
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 July 2020
Sec. Radiation Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.01170
This article is part of the Research Topic Influence of Radiation Therapies on Liver Cancer View all 15 articles
Stereotactic body radiotherapy (SBRT) is currently well-adopted as a curative treatment for primary and metastatic liver tumors. Among SBRT methods, dynamic conformal arc therapy (DCAT) and volumetric-modulated arc therapy (VMAT) are the most preferred methods. In this study, we report a comparison study measuring the dose distribution and delivery efficiency differences between DCAT and VMAT for liver SBRT. All patients who were treated with SBRT for primary or metastatic liver tumors with a curative aim between January 2016 and December 2017 at DIRAMS were enrolled in the study. For all patients, SBRT plans were designed using the Monte Carlo (MC) algorithm in Monaco treatment planning system (version 5.1). The planning goals were set according to the RTOG 0813, RTOG 0915, and RTOG 1112 protocols. A plan comparison was made on the metrics of dose volume histogram, planning and delivery efficiency, monitor unit (MU), and dosimetric indices. PTV coverage was evaluated using the following: Dmean, D95%, D98%, D2%, D50%, Dmax, V95%, heterogeneity index (HI), and conformality index (CI). For DCAT and VMAT, respectively, the Dmean was 5942.8 ± 409.3 cGy and 5890.6 ± 438.8 cGy, D50% was 5968.8 ± 413.1 cGy and 5954.3 ± 405.2 cGy, and CI was 1.05 ± 0.05 and 1.03 ± 0.04. The D98% and V95% were 5580.0 ± 465.3 cGy and 20.4 ± 12.0 mL for DCAT, and 5596.0 ± 478.7 cGy and 20.5 ± 12.0 mL for VMAT, respectively. For normal liver, V40, V30, V20, V17, V5, Dmean, Dmax were evaluated for comparison. The V30, V20, and V10 were significantly higher in DCAT; other parameters of normal livers showed no statistically significant differences. For evaluation of intermediate dose spillage, D2cm(%) and R50% of DCAT and VMAT were 45.8 ± 7.9 and 5.6 ± 0.9 and 45.1 ± 6.7 and 5.5 ± 1.2, respectively. Planning and delivery efficiency were evaluated using MU, Calculation time, and Delivery time. DCAT had shorter Calculation time and Delivery time with smaller MU. MU was smaller in DCAT and the average difference was 300.1 MU. For liver SBRT, DCAT is an effective alternative to VMAT plans that could meet the planning goals proposed by the RTOG SBRT protocol and increases plan and delivery effectiveness, while also ignoring the interplay effect.
Stereotactic body radiotherapy (SBRT) is currently well-adopted as a curative treatment for primary and metastatic liver tumors, especially for patients who are inoperable or undergoing systemic therapy (1–4). SBRT can be performed by various methods, including 3D conformal radiotherapy (3DCRT), intensity modulated radiotherapy (IMRT), dynamic conformal arc therapy (DCAT), volumetric-modulated arc therapy (VMAT), Tomotherapy, and CyberKnife. However, VMAT and DCAT are the most preferred methods (5–7). Both VMAT and DCAT are types of rotational radiotherapy, with each system having its own advantages and disadvantages. VMAT is typically considered superior to DCAT in terms of dose distribution since it has better target coverage. However, DCAT has potential advantages over VMAT in practical use. Reasons to consider DCAT instead of VMAT (8, 11) are as follows: (1) There is a concern about missing the target when VMAT is used for a moving target. Even if DCAT does not cover all targets of irregular movement, at least DCAT might offset this concern for the effect of MLC interplay since the target remains inside the open field with minimal modulation for the entire treatment. (2) VMAT requires a higher degree of quality assurance. (3) There is less concern with calculation accuracy for DCAT in an area of variable densities. (4) DCAT offers quicker plan and delivery times. (5) DCAT demands less monitor unit (MU) to deliver the same dose as VMAT. Furthermore, segment shape optimization (SSO) could supplement DCAT to maintain its inherent advantages while achieving results similar to VMAT. SSO is offered by the Monaco treatment planning system (TPS) (IMPAC Medical Systems, Inc., Maryland Heights, MO; a subsidiary of Elekta AB, Stockholm, Sweden) to improve plan quality by smoothing and clustering segments and optimizing beam weights and shapes. If DCAT for liver tumors is not inferior with regard to dose distribution and satisfies RTOG guidelines, DCAT could be a better option for SBRT plans than VMAT. Here, we report a comparison study measuring the dose distribution and delivery efficiency differences between DCAT and VMAT. Suggestions are offered for improved plan technique in primary and metastatic liver tumor radiotherapy.
This study was approved by the institutional review board of the Dongnam Institute of Radiological and Medical Sciences (DIRAMS).
All patients who were treated with SBRT for primary or metastatic liver tumors with a curative aim between January 2016 and December 2017 at DIRAMS were enrolled in the study. Patients with palliative aims, such as SBRT for portal vein tumor thrombosis, were excluded. All plans were built based on the prescribed dose at that time of treatment.
The target volumes and organs at risk (OARs) of all included patients were reviewed and re-contoured by one radiation oncologist. Gross target volume (GTV) was determined by merging the re-drawn tumor volumes for each phase of the four-dimensional computed tomography (4DCT). Clinical target volume was same as GTV, and plan target volume (PTV) was established with 3–8 mm expansion of the GTV. The total volume of the liver, heart, stomach, and duodenum were contoured. Other OARs were partially drawn as needed for treatment planning.
For all patients, SBRT plans were designed using the MC algorithm in Monaco TPS (version 5.1). Beam modeling of the MONACO TPS was performed by beam of Elekta Infinity linear accelerator (Elekta AB, Stockholm, Sweden). All plans were designed using a photon beam of energy 6 MV with nominal dose rate 600 MU/min. To accompany the prescription and dose criteria, constraints of the plan optimization were adjusted depending on the size and location of the tumor. However, the plans were developed with the same constraints to allow comparison of the DCAT plan with the VMAT plan for each patient. After planning optimization, all plans were normalized to 95% of the PTV coverage with the prescribed dose. The physical parameters for each plan are shown in Figure 1. Each application of DCAT was planned as a single arc with non-constant dose rate and SSO applied. The VMAT plan was designed using a dual arc with a maximum 150 control points per arc and 1.0 cm minimum segment width. The arc length of both plan types was the same, with a range of 360° rotating from 180° in increments of 10° without couch rotation using fluence-smoothing parameters in medium mode. The final dose calculation was performed using a calculation grid resolution of 2.0 mm and a statistical uncertainty of 3% per control point. Physical parameters such as calculation grid resolution, statistical uncertainty, arc length, number of arcs, increment, number of control point/arc, and fluence-smoothing parameters were identical for each case in the same type of plan. The planning goals were set according to the RTOG 0813, RTOG 0915, and RTOG 1112 protocols.
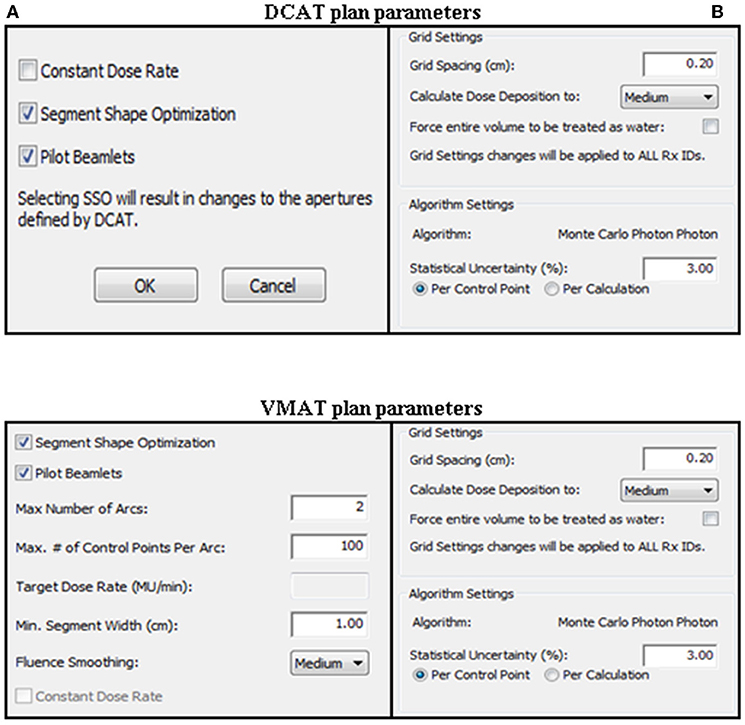
Figure 1. Physical parameters for DCAT and VMAT plans. (A) Sequencing parameters. (B) Calculation parameters.
Statistical analysis was performed using the SPSS 18.0 program. Data normality was assessed using the Shapiro-Wilk test. Student's paired t-test and the Wilcoxon signed-rank test were used, respectively, for parametric and non-parametric data analyses (9).
Twenty-five patients with a total of 31 lesions were enrolled. Two patients had 3 lesions, and 2 patients had 2 lesions, simultaneously. In one patient with 3 lesions, 2 of the lesions were located close to each other and were combined into a single PTV. Among the 25 patients, 17 had hepatocellular carcinomas (HCCs), 8 had metastatic liver cancers, and 7 had received previous radiotherapy (RT). A total of 30 plans for each arm were made and evaluated for comparison. The prescribed SBRT dose was 4,800–6,000 cGy in 4 or 5 fractions. The characteristics of the patients are summarized in Table 1.
All DCAT and VMAT plans were optimized with an identical optimization protocol, and all except 4 patient plans achieved the planning goals. According to plan type, the dose distribution and DVH for the same patient are shown in Figures 2, 3, respectively. Four patients' plans could not reach the objectives due to close proximity to OARs and some goals were compromised according to the physician's discretion to comply with the dose constraints.

Figure 2. The dose distribution for the same patient was shown in axial, coronal and sagittal planes according to the plan type. (A) DCAT. (B) VMAT.
PTV coverage was evaluated using the following: mean dose (Dmean), D95%, D98% (near-minimum absorbed dose), D2% (near-maximum absorbed dose), D50%, maximum point dose to 0.035 mL (Dmax), V95%, heterogeneity index (HI), and conformality index (CI). Among the evaluated parameters, Dmean, D98%, D50%, V95%, and CI showed statistically significant differences between DCAT and VMAT. The Dmean, D50%, and CI were higher, and D98% and V95% were lower in DCAT than in VMAT. For DCAT and VMAT, respectively, the Dmean was 5942.8 ± 409.3 cGy and 5890.6 ± 438.8 cGy, D50% was 5968.8 ± 413.1 cGy and 5954.3 ± 405.2 cGy, and CI was 1.05 ± 0.05 and 1.03 ± 0.04. The D98% and V95% were 5580.0 ± 465.3 cGy and 20.4 ± 12.0 mL for DCAT, and 5596.0 ± 478.7 cGy and 20.5 ± 12.0 mL for VMAT, respectively. The data for PTV dose distribution are presented in Table 2.
For normal livers (liver volume minus GTV), V40, V30, V20, V17, V5, Dmean, Dmax were evaluated for comparison. The V30, V20, and V10 were significantly higher in DCAT; other parameters of normal livers showed no statistically significant differences. Dmax, maximum dose to 1 mL (D1mL), maximum dose to 2 mL (D2mL), and Dmean were assessed for OARs such as the duodenum, stomach, small bowel, large bowel, spinal cord, heart, and esophagus. For the duodenum, all assessed parameters show statistically significant differences. Dmax, D1mL, and D2mL were higher in DCAT, but the Dmean was lower than in VMAT. For both the stomach and heart, Dmax, D1mL, D2mL were significantly higher in DCAT. The dose data for OARs that show statistically significant differences are listed in Table 3 and entire data in Supplementary Table 1.
The maximum dose to any point ≥2 cm away from the PTV in any direction (D2cm) and the ratio of the volume of 50% of the prescription dose isodose to the volume of the PTV (R50%) are reported for evaluation of intermediate dose spillage and to scrutinize the fall-off gradient beyond the PTV extending into normal tissue structures. The D2cm(%) and R50% of DCAT and VMAT were 45.8 ± 7.9 and 5.6 ± 0.9, and 45.1 ± 6.7 and 5.5 ± 1.2, respectively. The differences were not statistically significant. The D2cm(%) and R50% data for all patients are shown in Table 4.
We also evaluated plan and delivery efficiency using MU, the elapsed time to make a plan (Calculation time), and the estimated delivery time (Delivery time). The MU of DCAT and VMAT were 2440.5 ± 346.5 and 2741.4 ± 417.5, respectively, and the average difference was 300.9 ± 340.4. Calculation time for DCAT and VMAT was 14.4 ± 7.5 min and 29.0 ± 14.2 min, and delivery time was 3.6 ± 0.5 min and 4.5 ± 0.7 min, respectively. All efficiency parameters analyzed showed statistically significant differences (Table 5).
For plan comparison, we used 1 arc for DCAT and 2 arcs for VMAT. One-arc and 2-arc plans with both DCAT and VMAT were used for some patients to verify the ideal number of arcs for each method. Dose distribution in DCAT showed little improvement from increment of arc numbers. In contrast, VMAT showed better results with 2 arcs than with 1 arc. For comparison with the best plans, 1 arc in DCAT and 2 arcs in VMAT were used.
In this study, couch rotation was not used due to disadvantages of couch rotation. Even though the use of couch rotation can help improve dosimetric parameters, couch rotation should be used with caution because the theoretical advantage could be offset by errors caused by an increase in treatment time and position changes.
As expected, VMAT was superior to DCAT to build SBRT plan with better plan quality. However, although there were some statistically significant differences between DCAT and VMAT plans, we could not assert if VMAT was better than DCAT with only dose distribution in the absence of consideration for actual use. As mentioned above, DCAT has several advantages over VMAT that could offset the better dose-distribution of VMAT. Unless the differences of dose distribution and plan quality are substantial, DCAT could be reckoned prior to VMAT for SBRT in some specific cases. There are concerns regarding missing the targets in highly modulated treatment plan for moving targets such as liver tumors. Liver tumors might not only move up and down but also move sideways and twist. Moreover, liver tumors tend to grow in a relatively circular shape and this suggests that DCAT would be as appropriate as VMAT to achieve adequate target coverage in liver tumors. That is the one of reason why we should consider DCAT for liver tumors during SBRT if the differences between DCAT and VMAT are comparable. In addition, complex interplay between MLC motion, jaw movement, gantry rotation, and target motion during free-breathing treatments with VMAT could cause considerable dose discrepancies (7, 10). Our results indicated that several dosimetric parameters were significantly better in VMAT plans. However, DCAT also met the plan goals proposed by the RTOG SBRT protocol and the dose distribution differences were small enough to accept DCAT as a method of SBRT instead of VMAT.
D2cm(%) and R50% values are originally proposed for lung SBRT but not for the liver. However, we wanted to manage and minimize spillage dose and hence, D2cm(%) and R50% were evaluated. D2cm(%) achieved the suggested goal except in 1 case. In this 1 case where the D2cm(%) was not achieved, the tumor was located near the duodenum and D2cm(%) was compromised to obtain acceptable dose distribution to the duodenum. Meanwhile, R50% failed to meet the goal in 12 cases. This might be due to the different prescription goals for liver and lung PTV in RTOG protocols. To clarify the reason why R50% is hard to achieve, we tested dose spillage through CT scans with lung and liver density and there was no significant difference according to tissue densities. Stathakis et al. recently published the study of dosimetric comparison between VMAT and DCAT in SBRT for the lung and liver (10). In that study, R50% seemed to reach the desired goal; however, there appeared to be calculation errors in their R50 values. Though differences in protocols make it difficult to achieve R50 in the liver, there is still a need to evaluate controlling dose spillage outside the PTV.
In terms of efficiency, DCAT had shorter calculation times and delivery times with smaller MU. Since calculation time changes variably depending on the number of programs running on the TPS at the same time, measurement of accurate time for planning is not available. Nevertheless, calculation time is generally 2–3 times longer in VMAT, and DCAT could allow for quicker plans. Shorter delivery times would help patients maintain treatment postures and to control breathing which would reduce target misses during RT. Therefore, DCAT should be considered preferential to VMAT in patients with poor breathing or poor coordination. MU was smaller in DCAT; the average difference was 300.1 MU. The use of smaller MU would reduce both the load on the LINAC machine and concerns about leakage through the multi-leaf collimator. In addition, DCAT could help conserve resources, which might be important to institutions with many patients but limited resources.
Before SBRT, some patients receive prior surgery or RT and would need stricter dose control to avoid severe toxicities. VMAT would be more appropriate in such patients for minimizing the dose to OARs, although the dose differences are small. Moreover, since DCAT is not recommended for constructing plans for multiple lesions, VMAT should be considered when SBRT is administered for multiple lesions simultaneously. However, if multiple lesions are treated separately, DCAT should be given the priority.
As noted above, DCAT might be more advantageous than VMAT for liver SBRT with exception to specific cases. Although the method of treatment is at the physician's discretion, the physician needs to understand the pros and cons of each treatment prior to choosing a treatment method. We hope this study will help radiation oncologists make better decisions for selection of SBRT methods.
All datasets generated for this study are included in the article/Supplementary Material.
This study was retrospective and exempted from obtaining written informed consent. The study was also approved by the Institutional Review Board of the Dongnam Institute of Radiological and Medical Sciences (DIRAMS).
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This research was supported by National R&D Program through the Dongnam Institute of Radiological & Medical Sciences (DIRAMS) funded by the Ministry of Education, Science and Technology (50602-2019).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01170/full#supplementary-material
1. Jang WI, Bae SH, Kim M-S, Han CJ, Park SC, Kim SB, et al. A phase 2 multicenter study of stereotactic body radiotherapy for hepatocellular carcinoma: safety and efficacy. Cancer. (2020) 126:363–72. doi: 10.1002/cncr.32502
2. Doi H, Beppu N, Kitajima K, Kuribayashi K. Stereotactic body radiation therapy for liver tumors: current status and perspectives. Anticancer Res. (2018) 38:591–9. doi: 10.21873/anticanres.12263
3. Mahadevan A, Blanck O, Lanciano R, Peddada A, Sundararaman S, D'Ambrosio D, et al. Stereotactic Body Radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch(R) Patient Registry. Radiat Oncol. (2018) 13:26. doi: 10.1186/s13014-018-0969-2
4. Scorsetti M, Comito T, Clerici E, Franzese C, Tozzi A, Iftode C, et al. Phase II trial on SBRT for unresectable liver metastases: long-term outcome and prognostic factors of survival after 5 years of follow-up. Radiat Oncol. (2018) 13:234. doi: 10.1186/s13014-018-1185-9
5. Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. (2008) 35:310–7. doi: 10.1118/1.2818738
6. Poon DM, Kam M, Leung CM, Chau R, Wong S, Lee WY, et al. Dosimetric advantages and superior treatment delivery efficiency of RapidArc over conventional intensity-modulated radiotherapy in high-risk prostate cancer involving seminal vesicles and pelvic nodes. Clin Oncol. (2013) 25:706–12. doi: 10.1016/j.clon.2013.07.010
7. Rauschenbach BM, Mackowiak L, Malhotra HK. A dosimetric comparison of three-dimensional conformal radiotherapy, volumetric-modulated arc therapy, and dynamic conformal arc therapy in the treatment of non-small cell lung cancer using stereotactic body radiotherapy. J Appl Clin Med Phys. (2014) 15:4898. doi: 10.1120/jacmp.v15i5.4898
8. Stathakis S. Optimized DCAT for Lung and Liver SBRT Rapid and Accurate Delivery of Lung and Liver SBRT Using Optimized Dynamic Conformal Arc Treatment (DCAT) With Monaco® Treatment Planning System and Versa HD™. San Antonio, TX: University of Texas Health San Antonio Cancer Center (2018).
9. Chaikh A, Giraud J-Y, Perrin E, Bresciani J-P, Balosso J. The choice of statistical methods for comparisons of dosimetric data in radiotherapy. Radiat Oncol. (2014) 9:205. doi: 10.1186/1748-717X-9-205
10. Stathakis S, Narayanasamy G, Licon AL, Myers P, Li Y, Crownover R, et al. A dosimetric comparison between volumetric-modulated arc therapy and dynamic conformal arc therapy in SBRT. J Buon. (2019) 24:838–43.
Keywords: liver cancer, SBRT (stereotactic body radiotherapy), VMAT (volumetric modulated arc therapy), Monaco TPS (treatment planning system), DCAT (dynamic conformal arc therapy)
Citation: Moon YM, Jeon W, Yu T, Bae SI, Kim JY, Kang J-K and Choi CW (2020) Which Is Better for Liver SBRT: Dosimetric Comparison Between DCAT and VMAT for Liver Tumors. Front. Oncol. 10:1170. doi: 10.3389/fonc.2020.01170
Received: 02 January 2020; Accepted: 09 June 2020;
Published: 29 July 2020.
Edited by:
James M. Brindle, Rhode Island Hospital, United StatesReviewed by:
Fenghong Liu, UMass Memorial Medical Center, United StatesCopyright © 2020 Moon, Jeon, Yu, Bae, Kim, Kang and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chul Won Choi, ZHJhc2RmMTlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.