
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 August 2020
Sec. Pediatric Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01227
This article is part of the Research Topic Critical Complications In Pediatric Oncology and Hematopoietic Cell Transplant View all 31 articles
 Avis Harden1*†
Avis Harden1*† Dristhi Ragoonanan1†
Dristhi Ragoonanan1† Daryl Anildes-Gubman2
Daryl Anildes-Gubman2 David McCall1
David McCall1 Kathleen Faltus1
Kathleen Faltus1 Sarah Featherston1
Sarah Featherston1 Basirat Shoberu1
Basirat Shoberu1 Jerelyn R. Moffet3
Jerelyn R. Moffet3 Demetrios Petropoulos4
Demetrios Petropoulos4 Sajad J. Khazal4
Sajad J. Khazal4 Shehla Razvi1
Shehla Razvi1 Kris M. Mahadeo4†
Kris M. Mahadeo4† Priti Tewari4†
Priti Tewari4†Chimeric antigen receptor (CAR) therapies such as tisagenlecleucel, indicated for children and young adults with relapsed and/or refractory CD19+ acute lymphoblastic leukemia (ALL), have been associated with striking treatment outcomes and overall survival. Yet, they are also associated with unique and potentially life-threatening complications. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity (ICANS) are generally reversible complications of CAR therapies, but many patients may require critical care support especially if they are not promptly recognized and appropriately managed by frontline healthcare staff. As CAR therapies become more widely available, it is important that inter-professional staff members be aware of general principles regarding diagnosis and management. We hypothesized that an inter-professional education (IPE) simulation-based education intervention (CAR-TEAM) would improve knowledge base and confidence regarding complications of CAR therapies among inter-professional staff. Here, we demonstrate that following CAR-TEAM training, >90% of participants demonstrated knowledge proficiency and confidence in the IPE content area. CAR-TEAM training may serve as an important tool to establish initial and continued competency among sites introducing CAR therapies.
Cancer immunotherapies have been associated with remarkable response rates but are also associated with unique and potentially severe toxicities, which can lead to rapid and life-threatening cardiorespiratory and/or neurological clinical deterioration. In 2017, an autologous chimeric antigen receptor (CAR) T cell therapy (tisagenlecleucel) indicated for children and young adults with relapsed and/or refractory CD19+ acute lymphoblastic leukemia (ALL) became the first gene therapy to be approved in the USA (1). CAR T cell therapies are generated through genetic modification of the patient's own (autologous) T cells or those of an allogeneic donor. The isolated cells are activated and genetically modified via viral transduction or non-viral gene transfer, to express an engineered chimeric cell-surface receptor comprising an extracellular antigen-recognition domain; this is usually an antibody single-chain variable fragment (scFv), linked to at least one intracellular signaling domain—usually the CD3ζ chain of the T cell receptor plus one or more domains derived from co-stimulatory receptors, such as CD28 or 4-1BB ligand receptor (4-1BB; also known as TNFRSF9) (2, 3). The extracellular portion of the CAR enables recognition of a specific antigen (such as CD19), and the signaling domains stimulate T cell proliferation, cytolysis, and cytokine secretion to enable elimination of the target (4–6).
Tisagenlecleucel has been associated with ~76% overall survival at 12 months among patients with relapsed/refractory disease who previously had no curative options (7). Yet, unique toxicities such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity (ICANS) are potentially life-threatening complications of this therapy. The pathophysiological mechanisms of both CRS and ICANS remain poorly understood (8). CRS typically presents as a systemic inflammatory response associated with CAR cell proliferation, involving immune response-modulating proteins and cytokines, characterized by fever, hypoxia, tachycardia, hypotension, and multi-organ dysfunction (FDA)1 (9). ICANS can occur concurrently with CRS, following its resolution, or without associated CRS and is characterized by encephalopathy, delirium, seizures, and, at times, cerebral edema. Some patients who receive tisagenlecleucel may require intensive monitoring and critical care support, predominantly owing to these toxicities, especially if they are not promptly recognized and appropriately managed by frontline healthcare staff (6, 10).
We have previously collaborated with the Hematopoietic Cell Transplantation-Cancer Immunotherapy (HCT-CI) Subgroup of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network and the Pediatric Transplantation and Cellular Therapy Consortium (PTCTC) to develop guidelines for the grading and management of CRS and ICANS in pediatric patients (6), and key components were subsequently adopted by the American Society of Transplantation and Cellular Therapies (ASTCT) in their proposed consensus grading system (8).
As CAR therapies expand from select medical centers and become more widely available, it is essential that treating facilities and their local partners (who may, for example, see unanticipated patients in their local emergency rooms) have adequate clinical infrastructure in place. Inter-disciplinary staff should be appropriately trained to promptly recognize and manage complications of CAR therapies. While specific management algorithms may vary based on institutional preference, CAR product, and/or patient population, there are overall guiding principles that may be considered, as shown in Table 1 (6, 8). In 2019, we collaborated with the Association for Pediatric Hematology Oncology Nursing (APHON) and PALISI-HCT-CI to develop an inter-professional education (IPE) didactic and simulation (SIM) training intervention. We hypothesized that our interdisciplinary training intervention (CAR-TEAM) would improve knowledge base and confidence regarding complications of CAR therapies among interdisciplinary staff. We aimed for >90% of participants to demonstrate knowledge proficiency and confidence in the IPE content area. If successful, this could serve as an important tool to establish competency among sites introducing CAR therapies.
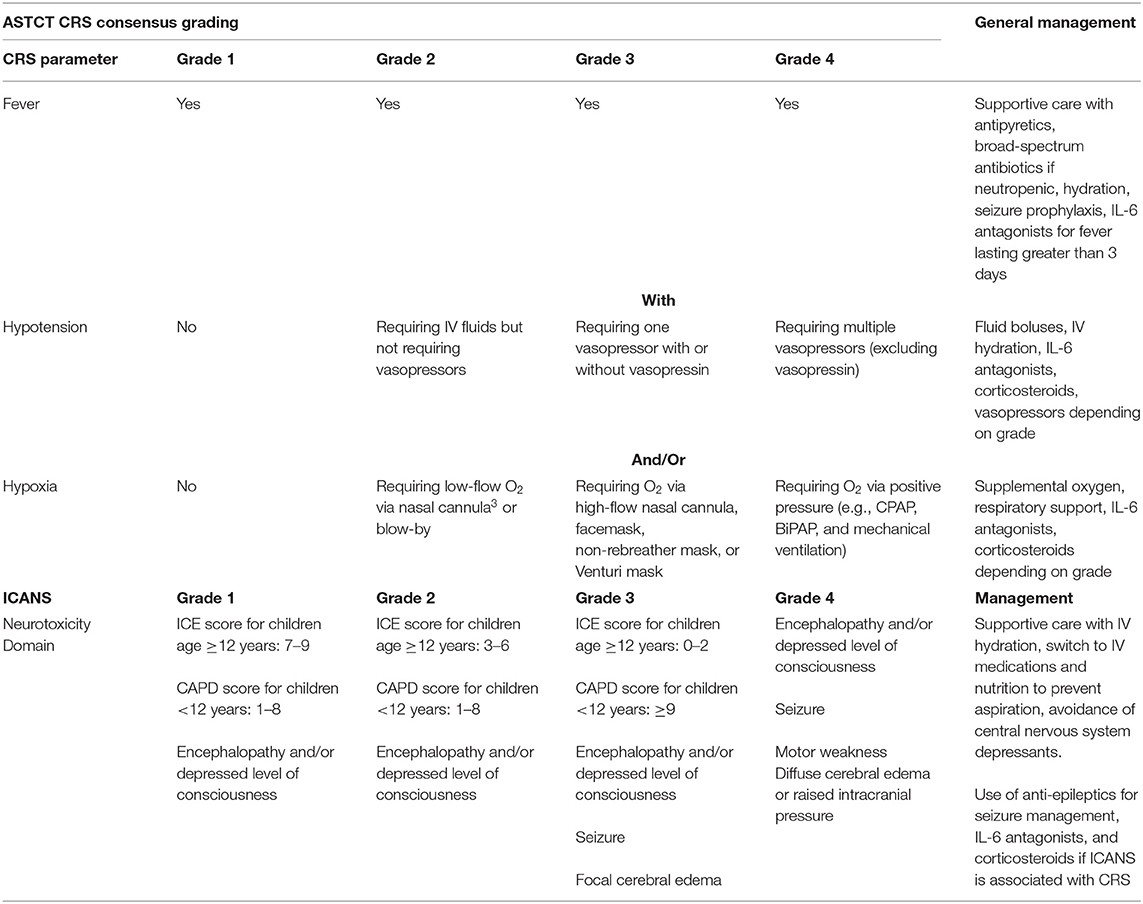
Table 1. ASTCT CRS and ICANS Consensus Grading and General Management Principles (8).
This retrospective study was approved by the institutional review board at the University of Texas at MD Anderson Cancer Center. We developed an IPE module in collaboration with content experts (faculty physicians, clinical fellows, pharmacists, and nursing from cellular therapy, and critical care units) from the MD Anderson CARTOX Program, APHON and PALISI, to address evidence-based management for CAR therapy-related toxicities in pediatric patients. We aimed to increase awareness of the risk factors for and improve recognition of CRS and ICANS and foster confidence in general management strategies for these complications. We hypothesized that as centers introduce CAR therapies at their centers and on-board new staff, simulation training may create a safe environment to learn and promote team confidence. If successful, teams could consider similar training exercises at their home centers.
The module included a pre-intervention knowledge and confidence assessment and didactic session followed by an IPE simulation exercise. The didactic lecture included information on indications for CAR therapy in pediatrics, clinical candidate selection, an overview of product collection and manufacture, infusion reactions and post-CART complications (including ASTCT grading and the Cornell Assessment of Pediatric Delirium—CAPD tool), along with general management principles (8, 11). Participants were also educated on the use of the SBAR (situation, background, assessment, and recommendation) communication tool to help facilitate improved communication and hand over quality between team members (12). Emphasis was placed on team dynamics and communication as well as appropriate escalation of care. Post-intervention knowledge and confidence assessments were performed immediately after and at 90 days. Our primary objective was to determine whether >90% of participants (i) either “agreed” or “strongly agreed” that they felt confident in specified CAR therapy diagnosis and management areas and (ii) demonstrated knowledge proficiency as determined by accurate responses to written questions.
MD Anderson's CARTOX Program provides oversight for the care for the hospital's CAR therapy patients and is the first stand-alone immune effector cellular therapy program to earn accreditation from the Foundation for the Accreditation of Cellular Therapy (FACT). The PALISI Network is a national organization devoted to identifying therapeutic and preventative strategies for acute respiratory distress syndrome, sepsis, multi-organ failure, and other acute life-threatening pulmonary or systemic inflammatory syndromes that affect infants and children through multi-center research. The Association of Pediatric Hematology/Oncology Nurses (APHON) is a professional organization for pediatric hematology/oncology nurses and other pediatric hematology/oncology healthcare professionals dedicated to promoting optimal nursing care for children, adolescents, and young adults with cancer and blood disorders.
IPE Module: Pre- and post-intervention confidence assessments were developed and reviewed by three content and/or education experts for content validity. A subject content expert led a didactic lecture on CRS and ICANS with course content reviewed and approved by the IPE committee. Inter-professional learners were then selected in small groups of 5–6 people to participate in a simulated exercise of a pediatric patient receiving CAR therapy who progressed through the various grades (per ASTCT criteria) of CRS and ICANS. These grades were separated into three phases of the simulated exercise. The goals of phase I and II were to recognize and effectively manage grades 1 and 2 CRS and grades 3 and 4 CRS, respectively. Phase 3 was aimed at recognition and management of ICANS. A patient caregiver, vital signs monitor, emergency equipment, medication dosing sheet, and a telephone to place a consult/escalate care were also provided. Participants were encouraged to approach the simulation from the perspective of their respective field (cellular therapy vs. critical care, physician, nurse etc.), but above all, to focus on teamwork and optimal communication. At the end of each phase, participants were asked to give a hand-off sheet summarizing the patient's clinical background, current clinical status, and, when appropriate, requirements for escalation of care. An objective checklist was used during each phase to ensure that key goals were achieved including the recognition and accurate grading of CRS and/or ICANS, appropriate general management based on CRS and/or ICANS grade, escalation of care when necessary, and use of effective and closed loop communication among team members as detailed in Table 2. All participants were then debriefed by a panel of experts from education, cellular therapy, critical care, renal, pharmacy, and nursing to identify further learning opportunities at the end of each phase. A post-IPE and simulation survey was distributed to assess participants' confidence of their knowledge base as well as assess general competency. Questions probed the participant's ability to appropriately identify risk factors and signs and symptoms of CRS and ICANS; general management principles were also assessed. Participants were encouraged to learn their institution-specific management algorithms as these may vary based on center.
Statistical Analysis: Descriptive statistics was used to summarize participants' demographics and baseline characteristics.
As shown in Table 3, participants (n = 70) represented diverse centers and disciplines from across the United States, including oncology, hematology, and critical care.
As shown in Figure 1A, pre- and post-IPE assessments indicate significant improvement in the confidence of the 47 participants (66% survey response rate) who responded to the post-assessment survey regarding (i) knowledge of risk factors for CRS and/or ICANS, (ii) recognition of initial signs/symptoms of CRS and/or ICANS, (iii) ability to conduct initial work-up of CRS and/or ICANS, and (iv) effective management of CRS and/or ICANS. In all areas assessed, >90% of the 47 respondents expressed confidence post-IPE intervention.
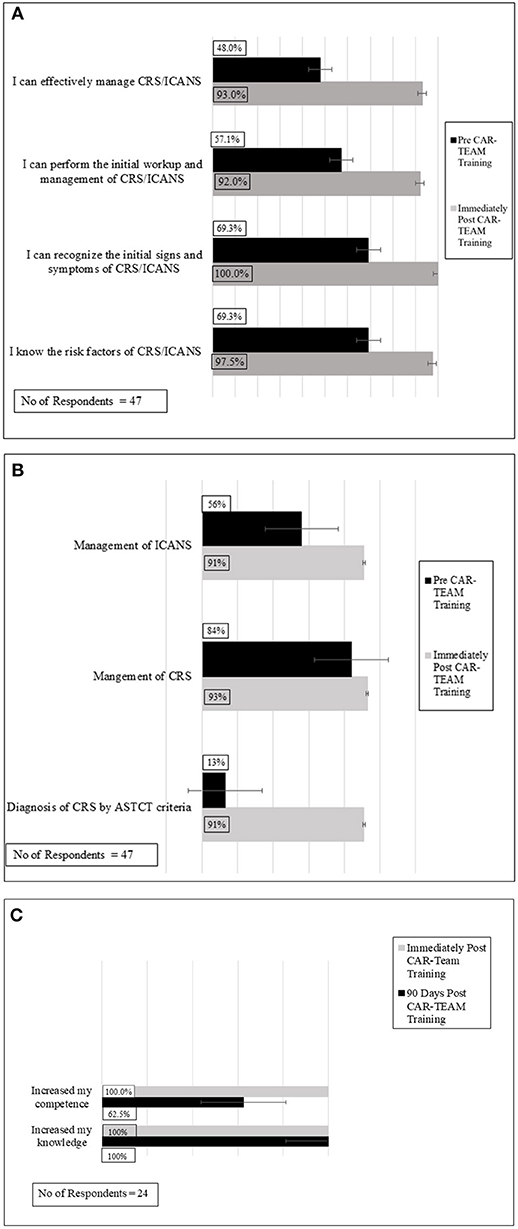
Figure 1. Confidence and knowledge assessments of participants pre and immediately post-CAR-TEAM Training: significant improvements were seen in both confidence (A) and knowledge base (B) following CAR-TEAM training. (C) Overall evaluation of participants immediately post-CAR-TEAM training and 90 day post- post-CAR-TEAM training.
Figure 1B shows significant improvements in knowledge assessments pre- and post-IPE intervention. Specifically, post SIM >90% of the 47 participants who responded to the survey demonstrated knowledge proficiency regarding (i) indications for admission to the intensive care unit, (ii) diagnosis of CRS by ASTCT criteria, (iii) management of CRS, and (iv) management of ICANS.
Immediately following completion of the IPE intervention, participants were surveyed regarding their overall perceptions. Of the 47 respondents, all 47 respondents “agreed” or “strongly agreed” that they felt more confident in (i) their patient management, (ii) their knowledge base, and (iii) their clinical performance following the IPE intervention (Figure 1C).
To assess durability of the IPE intervention, voluntary surveys were sent to participants by APHON, 90 days after the IPE intervention. The APHON survey consisted of three questions sent via electronic mail. There was a 34% response rate (n = 24). Of the 24 respondents, all respondents agreed that the IPE intervention had increased their knowledge (Figure 1C). When asked if they felt that their clinical practice had improved as a result of the IPE intervention, 22 of the 24 respondents of survey participants agreed. Further, 22 survey respondents agreed that that they were able to apply new and relevant information to their practice as a result of the IPE intervention. Overall, participants felt that the workshop helped improve clinical practice, increased knowledge, and improved the ability to apply the acquired knowledge to clinical practice.
As health institutions increasingly provide access to CAR therapies, adequate strategic and operational planning and preparation are needed to ensure the safe delivery of such treatments (6). CRS and ICANS are the most common toxicities with CAR therapies occurring in 60–94 and 70–80% of patients, respectively. While both syndromes are reversible, they can be severe and life-threatening if left untreated. Prompt and appropriate intervention is key (13). Immune effector cell (IEC) center accreditation by the FACT is a voluntary means of ensuring adherence to quality standards; it requires, among other components, initial education and ongoing competency training for inter-professional staff directly involved in the care of CAR therapy patients. This includes but is not limited to cellular therapy, critical care, triage and neurology, physicians, trainees, nurses, pharmacists, advance practice providers and medical assistants, and research and data personnel (6, 14). Additionally, emergency medical services, community hospitals, and local urgent care facilities may require high vigilance to recognize and promptly escalate care in the event that a patient treated with CAR therapy presents to their facility in an emergency (6).
IPE cultivates collaborative practice to provide patient-centered healthcare (15, 16). IPE programs are increasingly recognized as an important tool to reduce medical errors and improve communication (17, 18). Simulation-based training is a safe and effective means of educating inter-professional care providers (from novice to experienced levels) in various clinical settings, from surgical to critical care management in technical abilities and/or effective and practical non-technical skills such as problem-solving (19, 20). High-fidelity mannequin-based simulations provide training for smaller groups and there is evidence that technology-enhanced simulation can provide comparable results and may be useful for a larger number of trainees (21). Simulation-based training modules (SIM), such as those established by the American Heart and Lung Association for life support training, afford providers the opportunity to safely manage these challenging complications by providing lifelike clinical education opportunities (21–23). SIM training can provide real-time clinical education and subsequently bolster provider confidence without risking patient well-being (23). Most importantly, SIMs also allow for the practice of inter-professional provider communication and foster a sense of teamwork in a safe setting (24, 25). Further, computer-based virtual reality simulators may be accessible either on-site at an institution or via personal devices that can be accessed at a participant's leisure. This type of simulation education has been used successfully for years in adult, pediatric, and neonatal resuscitation courses (21).
Our IPE intervention facilitated critical care and cellular therapy providers from across the United States to learn jointly, which they may not otherwise have had the ability to do, as they likely attend more discipline-specific meetings. Positive overall confidence self-assessments suggest that the IPE intervention was not limited by fear of training in an inter-professional setting and/or in the presence of learners at different experience levels. Knowledge proficiency assessments suggest that the IPE intervention was effective in training learners regarding CAR therapy management.
Our study may have been limited by the survey response rates. The immediate post-assessments were completed electronically and required participants to use their smartphones. Not all participants were able to successfully access this survey system. Further, responder bias could have influenced our conclusions, in particular with our long-term follow-up surveys. As survey responses may sometimes be skewed by those who had strongly positive and/or negative perceptions, we find it encouraging that we did not receive any overwhelmingly negative responses. We expect the real-world value of our IPE to be at least satisfactory for consideration at individual centers. We did not intend to assess whether our IPE resulted in improved patient outcomes, but this can be assessed at individual centers in prospective studies. To increase survey responses in the future, the use of electronic tablets for those unable to access the survey on their smartphone at the education site, follow-up reminders to complete surveys via email, telephone surveys, or incentives may have improved the response rate if utilized.
Our institution has established integrated simulation training for CAR therapy toxicity recognition and management into a pediatric foundational orientation for all newly hired employees involved in the care of patients receiving IEC therapy. This simulation training is also being expanded to the adult patient care departments. We are currently exploring development of an internet-based simulation training that will augment our live module and facilitate larger-scale outreach.
All datasets presented in this study are included in the article/supplementary material.
KM conceptualized the project and obtained grant funding. AH, DR, DA-G, DM, KF, BS, JM, SF, SR, KM, and PT developed the IPE module. AH, DR, KM, and PT wrote the manuscript. All authors reviewed and/or edited the manuscript and made meaningful contributions before submission.
KM has received an unrestricted medical education grant from Jazz Pharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We sincerely thank the following for their invaluable contribution to this manuscript: Ms. Brandy Adams and Marisol Gonzalez for their administrative support. Joan O'Hanlon Curry, MS, RN, CPNP, CPON; the Association of Pediatric Hematology and Oncology Nurses (APHON); the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network; the Teaching, Inter-professional and Simulation (TIPS) Education Center; and the CARTOX Program at the University of Texas at MD Anderson Cancer Center are acknowledged for their assistance.
1. ^FDA Briefing Document: Oncologic Drugs Advisory Committee meeting; BLA 125646 Tisagenlecleucel. Available online at: https://www.fda.gov/media/106081/download (accessed 2020).
1. FDA. Package insert- KymriahTM (tisagenlecleucel) Novartis Pharmaceuticals Corporation. (2017) Available online at: https://www.fda.gov/downloads/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/UCM573941.pdf.
2. Dai H, Wang Y, Lu X, Han W. Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst. (2016) 108:djv439. doi: 10.1093/jnci/djv439
3. Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, et al. Phase I trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Invest. (2016) 126:3363–76. doi: 10.1172/JCI86721
4. Abate-Daga D, Davila ML. CAR models: next-generation CAR modifications for enhanced T-cell function. Mol Ther Oncolytics. (2016) 3:16014. doi: 10.1038/mto.2016.14
5. Maus MV, Levine BL. Chimeric antigen receptor T-cell therapy for the community. Oncologist. (2016) 21:608–17. doi: 10.1634/theoncologist.2015-0421
6. Mahadeo KM, Khazal SJ, Abdel-Azim H, Fitzgerald JC, Taraseviciute A, Bollard CM, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol. (2019) 16:45–63. doi: 10.1038/s41571-018-0075-2
7. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. (2018) 378:439–48. doi: 10.1056/NEJMoa1709866
8. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
9. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. (2018) 15:47–62. doi: 10.1038/nrclinonc.2017.148
10. Neelapu SS, Tummala S, Kebriaei P, Wierda W, Locke FL, Lin Y, et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit 'ALL'. Nat Rev Clin Oncol. (2018) 15:218. doi: 10.1038/nrclinonc.2018.20
11. Traube C, Silver G, Kearney J, Patel A, Atkinson TM, Yoon MJ, et al. Cornell assessment of pediatric delirium: a valid, rapid, observational tool for screening delirium in the PICU*. Crit Care Med. (2014) 42:656–63. doi: 10.1097/CCM.0b013e3182a66b76
12. Muller M, Jurgens J, Redaelli M, Klingberg K, Hautz WE, Stock S. Impact of the communication and patient hand-off tool SBAR on patient safety: a systematic review. BMJ Open. (2018) 8:e022202. doi: 10.1136/bmjopen-2018-022202
13. Gutierrez C, Mcevoy C, Munshi L, Stephens RS, Detsky ME, Nates JL, et al. Critical care management of toxicities associated with targeted agents and immunotherapies for cancer. Crit Care Med. (2020) 48:10–21. doi: 10.1097/CCM.0000000000004087
14. FACT. Foundation for the Accreditation of Cellular Therapy. Omaha, NE: Standards for Immune Effector Cells (2017).
15. Yan J, Gilbert JH, Hoffman SJ. World Health Organization study group on interprofessional education and collaborative practice. J Interprof Care. (2007) 21:588–9. doi: 10.1080/13561820701775830
16. Goldman J. (2011). Centre for the advancement of interprofessional education (CAIPE) website: www.caipe.org.uk. Journal of Interprofessional Care 25, 386–387. doi: 10.3109/13561820.2011.605618
17. Coalition-for-Patients'-Rights. Coalition members discuss value of collaboration in diabetes patient care. News Medical (2012).
19. Okuda Y, Bryson EO, Demaria S, Jacobson L, Quinones J, et al. The utility of simulation in medical education: what is the evidence? Mt Sinai J Med. (2009) 76:330–43. doi: 10.1002/msj.20127
20. Kim J, Park JH, Shin S. Effectiveness of simulation-based nursing education depending on fidelity: a meta-analysis. BMC Med Educ. (2016) 16:152. doi: 10.1186/s12909-016-0672-7
21. Cheng A, Lang TR, Starr SR, Pusic M, Cook DA. Technology-enhanced simulation and pediatric education: a meta-analysis. Pediatrics. (2014) 133:e1313–1323. doi: 10.1542/peds.2013-2139
22. Mcgaghie WC, Issenberg SB, Petrusa ER, Scalese RJ. A critical review of simulation-based medical education research: 2003-2009. Med Educ. (2010) 44:50–63. doi: 10.1111/j.1365-2923.2009.03547.x
23. Marker S, Mohr M, Ostergaard D. Simulation-based training of junior doctors in handling critically ill patients facilitates the transition to clinical practice: an interview study. BMC Med Educ. (2019) 19:11. doi: 10.1186/s12909-018-1447-0
24. Pasquale SJ. Educational science meets simulation. Best Pract Res Clin Anaesthesiol. (2015) 29:5–12. doi: 10.1016/j.bpa.2015.02.003
Keywords: inter-professional, education, simulation-based, cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity (ICANS)
Citation: Harden A, Ragoonanan D, Anildes-Gubman D, McCall D, Faltus K, Featherston S, Shoberu B, Moffet JR, Petropoulos D, Khazal SJ, Razvi S, Mahadeo KM and Tewari P (2020) Chimeric Antigen Receptor, Teamwork, Education, Assessment, and Management (CAR-TEAM): A Simulation-Based Inter-professional Education (IPE) Intervention for Management of CAR Toxicities. Front. Oncol. 10:1227. doi: 10.3389/fonc.2020.01227
Received: 25 April 2020; Accepted: 16 June 2020;
Published: 05 August 2020.
Edited by:
Rimas J. Orentas, Seattle Children's Research Institute, United StatesReviewed by:
Rebecca Gardner, University of Washington, United StatesCopyright © 2020 Harden, Ragoonanan, Anildes-Gubman, McCall, Faltus, Featherston, Shoberu, Moffet, Petropoulos, Khazal, Razvi, Mahadeo and Tewari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Avis Harden, QUhhcmRlbkBtZGFuZGVyc29uLm9yZw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.