- 1Department of Lung Cancer Surgery, Tianjin Medical University General Hospital, Tianjin, China
- 2Tianjin Key Laboratory of Lung Cancer Metastasis and Tumor Microenvironment, Lung Cancer Institute, Tianjin Medical University General Hospital, Tianjin, China
- 3Department of Clinical Trials Center, National Cancer Center, Cancer Hospital Chinese Academy of Medical Sciences, Beijing, China
- 4Department of Thoracic Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Introduction: Due to the introduction of low-dose computed tomography (CT) and screening procedures, the proportion of early-stage lung cancer with ground glass opacity (GGO) manifestation is increasing in clinical practice. However, its epidemiological characteristics is still not fully investigated.
Methods: We retrieved all solitary GGO adenocarcinoma lung cancer (ADLC) on the PubMed, Cochrane Library, and Embase databases until January 1, 2019 and extracted the general information to perform the meta-analysis, mainly focusing on age, gender, and smoking status.
Results: A total of 8,793 solitary GGO ADLC patients from 53 studies were included in this analysis. The final pooled analysis showed that the female proportion, average diagnosis age, and non-smoking proportion of solitary GGO ADLC was 0.62 (95% CI, 0.60–0.64), 56.97 (95% CI, 54.56–59.37), and 0.72 (95% CI, 0.66–0.77), respectively. The cumulative meta-analysis and meta-trend analysis confirmed that the average age at diagnosis has been decreasing while the non-smoking proportion significantly increased in the past two decades.
Conclusions: From our epidemiological analysis, it demonstrates that the clinical characteristics of GGO lung cancer patients may be out of the high-risk factors. Therefore, we propose to reconsider the risk assessment and current lung cancer screening criteria.
Introduction
Due to the introduction of low-dose computed tomography (CT) and screening procedures, the number of diagnoses of pulmonary ground glass opacity (GGO) lung cancer in clinical practice is increasing (1, 2). The GGO manifestation is generally caused by local airspace filling as a result of inflammation or neoplastic proliferation, and some studies reported that the malignancy rate of GGO was 63%, which has a higher malignant potential than solid nodules (3, 4). The GGO manifestation generally correlates with a lepidic, in situ, non-invasive growth pattern of cells along preexisting alveolar structures (4). A previous study has reported that GGO lung cancer may have several unique features, including an insignificant association with smoking history and a low degree of invasive biological characteristics (3). As the importance of GGO lung cancer is increasing, more researches have focused on the diagnosis and treatment of this early stage lung cancer; however, the epidemiology of lung cancer with GGO manifestation has not yet been fully elucidated. In this study, we summarized all of the publications concerning solitary GGO adenocarcinoma lung cancer (ADLC) and investigated the epidemiological data of this unique type of lung cancer by the use of a cumulative meta-analysis. The primary outcome is female proportion, and the secondary outcomes are average diagnosis age and non-smoking proportion. All analyses of our study were specified a priori in the protocol, and our study was registered and the protocol made available on the PROSPERO (the registration number CRD42019119240).
Methods
This study was reported on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Supplementary Table 1).
Two individual researchers conducted the platform searches on the PubMed, Cochrane Library, and Embase databases. Literature retrieving was carried out through a combined searching of subject terms (“MeSH” on PubMed and “Emtree” on “Embase”) and free terms on the platforms of PubMed and Embase, and through keywords searching on platform of Cochrane Library. Detailed searching criteria used in the three electronic platforms are available in Appendix 1.
All available studies that had been published in English until January 1, 2019 on patients with solitary GGO ADLC were included, and the inclusion and exclusion criteria were listed. The inclusion criteria of study were (1) GGO manifestation and (2) finally pathologically confirmed ADLC. The exclusion criteria were the following: (1) studies with a design of literature review, systematic review, basic research, letter to editors, diagnostic study, and so on; (2) studies that include the following cases and cannot be ruled out—multiple GGO, benign GGO, or pure solid nodules; (3) studies that did not involve basic information of patients; and (4) studies using repeated patients cohorts with any other study. There were no limitations on the participants' nationalities.
The Newcastle–Ottawa quality assessment scale (NOS) and National Institute for Clinical Excellence (NICE) quality assessment scale were performed to assess methodological quality and risk of bias for cohort studies and case series studies, respectively. We extracted the general characteristics of GGO patients (amount, age, gender, and smoking status) to perform the meta-analysis. For the proportions of GGO adenocarcinoma of the female gender and the smoking histories, the single rate was determined, and the single mean value was used for the calculation of the average diagnosis ages of the patients. Meta-analysis was performed on all the data using fixed or random effect through heterogeneity, which was tested by estimating value of I2 (significance level at I2 > 50%) or using the Cochrane Q-test (significance level at P < 0.100). The cumulative meta-analysis was also performed, and the trend test was performed to confirm the trend of cumulative meta-analysis, as sorted by years. The methods of Begg's and Egger's regression asymmetry test were performed to test publication bias, and P < 0.050 and P < 0.100 were considered to be statistically significant publication bias for Begg's and Egger's, respectively, (5). If the P-value indicates the existence of publication bias, the non-parametric trim and fill method would be performed to revise the result of meta-analysis (6). Sensitivity analysis was performed by omitting each individual study to check the stability of the result, and studies causing instability would be removed from the meta-analysis. The whole process of data analyses was performed by the software Stata version 13.0 (Stata Corp LLC, College Station, TX, USA).
Results
The process of eligible literature selection is presented in Figure 1, and a total of 8,793 solitary GGO ADLC patients from 53 studies until 2019 were recruited in the meta-analysis, mainly focusing on age, gender, and smoking status (7–59). No article was excluded by methodological quality and risk of bias and sensitivity analysis for significant heterogeneity (Supplementary Figures 1–3). The summary of individual study is listed in Table 1. All the meta-analyses were performed with a random-effect model (I2 > 50%).
For the female proportion of GGO ADLC, all 8,793 patients were included in the meta-analysis, and the results demonstrated that the female proportion was 0.62 (95% CI, 0.60–0.64), and the P-value of Begg's and Egger's test is > 0.1, indicating that there was no existence of publication bias (Figure 2). For average diagnosis age group, 24 articles involving 5,785 GGO ADLC patients were included for the meta-analysis of age (Figure 3A). The P-value of Egger's test was 0.015, which indicated the presence of publication bias, and the non-parametric trim-and-fill method was performed to adjust the effect value (5). Eleven studies were filled to rectify bias, and the final pooled average diagnosis age was 56.97 (95% CI, 54.56–59.37) (Figure 3C). A total of 4,330 GGO ADLC patients from 22 articles were assessed in the meta-analysis for smoking status (Figure 3B). The P-value of Egger's test was 0.003, and the non-parametric trim-and-fill method was performed. No studies were estimated to rectify the bias, and the final pooled non-smoking proportion of solitary GGO ADLC was 0.72 (95% CI, 0.66–0.77) (Figure 3D).
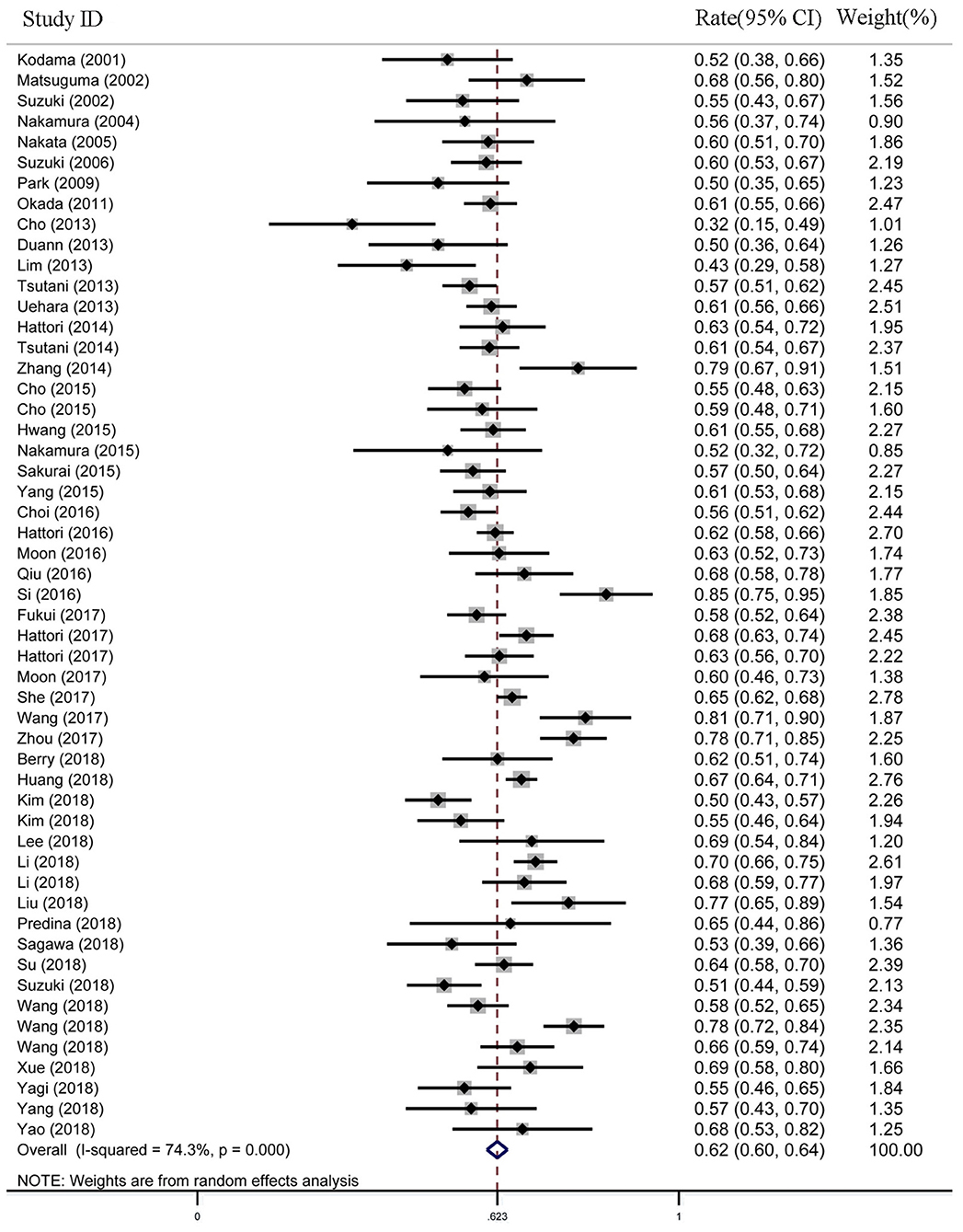
Figure 2. The meta-analysis forest map of the female rate of solitary ground glass opacity (GGO) adenocarcinoma lung cancer (ADLC).
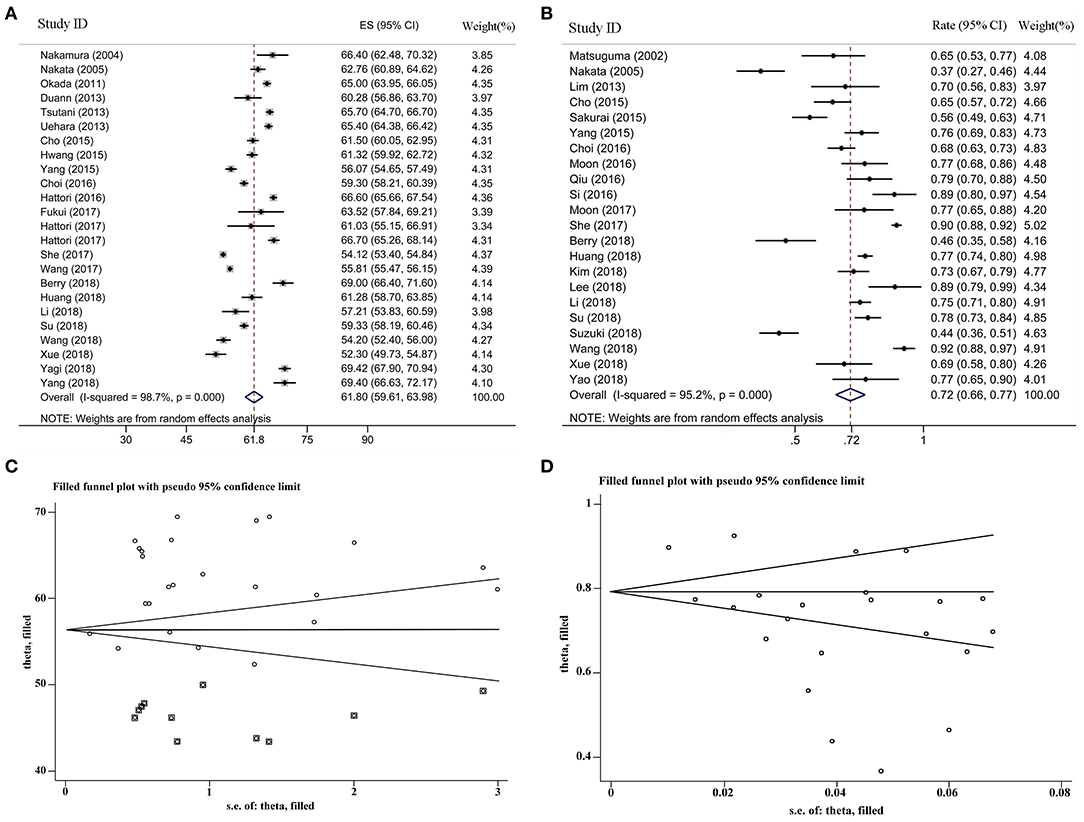
Figure 3. The meta-analysis forest maps, funnel plot with non-parametric trim-and-fill method for solitary ground glass opacity (GGO) adenocarcinoma lung cancer (ADLC). (A) The meta-analysis forest map of the average diagnosis age; (B) the meta-analysis forest map of the non-smoking rate; (C) the funnel plot of average age group with non-parametric trim and fill method; (D) the funnel plot of non-smoking rate group with non-parametric trim and fill method.
The cumulative meta-analysis of age group demonstrated that the average age had decreased from 66.40 to 59.06 years (95% CI, 58.84–59.28) (Figure 4A), and the meta-trend analysis confirmed that the decrease in age was statistically significant (P < 0.001) (Figure 4C). The cumulative meta-analysis of non-smoking group indicated that the non-smoking proportion in GGO patients has increased in the past two decades (Figure 4B), which was statistically significant in the meta-trend analysis (P < 0.001) (Figure 4D).
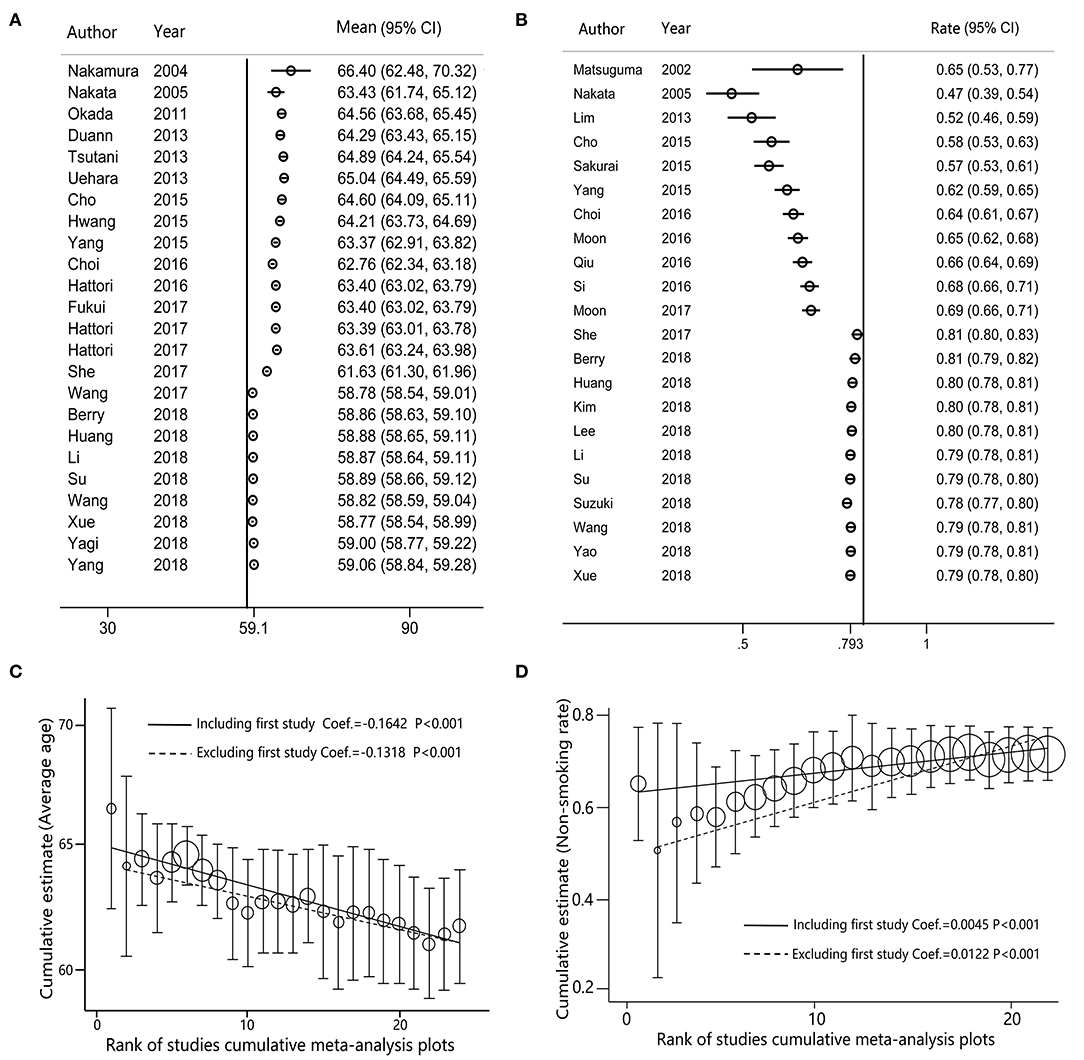
Figure 4. The cumulative meta-analysis forest maps and meta-trend analysis for solitary ground glass opacity (GGO) adenocarcinoma lung cancer (ADLC), as sorted by years. (A) The cumulative meta-analysis forest map of the female rate; (B) the cumulative meta-analysis forest map of the non-smoking rate; (C) the trend analysis for the cumulative meta-analysis of the female rate; (D) the trend analysis for the cumulative meta-analysis of the non-smoking rate.
Discussion
GGO-predominant lung cancers are typically characterized as non-invasively or minimally invasively low-grade adenocarcinomas and had good prognosis after surgical intervention (60). Early detection and therapeutic intervention for these early stage lung cancers is an important opportunity for decreasing overall mortality of lung cancer. Some lung cancer screening criteria have been proposed, which always consider heavy smoking history as a key factor for risk assessment (61, 62). The US Preventive Services Task Force (USPSTF) recommends lung cancer screening among individuals aged 55–80 years with a 30 pack-year cigarette smoking history (61). In addition, the latest Lung Cancer Screening from National Comprehensive Cancer Network (NCCN) Guidelines determines age <50 years and smoking history lower than 20 pack-year as low risk, in which lung cancer screening is not recommended (62). Our meta-analysis indicates that the pooled non-smoking proportion is 0.72. The majority of GGO lung cancer patients are female, and the average age at diagnosis has been significantly decreasing in the past two decades. Our data demonstrate that the clinical characteristics of GGO lung cancer patients may be out of the high-risk factors who are inappropriate for the lung cancer screening. Zhang et al. performed LDCT for 8,329 hospital employees from different regions, and 179 cases were pathologically confirmed lung cancer and 98.9% (171) cases presented with GGO (63). In Zhang's study, there was a higher lung cancer detection rate in female than male patients (2.5 vs. 1.3%), and the lung cancer detection rate of non-smokers was also high than smokers (2.2 vs. 1.4%). In subset analysis by age, the lung cancer detection rates were 1.0, 2.6, and 2.9% in the “age ≤ 40 years,” “40 < age ≤ 55 years,” and “age > 55 years” group, respectively (63). According to this substantial data, Zhang proposed that the “high-risk” population for lung cancer is changing, and more lung cancers from the traditionally “low-risk” groups, such as young female non-smokers, could be detected by LDCT (63). These finding are completely consistent with our study. More and more female younger non-smokers were diagnosed with lung cancer; however, the exact reasons of this phenomenon are still uncertain. Most researchers thought that the phenomenon may be caused by life pressure, living habits, and hormone levels; however, it needs to be further investigated. Luo et al. conducted a cohort study that demonstrated that younger and light smoker patients with lung cancer who are not recommended for screening have similar lung cancer survival to those lung cancer patients who meet all the USPSTF screening criteria (64). This study supports our findings that the individuals with low-risk factors should be concerned as well, and the criteria of current lung cancer screening might not be perfect. However, the cost effectiveness needs to be evaluated if more low risk individuals are included in low-dose computed tomography (CT) screening (65). A limitation of this study is that all of the included studies were retrospective studies that have a lower level of evidence compared to prospective studies.
Conclusions
Our study demonstrated that the majority of GGO ADLC patients are female with non-or light smoking history, and the average age at diagnosis has been significantly decreasing. This indicates that there are more lung cancers being detected from the traditionally “low-risk” groups, such as young female non-smokers. It is well-accepted that early detection of lung cancers is the most important procedure that contributes to improved survival outcomes and reduced lung cancer mortality. Therefore, we propose that, in order to identify these very early stage GGO lung cancer patients with low-risk factors, it is necessary to reconsider the risk assessment and current lung cancer screening criteria.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
XL, FR, and SW retrieved and analyzed all of the data in the study. ZH and ZS revised the manuscript for important intellectual contents. SX and JC designed, checked, and supervised the study process. All authors contributed to the article and approved the submitted version.
Funding
The present study was funded by the National Natural Science Foundation of China (Grant No. 81772464), the Tianjin Key Project of Natural Science Foundation (Grant No. 17JCZDJC36200), and Tianjin Science and Technology Plan Project (19ZXDBSY00060).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.01059/full#supplementary-material
Supplementary Figure 1. Sensitivity analysis for female rate of solitary GGO ADLC.
Supplementary Figure 2. Sensitivity analysis for average year of solitary GGO ADLC.
Supplementary Figure 3. Sensitivity analysis for non-smoking rate of solitary GGO ADLC.
Supplementary Table 1. The PRISMA checklist.
Appendix 1. Searching strategies performed for eligible study retrieval.
References
1. Pedersen JH, Saghir Z, Wille MM, Thomsen LH, Skov BG, Ashraf H. Ground-glass opacity lung nodules in the era of lung cancer CT screening: radiology, pathology, and clinical management. Oncology. (2016) 30:266–74.
2. Infante M, Lutman RF, Imparato S, Di Rocco M, Ceresoli GL, Torri V, et al. Differential diagnosis and management of focal ground-glass opacities. Eur Respir J. (2009) 33:821–7. doi: 10.1183/09031936.00047908
3. Migliore M, Fornito M, Palazzolo M, Criscione A, Gangemi M, Borrata F, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med. (2018) 6:90. doi: 10.21037/atm.2017.07.28
4. Park CM, Goo JM, Lee HJ, Lee CH, Chun EJ, Im JG. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics. (2007) 27:391–408. doi: 10.1148/rg.272065061
5. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
6. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
7. Yao F, Wang J, Yao J, Xu L, Qian J, Cao Y. Early experience with video-assisted thoracoscopic anatomic segmentectomy. J Laparoendosc Adv Surg Tech Part A. (2018) 28:819–26. doi: 10.1089/lap.2017.0680
8. Yang X, Ye X, Lin Z, Jin Y, Zhang K, Dong Y, et al. Computed tomography-guided percutaneous microwave ablation for treatment of peripheral ground-glass opacity-lung adenocarcinoma: a pilot study. J Cancer Res Ther. (2018) 14:764–71. doi: 10.4103/jcrt.JCRT_269_18
9. Yagi T, Yamazaki M, Ohashi R, Ogawa R, Ishikawa H, Yoshimura N, et al. HRCT texture analysis for pure or part-solid ground-glass nodules: distinguishability of adenocarcinoma in situ or minimally invasive adenocarcinoma from invasive adenocarcinoma. Jpn J Radiol. (2018) 36:113–21. doi: 10.1007/s11604-017-0711-2
10. Xue L, Fan H, Shi W, Ge D, Zhang Y, Wang Q, et al. Preoperative 3-dimensional computed tomography lung simulation before video-assisted thoracoscopic anatomic segmentectomy for ground glass opacity in lung. J Thorac Dis. (2018) 10:6598–605. doi: 10.21037/jtd.2018.10.126
11. Wang XW, Chen WF, He WJ, Yang ZM, Li M, Xiao L, et al. CT features differentiating pre- and minimally invasive from invasive adenocarcinoma appearing as mixed ground-glass nodules: mass is a potential imaging biomarker. Clin Radiol. (2018) 73:549–54. doi: 10.1016/j.crad.2018.01.017
12. Wang L, Shen W, Xi Y, Liu S, Zheng D, Jin C. Nomogram for predicting the risk of invasive pulmonary adenocarcinoma for pure ground-glass nodules. Ann Thorac Surg. (2018) 105:1058–64. doi: 10.1016/j.athoracsur.2017.11.012
13. Wang H, Yu W, Pan HY, Luo QQ, Le HB, Chen ZJ. Differentiating preinvasive from invasive lung adenocarcinoma appearing as part-solid ground-glass nodule using CT value and solid-part diameter. Iran J Radiol. (2018) 15:e61846. doi: 10.5812/iranjradiol.61846
14. Suzuki S, Sakurai H, Yotsukura M, Masai K, Asakura K, Nakagawa K, et al. Clinical features of ground glass opacity–dominant lung cancer exceeding 3.0 cm in the whole tumor size. Ann Thorac Surg. (2018) 105:1499–506. doi: 10.1016/j.athoracsur.2018.01.019
15. Su H, Dai C, Xie H, Ren Y, She Y, Kadeer X, et al. Risk factors of recurrence in patients with clinical stage IA adenocarcinoma presented as ground-glass nodule. Clin Lung Cancer. (2018) 19:e609–17. doi: 10.1016/j.cllc.2018.04.020
16. Sagawa M, Oizumi H, Suzuki H, Uramoto H, Usuda K, Sakurada A, et al. A prospective 5-year follow-up study after limited resection for lung cancer with ground-glass opacity. Eur J Cardiothorac Surg. (2018) 53:849–56. doi: 10.1093/ejcts/ezx418
17. Predina JD, Newton A, Corbett C, Xia L, Frenzel Sulyok L, Shin M, et al. A brief report: localization of pulmonary ground-glass opacities with folate receptor-targeted intraoperative molecular imaging. J Thorac Oncol. (2018) 13:1028–36. doi: 10.1016/j.jtho.2018.03.023
18. Liu G, Li M, Li G, Li Z, Liu A, Pu R, et al. Assessing the blood supply status of the focal ground-glass opacity in lungs using spectral computed tomography. Korean J Radiol. (2018) 19:130–8. doi: 10.3348/kjr.2018.19.1.130
19. Li W, Wang X, Zhang Y, Li X, Li Q, Ye Z. Radiomic analysis of pulmonary ground-glass opacity nodules for distinction of preinvasive lesions, invasive pulmonary adenocarcinoma and minimally invasive adenocarcinoma based on quantitative texture analysis of CT. Chin J Cancer Res. (2018) 30:415–27. doi: 10.21147/j.issn.1000-9604.2018.04.04
20. Li M, Wang Y, Chen Y, Zhang Z. Identification of preoperative prediction factors of tumor subtypes for patients with solitary ground-glass opacity pulmonary nodules. J Cardiothorac Surg. (2018) 13:9. doi: 10.1186/s13019-018-0696-7
21. Lee GD, Park CH, Park HS, Byun MK, Lee IJ, Kim TH, et al. Lung adenocarcinoma invasiveness risk in pure ground-glass opacity lung nodules smaller than 2 cm. Thorac Cardiovasc Surg. (2018) 67:321–8. doi: 10.1055/s-0037-1612615
22. Kim H, Goo JM. Evaluation of T categories for pure ground-glass nodules with semi-automatic volumetry: is mass a better predictor of invasive part size than other volumetric parameters? Eur Radiol. (2018) 28:4288–95. doi: 10.1007/s00330-018-5440-0
23. Kim D, Kim HK, Kim SH, Lee HY, Cho JH, Choi YS, et al. Prognostic significance of histologic classification and tumor disappearance rate by computed tomography in lung cancer. J Thorac Dis. (2018) 10:388–97. doi: 10.21037/jtd.2017.12.38
24. Huang TW, Lin KH, Huang HK, Chen YI, Ko KH, Chang CK, et al. The role of the ground-glass opacity ratio in resected lung adenocarcinoma. Eur J Cardio Thorac Surg. (2018) 54:229–34. doi: 10.1093/ejcts/ezy040
25. Berry MF, Gao R, Kunder CA, Backhus L, Khuong A, Kadoch M, et al. Presence of even a small ground-glass component in lung adenocarcinoma predicts better survival. Clin Lung Cancer. (2018) 19:e47–51. doi: 10.1016/j.cllc.2017.06.020
26. Zhou QJ, Zheng ZC, Zhu YQ, Lu PJ, Huang J, Ye JD, et al. Tumor invasiveness defined by IASLC/ATS/ERS classification of ground-glass nodules can be predicted by quantitative CT parameters. J Thorac Dis. (2017) 9:1190–200. doi: 10.21037/jtd.2017.03.170
27. Wang X, Wang L, Zhang W, Zhao H, Li F. Can we differentiate minimally invasive adenocarcinoma and non-invasive neoplasms based on high-resolution computed tomography features of pure ground glass nodules? PLoS ONE. (2017) 12:e0180502. doi: 10.1371/journal.pone.0180502
28. She Y, Zhao L, Dai C, Ren Y, Zha J, Xie H, et al. Preoperative nomogram for identifying invasive pulmonary adenocarcinoma in patients with pure ground-glass nodule: a multi-institutional study. Oncotarget. (2017) 8:17229–38. doi: 10.18632/oncotarget.11236
29. Moon Y, Lee KY, Moon SW, Park JK. Sublobar resection margin width does not affect recurrence of clinical n0 non-small cell lung cancer presenting as GGO-predominant nodule of 3 cm or less. World J Surg. (2017) 41:472–9. doi: 10.1007/s00268-016-3743-3
30. Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Importance of ground glass opacity component in clinical stage ia radiologic invasive lung cancer. Ann Thorac Surg. (2017) 104:313–20. doi: 10.1016/j.athoracsur.2017.01.076
31. Hattori A, Matsunaga T, Hayashi T, Takamochi K, Oh S, Suzuki K. Prognostic impact of the findings on thin-section computed tomography in patients with subcentimeter non-small cell lung cancer. J Thorac Oncol. (2017) 12:954–62. doi: 10.1016/j.jtho.2017.02.015
32. Fukui M, Suzuki K, Matsunaga T, Oh S, Takamochi K. Surgical intervention for ground glass dominant lesions: observation or outright resection? Jpn J Clin Oncol. (2017) 47:749–54. doi: 10.1093/jjco/hyx053
33. Si MJ, Tao XF, Du GY, Cai LL, Han HX, Liang XZ, et al. Thin-section computed tomography–histopathologic comparisons of pulmonary focal interstitial fibrosis, atypical adenomatous hyperplasia, adenocarcinoma in situ, and minimally invasive adenocarcinoma with pure ground-glass opacity. Eur J Radiol. (2016) 85:1708–15. doi: 10.1016/j.ejrad.2016.07.012
34. Qiu ZX, Cheng Y, Liu D, Wang WY, Wu X, Wu WL, et al. Clinical, pathological, and radiological characteristics of solitary ground-glass opacity lung nodules on high-resolution computed tomography. Ther Clin Risk Manage. (2016) 12:1445–53. doi: 10.2147/TCRM.S110363
35. Moon Y, Sung SW, Lee KY, Sim SB, Park JK. Pure ground-glass opacity on chest computed tomography: predictive factors for invasive adenocarcinoma. J Thorac Dis. (2016) 8:1561–70. doi: 10.21037/jtd.2016.06.34
36. Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Neither maximum tumor size nor solid component size is prognostic in part-solid lung cancer: impact of tumor size should be applied exclusively to solid lung cancer. Ann Thorac Surg. (2016) 102:407–15. doi: 10.1016/j.athoracsur.2016.02.074
37. Choi SH, Chae EJ, Shin SY, Kim EY, Kim JE, Lee HJ, et al. Comparisons of clinical outcomes in patients with and without a preoperative tissue diagnosis in the persistent malignant-looking, ground-glass-opacity nodules. Medicine. (2016) 95:e4359. doi: 10.1097/MD.0000000000004359
38. Yang Y, Yang Y, Zhou X, Song X, Liu M, He W, et al. EGFR L858R mutation is associated with lung adenocarcinoma patients with dominant ground-glass opacity. Lung Cancer. (2015) 87:272–7. doi: 10.1016/j.lungcan.2014.12.016
39. Sakurai H, Nakagawa K, Watanabe SI, Asamura H. Clinicopathologic features of resected subcentimeter lung cancer. Ann Thorac Surg. (2015) 99:1731–8. doi: 10.1016/j.athoracsur.2015.01.034
40. Nakamura S, Fukui T, Kawaguchi K, Fukumoto K, Hirakawa A, Yokoi K. Does ground glass opacity-dominant feature have a prognostic significance even in clinical T2aN0M0 lung adenocarcinoma? Lung Cancer. (2015) 89:38–42. doi: 10.1016/j.lungcan.2015.04.011
41. Hwang EJ, Park CM, Ryu Y, Lee SM, Kim YT, Kim YW, et al. Pulmonary adenocarcinomas appearing as part-solid ground-glass nodules: is measuring solid component size a better prognostic indicator? Eur Radiol. (2015) 25:558–67. doi: 10.1007/s00330-014-3441-1
42. Cho JH, Choi YS, Kim J, Kim HK, Zo JI, Shim YM. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg. (2015) 99:218–22. doi: 10.1016/j.athoracsur.2014.07.068
43. Cho H, Lee HY, Kim J, Kim HK, Choi JY, Um SW, et al. Pure ground glass nodular adenocarcinomas: are preoperative positron emission tomography/computed tomography and brain magnetic resonance imaging useful or necessary? J Thorac Cardiovasc Surg. (2015) 150:514–20. doi: 10.1016/j.jtcvs.2015.06.024
44. Zhang H, Duan J, Li ZJ, He ZF, Chen ZM, Xu Y, et al. Analysis on minimally invasive diagnosis and treatment of 49 cases with solitary nodular ground-glass opacity. J Thorac Dis. (2014) 6:1452–7. doi: 10.3978/j.issn.2072-1439.2014.10.09
45. Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, et al. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest. (2014) 145:66–71. doi: 10.1378/chest.13-1094
46. Hattori A, Suzuki K, Matsunaga T, Fukui M, Tsushima Y, Takamochi K, et al. Tumour standardized uptake value on positron emission tomography is a novel predictor of adenocarcinoma in situ for c-Stage IA lung cancer patients with a part-solid nodule on thin-section computed tomography scan. Interactive Cardiovasc Thorac Surg. (2014) 18:329–34. doi: 10.1093/icvts/ivt500
47. Uehara H, Tsutani Y, Okumura S, Nakayama H, Adachi S, Yoshimura M, et al. Prognostic role of positron emission tomography and high-resolution computed tomography in clinical stage IA lung adenocarcinoma. Ann Thorac Surg. (2013) 96:1958–65. doi: 10.1016/j.athoracsur.2013.06.086
48. Tsutani Y, Miyata Y, Yamanaka T, Nakayama H, Okumura S, Adachi S, et al. Solid tumors versus mixed tumors with a ground-glass opacity component in patients with clinical stage IA lung adenocarcinoma: prognostic comparison using high-resolution computed tomography findings. J Thorac Cardiovasc Surg. (2013) 146:17–23. doi: 10.1016/j.jtcvs.2012.11.019
49. Lim HJ, Ahn S, Lee KS, Han J, Shim YM, Woo S, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest. (2013) 144:1291–9. doi: 10.1378/chest.12-2987
50. Duann CW, Hung JJ, Hsu PK, Huang CS, Hsieh CC, Hsu HS, et al. Surgical outcomes in lung cancer presenting as ground-glass opacities of 3cm or less: a review of 5 years' experience. J Chin Med Assoc. (2013) 76:693–7. doi: 10.1016/j.jcma.2013.08.005
51. Cho S, Yang H, Kim K, Jheon S. Pathology and prognosis of persistent stable pure ground-glass opacity nodules after surgical resection. Ann Thorac Surg. (2013) 96:1190–5. doi: 10.1016/j.athoracsur.2013.05.062
52. Okada M, Nakayama H, Okumura S, Daisaki H, Adachi S, Yoshimura M, et al. Multicenter analysis of high-resolution computed tomography and positron emission tomography/computed tomography findings to choose therapeutic strategies for clinical stage IA lung adenocarcinoma. J Thorac Cardiovasc Surg. (2011) 141:1384–91. doi: 10.1016/j.jtcvs.2011.02.007
53. Park JH, Lee KS, Kim JH, Shim YM, Kim J, Choi YS, et al. Malignant pure pulmonary ground-glass opacity nodules: prognostic implications. Korean J Radiol. (2009) 10:12–20. doi: 10.3348/kjr.2009.10.1.12
54. Suzuki K, Kusumoto M, Watanabe SI, Tsuchiya R, Asamura H. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg. (2006) 81:413–9. doi: 10.1016/j.athoracsur.2005.07.058
55. Nakata M, Sawada S, Yamashita M, Saeki H, Kurita A, Takashima S, et al. Objective radiologic analysis of ground-glass opacity aimed at curative limited resection for small peripheral non-small cell lung cancer. J Thorac Cardiovasc Surg. (2005) 129:1226–31. doi: 10.1016/j.jtcvs.2004.10.032
56. Nakamura H, Saji H, Ogata A, Saijo T, Okada S, Kato H. Lung cancer patients showing pure ground-glass opacity on computed tomography are good candidates for wedge resection. Lung Cancer. (2004) 44:61–8. doi: 10.1016/j.lungcan.2003.09.025
57. Suzuki K, Asamura H, Kusumoto M, Kondo H, Tsuchiya R. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg. (2002) 74:1635–9. doi: 10.1016/S0003-4975(02)03895-X
58. Matsuguma H, Yokoi K, Anraku M, Kondo T, Kamiyama Y, Mori K, et al. Proportion of ground-glass opacity on high-resolution computed tomography in clinical T1 N0 M0 adenocarcinoma of the lung: a predictor of lymph node metastasis. J Thorac Cardiovasc Surg. (2002) 124:278–84. doi: 10.1067/mtc.2002.122298
59. Kodama K, Higashiyama M, Yokouchi H, Takami K, Kuriyama K, Mano M, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer. (2001) 33:17–25. doi: 10.1016/S0169-5002(01)00185-4
60. Detterbeck FC, Marom EM, Arenberg DA, Franklin WA, Nicholson AG, Travis WD, et al. The IASLC lung cancer staging project: background data and proposals for the application of TNM staging rules to lung cancer presenting as multiple nodules with ground glass or lepidic features or a pneumonic type of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol. (2016) 11:666–80. doi: 10.1016/j.jtho.2015.12.113
61. Moyer VA. Screening for lung cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. (2014) 160:330–8. doi: 10.7326/M13-2771
62. National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Lung Cancer Screening, Version 4. (2019) Available Online at: https://www.nccn.org/professionals/physician_gls/default.aspx#lung_screening (accessed April 29, 2019).
63. Zhang Y, Jheon S, Li H, Zhang H, Xie Y, Qian B, et al. Results of low-dose computed tomography as a regular health examination among Chinese hospital employees. J Thorac Cardiovasc Surg. (2019). doi: 10.1016/j.jtcvs.2019.10.145. [Epub ahead of print].
64. Luo YH, Luo L, Wampfler JA, Wang Y, Liu D, Chen YM, et al. 5-year overall survival in patients with lung cancer eligible or ineligible for screening according to US preventive services task force criteria: a prospective, observational cohort study. Lancet Oncol. (2019) 20:1098–108. doi: 10.1016/S1470-2045(19)30329-8
65. Ten Haaf K, Tammemagi MC, Bondy SJ, van der Aalst CM, Gu S, McGregor SE. Performance and cost-effectiveness of computed tomography lung cancer screening scenarios in a population-based setting: a microsimulation modeling analysis in Ontario, Canada. PLoS Med. (2017) 14:e1002225. doi: 10.1371/journal.pmed.1002225
Keywords: ground glass opacity, lung adenocarcinoma, cumulative meta-analysis, epidemiological trends, lung cancer screening criteria
Citation: Li X, Ren F, Wang S, He Z, Song Z, Chen J and Xu S (2020) The Epidemiology of Ground Glass Opacity Lung Adenocarcinoma: A Network-Based Cumulative Meta-Analysis. Front. Oncol. 10:1059. doi: 10.3389/fonc.2020.01059
Received: 06 March 2020; Accepted: 27 May 2020;
Published: 21 July 2020.
Edited by:
Jill Barnholtz-Sloan, Case Western Reserve University, United StatesReviewed by:
Alejandra Castanon, King's College London, United KingdomXiaojie Tan, Second Military Medical University, China
Copyright © 2020 Li, Ren, Wang, He, Song, Chen and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Chen, aHVudGVyY2oyMDA0QHlhaG9vLmNvbQ==; Song Xu, eHVzb25nMTk4QGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Xiongfei Li
Xiongfei Li Fan Ren
Fan Ren Shuhang Wang
Shuhang Wang Zhicheng He
Zhicheng He Zuoqing Song
Zuoqing Song Jun Chen
Jun Chen Song Xu
Song Xu