- 1Department of Urology, Yichun People's Hospital, Yichun, China
- 2Department of Medical Record Management, Chinese Air Force Specialty Medical Center, Beijing, China
Background: To investigate the potential prognostic role of serum lactate dehydrogenase (LDH) in patients with urothelial carcinoma (UC) using the method of systematic review and meta-analysis.
Materials and Methods: We searched PubMed, Embase, Cochrane Library, and Web of Science for eligible studies up to February 2020. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were used to estimate the relationship.
Results: A total of 14 studies including 4,009 patients with UC were incorporated. The results showed that a high pretreatment serum LDH was associated with an inferior overall survival (OS, HR 1.61, 95% CI 1.39–1.87, p < 0.001), cancer-specific survival (CSS, HR 1.41, 95% CI 1.05–1.90, p = 0.022), and disease-free survival (DFS, HR 1.64, 95% CI 1.04–2.59, p = 0.034) in UC. Subgroup analyses identified that a high pretreatment serum LDH was associated with a poor OS (HR 1.97, 95% CI 1.02–3.81, p = 0.042) and DFS (HR 1.64, 95% CI 1.04–2.59, p = 0.034) in upper tract urothelial carcinoma, a short OS (HR 1.71, 95% CI 1.37–2.15, p < 0.001) in urothelial carcinoma of bladder.
Conclusion: Our findings indicated that a high level of pretreatment serum LDH was associated with inferior OS, CSS, and DFS in patients with UC. This biomarker can be an important factor incorporated into the prognostic models for UC.
Introduction
Urothelial carcinoma (UC), mainly consisting of upper tract urothelial carcinoma (UTUC) and urothelial carcinoma of the bladder (UCB), is, respectively estimated to have 85,000 new incidences and 18,000 related mortality in the United States in 2018. These made UC become the 4th and 12th most common malignance for males and females (1). Multiple lesions, high rate of recurrences, and distant metastasis are typical features of UC. Despite advances in surgical techniques and developments of preoperative and postoperative adjuvant therapy, the long-term survival of cases with UC have not significantly changed over these years (2). Especially, for patients with advanced UC, median overall survival was only 3 to 6 months without therapy, and prolonged to 13–16 months when receiving systematic chemotherapy (3). Therefore, it is important to determine prognostic factors for timely adjustment of treatment.
Previous literatures have found that cancer cells metabolize differently from normal cells, which means more lactate seems to be needed for cancer cells (4). Lactate dehydrogenase (LDH), an enzyme that catalyzes lactic acid into pyruvate, may exert a crucial role in the metabolism of tumor cells. High level of serum LDH has been reported to serve as an unfavorable prognostic factor in kinds of malignances, including prostate cancer, renal cell carcinoma, lymphoma, colorectal cancer, and lung cancer (5). Moreover, many studies have identified the prognostic role of pretreatment serum LDH in cases with UC. Because circulating blood LDH is easy to be measured clinically, it can be used as an indicator of cancer burden and a useful biomarker in clinical management. In the present study, we aimed to systematic review literatures studying the prognostic role of pretreatment LDH in UC and merged the quantitative data.
Methods
Study Design
The present study was performed according to the PRISMA statements (Supplementary Material) (6). The protocol has been registered on PROSPERO (No. CRD42019147216).
Literature Searching
In order to examine the prognostic significance of serum lactate dehydrogenase in urothelial carcinoma, we searched databases PubMed, Embase, Cochrane Library, and Web of Science up to February 2020 to identify related literatures. The following major terms were used to constitute the search strategy: “lactate dehydrogenase” (e.g., “lactate dehydrogenase,” “LDH,” “lactic dehydrogenase”), “urothelial carcinoma” (e.g., “urothelial carcinoma,” “transitional cell carcinoma,” “urothelial tumor,” “urothelial cancer”), and “prognosis” (e.g., “prognosis,” “survival,” “progression,” “recurrence,” “mortality,” “outcome”). Additionally, we manually screened literature references to identify relevant studies. There was no language restriction in the process of literature searching.
Selection Criteria
In general, the present study included literatures investigating the prognostic role of pretreatment of serum lactate dehydrogenase (LDH) in patients with urothelial carcinoma, and detailed criteria were shown as follows. Inclusion criteria: (1) studies involving patients confirmed diagnosed with urothelial carcinoma; (2) studies that measured serum lactate dehydrogenase of patients before treatment; (3) studies that reported the results of oncological outcomes including overall survival (OS), cancer-specific survival (CSS), and disease-free survival (DFS); (4) studies that directly provided hazard ratios (HRs) and 95% confidence interval (CI) or required data for calculating them. The methods for calculation were reported by Tierney et al. (7). Exclusion criteria: (1) studies wherein we cannot extract HRs and 95% CI; (2) studies that did not analyze serum lactate dehydrogenase as dichotomous variable or did not clearly report the cut-off value; (3) not original article, such as review, abstract, opinion, letter, and so on; (4) duplicated results from the same cohort. Two researchers independently screened and evaluated the literature; the disputes were resolved by discussion.
Data Extraction and Quality Assessment
These two important steps were independently performed by two researchers, and the disputes were resolved by discussion. According to a pre-designed table, items of data extraction included the first author's last name, publication year, belonging country, number of subjects included, patient's age, cancer type (urothelial carcinoma, upper tract urothelial carcinoma, or urothelial carcinoma of bladder), cancer stage, cut-off value and decision method, therapies that patients underwent, endpoints of oncological outcomes, HRs and 95% CIs (from univariate or multivariate Cox analysis), follow-up durations, and adjusted variables in multivariate Cox analysis.
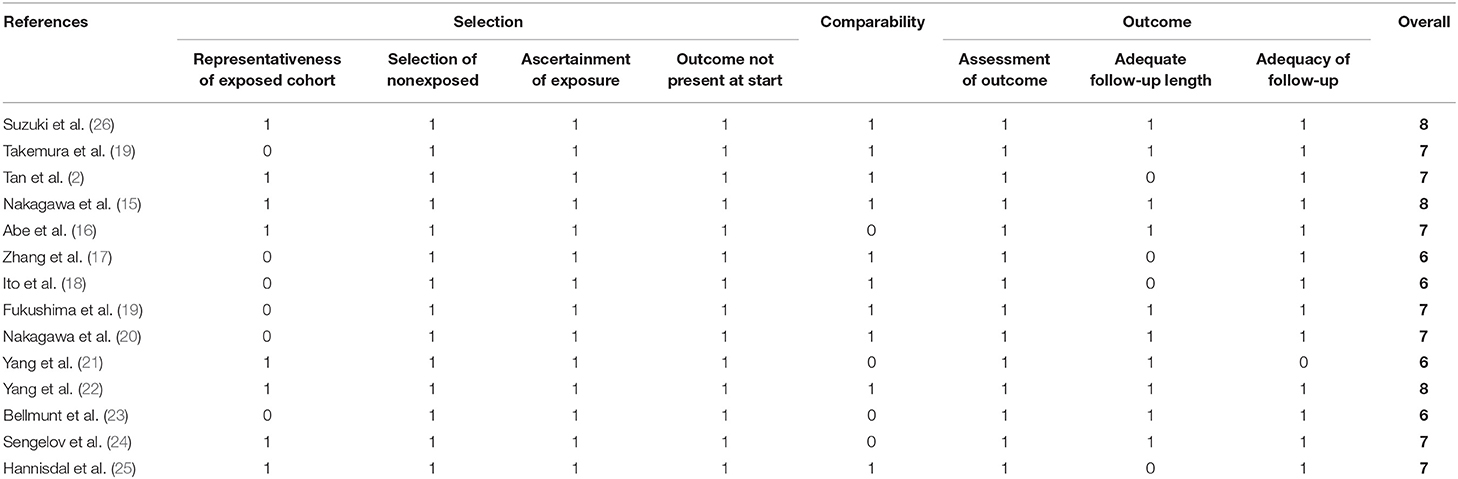
The quality of each study was evaluated using the Newcastle–Ottawa scale, which embraced three aspects including patient selection, comparability, assessment of outcome, and follow-ups.
Statistical Analysis
We extracted all HRs and 95% CIs of related endpoints from included studies. Subgroup analyses of overall survival were also conducted. The grouping variables included publication year, region, site of malignance, number of patients, cancer stage, cut-off value, and NOS score. Meta-regression was also performed to identify the resources of interstudy heterogeneity. Subgroup analyses of upper tract urothelial carcinoma (UTUC) and urothelial carcinoma of the bladder (UCB) were also performed. HRs and the corresponding 95% CI were used to assess the significance of the prognostic value of serum lactate dehydrogenase for urothelial carcinoma. A pooled value >1 was regarded as indicating an unfavorable outcome for the subjects having a high level of serum lactate dehydrogenase. Cochran's Q test and Higgins I2 statistic were both conducted to examine inter-study heterogeneity. A random effects model was applied for all analyses. Publication bias was assessed by funnel plots, Egger's, and Begg's tests. Sensitivity analysis was performed to check the stability of the results. All statistical analyses were performed with Stata 12.0 (STATA Corporation, College Station, TX, USA).
Results
Study Searching and Screening
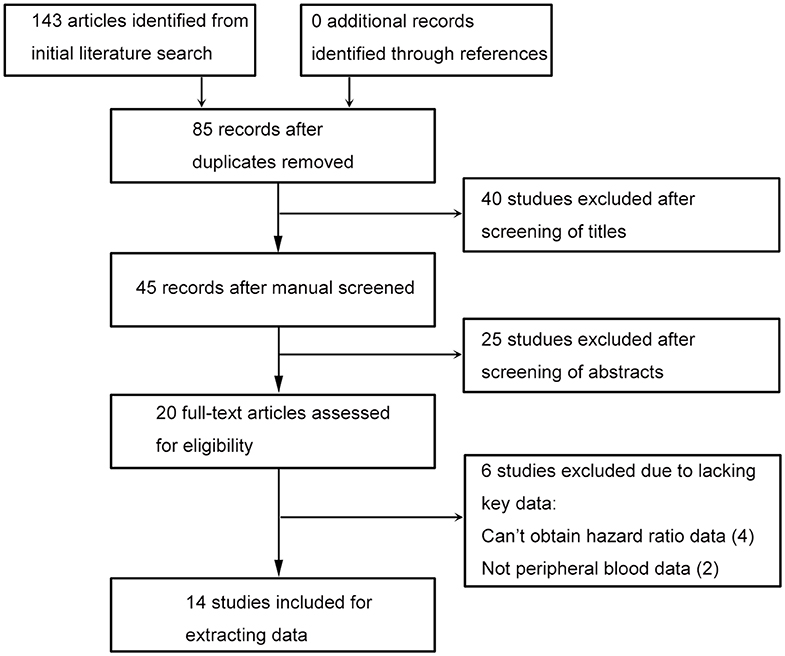
The flowchart of this process is presented in (Figure 1). Database searching identified 143 articles, and no additional study was found by checking the references. Eighty-five studies were remained after excluding duplicated records. Title and abstract screening, respectively, excluded 40 and 25 literatures, then 20 studies were assessed with full text. Since there were four studies wherein hazard ratio data cannot be obtained (8–11), and two studies had no peripheral blood date (12, 13), 14 literatures were included last for data extraction (2, 14–25).
Baseline features of the included studies are shown in (Table 1). Nine studies were published recently (2013–2020) by Chinese and Japanese researchers (2, 14–20, 26). Five studies were published 16 years (1993–2002) ago by Chinese and European researchers (21–25). The median or mean age of patients ranged from 60.3 to 73 years, and the sample size ranged from 56 to 1,087. Seven studies focused on urothelial carcinoma (14, 16, 19, 22–24, 26), three focused on UTUC (2, 17, 18), and four focused on UCB (15, 20, 21, 25). Of all 40 studies, 10 provided results of multivariable Cox analysis (2, 14, 15, 17–20, 22, 25, 26). The adjusted factors embraced clinical, pathological, and laboratorial variables, which are detailed in (Table 2). All studies were of low-to-moderate risk of bias, the NOS score ranged from 6 to 8, which are presented in (Table 3).
Urothelial Carcinoma
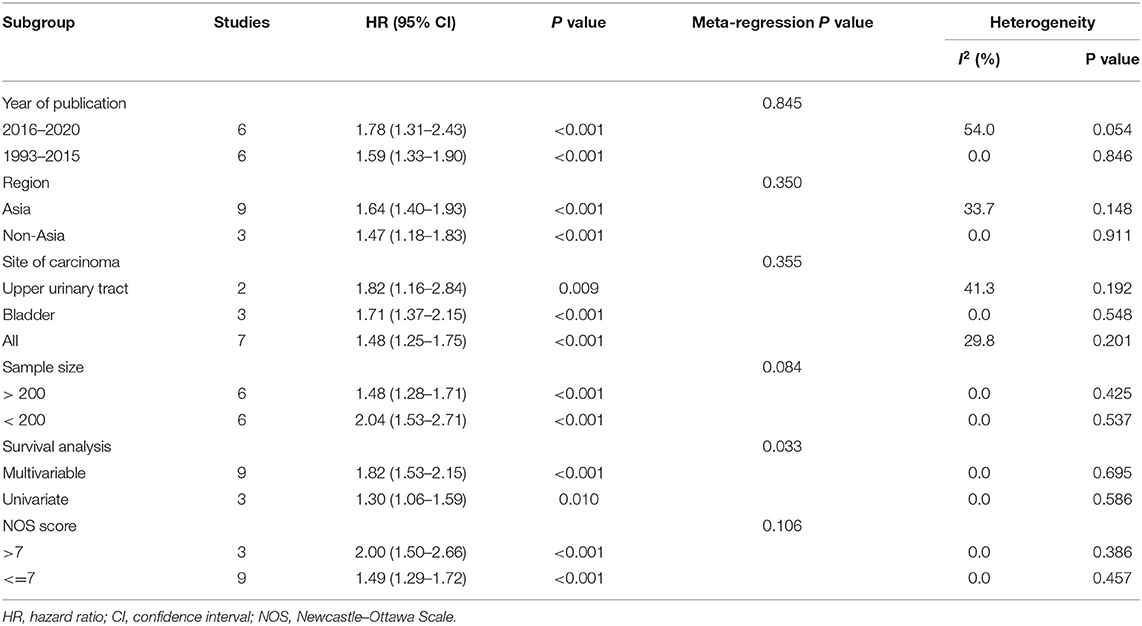
First, all included studies were analyzed together. For the three endpoints (overall survival, cancer-specific survival, disease-free survival), there were 12 (2, 14–17, 19, 20, 22–26), 2 (2, 21), and 3 (2, 17, 18) studies, respectively, that reported related results. After merging HRs and 95% CIs, we identified that a high pretreatment serum LDH was associated with an inferior overall survival (HR 1.61, 95% CI 1.39–1.87, p < 0.001), cancer-specific survival (HR 1.41, 95% CI 1.05–1.90, p = 0.022), and disease-free survival (HR 1.64, 95% CI 1.04–2.59, p = 0.034) in patients with urothelial carcinoma (Figure 2). Subgroup analyses of overall survival were also conducted, and the results are presented in (Table 4). The subgroup variables included year of publication, region, site of carcinoma, sample size, survival analysis, and NOS score, which did not obviously change the results. Moreover, except for survival analysis (p = 0.033), other subgroup variables were not the resources of inter-study heterogeneity (P > 0.05 for all).
UTUC and UCB
Subgroup analyses of UTUC and UCB were also performed. There were three and three studies, respectively, that were included in the subgroup analyses of UTUC (2, 17, 18) and UCB (15, 20, 25). After merging HRs and 95% CIs, we identified that a high pretreatment serum LDH was associated with a poor overall survival (HR 1.97, 95% CI 1.02–3.81, p = 0.042) and disease-free survival (HR 1.64, 95% CI 1.04–2.59, p = 0.034) in patients with UTUC (Figure 3A). A high pretreatment serum LDH was associated with a short overall survival (HR 1.71, 95% CI 1.37–2.15, p < 0.001) in patients with UCB (Figure 3B).
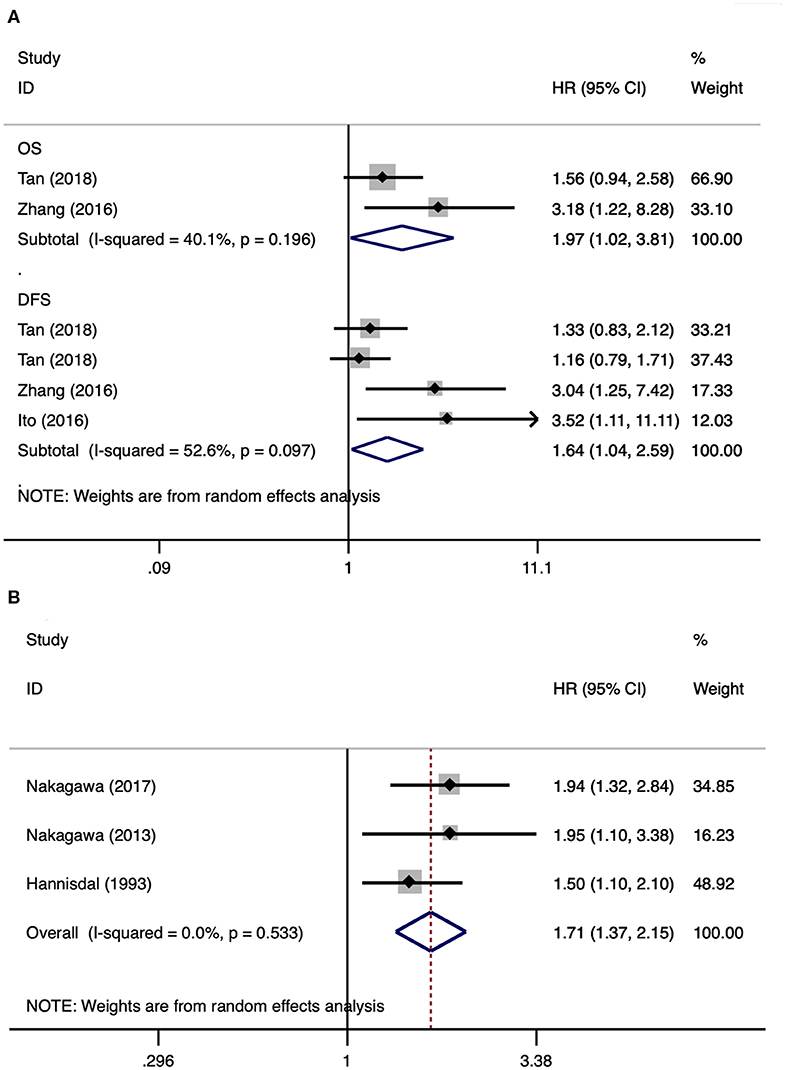
Figure 3. Forest plots of studies about upper tract urothelial carcinoma and urothelial carcinoma of the bladder. (A) Upper tract urothelial carcinoma; (B) urothelial carcinoma of the bladder.
Publication Bias and Sensitivity Analysis
Since most analyses embraced insufficient literatures, we only checked publication bias and performed sensitivity analysis for overall survival of patients with urothelial carcinoma. The funnel plot showed an approximately asymmetric result, and quantitative tests identified significant differences (Begg's test: p = 0.024; Egger's test: p = 0.005) (Figure 4A). The result of sensitivity analysis showed that excluding any study did not significantly change the merged data (Figure 4B).
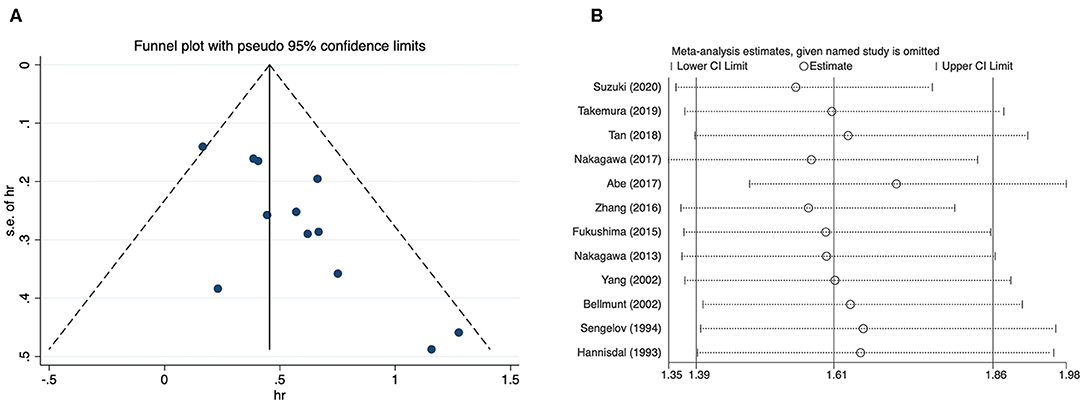
Figure 4. Funnel plot and sensitivity analysis for overall survival of urothelial carcinoma. (A) Funnel plot; (B) sensitivity analysis.
Discussion
Previous literatures have found that cancer cells metabolize differently from normal cells. Even with sufficient oxygen, malignant cells will preferentially metabolize glucose through glycolysis to produce adequate energy for growth, which is known as the Warburg effect and is one of the major metabolic changes in the process of malignant transformation (4). The serum level of LDH, the enzyme involved in the glycolytic pathway, can reflect the metabolic alterations (27). A high level of serum LDH has been reported to serve as an unfavorable prognostic factor in several types of malignances. Several studies have examined the prognostic role of LDH in subjects with UC and have reported inconsistent results. Hence, the present systematic review and meta-analysis about this issue was performed.
After systematic literature searching and screening, 14 studies embracing 4,009 patients were included. All studies were of low-to-moderate risk of bias, and the NOS score ranged from 6 to 8. The HRs and 95% CIs extracted from these studies were merged. The results showed that a high pretreatment serum LDH was associated with an inferior overall survival, cancer-specific survival, and disease-free survival in UC. Subgroup analyses of OS showed that grouping variable did not obviously change the results. Moreover, sensitivity analysis further confirmed the stability and reliability of the results. Subgroup analyses of UTUC and UCB were also performed. We identified that a high pretreatment serum LDH was associated with a poor overall survival and disease-free survival in UTUC, a short overall survival in UCB. Since circulating blood LDH is easy to be measured clinically, it can be used as an indicator of cancer burden and a useful biomarker in management of UC.
For the prognostic significance of pretreatment LDH in cases with UC, only one meta-analysis previously was reported. In 2016, Zhang et al. (28) performed a meta-analysis to evaluate the prognostic role of LDH for cases with urological cancer. They included two studies about bladder cancer and one study about UTUC. A high level of serum LDH has been reported to serve as an unfavorable prognostic factor. With more and later studies being included, the present study may provide more comprehensive and reliable results for this issue.
Metabolically, the most prominent feature of cancer cells is an increase in lactic acid production due to their increased glucose uptake rate and reduced oxidative phosphorylation rate, regardless of the availability of oxygen. The phenomenon, called aerobic glycolysis, was first discovered 70 years ago by Otto Warburg (4). As an important substance of the Warburg effect, LDH exists in nearly all kinds of tissues, which has six different isoenzymes. These isoenzymes are assembled from two protein subunits, LDHA and LDHB, into a homo- or hetero-tetramer structure (29). The third protein subunit, called LDHC, forms the testicular specific subtype LDH-6 (30). The main function of lactate dehydrogenase is to catalyze the reversible reaction between pyruvate and lactic acid. NAD+ is produced along with this process and is necessary for the continuous production of ATP to maintain glycolysis. Among the LDH isoforms, LDH-5 has the highest catalytic efficiency (31). When cancer tissue is necrotic, high intracellular LDH levels are released into the blood, increasing serum LDH concentrations (32). In addition, when distant metastases occur, tumor cells can damage adjacent organs, such as the lung, liver, and bone. Damage to these organs can also increase serum LDH levels (31, 33–35). In conclusion, serum LDH seems to be a significant factor in the development of malignance. Its level can reflect tumor burden and serve as cancer biomarker.
There were several limitations for the present study. First, due to limited literatures, we studied all types and stages of UC together. The differences in type, stage, and treatment for UC may be the sources of inter-study heterogeneity. Despite both belonging to UC, UTUC, and UCB may have different biological behaviors and disease characteristics. Hence, subgroup analyses of UTUC and UCB were also performed. Second, serum LDH may be affected by other non-tumor diseases, such as anemia, hepatic disease, muscular dystrophy, and heart failure, which have not been examined in these studies. Moreover, the present study also included results from univariable analysis. These uncontrolled factors may affect the results, and the methods of survival analysis have been found to be the source of inter-study heterogeneity. Third, taken that different cut-off values were used in the included literatures, it is a problem to choose the best one, which may affect the application of this biomarker in clinical practice. Fourth, a significant publication bias was found in the meta-analysis of overall survival, which cannot be overcome by statistical methods. Regardless, the present study represents the most comprehensive and latest systematic review and meta-analysis about the prognostic role of LDH in patients with UC.
In general, the present study proved that a high level of pretreatment serum LDH was associated with inferior OS, CSS, and DFS in patients with UC. Moreover, an elevated level of serum LDH was associated with poor OS and DFS in patients with UTUC, a short OS in patients with UCB. Despite more high-level studies were needed to verify our results, the level of serum LDH can be an important factor incorporated into the prognostic models for UC.
Author Contributions
MW and PL: protocol/project development. LX, ZY, and QC: data collection or management. MW, HG, and CL: data analysis. MW and PL: manuscript writing/editing. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00677/full#supplementary-material
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics 2018. CA Cancer J Clin. (2018) 68:7–30. doi: 10.3322/caac.21442
2. Tan P, Chen J, Xie N, Xu H, Ai J, Xu H, et al. Is preoperative serum lactate dehydrogenase useful in predicting the outcomes of patients with upper tract urothelial carcinoma? Cancer Med. (2018) 7:5096–106. doi: 10.1002/cam4.1751
3. Bellmunt J, von der Maase H, Mead GM, Skoneczna I, De Santis M, Daugaard G, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. (2012) 30:1107–13. doi: 10.1200/JCO.2011.38.6979
4. Warburg O. On the origin of cancer cells. Science. (1956) 123:309–14. doi: 10.1126/science.123.3191.309
5. Zhang J, Yao YH, Li BG, Yang Q, Zhang PY, Wang HT. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: a systematic review and meta-analysis. Sci Rep. (2015) 5:9800. doi: 10.1038/srep09800
6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
7. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
8. Inamoto T, Matsuyama H, Ibuki N, Komura K, Fujimoto K, Shiina H, et al. Risk stratification by means of biological age-related factors better predicts cancer-specific survival than chronological age in patients with upper tract urothelial carcinoma: a multi-institutional database study. Ther Adv Urol. (2018) 10:403–10. doi: 10.1177/1756287218811050
9. Koie T, Ohyama C, Yamamoto H, Hatakeyama S, Imai A, Yoneyama T, et al. Significance of preoperative butyrylcholinesterase as an independent predictor of survival in patients with muscle-invasive bladder cancer treated with radical cystectomy. Urol Oncol. (2014) 32:820–5. doi: 10.1016/j.urolonc.2014.03.010
10. Deshpande N, Mitchell IP, Hayward SW, Love S, Towler JM. Tumor enzymes and prognosis in transitional cell carcinoma of the bladder: prediction of risk of progression in patients with superficial disease. J Urol. (1991) 146:1247–51. doi: 10.1016/S0022-5347(17)38060-6
11. Mitchell I, Hayward S, Deshpande N, Towler JM. Enzyme studies in human transitional cell carcinoma of the urinary bladder. J Urol. (1989) 141:1234–7. doi: 10.1016/S0022-5347(17)41228-6
12. Wang Z, Zhou Q, Li A, Huang W, Cai Z, Chen W. Extracellular matrix protein 1 (ECM1) is associated with carcinogenesis potential of human bladder cancer. OncoTarg Ther. (2019) 12:1423–32. doi: 10.2147/OTT.S191321
13. Liu X, Yao D, Liu C, Cao Y, Yang Q, Sun Z, et al. Overexpression of ABCC3 promotes cell proliferation, drug resistance, and aerobic glycolysis and is associated with poor prognosis in urinary bladder cancer patients. Tum Biol. (2016) 37:8367–74. doi: 10.1007/s13277-015-4703-5
14. Takemura K, Fukushima H, Ito M, Kataoka M, Nakanishi Y, Sakamoto K, et al. Prognostic significance of serum gamma-glutamyltransferase in patients with advanced urothelial carcinoma. Urol Oncol. (2019) 37:108–15. doi: 10.1016/j.urolonc.2018.11.002
15. Nakagawa T, Taguchi S, Uemura Y, Kanatani A, Ikeda M, Matsumoto A, et al. Nomogram for predicting survival of postcystectomy recurrent urothelial carcinoma of the bladder. Urol Oncol. (2017) 35:457.e15-.e21. doi: 10.1016/j.urolonc.2016.12.010
16. Abe T, Ishizaki J, Kikuchi H, Minami K, Matsumoto R, Harabayashi T, et al. Outcome of metastatic urothelial carcinoma treated by systemic chemotherapy: Prognostic factors based on real-world clinical practice in Japan. Urol Oncol. (2017) 35:38.e1–e8. doi: 10.1016/j.urolonc.2016.08.016
17. Zhang XK, Zhang ZL, Lu X, Yang P, Cai MY, Hu WM, et al. Prognostic significance of preoperative serum lactate dehydrogenase in upper urinary tract urothelial carcinoma. Clin Genit Cancer. (2016) 14:341–5.e3. doi: 10.1016/j.clgc.2016.01.003
18. Ito K, Asakuma J, Kuroda K, Tachi K, Sato A, Horiguchi A, et al. Preoperative risk factors for extraurothelial recurrence in N0M0 patients with renal pelvic cancer treated by radical nephroureterectomy. Mol Clin Oncol. (2016) 4:530–6. doi: 10.3892/mco.2016.754
19. Fukushima H, Yokoyama M, Nakanishi Y, Tobisu K, Koga F. Sarcopenia as a prognostic biomarker of advanced urothelial carcinoma. PLoS ONE. (2015) 10:e0115895. doi: 10.1371/journal.pone.0115895
20. Nakagawa T, Hara T, Kawahara T, Ogata Y, Nakanishi H, Komiyama M, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol. (2013) 189:1275–81. doi: 10.1016/j.juro.2012.10.065
21. Yang MH, Yen CC, Chen PM, Wang WS, Chang YH, Huang WJ, et al. Prognostic-factors-based risk-stratification model for invasive urothelial carcinoma of the urinary bladder in Taiwan. Urology. (2002) 59:232–8; discussion 8–9. doi: 10.1016/S0090-4295(01)01590-4
22. Yang MH, Chen KK, Yen CC, Wang WS, Chang YH, Huang WJ, et al. Unusually high incidence of upper urinary tract urothelial carcinoma in Taiwan. Urology. (2002) 59:681–7. doi: 10.1016/S0090-4295(02)01529-7
23. Bellmunt J, Albanell J, Paz-Ares L, Climent MA, Gonzalez-Larriba JL, Carles J, et al. Pretreatment prognostic factors for survival in patients with advanced urothelial tumors treated in a phase I/II trial with paclitaxel, cisplatin, and gemcitabine. Cancer. (2002) 95:751–7. doi: 10.1002/cncr.10762
24. Sengelov L, Kamby C, Schou G, von der Maase H. Prognostic factors and significance of chemotherapy in patients with recurrent or metastatic transitional cell cancer of the urinary tract. Cancer. (1994) 74:123–33. doi: 10.1002/1097-0142(19940701)74:1<123::AID-CNCR2820740121>3.0.CO;2-T
25. Hannisdal E, Fossa SD, Host H. Blood tests and prognosis in bladder carcinomas treated with definitive radiotherapy. Radiother Oncol. (1993) 27:117–22. doi: 10.1016/0167-8140(93)90131-Q
26. Suzuki H, Ito M, Takemura K, Nakanishi Y, Kataoka M, Sakamoto K, et al. Prognostic significance of the controlling nutritional status (CONUT) score in advanced urothelial carcinoma patients. Urol Oncol. (2020) 38:76.e11-76.e17. doi: 10.1016/j.urolonc.2019.10.014
27. Serganova I, Rizwan A, Ni X, Thakur SB, Vider J, Russell J, et al. Metabolic imaging: a link between lactate dehydrogenase A, lactate, and tumor phenotype. Clin Cancer Res. (2011) 17:6250–61. doi: 10.1158/1078-0432.CCR-11-0397
28. Zhang Y, Xu T, Wang Y, Zhang H, Zhao Y, Yang X, et al. Prognostic role of lactate dehydrogenase expression in urologic cancers: a systematic review and meta-analysis. Oncol Res Treat. (2016) 39:592–604. doi: 10.1159/000449138
29. Krieg AF, Rosenblum LJ, Henry JB. Lactate dehydrogenase isoenzymes a comparison of pyruvate-to-lactate and lactate-to-pyruvate assays. Clin Chem. (1967) 13:196–203. doi: 10.1093/clinchem/13.3.196
30. Goldberg E. Immunochemical specificity of lactate dehydrogenase-X. Proc Natl Acad Sci USA. (1971) 68:349–52. doi: 10.1073/pnas.68.2.349
31. Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett. (2015) 358:1–7. doi: 10.1016/j.canlet.2014.12.035
32. Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. (2004) 4:891–9. doi: 10.1038/nrc1478
33. Hermes A, Gatzemeier U, Waschki B, Reck M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer - a retrospective single institution analysis. Resp Med. (2010) 104:1937–42. doi: 10.1016/j.rmed.2010.07.013
34. Gatenby RA, Gawlinski ET. A reaction-diffusion model of cancer invasion. Cancer Res. (1996) 56:5745–53.
Keywords: urothelial carcinoma, lactate dehydrogenase, prognosis, systematic review, meta-analysis
Citation: Wu M, Lin P, Xu L, Yu Z, Chen Q, Gu H and Liu C (2020) Prognostic Role of Serum Lactate Dehydrogenase in Patients With Urothelial Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 10:677. doi: 10.3389/fonc.2020.00677
Received: 13 January 2020; Accepted: 09 April 2020;
Published: 20 May 2020.
Edited by:
Woonyoung Choi, The Johns Hopkins Hospital, United StatesCopyright © 2020 Wu, Lin, Xu, Yu, Chen, Gu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengxiu Lin, bHB4d21oMjAxOUBzaW5hLmNvbQ==
 Minhong Wu1
Minhong Wu1 Pengxiu Lin
Pengxiu Lin