spacing of 0.985. The patients had fasted for 4–6 h before the scan. Twenty minutes prior to the scan, the patients were given 500–1,000 ml of drinking water. For enhanced scanning, 90 ml of the nonionic contrast agent iohexol was injected into the antecubital vein at a rate of 3 ml/s using a high-pressure syringe. The scanning procedure was carried out as follows: after the injection of the intravenous contrast agent, there was a delay of 33 s in the arterial phase, 65 s in the venous phase, and 120 s in the equilibrium phase. The scan range was from the diaphragm to the umbilical plane.
We used the labeling software LabelImg to identify and label the images. Two senior radiologists independently interpreted the CT images and labeled the tumor lesions. The tumor segmentation method was used for labeling. According to the relevant literature, compared with the adjacent stomach wall, focal gastric wall thickening ≥ 6 mm was identified as abnormal thickening and canceration (4). Because the purpose of this study was to accurately identify the T stage of advanced gastric cancer, not to assess the detection ability of upper abdominal enhanced CT, the two radiologists only labeled the deepest position that the gastric lesion infiltrated into the stomach wall in the images, taking into consideration the patient's gastroscopy report and the final pathological results after surgery. According to the postoperative pathological results, a third radiologist examined the labeled tumor site on the upper abdominal enhanced CT images to ensure the accuracy and consistency of the lesions in the two enhanced CT images.
We used a data augmentation model to extract different regions of interest (ROIs) on the upper abdominal enhanced CT images and then used cropping, flipping, and other data enhancement methods to obtain additional images. This method enhanced the research data set while reducing the overfitting problem that could arise when the model processed the data set (12). After effective data augmentation, 5,855 advanced gastric cancer images were obtained.
The automatic detection of T stage in gastric cancer images using a Faster R-CNN was studied (Supplementary Methods). The methods included a region proposal network (RPN) and the Fast R-CNN (Figure 3). In this experiment, RPN and Fast R-CNN were alternately trained in two stages, and the parameters were listed in Table 2.
Prior to establishing the diagnostic system, we pretreated the images, which included normalization of the image intensity range and image processing by histogram equalization (13). We uniformly scaled the images to 512 × 557 and then used a random sampling method to divide patients and their labeled images of various stages of advanced gastric cancer into a training set and a verification set in a ratio of 4:1; the consistency of the grouping was ensured. The images in the training set were then input into the diagnostic network for training. The skeleton of the CNN used in this study was a 50-layer deep CNN that could extract image features. Each level of the model was trained by 600 epochs. The stochastic gradient descent (SGD) optimizer was used with an initial learning rate of 0.0002.
The classification performance of the system was verified using a verification set consisting of a subset of the same original data set. We conducted comparisons of the gastric cancer tumor area labeled by the radiologists with that by the system and determined the accuracy of the classification results for the verification set. A ROC curve was used to evaluate the area under the curve (AUC) of the CNN system for image accuracy, and the sensitivity, specificity, positive predictive value, and negative predictive value were determined. The flow chart of the test platform is shown in Figure 3.
The count data were descriptively analyzed using the number of cases (n) and percentage (%). According to the results of the Kolmogorov–Smirnov test, measurement data with a normal distribution were subjected to descriptive analysis using the mean ± standard deviation (mean ± SD). SPSS 20.0 software (SPSS, Chicago, IL) was used for statistical analysis. The computation learning results were analyzed using the Python programming language. Classification-report in the Metric module was used to generate multiclass conclusions, and the accuracy, recall, F1-score, and overall micro average, macro average, and weighted average in each class were recorded. The numbers of true positives and false positives at all nodes were counted, and the true positive rate and false positive rate under different probability thresholds were calculated to plot the ROC curve. The AUC value was calculated to obtain the accuracy by which the model predicts gastric cancer T staging.
To assess the learning effects of the Faster RCNN deep neural network, we input the training set into the trained Faster RCNN. The training loss result for the learning curve of the diagnostic platform suggested that the diagnostic platform achieved optimal optimization parameters after 600 learning epochs (Figure 4A). The AUC of the ROC curve for the Faster RCNN operating system in identifying gastric cancer tumors was 0.93, with an accuracy of 0.93 and a specificity of 0.95 comparable to that of the radiologists (Figure 4B).
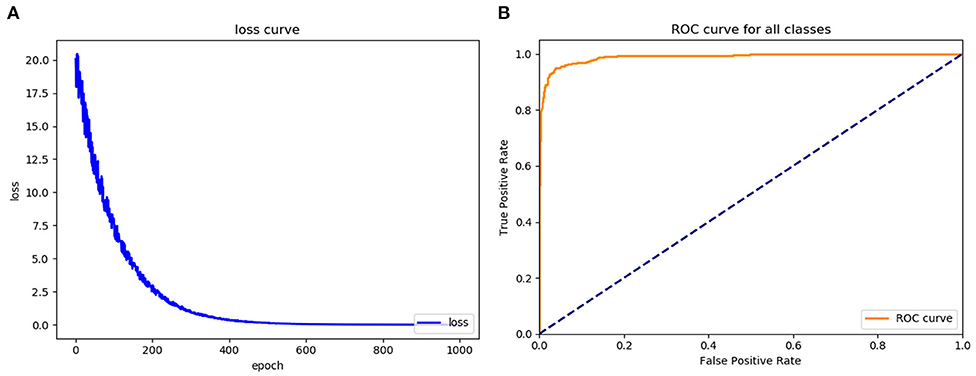
Figure 4. Assess the learning effects of the Faster RCNN operating system. (A) LOSS learning curve of the experimental system, (B) the ROC curve of the automatic recognition model for advanced gastric cancer recognition had AUC of 0.93.
The left side of Figure 5 is the image wherein the physician manually identified tumor position based on the pathological results for the training and testing of the model. The right side is the segmentation of the tumor and the identification of the T stage by the recognition model. As shown in Figure 5, the accuracy of the T2, T3, and T4 stage identified by the trained Faster RCNN approach were 0.91, 0.94, and 0.95. It can be concluded that the operating platform has high accuracy in recognizing the gastric cancer T stage based on enhanced CT images. Therefore, Faster RCNN operating system had been effectively trained for images of T stage in gastric cancer.
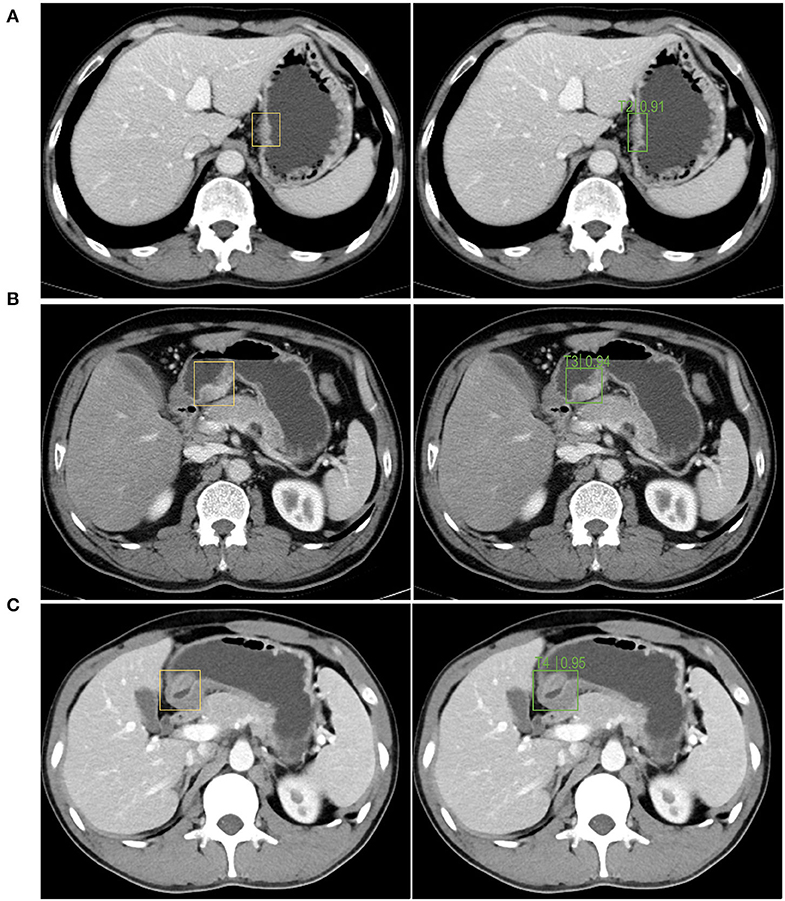
Figure 5. The autorecognition model recognizes the T2 (A), T3 (B), T4 (C) stage of the tumor in the image after segmenting and identifying the tumor. The left side of figures were the images wherein the physician manually identified the tumor position based on the pathological results for the training and testing of the model. The right side were the segmentation of the tumor and the identification of the T stage by the recognition model.
After the validation were completed, the recognition rate of T2 stage gastric cancer was 90%, the recognition rate of T3 stage gastric cancer was 93%, and the recognition rate of T4 stage gastric cancer was 95% (Figure 6). The results of classification-report in the Metric module were shown in Table 3. It can be concluded that the automatic recognition model has high recognition performance for gastric cancer.
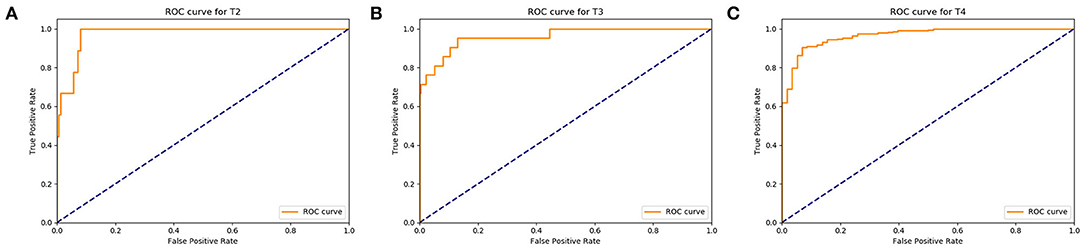
Figure 6. Assess the diagnosis effects of the Faster RCNN operating system. (A) The ROC curve of the automatic recognition model for T2 stage gastric cancer recognition had AUC of 0.90, (B) the ROC curve of the automatic recognition model for T3 stage gastric cancer recognition had AUC of 0.93, (C) the ROC curve of the automatic recognition model for T4 stage gastric cancer recognition had AUC of 0.95.
Gastric cancer T staging is an important basis to guide the treatment of gastric cancer. The eighth edition of the National Comprehensive Cancer Network (NCCN) guidelines proposed that upper abdominal enhanced CT is the main diagnostic imaging method for gastric cancer T staging. Patients with early-stage gastric cancer can be treated with endoscopic or surgical resection; patients with T3 and above gastric cancer should be treated first with neoadjuvant therapy and then surgical resection. Accurate preoperative staging is critical for selecting perioperative treatment options and for predicting the prognosis of patients with gastric cancer (12, 14, 15). Radiologists mainly determine the tumor–node–metastasis (TNM) stage of gastric cancer based on preoperative abdominal CT and other imaging data, after which a treatment plan is selected. However, in current clinical practice, there are still some issues regarding TNM staging by preoperative abdominal CT. Different radiologists may determine different TNM stages based on the same abdominal CT data. In the context of the large patient population cared for by three-tier hospitals in China, radiologists have tremendous workloads while being challenged by the complexity of clinical staging of gastric cancer through CT and other imaging data. Staging by doctors is a subjective assessment, to a certain extent, and lacks objectivity. Therefore, a new approach is needed to strike a balance between the increasing workload of radiologists and the need for efficient clinical diagnoses.
The development of deep learning network technology has provided a possible solution to these problems. A CNN computer-aided diagnosis (CAD) system has been applied to the classification and detection of breast histopathological images (16) and colorectal cancer detection (17). Early studies from this research group developed an automatic magnetic resonance image recognition system for metastatic lymph nodes in rectal cancer based on a deep learning network (3, 17, 18). Based on the above research experience, a T stage diagnostic platform for advanced gastric cancer based on a CNN was established, and its clinical value was evaluated.
In this study, enhanced CT images labeled by senior radiologists were used to train the T stage diagnostic platform for advanced gastric cancer, and the results were verified using clinical pathological section results. The diagnosis based on continuous venous phase images of gastric cancer was consistent with the T staging based on the postoperative pathological results. The area under the ROC curve for the diagnostic platform was 0.93, and the accuracy rates for T2, T3, and T4 gastric cancer were 90, 93, and 95%, respectively, which were close to the diagnostic levels of the senior radiologists. The results of this experiment can be explained as follows. T4 tumors are relatively larger than T2 and T3 tumors and infiltrate the serosal layer; therefore, they are easily identified in CT images. In contrast, T2 and T3 tumor invasion affects the submucosa and muscles of the stomach wall, respectively, and these two layers together constitute a low-density striped layer under enhanced CT. Given that gastric cancer, infiltration is accompanied by changes in inflammation and edema, the accurate identification of the gastric cancer T stage is challenging, leading to ambiguous conclusions. There were more images of T4 tumors, and the parameters for the diagnostic platform were optimized better. There were fewer images of T2 and T3 gastric tumors than there were of T4 gastric tumors, and the optimization of the relevant parameters of the diagnostic platform was relatively poorer. The final T stage determinations made by the diagnostic platform based on continuous venous phase images were completely consistent with the postoperative pathological T stage diagnosis. These results suggest that the diagnostic platform has high feasibility, accuracy, objectivity, and efficiency. It is expected that the developed platform will assist radiologists during screening and reduce their workload. Furthermore, the platform will help guide clinicians in determining diagnoses and developing treatment plans and enable patients with gastric cancer to receive more precise and personalized treatment.
The limitations of this study are as follows. This study was a single-center trial with limited data. The artificial intelligence platform constructed in this study can reliably determine T stages, but the T stage evaluation accuracy for single images is not ideal. Increased data volume and algorithm optimization are needed to improve the diagnostic performance. This study is based on the supervised learning of a CNN. The training accuracy of the platform depends on accurate tumor area identification in enhanced CT images by radiologists. We aim to develop and utilize highly efficient data labeling methods with weak supervision or using unsupervised techniques. We explored the feasibility of applying deep learning in this study, and it is necessary to further verify the clinical application value of this diagnostic platform in clinical practice. Therefore, to further improve the reliability of the artificial intelligence-assisted platform, the volume of data will need to be increased using a multicenter approach, and the algorithm and the labeling efficiency will need to be optimized; with those improvements, clinical verification will be realized to achieve the goal of assisting clinicians in diagnosis and treatment.
This study show that the convolutional neural network computer-aided system established in this study can automatically segment and recognize T stages based on enhanced CT images of advanced gastric cancer, with an accuracy comparable to that of experienced radiologists. The platform is expected to assist radiologists in making more accurate, intuitive, and efficient diagnoses, greatly reducing the workload of radiologists, guiding clinicians in determining diagnoses and developing treatment plans, and helping patients receive more precise and personalized treatment.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by ethics committee of Qingdao University affiliated Hospital. The patients/participants provided their written informed consent to participate in this study.
DW and YL: study concept and design. JH, YG, and XiaoZ: acquisition of data. XiZ, MZ, and TN: analysis and interpretation of data. LZ, XuZ, and DW: critical revision of the manuscript and obtained funding. LZ and XuZ: statistical analysis. All authors contributed to the article and approved the submitted version.
This work was financially supported by National Science Foundation of China (Grant No. 81802473).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01238/full#supplementary-material
1. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. (2017) 3:524–48. doi: 10.1001/jamaoncol.2016.5688
2. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. (2011) 29:4387–93. doi: 10.1200/JCO.2011.36.5908
3. Lu Y, Yu Q, Gao Y, Zhou Y, Liu G, Dong Q, et al. Identification of metastatic lymph nodes in MR imaging with faster region-based convolutional neural networks. Cancer Res. (2018) 78:5135–43. doi: 10.1158/0008-5472.CAN-18-0494
4. Kim HJ, Kim AY, Oh ST, Kim JS, Kim KW, Kim PN, et al. Gastric cancer staging at multi-detector row CT gastrography: comparison of transverse and volumetric CT scanning. Radiology. (2005) 236:879–85. doi: 10.1148/radiol.2363041101
5. Yan C, Zhu ZG, Yan M, Zhang H, Pan ZL, Chen J, et al. Value of multidetector-row computed tomography in the preoperative T and N staging of gastric carcinoma: a large-scale Chinese study. J Surg Oncol. (2009) 100:205–14. doi: 10.1002/jso.21316
6. Kubota K, Suzuki A, Shiozaki H, Wada T, Kyosaka T, Kishida A. Accuracy of multidetector-row computed tomography in the preoperative diagnosis of lymph node metastasis in patients with gastric cancer. Gastrointest Tumors. (2017) 3:163–70. doi: 10.1159/000454923
7. Joo I, Lee JM, Kim JH, Shin CI, Han JK, Choi BI. Prospective comparison of 3T MRI with diffusion-weighted imaging and MDCT for the preoperative TNM staging of gastric cancer. J Magn Reson Imaging. (2015) 41:814–21. doi: 10.1002/jmri.24586
8. Ng F, Ganeshan B, Kozarski R, Miles KA, Goh V. Assessment of primary colorectal cancer heterogeneity by using whole-tumor texture analysis: contrast-enhanced CT texture as a biomarker of 5-year survival. Radiology. (2013) 266:177–84. doi: 10.1148/radiol.12120254
9. Tatsugami F, Higaki T, Nakamura Y, Yu Z, Zhou J, Lu Y, et al. Deep learning-based image restoration algorithm for coronary CT angiography. Eur Radiol. (2019) 29:5322–9. doi: 10.1007/s00330-019-06183-y
10. Geras KJ, Mann RM, Moy L. Artificial intelligence for mammography and digital breast tomosynthesis: current concepts and future perspectives. Radiology. (2019) 293:246–59. doi: 10.1148/radiol.2019182627
11. Chen XH, Ren K, Liang P, Chai YR, Chen KS, Gao JB. Spectral computed tomography in advanced gastric cancer: Can iodine concentration non-invasively assess angiogenesis? World J Gastroenterol. (2017) 23:1666–75. doi: 10.3748/wjg.v23.i9.1666
12. Zhong Z, Zheng L, Kang G, Li S, Yang Y. Random erasing data augmentation. arXiv:1708.04896 (2017).
13. Drozdzal M, Chartrand G, Vorontsov E, Shakeri M, Di Jorio L, Tang A, et al. Learning normalized inputs for iterative estimation in medical image segmentation. Med Image Anal. (2018) 44:1–13. doi: 10.1016/j.media.2017.11.005
14. Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2016) 14:1286–312. doi: 10.6004/jnccn.2016.0137
15. Chae S, Lee A, Lee JH. The effectiveness of the new (7th) UICC N classification in the prognosis evaluation of gastric cancer patients: a comparative study between the 5th/6th and 7th UICC N classification. Gastric Cancer. (2011) 14:166–71. doi: 10.1007/s10120-011-0024-6
16. Gandomkar Z, Brennan PC, Mello-Thoms C. MuDeRN: Multi-category classification of breast histopathological image using deep residual networks. Artif Intell Med. (2018) 88:14–24. doi: 10.1016/j.artmed.2018.04.005
17. Hornbrook MC, Goshen R, Choman E, O'Keeffe-Rosetti M, Kinar Y, Liles EG, et al. Early colorectal cancer detected by machine learning model using gender, age, and complete blood count data. Dig Dis Sci. (2017) 62:2719–27. doi: 10.1007/s10620-017-4722-8
Keywords: convolutional neural network, advanced gastric cancer, T staging, faster RCNN, artificial intelligence
Citation: Zheng L, Zhang X, Hu J, Gao Y, Zhang X, Zhang M, Li S, Zhou X, Niu T, Lu Y and Wang D (2020) Establishment and Applicability of a Diagnostic System for Advanced Gastric Cancer T Staging Based on a Faster Region-Based Convolutional Neural Network. Front. Oncol. 10:1238. doi: 10.3389/fonc.2020.01238
Received: 02 March 2020; Accepted: 16 June 2020;
Published: 28 July 2020.
Edited by:
Bo Gao, Affiliated Hospital of Guizhou Medical University, ChinaReviewed by:
Laurence Gluch, The Strathfield Breast Centre, AustraliaCopyright © 2020 Zheng, Zhang, Hu, Gao, Zhang, Zhang, Li, Zhou, Niu, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Lu, bHV5dW5AcWR5eS5jbg==; Dongsheng Wang, d2FuZ2RzMDUzOEBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.