
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 25 June 2020
Sec. Thoracic Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00904
Background: Tyrosine kinase inhibitors (TKIs) are standard treatment options for non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutations. Increasing clinical investigations have explored the value of EGFR-TKIs plus antiangiogenic drugs as the first-line treatment for EGFR-mutated NSCLC.
Methods: We systematically searched PubMed, Cochrane Library, and EMBASE for randomized controlled trials (RCTs) investigating EGFR-TKIs administered with or without antiangiogenic agents for advanced EGFR-mutated NSCLC. The latest RCT that was presented orally at the 2019 European Society for Medical Oncology Congress was obtained online. The endpoints included progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rates (DCRs), and grade 3 or higher adverse events (AEs).
Results: We included seven articles on five trials with 1,226 patients. The interventions for the experimental group were the first-generation EGFR-TKI erlotinib combined with bevacizumab (four studies) or ramucirumab (one study), and erlotinib monotherapy (four studies) or erlotinib plus placebo (one study) for the control group. All studies reached their primary study endpoints (i.e., PFS). Compared to erlotinib monotherapy, erlotinib plus antiangiogenic agents remarkably prolonged PFS [hazard ratio (HR) = 0.59, 95% confidence interval (CI) = 0.51–0.69, P = 0.000]; however, ORR, DCR, and OS were similar between the two groups. The overall grade 3–5 AEs increased in combination group (OR = 5.772, 95% CI = 2.38–13.94, P = 0.000), particularly the incidence of diarrhea (OR = 2.51, 95% CI = 1.21–5.23, P = 0.014), acneiform (OR = 1.815, 95% CI = 1.084–3.037, P = 0.023), hypertension (OR = 6.77, 95% CI = 3.62–12.66, P = 0.000), and proteinuria (OR = 13.48, 95% CI = 4.11–44.22, P = 0.000). Additionally, subgroup analysis demonstrated that Asian patients could significantly benefit from combination therapy (HR = 0.59, 95% CI = 0.50–0.69, P = 0.000). Patients with exon 19 deletions (HR = 0.61, 95% CI = 0.49–0.75, P = 0.000) and 21 Leu858Arg mutations (HR = 0.59, 95% CI = 0.47–0.73, P = 0.000) had almost equivalent PFS benefits when treated with double-blocking therapy. Patients with brain metastases at baseline in the combination group had a trend toward better PFS (HR = 0.55, 95% CI = 0.30–1.01, P = 0.001).
Conclusions: Erlotinib plus bevacizumab or ramucirumab in EFGR-mutated NSCLC first-line setting yielded remarkable PFS benefits; however, this was accompanied by higher AEs. Epidermal growth factor receptor–TKI plus antiangiogenic agent therapy may be considered a new option for advanced EGFR-mutated NSCLC patients.
Lung cancer is the most common malignant tumor. In addition, it has the highest morbidity and mortality worldwide (1). Approximately 85% of primary lung cancers are non-small cell lung cancer (NSCLC). Epidermal growth factor receptor (EGFR) mutations are found in 11–16% of lung adenocarcinomas in western countries and 50% in Asia (2). Since 2014, EGFR tyrosine kinase inhibitors (EGFR-TKIs) have become the standard first-line therapy for NSCLC patients harboring EGFR-activating mutations (3, 4). When treated with first- or second-generation TKIs, the median progression-free survival (PFS) is ~1 year, whereas with the third-generation TKI osimertinib, it is 18.9 months (5–7). Despite the high disease control rates, almost all patients eventually experience acquired resistance-mediated disease progression. Therefore, new combination regimens are needed to delay or overcome acquired TKI resistance. Neovascularization plays an essential role in supplying tumors with oxygen and nutrients, eliminating metabolic waste and facilitating tumor growth, progression, and metastasis (8–10). Hypoxia-driven vascular endothelial growth factor (VEGF) is a primary angiogenesis regulator that can activate proangiogenic signaling by binding to the vascular endothelial growth factor receptor (VEGFR) (11). Preclinical studies have shown that the EGFR signaling pathway can up-regulate VEGF expression (12–14). Epidermal growth factor receptor and VEGF share a common downstream signaling pathway. Further, VEGF/VEGFR plays a role in EGFR-TKI resistance (15–21). Antiangiogenic agents can increase the delivery of antitumor drugs by normalizing the blood flow of tumor blood vessels (22, 23). Lower doses of EGFR-TKIs were associated with earlier resistance (24, 25). Blocking the VEGF/VEGFR signaling pathway can abrogate EGFR-TKI primary or acquired resistance (15, 26). Increasing trials have sought to determine whether EGFR-TKIs plus antivascular drugs could elicit stronger antitumor effects than EGFR-TKIs alone for EGFR-mutant advanced NSCLC patients in the first-line setting. Therefore, we aimed to synthesize published randomized controlled trial (RCT) data to assess the efficacy and safety of this emerging remedy and to provide guidance for clinical decision-making and future researches.
This meta-analysis was conducted in conformity with the PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analysis) guidelines (Table S1).
PubMed, EMBASE, and Cochrane Library databases were searched to identify potential studies up to November 2019 that assessed the use of EFGR-TKIs plus angiogenesis inhibitors as a first-line therapy for NSCLC. Mesh and entry terms were both used for a comprehensive lookup. Additionally, the reference lists of included studies, and several commonly prescribed drugs were checked to further derive qualified publications. Further, we searched online for recently updated reports presented at meetings only and not presented in the databases (Supplementary Material).
We included studies that met the following conditions: (1) recruited patients with cytologically or histologically confirmed advanced EGFR-mutant NSCLC; (2) compared EGFR-TKIs plus antiangiogenic drugs with EGFR-TKI monotherapy in the first-line setting; (3) reported outcomes: PFS, overall survival (OS), objective response rate (ORR), disease control rate (DCR), and grade ≥3 adverse events (AEs); and (4) were designed as RCTs. Articles or abstracts lacking any of the above criteria were excluded.
Two investigators (Fei Chen and Naifei Chen) extracted the main characteristic information, including the study name, sample size, median age, sex percentage, disease stage, treatment regimens, and primary and secondary endpoints. The latest results from the identical cohort of patients were considered. If disagreements arose, a third researcher (Jiuwei Cui) made the final decisions.
The risk of bias of an individual study was assessed using the Cochrane Risk of Bias Tool, built into the Review Manager software (version 5.3), using the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias sources. Each item was marked as low, high, or unclear risk of bias.
We performed this meta-analysis using Stata/SE software (College station, TX, USA) (version 15.0). The pooled hazard ratio (HR) and 95% CI were generated to analyze the PFS advantage, while the pooled OR and 95% CI were calculated to compare ORR, DCR, and the rate of grades 3–5 AEs. I2 and χ2 tests were utilized to assess the heterogeneity among the included studies. Either I2 > 50% or P < 0.1 illustrated significant heterogeneity. Accordingly, the randomized-effects model was employed; otherwise, the fixed-effects model was employed. Statistical differences were defined as P < 0.05. Sensitivity analyses of PFS, ORR, DCR, OS, and grades 3–5 AEs were conducted to detect the robustness of the meta-analysis results. Because the number of articles is <10, we did not conduct publication bias tests.
A total of 2,491 records were identified from three pivotal databases—PubMed, EMBASE, and Cochrane Library. The data from the CTONG 1,509 study presented at the 2019 European Society for Medical Oncology (ESMO) Congress were also made available online. A total of 248 duplicate records were removed from the 2,492 records. Subsequently, by screening titles and abstracts, 24 promising publications were fully reviewed. Aligning with the predefined inclusion criteria, five RCTs involving 1,226 patients were used for the analyses (Figure 1). The excluded publications include many trial protocols (interventions include the first-generation EGFR-TKI gefitinib in combination with bevacizumab or anlotinib or fruquintinib, second-generation EGFR-TKI afatinib in combination with bevacizumab, and third-generation EGFR-TKI osimertinib combined with bevacizumab).
Five journal articles (27–31), one conference abstract (32), and an oral presentation at the 2019 ESMO congress (33) met the inclusion criteria, including two Japanese studies, one Chinese study, one American study, and one international multicenter study. Three trials included brain metastatic patients. The EGFR mutation types for patients were exon 19 deletions and 21 Leu858Arg mutations. The intervention for the experimental group was the first-generation EGFR-TKI erlotinib plus bevacizumab (anti-VEGF antibody) (27, 30, 31, 33) or erlotinib plus ramucirumab (anti-VEGFR antibody) (29), whereas the control group was administered erlotinib alone or erlotinib with placebo. Table 1 lists the primary features of all included studies. The Cochrane Risk of Bias Tool was employed to appraise the research quality (Figure 2).
In terms of PFS (heterogeneity: P = 0.721, I2 = 0), the pooled results from five studies demonstrated that erlotinib plus anti-VEGF/anti-VEGFR antibody could improve PFS compared to the erlotinib monotherapy (HR = 0.59, 95% CI = 0.51–0.69, P = 0.000; Figure 3A).
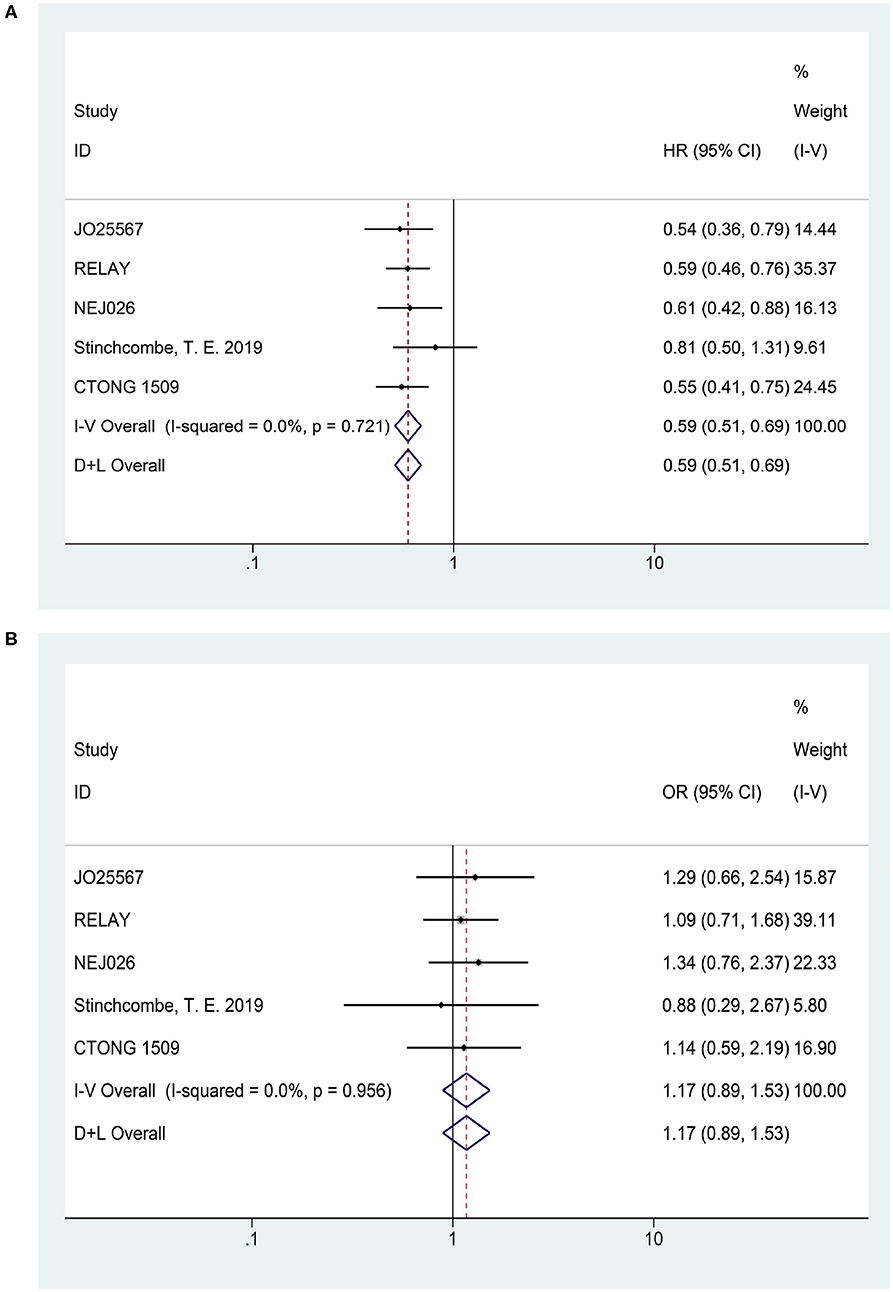
Figure 3. Forest plots of the PFS (A) and ORR (B) that are associated with erlotinib plus antiangiogenic agents vs. erlotinib.
Five articles reported the ORR (heterogeneity: P = 0.956, I2 = 0). The pooled results suggested that there was no significant difference in ORR between combination therapy and erlotinib alone (OR = 1.17, 95% CI = 0.89–1.53, P = 0.258; Figure 3B).
Four articles presented DCR (heterogeneity: P = 0.163, I2 = 41.4%). Patients could not receive more benefit from erlotinib plus anti-VEGF therapy than erlotinib monotherapy (OR = 1.00, 95% CI = 0.55–1.82, P = 0.989; Figure 4A).
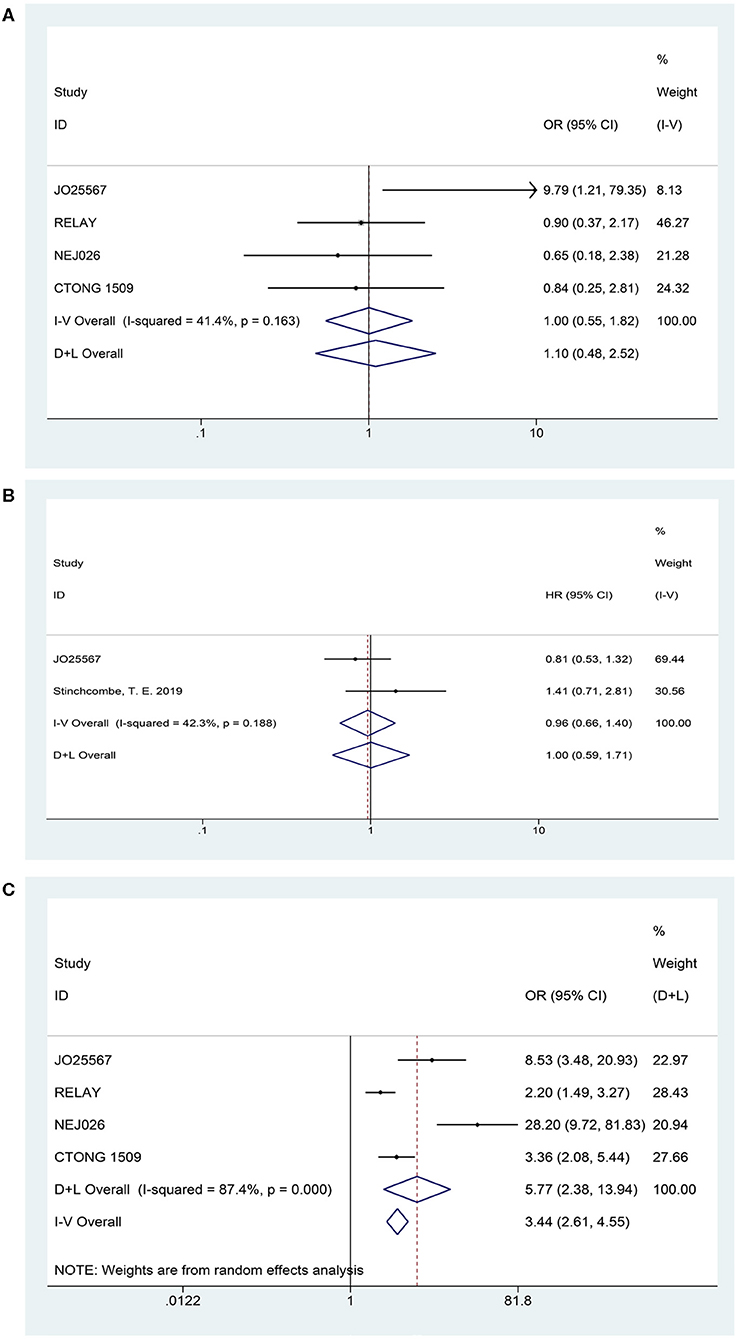
Figure 4. Forest plots of the DCR (A), OS (B), and grade ≥3 AEs (C) that are associated with erlotinib plus antiangiogenic agents vs. erlotinib.
Two studies published their OS findings (heterogeneity: P = 0.178, I2 = 44.9%). However, current data have not demonstrated that patients experienced an OS prolongation benefit when treated with combination therapy (HR = 0.94, 95% CI = 0.66–1.35, P = 0.745; Figure 4B).
Based on the total number of patients who experienced AEs, we analyzed the difference in the incidence rate of grades 3–5 AEs between combination therapy and erlotinib alone. The data for the overall grades 3–5 AEs originated from four trials (heterogeneity: P = 0.000, I2 = 87.4%). Results demonstrated that the dual-blocking strategy caused substantial increase in grades 3–5 AEs relative to erlotinib monotherapy (OR = 5.772, 95% CI = 2.38–13.94, and P = 0.000; Figure 4C). Additionally, we performed subgroup analysis of the 10 primarily mentioned AEs and found that combination therapy led to higher incidence of diarrhea (OR = 2.51, 95% CI = 1.21–5.23, P = 0.014), acneiform (OR = 1.82, 95% CI = 1.08–3.04, P = 0.023), hypertension (OR = 6.77, 95% CI = 3.62–12.66, P = 0.000), and proteinuria (OR = 13.48, 95% CI = 4.11–44.22, P = 0.000; Figure 5).
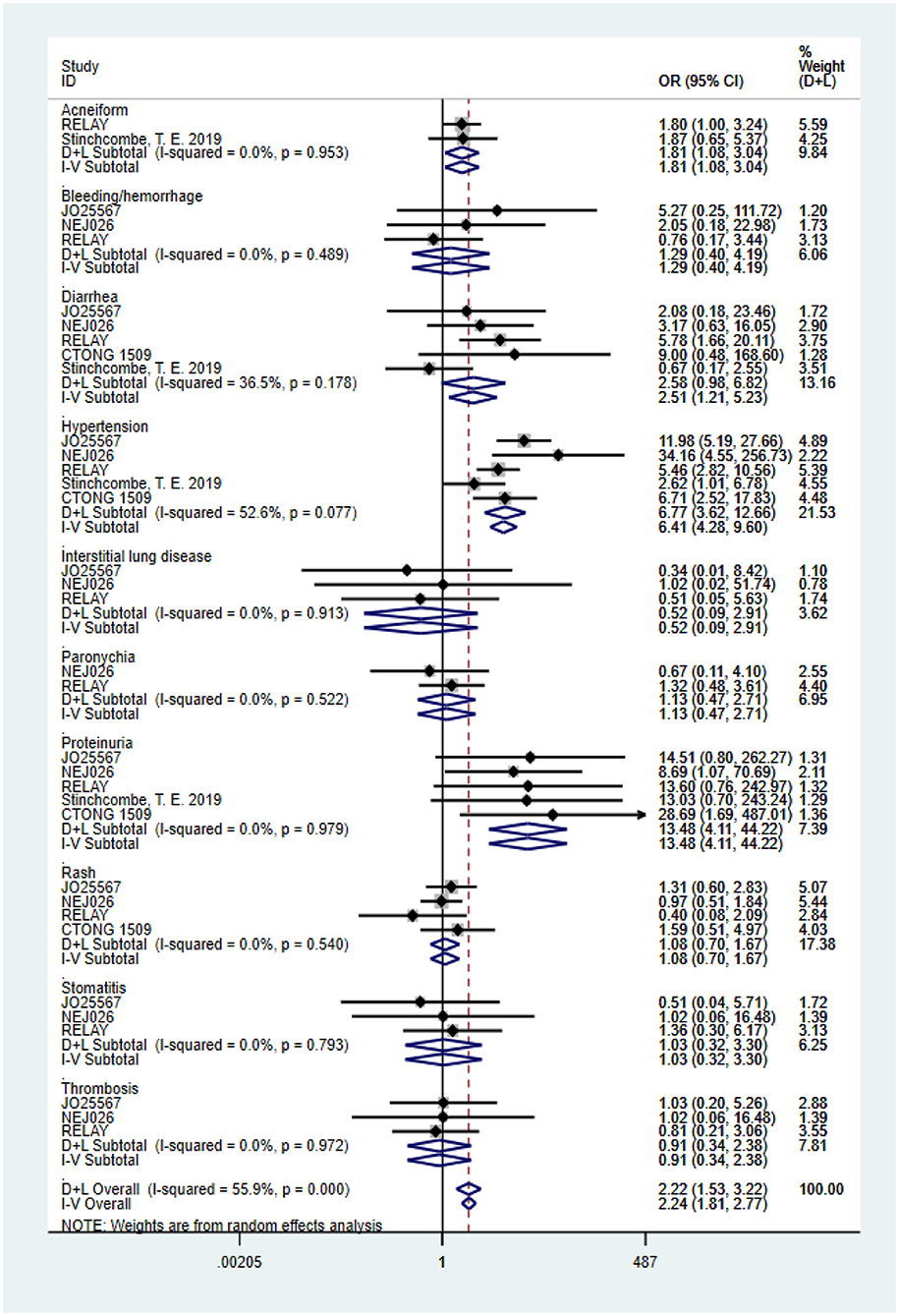
Figure 5. Forest plots of the 10 primarily mentioned grade ≥3 AEs that are associated with erlotinib plus antiangiogenic agents vs. erlotinib.
Considering the influence of race on efficacy, we first conducted a subgroup analysis of the patient population. The ethnic stratification of each study included Japanese (JO25567 and NEJ026 study), Chinese (CTONG 1509 study), East Asian, and other races (RELAY study), and white and nonwhite (phase II study of the United States). Accordingly, we grouped the total population into two categories: Asian and non-Asian. A phase II study in the United States only reported the intergroup HR of the white and nonwhite groups (HR = 0.86, 95% CI = 0.41–1.81), whereas the RELAY study reported the HR of East Asian patients and other patients (0.64 and 0.61, respectively). Therefore, only the pooled PFS value of Asian patients was retrieved. The result showed that the risk of disease progression of Asian patients in the combined treatment group was reduced by 41% (HR = 0.59, 95% CI = 0.50–0.69, P = 0.000), which is consistent with the result for the overall populations. To ascertain the possible EGFR mutation type–mediated discrepancies in treatment efficacy, we performed the subgroup analysis of exon 19 deletions and 21 Leu858Arg mutations. Combination therapy yielded better PFS benefits irrespective of the mutation type. Further, disease progression risk was reduced by 39% in the exon 19 deletions subgroup (HR = 0.61, 95% CI = 0.49–0.75, P = 0.000) and 41% in the 21 Leu858Arg mutation subgroup (HR = 0.59, 95% CI = 0.47–0.73, P = 0.000). Furthermore, we analyzed the pooled PFS for the brain metastasis subgroups. One study reported only the HR between groups with and without brain metastases. The pooled result of the two trials showed that erlotinib plus bevacizumab had better efficacy for patients without brain metastasis at baseline (HR = 0.61, 95% CI = 0.46–0.79, P= 0.000). For patients with brain metastases at baseline, the current data did not indicate there was a difference between the two regimens (HR = 0.55, 95% CI = 0.30–1.01, P= 0.001; Figure 6).
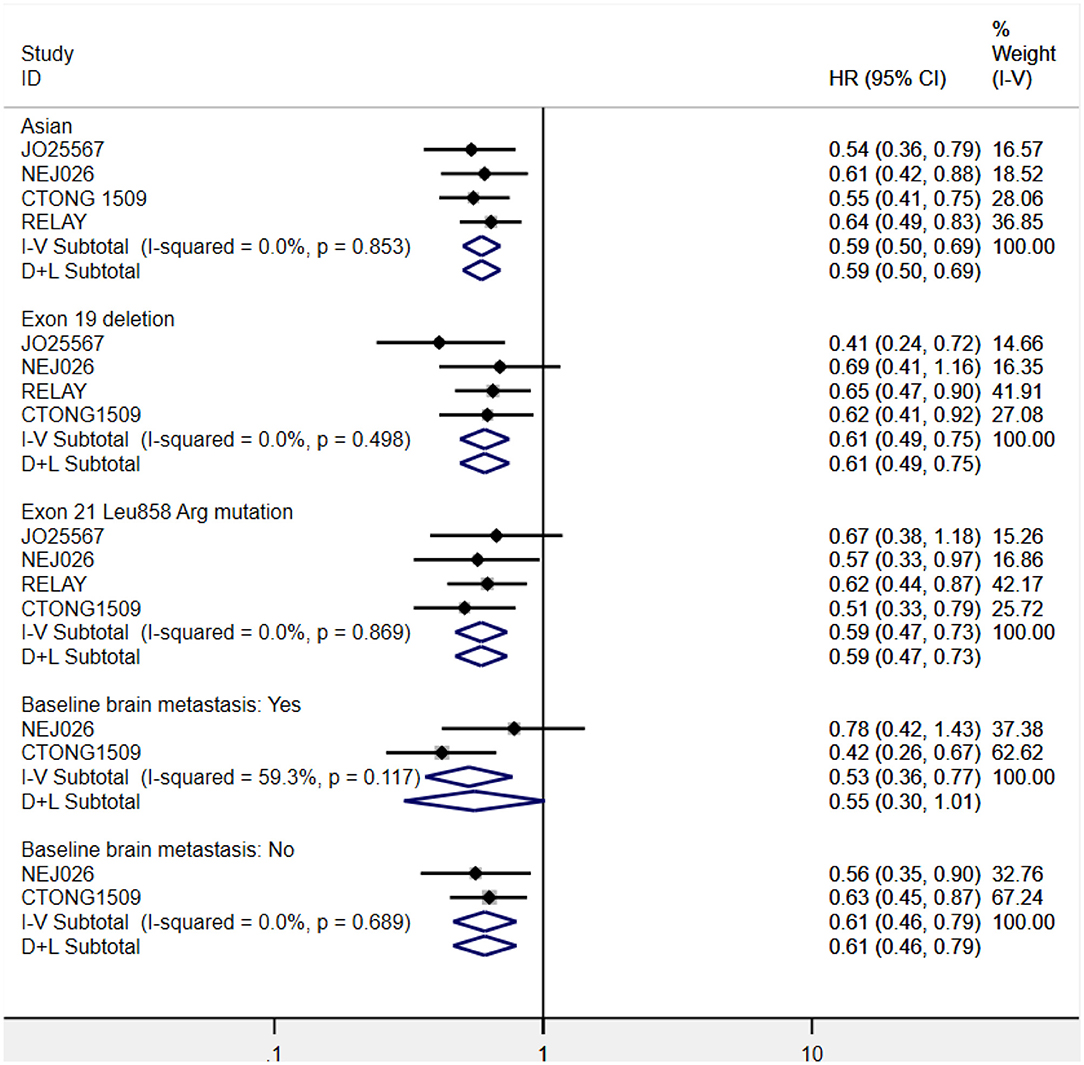
Figure 6. Forest plots of the PFS subgroup of Asian patients, exon 19 deletions and 21 Leu858Arg mutations, and brain metastasis status at baseline that are associated with erlotinib plus antiangiogenic agents vs. erlotinib.
The pooled PFS, ORR, DCR, AE, and PFS subgroup analyses of the Asian population and two mutation types were all robust, with concordant conclusions derived from the sensitivity analysis and no estimates beyond the 95% CIs. However, the PFS subgroup of patients with brain metastasis and OS results were not persuasive. This might be due to the small number of eligible trials for analyses. In terms of overall grades 3–5 AEs, the RELAY study dramatically differed from other studies. Additionally, replacing the effect model did not render any opposite findings except for the incidence of diarrhea and the PFS subgroup of brain metastatic patients (Figures S1–S6; Figures 3–6).
To the best of our knowledge, this is the first meta-analysis to compare the efficacy and safety of EGFR-TKIs plus antiangiogenesis therapy to that of EGFR-TKIs alone as a first-line treatment for NSCLC. Through systematic and comprehensive literature research, we found multiple trials that have sought to determine the efficacy of EGFR-TKIs combined with antiangiogenic drugs as a first-line treatment (including the first-, second-, and third-generation EGFR-TKIs combined with anti-VEGF/VEGFR monoclonal antibodies or small molecule TKIs) (34–38). However, only RCTs on the first-generation EGFR-TKI erlotinib combined with anti-VEGF/VEGFR antibodies reported the endpoint results. Analyses of these large-scale, high-quality phase II/III RCTs illustrated that erlotinib combined with bevacizumab or ramucirumab had the advantage of PFS extension relative to erlotinib. For Asian patients, combination therapy had evident PFS advantages. Patients with exon 19 deletions and 21 Leu858Arg mutations could gain similar PFS benefits from dual-blocking therapy. However, grade 3–5 AEs, especially acneiform, hypertension, proteinuria, and diarrhea, were increased in the combination group.
We proved that the first-generation EGFR-TKI erlotinib plus anti-VEGF/VEGFR antibody has PFS advantage over erlotinib alone for EGFR-mutant advanced NSCLC patients. This meta-analysis provides evidence to support the use of EGFR-TKIs combined with antiangiogenesis therapy as a first-line treatment for EGFR-mutant NSCLC patients. Among the five trials included in our meta-analysis, PFS prolongation ranged from 3.6 to 7.0 months in the combination arm (27, 29–31, 33). A single-arm phase II study in Japan suggested that the first-generation EGFR-TKI gefitinib combined with bevacizumab as a first-line therapy for EGFR-mutant NSCLC patients increased PFS by 4–5 months relative to the historic EGFR-TKI monotherapy (39). In the EGFR-mutant subgroup of the phase III BeTa trial, erlotinib plus bevacizumab increased the PFS by 7.2 months compared to erlotinib for advanced NSCLC, which did not respond to standard first-line chemotherapy (40). A retrospective study revealed that erlotinib/gefitinib plus bevacizumab yielded PFS of 16.0 months in the first-line setting, which is similar to that in the JO25567 study (41). Notably, the FLAURA study confirmed the superiority of the third-generation EGFR-TKI osimertinib over other EGFR-TKIs as a first-line treatment for EGFR-sensitive mutations in NSCLC patients, with PFS of 18.9 months and OS of 38.6 months (7, 42). As a result, the following questions arose: what are the differences between osimertinib and first-generation EGFR-TKIs plus antivascular drugs, in terms of efficacy, and could osimertinib plus antivascular drugs improve survival, compared to osimertinib alone? The ongoing single-arm phase I/II clinical trial of osimertinib plus bevacizumab (34) and the phase II RCT of osimertinib plus bevacizumab vs. osimertinib (43) are expected to answer these questions. Osimertinib is effective for patients with T790M mutation in EGFR exon 20, which accounts for 40–60% of resistant mutations (44–46). Epidermal growth factor receptor–TKIs plus antiangiogenic agents can delay the occurrence of resistance (15). If the combination of first-generation EGFR-TKIs and antiangiogenic agents could yield a similar survival benefit relative to osimertinib in advanced patients with EGFR-sensitive mutations, first-generation TKIs plus antiangiogenic therapy could be employed in the first-line setting, and osimertinib could be considered a subsequent option when T790M mutation occurred, which is expected to maximize patient survival. Moreover, a variety of EGFR-TKIs and different classes of antivascular drugs currently exist. Are there any differences in the effects of different combinations of these drugs? More research data, head-to-head comparisons, and network meta-analyses are needed to answer this question. In addition, in the case of multiple treatment options, a cost-effectiveness analysis is of marked importance for therapy selection.
Notably, the efficacy of combination therapy may be discrepant in different populations. Patients recruited in the JO25567 and NEJ026 studies were all Japanese. Further, the PFS was found to significantly increase in the combination group (27, 30). The CTONG 1509 study focused on Chinese patients and achieved positive results (33). In the RELAY study, most of the population was East Asians (75%), and their PFS was remarkably increased (HR = 0.64, 95% CI = 0.49–0.83). However, no statistically significant difference in PFS was found in other populations (white, American Indian or Alaska Native, black or African–American, or missing) (HR = 0.61, 95% CI = 0.36–1.01) (29). The phase II study in the United States revealed no difference in PFS between white and nonwhite subgroups (African American, Asian, not available) (HR = 0.86, 95% CI = 0.41–1.81) in two groups and increased risk of death in the combination group (31). As the existing data format for non-Asian patients is limited, we analyzed data from Asian patients and found that combination therapy significantly improved the PFS of these patients to the same extent as that in general populations, with equal HR. Therefore, Asian patients can benefit from combination therapy; however, the data for other races remain insufficient to draw relevant conclusions. It is hoped that future studies with large sample sizes will elucidate the effect of race on efficacy.
Exon 19 deletions and 21 Leu858Arg mutations account for 50 and 40% of all EGFR gene mutation types, respectively (47). Patients that harbor these two most common types of driving mutations are sensitive to EGFR-TKIs (3). A meta-analysis revealed that patients with exon 19 deletions had a better prognosis than those with 21 Leu858Arg mutations (48). However, our generated data revealed similar PFS benefits between the two mutation types in the combination arm. The risk of disease progression was reduced by 39 and 41% for exon 19 deletions and 21 Leu858Arg, respectively. Three phase III trials (NET026, RELAY, and CTONG 1509) demonstrated that patients with exon 21 Leu858Arg mutations had greater reduction in disease progression risk than those with exon 19 deletions in the combination group (29, 30, 33), whereas only one phase II trial (JO25567) showed contrasting results (27). In the subgroup analysis in the CTONG 1509 study, the PFS of patients with exon 21 Leu858Arg mutations was 19.5 months in the erlotinib plus bevacizumab group (33), which is the longest PFS observed to date. This result exceeded the 14.4 months PFS of patients receiving monotherapy with the third-generation TKI osimertinib (7). Apparently, the combination of erlotinib plus anti-VEGF/VEGFR agents is more beneficial for patients with 21 Leu858Arg mutations. Therefore, the synergistic antiproliferative effects of EGFR-TKIs and antiangiogenic treatment might eliminate the prognosis differences caused by genetic mutations. Nevertheless, we await the OS data to verify this hypothesis.
Patients with brain metastasis have poor prognosis. The probability of brain metastasis in patients harboring EGFR mutations is three-fold greater than that in patients without EGFR mutations. Among the five studies included in this meta-analysis, only three (NEJ026, CTONG 1509, and Stinchcombe, T. E. 2019) (30, 31, 33) recruited patients with brain metastasis at baseline. The phase II trial in the United States (31) reported that baseline brain metastasis status was not related to PFS (HR = 1.72, 95% CI = 0.92–3.23). Although pooled PFS of patients with brain metastasis at baseline was not statistically different, a tendency of PFS benefit could be observed using combination therapy. In the CTONG 1509 study, combination therapy reduced the risk of progression by half in brain metastatic patients (33). Another study demonstrated that the intracranial tumor control rate in brain metastatic patients in the erlotinib and bevacizumab combination group was almost twice that in the erlotinib monotherapy group (49). However, current data are limited to reach a definitive conclusion. Future studies of EGFR-TKIs combined with antiangiogenic agents could consider including patients with brain metastasis.
Objective response rate and DCR are both implicated in tumor response to drugs. In the combination group, the proportion of patients who reached complete response was low, with only 3 of the 224 patients (1%) in the RELAY study reaching complete response (29). According to our meta-analysis results, there were no differences in ORR and DCR improvement between erlotinib plus anti-VEGF/VEGFR drugs and erlotinib alone. Overall survival extension is the ultimate goal of antitumor therapy. Our meta-analysis result of OS was negative, which might be attributed to the limited data from the two phase II RCTs. After OS is reached in the other three phase III RCTs, we will update the pooled results. Overall survival is affected by different factors, such as the statistical design of the trials, posterior treatments, and complicated biological mechanisms. A preclinical study found that anti-VEGF therapy increased tumor hypoxia, hypoxia-inducible factor 1α, and c-Met activation and facilitated cancer invasion and metastasis (50). More in-depth research and clinical data are required to better assess the long-term benefits of combination therapy.
Furthermore, safety should be considered for treatment evaluation. Adverse events, especially grades 3–5 AEs, remarkably impair patients' treatment compliance and quality of life, thereby indirectly undermining antitumor efficacy. Our pooled results demonstrate that acneiform, hypertension, proteinuria, and diarrhea evidently increased in the combination group. However, the difference in side effects caused by different combinations of EGFR-TKI and antivascular drugs is unclear. Following the publication of more trial results, a network meta-analysis may be a good approach to comprehensively compare AEs. Moreover, we found that increased AEs were associated with better PFS. Concurrently, two other studies found that antiangiogenesis-related AEs were associated with prolonged PFS and OS (51, 52). Therefore, on the basis of close monitoring, timely identification, and instant management of AEs, EGFR-TKI combined with anti-VEGF/VEGFR agents may be a favorable strategy in the first-line setting for EGFR mutant NSCLC.
This study had several limitations. First, the reliability of our results is reduced by the limited number of studies. Second, some trials are ongoing and the final OS data have not been announced. Third, insufficient data on brain metastasis and race led to unreliable analyses results. Fourth, a test of publication bias was not performed as the number of included trials was fewer than 10. Several clinical investigations that aim to explore the role of EGFR-TKIs plus antiangiogenic drugs in the first-line setting for advanced NSCLC are underway (35–38, 43). Thus, we anticipate the publication of new data and will update the meta-analysis results to better guide clinical decisions.
In summary, this meta-analysis demonstrates that the first-generation EGFR-TKI erlotinib plus antiangiogenesis therapy yields more benefits in terms of PFS when administered as a first-line therapy for EGFR-sensitive mutations NSCLC than erlotinib alone. The combination therapy resulted in evident PFS benefits for Asian patients. Further, exon 19 deletions and 21 Leu858Arg mutations were found to gain similar benefits from the administration of combination therapy. Patients with brain metastases at baseline also tended to display PFS benefits. The combination strategy causes a higher incidence of grades 3–5 diarrhea, acneiform, hypertension, and proteinuria. Our work provided evidence to support the addition of antiangiogenesis agents to anti-EGFR therapy for use as a first-line treatment for EGFR-mutant NSCLC patients. Several studies on combination therapy are ongoing, and an update of the pooled results is required after the data are updated to better determine the practical application of EGFR-TKIs in combination with antiangiogenic agents.
All datasets analyzed for this study are included in the article/Supplementary Material.
FC and JC conceived and designed the study. FC and NC performed the literature search, data extraction, quality assessment of the included studies, and statistical analysis. FC wrote the paper. NC, YY, and JC reviewed and edited the manuscript. All authors read and approved the manuscript.
This study was supported by the National Key R&D Program of China (No. 2016YFC1303800), Thirteenth Five-Year Plan Science and Technology Project of Education Department of Jilin Province (JJKH20190020KJ), and Health Technology Innovation Project of Jilin Province (2017J064).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to all authors that participated in this study. We thank Dr. C. T. Zhu for providing outstanding technical assistance. Additionally, we would like to thank Editage for English language editing.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00904/full#supplementary-material
Table S1. PRISMA 2009 checklist.
Figure S1. Sensitivity analysis of PFS (A) and ORR (B) associated with erlotinib plus antiangiogenic agents vs. erlotinib.
Figure S2. Sensitivity analysis of DCR (A), OS (B), and the grade ≥3 AEs (C) associated with erlotinib plus antiangiogenic agent vs. erlotinib.
Figure S3. Sensitivity analysis of diarrhea (A), acneiform (B), hypertension (C), and proteinuria (D) associated with erlotinib plus antiangiogenic agent vs. erlotinib.
Figure S4. Sensitivity analysis of the Asian population and its association with erlotinib plus antiangiogenic agent vs. erlotinib.
Figure S5. Sensitivity analysis of Exon 19 deletions (A) and Exon 21 Leu858Arg mutations (B) and their association with erlotinib plus antiangiogenic agents vs. erlotinib.
Figure S6. Sensitivity analysis of brain metastasis at baseline (A) and no brain metastases at baseline (B) and their association with erlotinib plus antiangiogenic agent vs. erlotinib.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol. (2018) 15:694–708. doi: 10.1038/s41571-018-0081-4
3. Hsu W-H, Yang J-H, Mok T, Loong H. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. (2018) 29:i3–i9. doi: 10.1093/annonc/mdx702
4. Carlisle JW, Ramalingam SS. Role of osimertinib in the treatment of EGFR-mutation positive non-small-cell lung cancer. Future Oncol. (2019) 15:805–16. doi: 10.2217/fon-2018-0626
5. Park K, Tan EH, O'byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. (2016) 17:577–89. doi: 10.1016/S1470-2045(16)30033-X
6. Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. (2017) 18:1454–66. doi: 10.1016/S1470-2045(17)30608-3
7. Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
8. Hanahan D, Weinberg R A. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
9. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. (2011) 146:873–87. doi: 10.1016/j.cell.2011.08.039
10. Martino E, Misso G, Pastina P, Costantini S, Vanni F, Gandolfo C, et al. Immune-modulating effects of bevacizumab in metastatic non-small-cell lung cancer patients. Cell Death Dis. (2016) 2:16025. doi: 10.1038/cddiscovery.2016.25
11. Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. (2013) 123:3190–200. doi: 10.1172/JCI70212
12. Gille J, Swerlick RA, Caughman SW. Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J. (1997) 16:750–9. doi: 10.1093/emboj/16.4.750
13. Goldman CK, Kim J, Wong W, King V, Brock T, Gillespie G. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. (1993) 4:121–33. doi: 10.1091/mbc.4.1.121
14. Yen L, You X-L, Al Moustafa A-E, Batist G, Hynes NE, Mader S, et al. Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis. Oncogene. (2000) 19:3460. doi: 10.1038/sj.onc.1203685
15. Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. (2009) 15:3484–94. doi: 10.1158/1078-0432.CCR-08-2904
16. Larsen AK, Ouaret D, El Ouadrani K, Petitprez A. Targeting EGFR and VEGF(R) pathway cross-talk in tumor survival and angiogenesis. Pharmacol Ther. (2011) 131:80–90. doi: 10.1016/j.pharmthera.2011.03.012
17. Ciardiello F, Bianco R, Caputo R, Caputo R, Damiano V, Troiani T, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res. (2004) 10:784–93. doi: 10.1158/1078-0432.CCR-1100-03
18. Li H, Takayama K, Wang S, Shiraishi Y, Gotanda K, Harada T, et al. Addition of bevacizumab enhances antitumor activity of erlotinib against non-small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother Pharmacol. (2014) 74:1297–305. doi: 10.1007/s00280-014-2610-x
19. Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. (2001) 61:5090–101. Available online at: https://cancerres.aacrjournals.org/content/61/13/5090.short
20. Masuda C, Yanagisawa M, Yorozu K, Kurasawa M, Furugaki K, Ishikura N, et al. Bevacizumab counteracts VEGF-dependent resistance to erlotinib in an EGFR-mutated NSCLC xenograft model. Int J Oncol. (2017) 51:425–34. doi: 10.3892/ijo.2017.4036
21. Furugaki K, Fukumura J, Iwai T, Yorozu K, Kurasawa M, Yanagisawa M, et al. Impact of bevacizumab in combination with erlotinib on EGFR-mutated non-small cell lung cancer xenograft models with T790M mutation or MET amplification. Int J Cancer. (2016) 138:1024–32. doi: 10.1002/ijc.29848
22. Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. (2003) 88:1979–86. doi: 10.1038/sj.bjc.6601005
23. Dickson PV, Hamner JB, Sims TL, Fraga CH, Ng CY, Rajasekeran S, et al. Bevacizumab-induced transient remodeling of the vasculature in neuroblastoma xenografts results in improved delivery and efficacy of systemically administered chemotherapy. Clin Cancer Res. (2007) 13:3942–50. doi: 10.1158/1078-0432.CCR-07-0278
24. Furugaki K, Iwai T, Moriya Y, Harada N, Fujimoto-Ouchi K. Loss of an EGFR-amplified chromosome 7 as a novel mechanism of acquired resistance to EGFR-TKIs in EGFR-mutated NSCLC cells. Lung Cancer. (2014) 83:44–50. doi: 10.1016/j.lungcan.2013.10.003
25. Hayakawa H, Ichihara E, Ohashi K, Ninomiya T, Yasugi M, Takata S, et al. Lower gefitinib dose led to earlier resistance acquisition before emergence of T790M mutation in epidermal growth factor receptor-mutated lung cancer model. Cancer Sci. (2013) 104:1440–6. doi: 10.1111/cas.12284
26. Ichihara E, Ohashi K, Takigawa N, Osawa M, Ogino A, Tanimoto M, et al. Effects of vandetanib on lung adenocarcinoma cells harboring epidermal growth factor receptor T790M mutation in vivo. Cancer Res. (2009) 69:5091–8. doi: 10.1158/0008-5472.CAN-08-4204
27. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol. (2014) 15:1236–44. doi: 10.1016/S1470-2045(14)70381-X
28. Kato T, Seto T, Nishio M, Goto K, Yamamoto N, Okamoto I, et al. Erlotinib plus bevacizumab phase II study in patients with advanced non-small-cell lung cancer (JO25567): updated safety results. Drug Saf. (2018) 41:229–37. doi: 10.1007/s40264-017-0596-0
29. Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. (2019) 20:1655–69. doi: 10.1016/S1470-2045(19)30634-5
30. Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. (2019) 20:625–35. doi: 10.1016/S1470-2045(19)30035-X
31. Stinchcombe TE, Janne PA, Wang X, Bertino EM, Weiss J, Bazhenova L, et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. (2019) 5:1448–55. doi: 10.1001/jamaoncol.2019.1847
32. Yamamoto N, Seto T, Nishio M, Goto K, Okamoto I, Yamanaka T, et al. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced EGFR mutation-positive nonsquamous non-small-cell lung cancer (NSCLC): survival follow-up results of JO25567. J Clin Oncol. (2018) 36:9007. doi: 10.1200/JCO.2018.36.15_suppl.9007
33. Zhou Q, Wu YL, Cheng Y, Liu Y, Chen G. ARTEMIS(CTONG 1509): Phase 3 study of bevacizumab with or without erlotinib in untreated chinese patients with advanced EGFR-mutated NSCLC. Ann Oncol. (2019) 30(Suppl 5):V603. doi: 10.1093/annonc/mdz260.002
34. Yu HA, Kim R, Makhnin A, Ahn LSH, Ni A, Hayes SA, et al. A phase 1/2 study of osimertinib and bevacizumab as initial treatment for patients with metastatic EGFR-mutant lung cancers [M]. Am Soc Clin Oncol. (2019) 37(15 Suppl):9086. doi: 10.1200/JCO.2019.37.15_suppl.9086
35. Nct. Gefitinib in Combination with Anlotinib or Placebo in Previously Untreated EGFR-mutant NSCLC. (2019). Available online at: https://clinicaltrialsgov/show/NCT04028778
36. Ninomiya T, Ishikawa N, Inoue K, Kubo T, Yasugi M, Shibayama T, et al. Phase 2 study of afatinib alone or combined with bevacizumab in chemonaive patients with advanced non-small-cell lung cancer harboring EGFR mutations: AfaBev-CS study protocol. Clin Lung Cancer. (2019) 20:134–8. doi: 10.1016/j.cllc.2018.10.008
37. Kitagawa C, Kada A, Saito AM, Ichinose Y, Saka H. Rationale and design of a randomized phase 2 trial of gefitinib plus bevacizumab vs gefitinib alone in patients with epidermal growth factor receptor mutant non-squamous non-small-cell lung cancer: study protocol. Kurume Med J. (2019) 65:77–81. doi: 10.2739/kurumemedj.MS652001
38. Lu S, Zhou J, Niu X, Chen M, Hua Y, Su WA. Phase II study of fruquintinib in combination with gefitinib in stage IIIb/IV NSCLC patients harboring EGFR activating mutations. J Thorac Oncol. (2017) 12:S1735. doi: 10.1016/j.jtho.2017.09.301
39. Ichihara E, Hotta K, Nogami N, Kuyama S, Kishino D, Fujii M, et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial (1001). J Thorac Oncol. (2015) 10:486–91. doi: 10.1097/JTO.0000000000000434
40. Herbst RS, Ansari R, Bustin F, Flynn P, Hart L, Otterson GA, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet. (2011) 377:1846–54. doi: 10.1016/S0140-6736(11)60545-X
41. Zhang Y, Zeng L, Yang N, Li Y, Hu D. Real world data of 1st line A+T in advanced EGFR+ NSCLC: clinical outcome, PFS influential factors and acquired resistance. J Thorac Onco. (2018) 13:S1090. doi: 10.1016/j.jtho.2018.10.142
42. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. (2020) 382:41–50. doi: 10.1056/NEJMoa1913662
43. Nct. Osimertinib and Bevacizumab Versus Osimertinib Alone as Second-line Treatment in Stage IIIb-IVb NSCLC with Confirmed EGFRm and T790M (BOOSTER). (2017). Available online at: https://clinicaltrialsgov/show/NCT03133546
44. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. (2011) 3:75ra26. doi: 10.1126/scitranslmed.3002003
45. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. (2013) 19:2240–7. doi: 10.1158/1078-0432.CCR-12-2246
46. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. (2014) 11:473–81. doi: 10.1038/nrclinonc.2014.104
47. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. (2005) 97:339–46. doi: 10.1093/jnci/dji055
48. Sheng M, Wang F, Zhao Y, Li S, Wang X, Shou T, et al. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: a meta-analysis. Eur J Clin Pharmacol. (2016) 72:1–11. doi: 10.1007/s00228-015-1966-0
49. Feng PH, Chen KY, Huang YC, Luo CS, Wu SM, Chen TT, et al. Bevacizumab reduces S100A9-positive MDSCs linked to intracranial control in patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol. (2018) 13:958–67. doi: 10.1016/j.jtho.2018.03.032
50. Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. (2012) 2:270–87. doi: 10.1158/2159-8290.CD-11-0240
51. Liu X, Qin S, Wang Z, Xu J, Xiong J, Bai Y, et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol. (2017) 10:153. doi: 10.1186/s13045-017-0521-0
Keywords: epidermal growth factor receptor inhibitors, angiogenesis inhibitors, non-small cell lung cancer, EGFR mutation, anti-VEGF, targeted treatment, first line, meta-analysis
Citation: Chen F, Chen N, Yu Y and Cui J (2020) Efficacy and Safety of Epidermal Growth Factor Receptor (EGFR) Inhibitors Plus Antiangiogenic Agents as First-Line Treatments for Patients With Advanced EGFR-Mutated Non-small Cell Lung Cancer: A Meta-Analysis. Front. Oncol. 10:904. doi: 10.3389/fonc.2020.00904
Received: 07 March 2020; Accepted: 11 May 2020;
Published: 25 June 2020.
Edited by:
Karen L. Reckamp, Cedars-Sinai Samuel Oschin Comprehensive Cancer Institute, United StatesReviewed by:
Jared Weiss, University of North Carolina at Chapel Hill, United StatesCopyright © 2020 Chen, Chen, Yu and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiuwei Cui, Y3VpandAamx1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.