- 1Division of Hematology-Oncology, Department of Internal Medicine, Chang Gung Memorial Hospital at Linkou, Chang Gung University College of Medicine, Taoyuan, Taiwan
- 2Immuno-Oncology Center of Excellence, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
- 3Department of Nursing, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
- 4Department of Pharmacy, Chang Gung Memorial Hospital at Linkou, Chang Gung University College of Medicine, Taoyuan, Taiwan
- 5Department of Dermatology, Chang Gung Memorial Hospital at Linkou, Chang Gung University College of Medicine, Taoyuan, Taiwan
- 6Department of Medical Imaging and Intervention, Chang Gung Memorial Hospital at Linkou, Chang Gung University College of Medicine, Taoyuan, Taiwan
Background: Immune checkpoint inhibitors (ICIs) have significantly changed the current approach to cancer treatment. Although the use of ICIs has become the standard of care for advanced melanoma, reports of ICI use among Asian populations with melanoma are limited. Therefore, we conducted this retrospective study to assess the efficacy and safety of ICI use in Taiwanese patients.
Patients: Patients with histologically confirmed melanoma treated with ICIs at Linkou Chang Gung Memorial Hospital from January 2014 to July 2019 were retrospectively reviewed. Univariant and multivariant analyses were performed to identify possible prognostic factors.
Results: Among 80 patients, 45 were treatment-naïve (56.3%), and 35 received prior systemic drugs other than ICIs. Regarding treatment regimens, patients were treated with ipilimumab (n = 9), nivolumab (n = 33), pembrolizumab (n = 16), or combination drugs (n = 22). Nine patients achieved either a complete (n = 2) or partial (n = 7) response and 13 patients were stable, with a resulting response rate of 11.3% and disease control rate of 27.5%. As of the last follow-up in January 2020, patients treated with combination drugs had longer median progression-free survival (PFS) of 5.6 (95% confidence interval [CI]: 1.6–9.6) months than nivolumab (2.9 months, 95% CI: 1.9–3.9 months), pembrolizumab (3.2 months, 95% CI: 2.6–3.8 months), and ipilimumab (2.6 months, 95% CI: 2.4–2.8 months; p = 0.011). No significant differences in overall survival (OS) among the four regimens (p = 0.891) were noted. In the multivariate analysis, combination treatment, disease control, and performance ≤ 1 were independent prognostic factors for PFS. Liver metastases and no disease control were independent unfavorable prognostic factors for OS. The most common factor was skin toxicity (45%), followed by endocrine toxicity (18.8%). Patients undergoing combination treatment experienced more frequent and serious adverse events than patients undergoing monotherapy.
Conclusion: ICIs demonstrated efficacy and safety in Taiwanese patients with melanoma. Combination treatment showed the greatest efficacy, but this was also accompanied by greater toxicity among the four regimens. In addition, we identified important prognostic factors, such as liver metastases, performance status, and tumor response, for both PFS and OS. These findings could provide physicians with more information to justify clinical outcomes observed in Asian patients with advanced melanoma.
Introduction
Immune checkpoint inhibitors (ICIs) aim to target the interaction between cancer and immune cells, thus enhancing immunity against tumors. Currently, the availability of ICIs, such as anti-cytotoxic T lymphocyte antigen 4 (CTLA-4) and anti-programmed cell death 1(PD-1)/programmed cell death-ligand 1 monoclonal antibodies, has significantly changed the approach to cancer treatment. Ipilimumab, an anti-CTLA-4 monoclonal antibody, was the first approved ICI that showed efficacy in advanced/metastatic melanoma (1). Subsequently, nivolumab (2) and pembrolizumab (3), both of which are anti-PD-1 antibodies, were approved for advanced melanoma treatment. Combination therapy of ipilimumab and nivolumab has demonstrated better efficacy than either nivolumab or ipilimumab alone, particularly in BRAF-mutant melanoma, but with significantly greater adverse events (AEs) as evidenced in the CheckMate 067 trial (2, 4, 5). The CheckMate 511 study demonstrated a significantly lower incidence of treatment-related grade 3–5 AEs with N3I1 (3-mg/kg nivolumab plus 1-mg/kg ipilimumab) vs. N1I3 (1-mg/kg nivolumab plus 3-mg/kg ipilimumab) without compromising efficacy (6); therefore, N3I1 is widely used in clinical practice for patients undergoing combination treatment. However, the aforementioned studies were conducted in Western countries where cases of acral or mucosal melanoma are rare and thus account for a very small proportion of melanomas.
The spectrum of melanoma in Asians is distinct compared with that in Western population, as acral melanoma and mucosal melanoma account for the majority of melanoma cases (7–11). Tumor mutation burden (TMB) is possibly one of the best biomarkers to predict the response to ICIs (12), and has been validated in melanoma (13). However, TMB of acral and mucosal melanoma was not as high as TMB of cutaneous melanoma (14), so the question arises as to whether ICIs exhibit similar efficacy in Asian melanoma as in Western melanoma, particularly for acral and mucosal melanoma. Previous studies have shown conflicting results, with some reporting no differences with cutaneous melanoma (15, 16) and others showing contrary results (17–19).
To address this issue, we retrospectively reviewed patients with melanoma undergoing ICI treatment in a high-volume tertiary-care cancer center in Taiwan, and analyzed possible prognostic factors.
Materials and Methods
Patients
All patients with histologically confirmed melanoma treated at the Chang Gung Memorial Hospital (CGMH), Linkou, from 2014 to 2019 were retrospectively reviewed. A total of 80 ICI-naïve patients with advanced melanoma undergoing ICI treatment with either nivolumab, pembrolizumab, ipilimumab, or combination were included in the study.
Treatment Regimens and Response Evaluation
The treatment regimens consisted of ipilimumab (3 mg/kg every 3 weeks for maximum of four cycles), nivolumab (3 mg/kg every 2 weeks), pembrolizumab, (2 mg/kg every 3 weeks), or combination of ipilimumab and nivolumab/pembrolizumab until disease progression or intolerant toxicities. The dosing schedule of ICIs was adjusted at the physician's discretion according to the patient's clinical status and toxicity to ICIs. Tumor response was evaluated regularly by physical examination, chest X ray, computed tomography scan, or positron emission tomography scan.
Patient Characteristics and Evaluation of Outcomes
All patients with advanced melanoma treated from 2014 to 2019 were retrospectively reviewed, and ICI-naïve patients undergoing first-time treatment were included in the current study. Patients who received other systemic treatments prior to ICI therapy, such as chemotherapy, targeted therapy, or cytokine therapy, were also included. The last follow-up timepoint included in the study was January 31, 2020. Patient characteristics, including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, systemic treatment prior to ICIs, stage of melanoma, and tumor involvement of distant metastases, were recorded.
RECIST 1.1 criteria was used to evaluate the best tumor response as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Patients who experienced rapid deterioration or lacked radiological evaluation data before death were recorded as not assessed (N/A). Objective response rate (ORR) was the sum of CR and PR; disease control rate (DCR) was the sum of CR, PR, and SD. Progression-free survival (PFS) was defined as the length of time from the first day of ICI treatment until the first clinical or radiological evidence of disease progression, death, or latest follow-up timepoint. Overall survival (OS) was defined as the length of time from the first day of ICI treatment until the date of death or last follow-up.
Statistical Analysis
To assess the differences among the four regimens, Fisher–Freeman–Halton test of independence was used for categorical variables. Kruskal–Wallis test, a non-parametric (distribution-free) test, was used for continuous variables. Survival was estimated using the Kaplan–Meier method and was compared using the log-rank test. Univariate and multivariate analyses were performed to evaluate possible prognostic factors. Only significant prognostic factors from univariate analysis were further analyzed using multivariate analysis. IBM SPSS Statistics for Windows (Version 20.0, Armonk, NY, USA) was used for statistical analyses, where P < 0.05 was considered statistically significant. This study was approved by the Institutional Review Board of CGMH (202000182B0). Patient consent to participate was not required because of the retrospective nature of this study, which was approved by the Institutional Review Board of CGMH.
Results
Patient Characteristics
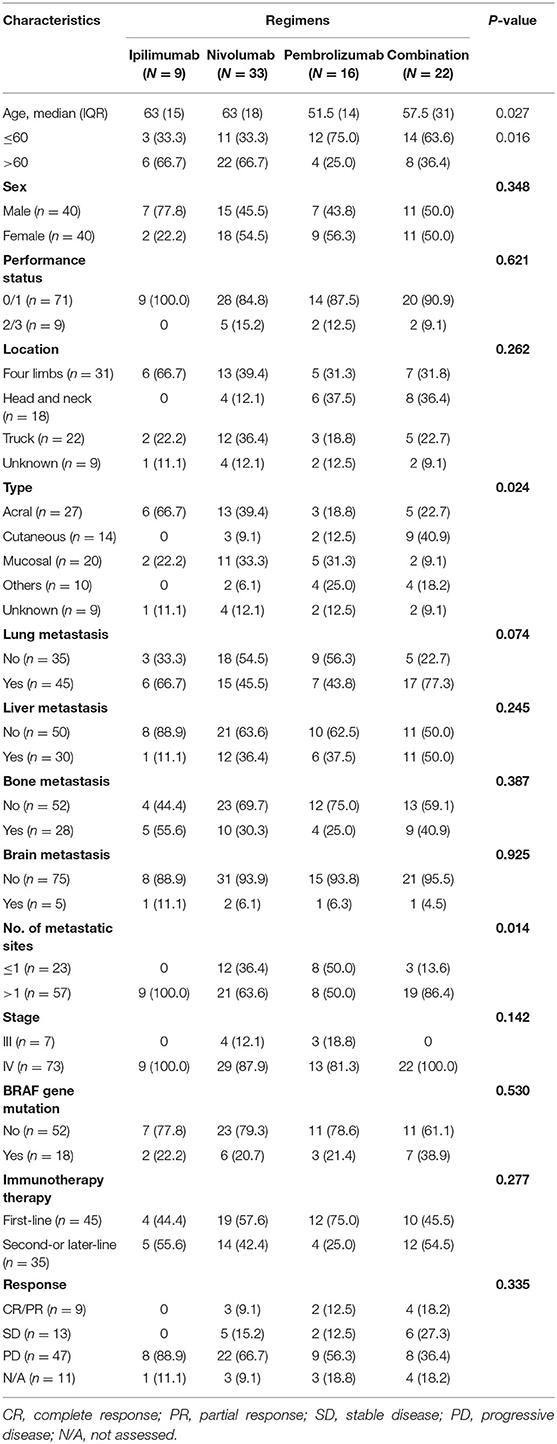
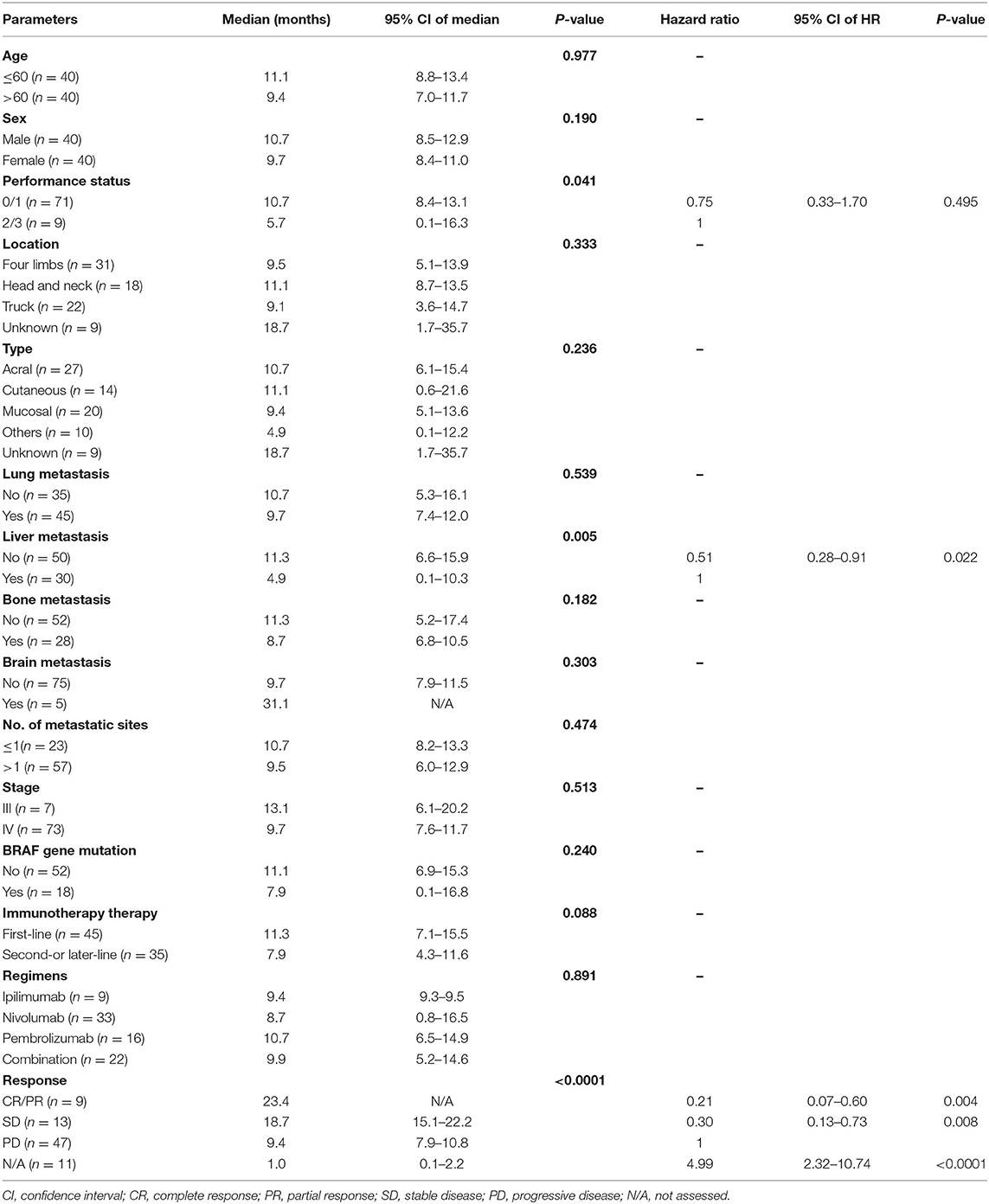
A total of 80 patients with advanced ICI-naïve melanoma undergoing ICIs were included in the study. In terms of treatment regimens, patients received ipilimumab (n = 9), nivolumab (n = 33), pembrolizumab (n = 16), or combination (n = 22). Among 22 patients undergoing combination treatment, 17 patients received ipilimumab plus nivolumab, and 5 patients received ipilimumab plus pembrolizumab. The median age was 59.6 years, with a range from 22.5 to 82.4 years. Forty patients (50%) were male and 40 patients (50%) were female. Most patients had an ECOG performance status ≤ 1 (n = 71, 88.8%). Twenty-seven patients had acral melanoma, 14 patients had cutaneous melanoma, 20 patients had mucosal melanoma, 10 patients had other types of melanoma (including eyes and soft tissue), and 9 patients had unknown primary melanoma. Most patients (n = 73, 91.3%) had been diagnosed as stage IV. Lung (n = 45) was the most common metastatic site, followed by liver (n = 30), bone (n = 28), and brain (n = 5). Eighteen of 70 patients (25.7%) had a BRAF mutation, and mutation status was unknown in 10 patients.
Except for age, tumor type, and number of metastatic sites, no significant differences of clinical characteristics among different ICI treatment groups were identified. The clinical features and tumor involvement with different regimens are summarized in Table 1.
Prior Treatments Before ICIs
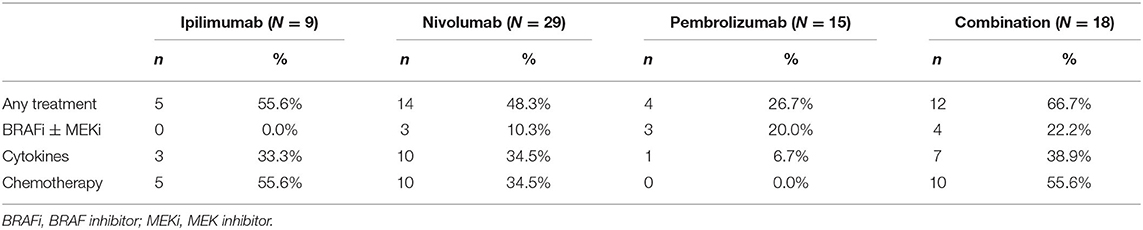
Forty-five (56.3%) patients were treated with ICIs as a first-line systemic treatment, and 35 (43.7%) patients were treated with these as second- or late-line treatment. The details of prior treatments are summarized in Table 2.
Efficacy of ICIs With Different Regimens
Among the patients with an evaluable response, 2 patients achieved CR, 7 achieved PR, 13 achieved SD, and 47 had PD as their best response. Eleven patients had no response evaluation data, and most experienced rapid progression without radiological confirmation. The ORR and DCR were 11.3 and 27.5% in the entire cohort and 12.7 and 31.0% in evaluable patients, respectively. Patients treated with combination drugs had a numerically but non-significantly higher ORR and DCR (Table 1, Figure 1A).
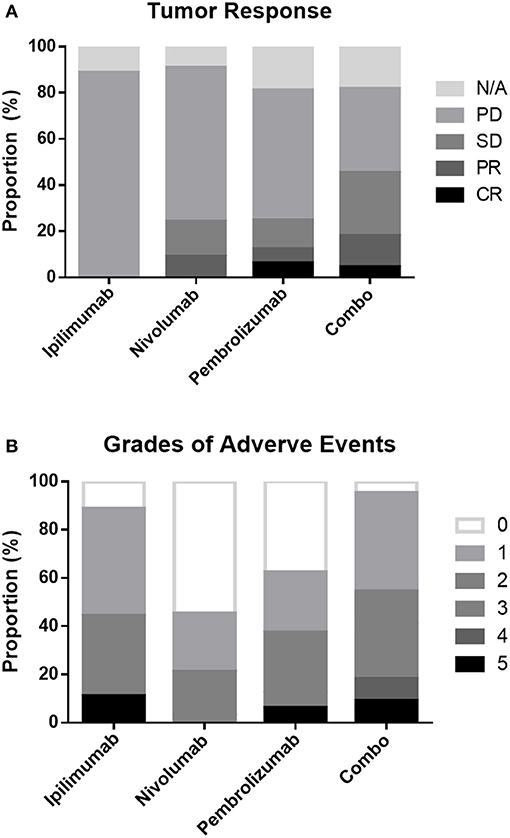
Figure 1. Proportion of tumor responses (A) and adverse events (B) following different treatment regimens.
Patients in the combination treatment group had a longer PFS of 5.6 (95% CI: 1.6–9.6) months than that of nivolumab (2.9 months, 95% CI: 1.9–3.9 months), pembrolizumab (3.2 months, 95% CI: 2.6–3.8 months), and ipilimumab (2.6 months, 95% CI: 2.4–2.8 months) treatment groups (p = 0.011, Table 2, Figure 2A). However, there were no significant differences in OS observed among the four regimens (p = 0.891; Figure 2D), possibly because most patients received sequential systemic treatment after progression (Table 3).
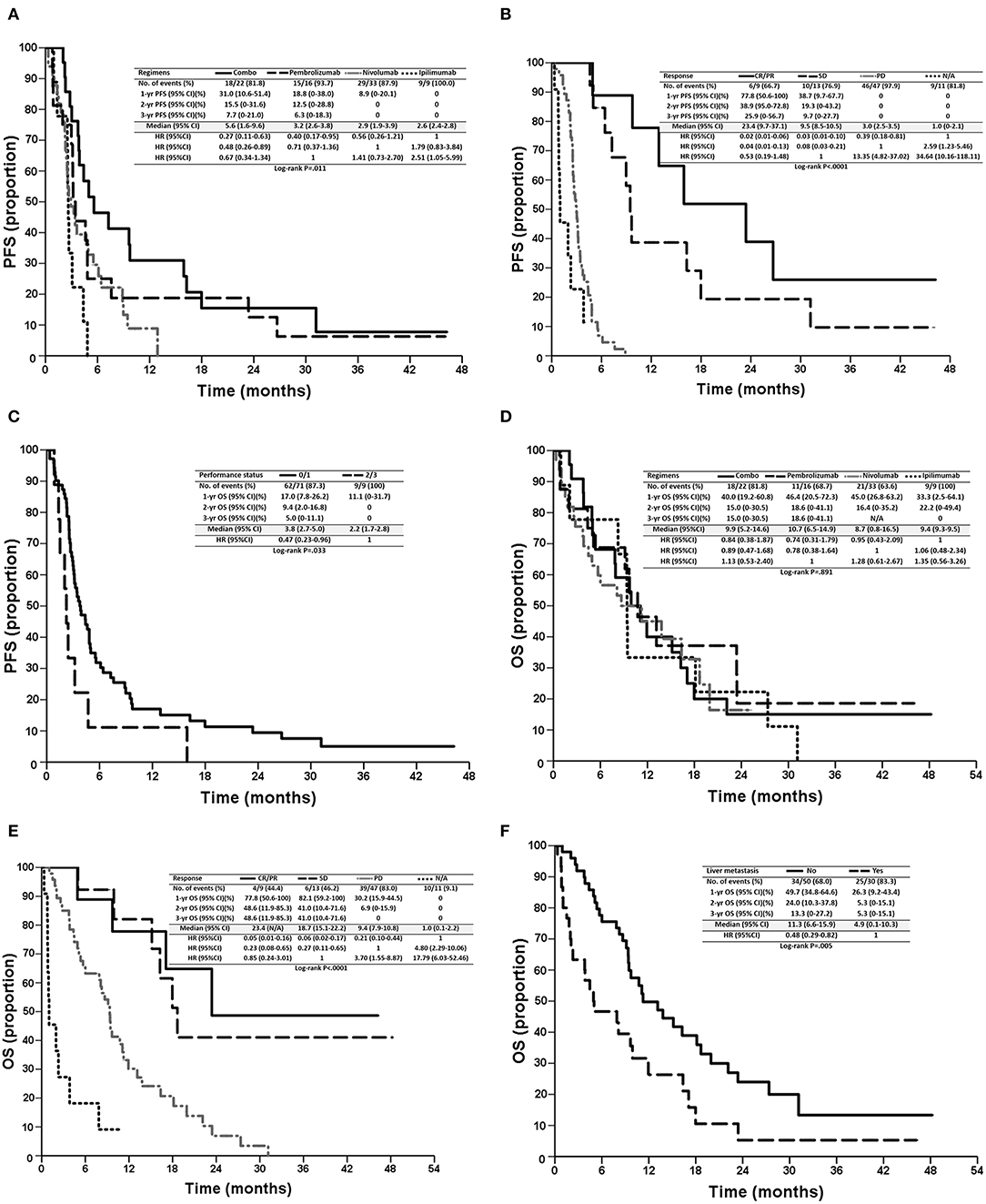
Figure 2. Kaplan–Meier survival curves of PFS (A–C) and OS (D–F) of patients, stratified according to prognostic factors, regimens (A,D), tumor responses (B,E), performance status (E), and liver metastasis (F). PFS, progression-free survival; OS, overall survival; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; N/A, not assessed.
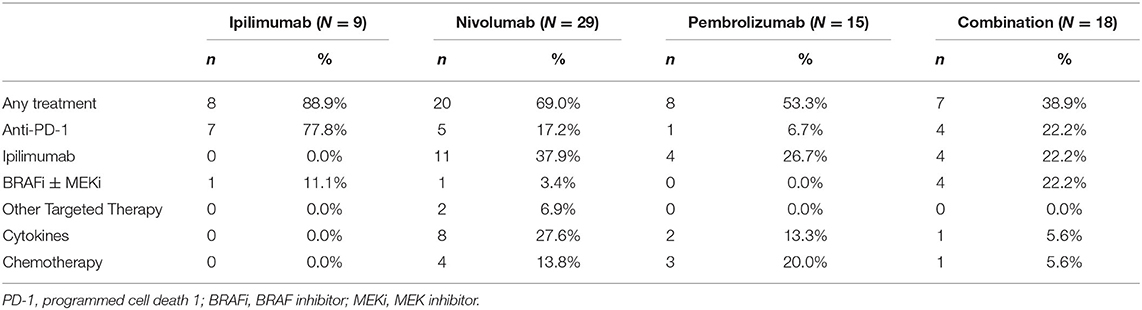
Subsequent Systemic Treatment After ICIs
Forty-three (53.8%) patients received systemic treatment after ICIs, including pembrolizumab (n = 8, 53.3%), nivolumab (n = 20, 69.0%), ipilimumab (n = 8, 88.9%), and combination (n = 7, 38.9%). The details of systemic treatments are summarized in Table 3.
Identification of Prognostic Factors for PFS
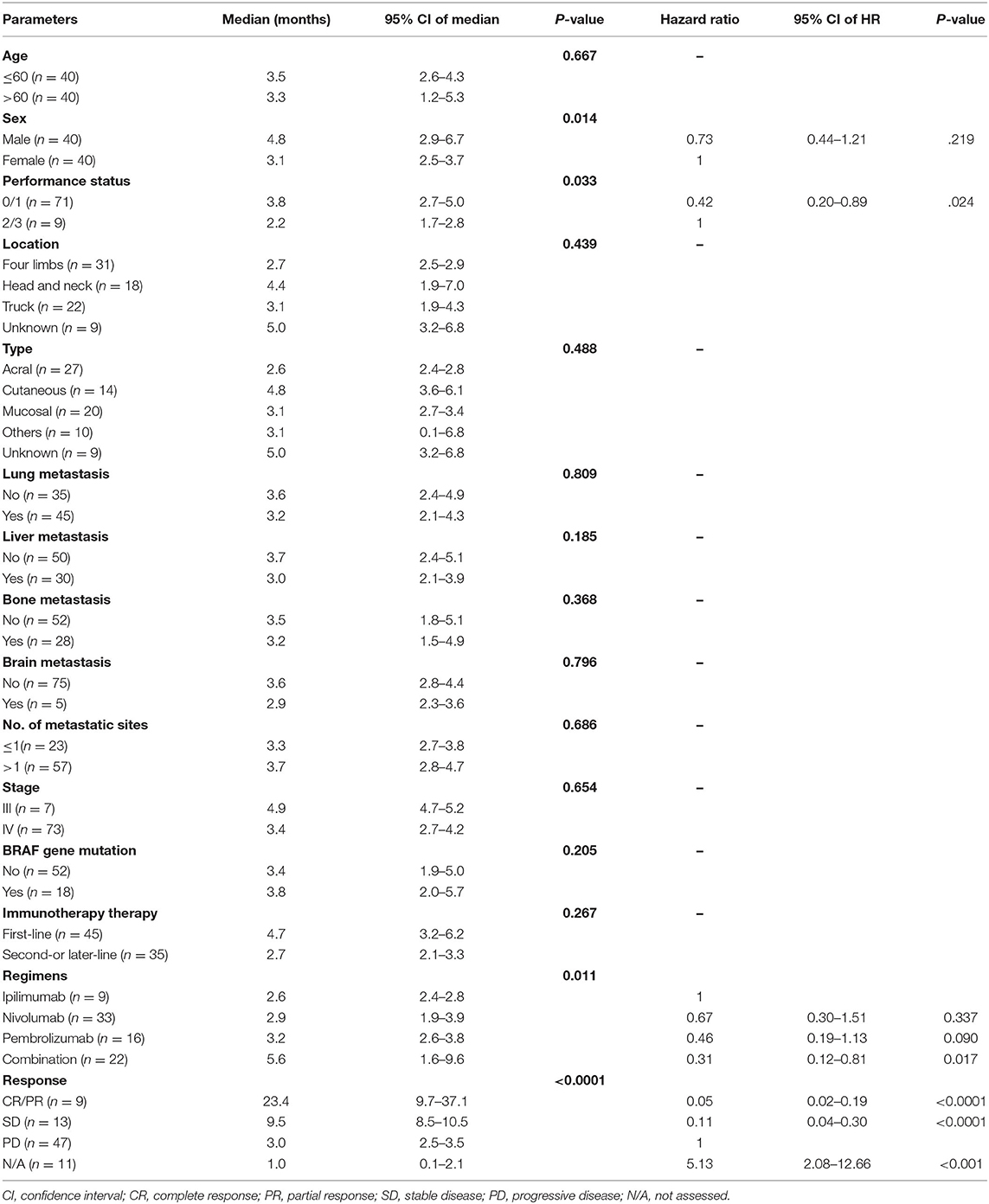
In the univariate analysis, sex (p = 0.014), performance status (p = 0.033), treatment regimens (p = 0.011), and tumor response (p < 0.001) were significant prognostic factors of PFS. In the multivariate analysis, combination treatment [vs. ipilimumab, hazards ratio (HR): 0.31, 95% CI: 0.12–0.81, p = 0.017, Figure 2A], tumor responses with CR/PR (vs. PD, HR: 0.05, 95% CI: 0.02–0.19, p < 0.0001), SD (vs. PD, HR: 0.11, 95% CI: 0.04–0.30, p < 0.0001), N/A (vs. PD, HR: 5.13, 95% CI: 2.08–12.66, p < 0.001, Figure 2B), and a performance status ≤ 1 (vs. > 2, HR: 0.42, 95% CI: 0.20–0.89, p = 0.024, Figure 2C) were independent favorable prognostic factors for PFS (Table 4). In addition, pembrolizumab showed a tendency for longer PFS than ipilimumab (HR: 0.46, 95% CI: 0.19–1.13, p = 0.090), and nivolumab had numerically longer PFS than ipilimumab (HR: 0.67, 95% CI: 0.30–1.51, p = 0.337).
Identification of Prognostic Factors for OS
In the univariate analysis, liver metastasis (p = 0.005), performance status (p = 0.041), and tumor response (p < 0.001) were significant prognostic factors for OS. In the multivariate analysis, tumor responses with CR/PR (vs. PD, HR: 0.21, 95% CI: 0.07–0.60, p = 0.004), SD (vs. PD, HR: 0.30, 95% CI: 0.13–0.73, p = 0.008), N/A (vs. PD, HR: 4.99, 95% CI: 2.32–10.74, p < 0.001, Figure 2E), and no liver metastasis (vs. liver metastasis, HR: 0.51, 95% CI: 0.28–0.91, p = 0.022, Figure 2F) were independent favorable prognostic factors for OS (Table 5). Among 30 patients with liver metastasis, 3 patients had PR and 4 patients had SD resulting in ORR of 10% and DCR of 23.3% which were not statistically different from the patients without liver metastasis.
AEs
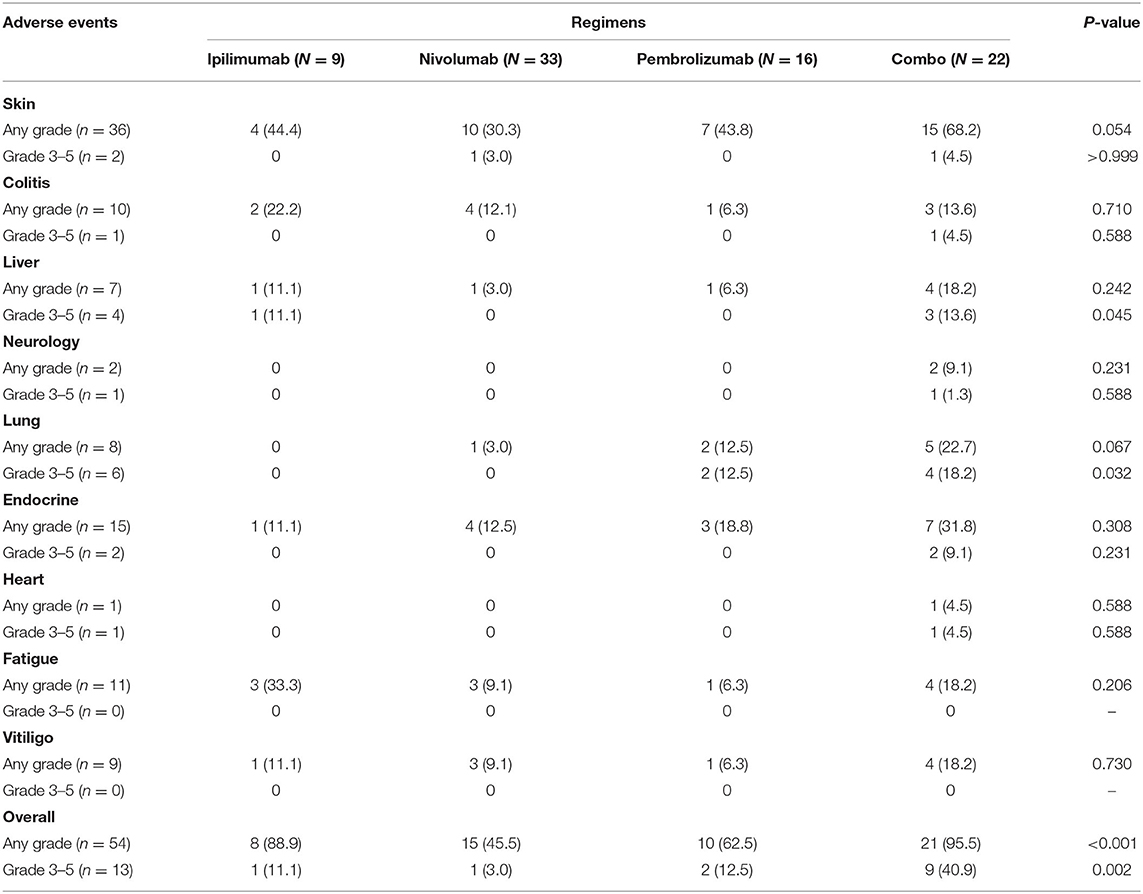
Overall, patients treated with combination treatment had the most frequent AEs across all grades (p < 0.001) and more grade 3 AEs (p = 0.002) than other ICIs treatment. Grade 3–5 treatment-related AEs occurred in 11.1, 3, 12.5, and 40.9% of patients in the ipilimumab, nivolumab, pembrolizumab, and combination groups, respectively. Skin-related AEs were the most common, followed by endocrine and pulmonary AEs. Patients treated with combination displayed a trend for skin, endocrine, and lung related AEs, although these results did not reach statistical significance. The details of all grades of AE are summarized in Table 6 and Figure 1B.
Discussion
We retrospectively reviewed ICI-naïve patients with melanoma undergoing various ICI treatments in Taiwan. Overall, the ORR was 11.3%, and DCR was 27.5%. Combination treatment with anti-CTLA-4 and anti-PD-1 antibodies provided numerically higher ORR and DCR, but this result was not statistically significant. In addition, combination treatment was associated with similar OS but significantly longer PFS with a greater risk of AEs than monotreatment, possibly because of subsequent treatments. In addition, we identified important prognostic factors, including liver metastases, performance status, and tumor response, for both PFS and OS. These findings could provide physicians with more information to justify clinical outcomes in patients with advanced melanoma in acral/mucosal-melanoma-predominant areas.
Combination treatment demonstrated the greatest ORR of 18.2% and DCR of 45.5%, and anti-PD-1 (nivolumab or pembrolizumab) showed an ORR of ~10% and DCR of ~25%. Both ORR and DCR were lower than those in previous prospective studies (4, 6, 20–24). In the CheckMate 067 study, the ORR and DCR were 48 and 70%, respectively, in the N3I1 group, 44 and 54%, respectively, in the nivolumab group, and 19 and 41%, respectively, in the ipilimumab group (4). Additionally, In three clinical trials for advanced melanoma (KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006), pembrolizumab provided ORRs of 30–40% in previously treated and treatment-naïve patients (6, 20–24). The low ORR and DCR seen in the current study may be a result of a high proportion of acral/mucosal melanoma with lower TMB than cutaneous melanoma (14). However, the response of ICIs in cutaneous melanoma was not as good as the results reported in clinical trials. The genetic alterations such as copy number variations of CDK4 pathway-related genes differ in Asian and Western melanoma which may contribute low response in Asian melanoma (25, 26). These findings support our hypothesis that ICIs function differently in Asian patients with melanoma than in Western patients with melanoma. Further studies are needed to explore possible mechanism and investigate novel treatment to improve the efficacy of ICIs in Asian melanoma particularly for acral and mucosal melanomas.
Furthermore, median PFS for acral melanoma (2.6 months) and mucosal melanoma (3.1 months) was shorter than that for cutaneous melanoma (4.8 months), indicating that tumor histology plays some sort of role in response to ICIs (Table 4). In a retrospective study of 60 individuals with acral (n = 25)/mucosal (n = 35) melanoma treated with anti-PD-1 (either nivolumab or pembrolizumab), ORR was 32% and median PFS was 4.1 months in patients with acral melanomas and ORR was 23% and median PFS was 3.9 months in patients with mucosal melanomas (15). Although the authors concluded that the ORR was comparable to published rates in cutaneous melanoma, the numerically lower ORR and shorter PFS of patients with acral/mucosal melanoma should be concerning in such subtypes of melanoma. Similar findings were reported in various melanoma studies, including a phase II study of Japanese patients treated with nivolumab (27), another phase II study of 30 Japanese patients treated with N1I3 combination (28), an observational study of 124 Japanese patients treated with nivolumab (29), and a phase 1b study (Keynote 151) of 103 Chinese melanoma patients treated with pembrolizumab as a second-line therapy (30). The post-hoc analysis of KEYNOTE 001, 002, and 006 trials reported pembrolizumab use in 84 patients with advance mucosal melanoma, with an ORR of 19% (compared with 33% in patients with a cutaneous melanoma), a DCR of 31%, a median PFS of 2.8 months, and a median OS of 11.3 months, which were inferior than those reported for patients with non-mucosal melanoma, thus supporting previous findings (17).
Combination treatment lead to a significantly higher frequency of any grade (95.5%) and grade ≥ 3 (40.9%) AEs than monotherapy, which was comparable with AE rates reported in previous phase III (2, 4–6) and phase II (28) studies. Unfortunately, four patients experienced AE-related death in the current study, and all occurred in the early stages of ICI treatment (prior to 2016). Presently, with a combination of comprehensive understanding, early reorganization and diagnosis, and adequate management of immune-related AEs (irAEs), most irAEs can be diagnosed and treated in early stages (6). Thus, there were no more deaths from AEs since 2017 in the current series. Recent studies found the occurrence of AEs might impact the therapeutic efficacy of ICIs. Yamazaki et al. (29) reported that the occurrence of skin-related and endocrine-related irAEs had a significant impact on PFS of patients with melanoma treated with nivolumab. Moreover, Fujisawa et al. (31) demonstrated that occurrences of endocrine-related irAEs were associated with longer OS of patients treated with ipilimumab after nivolumab. Interestingly, the development of vitiligo was correlated with better responses to ICIs, particularly in patients with melanoma, possibly because both melanocytes and melanomas share common antigens that are recognized by the activated immune response (32). Therefore, it is critical to manage the occurrence of irAEs appropriately, as patients with particular irAEs experience better survival.
The current retrospective analysis has some limitations. The nature of a retrospective study always involves biases. The limited number of patients with melanoma and imbalanced characteristics among different ICIs were the major limitations owing to a low prevalence of melanoma in the area. This made it difficult to do further subgroup analysis such as the influence of different regimens in subtypes of melanoma. Indeed, the economic burden to utilize ICIs as a therapeutic option has made this therapy unaffordable for most patients until recently, as reimbursement by national health insurance in Taiwan has been made available since April 2019. Upon consideration of all prognostic factors investigated for PFS and OS, we did not include few factors such as LDH and CRP reported by previous studies as these were not available for some patients before ICI administration. Furthermore, these patients were treated in a single, high-volume tertiary-care institute, which could not fully capture the type of real-world practice observed in smaller, peripheral clinics. However, the homogeneity of standardized treatment by medical oncologists in such a cancer institute could attenuate the weight of confounding factors.
In conclusion, the current study demonstrated clinical experience of ICI use in Taiwanese patients with melanoma. To our knowledge, this is the first report to compare different ICIs, including anti-PD-1, anti-CTLA-4, and combination treatment, in Asian population. Although ICIs were less efficacious in Taiwanese patients with melanoma, ICIs still provide an alternative option for Taiwanese patients seeking a robust response profile with tolerable toxicity.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of CGMH (202000182B0). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
C-EW, C-KY, M-TP, P-WH, C-YC, and JC contributed conception and design of the study. C-EW, Y-FL, and JC organized the database. C-EW, C-YC, Y-YC, H-WC, and JH performed the statistical analysis. C-EW wrote the first draft of the manuscript. C-EW, Y-YC, H-WC, and C-EW wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Immuno-Oncology Center of Excellence, Chang-Gung Memorial Hospital at Linkou. This work was supported by grants from Linkou Chang-Gung Memorial Hospital (CIRPG3H0061–2 and CORPG3J0151–2 to JC; CMRPG3J0971 and NMRPG3J0011 to C-EW), the Ministry of Science and Technology (108-2314-B-182A-007 to C-EW).
References
1. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
2. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. (2018) 19:1480–92. doi: 10.1016/S1470-2045(18)30700-9
3. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. (2019) 30:582–8. doi: 10.1093/annonc/mdz011
4. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2017) 377:1345–56. doi: 10.1056/NEJMoa1709684
5. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
6. Lebbe C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV CHECKMATE 511 trial. J Clin Oncol. (2019) 37:867–75. doi: 10.1200/JCO.18.01998
7. Chang JW, Cutaneous melanoma: Taiwan experience and literature review. Chang Gung Med J. (2010) 33:602–12.
8. Chang JW, Guo J, Hung CY, Lu S, Shin SJ, Quek R, et al. Sunrise in melanoma management: time to focus on melanoma burden in Asia. Asia Pac J Clin Oncol. (2017) 13:423–7. doi: 10.1111/ajco.12670
9. Chi Z, Li S, Sheng X, Si L, Cui C, Han M, et al. Clinical presentation, histology, and prognoses of malignant melanoma in ethnic Chinese: a study of 522 consecutive cases. Bmc Cancer. (2011) 11:85. doi: 10.1186/1471-2407-11-85
10. Chang JW, Huang YL, Chang YY, Lo YF, Ho TY, Huang YT, et al. Sentinel lymph node biopsy was associated with favorable survival outcomes for patients with clinically node-negative Asian melanoma. Cancer Manag Res. (2019) 11:9655–64. doi: 10.2147/CMAR.S227837
11. Wu CE, Hsieh CH, Chang CJ, Yeh JT, Kuo TT, Yang CH, et al. Prognostic factors for Taiwanese patients with cutaneous melanoma undergoing sentinel lymph node biopsy. J Formos Med Assoc. (2015) 114:415–21. doi: 10.1016/j.jfma.2013.06.018
12. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. (2019) 19:133–50. doi: 10.1038/s41568-019-0116-x
13. Wu Y, Xu J, Du C, Wu Y, Xia D, Lv W, et al. The predictive value of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers: a systematic review and meta-analysis. Front Oncol. (2019) 9:1161. doi: 10.3389/fonc.2019.01161
14. Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K, et al. Whole-genome landscapes of major melanoma subtypes. Nature. (2017) 545:175–80. doi: 10.1038/nature22071
15. Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, et al. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. (2016) 122:3354–62. doi: 10.1002/cncr.30259
16. Johnson DB, Peng C, Abramson RG, Ye F, Zhao S, Wolchok JD, et al. Clinical activity of ipilimumab in acral melanoma: a retrospective review. Oncologist. (2015) 20:648–52. doi: 10.1634/theoncologist.2014-0468
17. Hamid O, Robert C, Ribas A, Hodi FS, Walpole E, Daud A, et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: a post-hoc analysis of KEYNOTE-001, 002, 006. Br J Cancer. (2018) 119:670–4. doi: 10.1038/s41416-018-0207-6
18. D'Angelo SP, Larkin J, Sosman JA, Lebbe C, Brady B, Neyns B, et al., Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. (2017) 35:226–35. doi: 10.1200/JCO.2016.67.9258
19. Nathan P, Ascierto PA, Haanen J, Espinosa E, Demidov L, Garbe C, et al. Safety and efficacy of nivolumab in patients with rare melanoma subtypes who progressed on or after ipilimumab treatment: a single-arm, open-label, phase II study (CheckMate 172). Eur J Cancer. (2019) 119:168–78. doi: 10.1016/j.ejca.2019.07.010
20. Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. (2019) 20:1239–51. doi: 10.1016/S1470-2045(19)30388-2
21. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Investigators pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. (2015) 372:2521–32. doi: 10.1056/NEJMoa1503093
22. Puzanov I, Dummer R, Schachter J, Pavlick AC, Gonzalez R, Ascierto PA, et al. Efficacy based on tumor PD-L1 expression in KEYNOTE-002, a randomized comparison of pembrolizumab (pembro; MK-3475) versus chemotherapy in patients (pts) with ipilimumab-refractory (IPI-R) advanced melanoma (MEL). J Clin Oncol. (2015) 33:3012. doi: 10.1200/jco.2015.33.15_suppl.3012
23. Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Three-year overall survival for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. J Clin Oncol. (2016) 34:9503. doi: 10.1200/JCO.2016.34.15_suppl.9503
24. Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol. (2016) 34:1510–7. doi: 10.1200/JCO.2015.64.0391
25. Sheen YS, Tan KT, Tse KP, Liao YH, Lin MH, Chen JS, et al. Genetic alterations in primary melanoma in Taiwan. Br J Dermatol. (2019) 182:1205–13. doi: 10.1111/bjd.18425
26. Kong Y, Sheng X, Wu X, Yan J, Ma M, Yu J, et al. Frequent genetic aberrations in the CDK4 pathway in acral melanoma indicate the potential for CDK4/6 inhibitors in targeted therapy. Clin Cancer Res. (2017) 23:6946–57. doi: 10.1158/1078-0432.CCR-17-0070
27. Yamazaki N, Kiyohara Y, Uhara H, Uehara J, Fujisawa Y, Takenouchi T, et al. Long-term follow up of nivolumab in previously untreated Japanese patients with advanced or recurrent malignant melanoma. Cancer Sci. (2019) 110:1995–2003. doi: 10.1111/cas.14015
28. Namikawa K, Kiyohara Y, Takenouchi T, Uhara H, Uchi H, Yoshikawa S, et al. Efficacy and safety of nivolumab in combination with ipilimumab in Japanese patients with advanced melanoma: an open-label, single-arm, multicentre phase II study. Eur J Cancer. (2018) 105:114–26. doi: 10.1016/j.ejca.2018.09.025
29. Yamazaki N, Takahashi A, Namikawa K, Takenouchi T, Nakamura Y, Kitano S, et al. Response of nivolumab monotherapy in 124 Japanese patients with advanced melanoma: interim analysis of prospective observational study (CREATIVE study). Ann Oncol. (2019) 30(Suppl 9):ix115. doi: 10.1093/annonc/mdz429.001
30. Si L, Zhang XS, Shu YQ, Pan HM, Wu D, Liu JW, et al. A Phase Ib study of pembrolizumab as second-line therapy for chinese patients with advanced or metastatic melanoma (KEYNOTE-151). Transl Oncol. (2019) 12:828–35. doi: 10.1016/j.tranon.2019.02.007
31. Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Uchi H, Fujimura T, et al. Retrospective study of advanced melanoma patients treated with ipilimumab after nivolumab: analysis of 60 Japanese patients. J Dermatol Sci. (2018) 89:60–6. doi: 10.1016/j.jdermsci.2017.10.009
32. Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. (2015) 33:773–81. doi: 10.1200/JCO.2014.57.4756
Keywords: melanoma, immunotherapy, nivolumab, ipilimumab, pembrolizumab, immune checkpoint inhibitors
Citation: Wu C-E, Yang C-K, Peng M-T, Huang P-W, Lin Y-F, Cheng C-Y, Chang Y-Y, Chen H-W, Hsieh J-J and Chang JW-C (2020) Immune Checkpoint Inhibitors for Advanced Melanoma: Experience at a Single Institution in Taiwan. Front. Oncol. 10:905. doi: 10.3389/fonc.2020.00905
Received: 13 March 2020; Accepted: 11 May 2020;
Published: 04 June 2020.
Edited by:
Sapna Patel, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Lu Si, Peking University Cancer Hospital, ChinaMahendra Pratap Kashyap, University of Alabama at Birmingham, United States
Copyright © 2020 Wu, Yang, Peng, Huang, Lin, Cheng, Chang, Chen, Hsieh and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Wen-Cheng Chang, d2VuMTkwMkBob3RtYWlsLmNvbQ==
 Chiao-En Wu
Chiao-En Wu Chan-Keng Yang1,2
Chan-Keng Yang1,2 Jia-Juan Hsieh
Jia-Juan Hsieh