- Department of Gastrointestinal Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Background: Totally laparoscopic total gastrectomy (TLTG) not only is difficult to operate but also has high technical requirements and a long learning curve. Therefore, it has not been widely carried out yet, and esophagojejunostomy is one of its difficulties. Relevant studies have shown that intracorporeal hand-sewn esophagojejunostomy is safe, feasible and low-cost, but it is complicated and time-consuming and requires a high-suture technique. This study introduces a simple, safe and feasible hand-sewn technique.
Methods: The clinical data of 32 patients with the esophageal suspension method for hand-sewn esophagojejunostomy (suspension group) after TLTG were collected from February 2018 to June 2019. During the same period, 32 patients with traditional hand-sewn esophagojejunostomy (traditional group) after TLTG were used as the control group.
Results: The operative time, anastomosis time, exhaust time and hospitalization time of the suspension group were shorter than those of the traditional group. The intraoperative blood loss in the suspension group was less than that in the traditional group. There were no postoperative complications associated with the suspension group.
Conclusion: For those who have some experience in laparoscopic suture technique, the esophageal suspension method for hand-sewn esophagojejunostomy after TLTG is a simple, safe, and feasible suture technique.
Introduction
Since 1994, when Kitano et al. (1) first reported laparoscopic assisted distal gastrectomy (LADG) for early gastric cancer, laparoscopic technology has continuously matured and improved. At present, laparoscopic distal gastrectomy (LDG) is a way to treat gastric cancer, and its safety and feasibility have been indicated (2–4). However, totally laparoscopic total gastrectomy (TLTG) is a difficult operation, has high technical requirements and has a long learning curve. Therefore, it has not been widely carried out yet, and esophagojejunostomy is its main difficulty (5, 6). Roux-en-Y anastomosis is the main anastomosis of digestive tract reconstruction after total gastrectomy (7, 8). Currently, relevant literature (9–13) reports that esophagojejunostomy is mainly performed with circular and linear staplers. The former mainly includes the transorally inserted anvil (OrVil™), reverse puncture device (RPD), and purse-string suture method; the latter mainly includes functional end-to-end (FETE), overlap anastomosis, π-shaped esophagojejunostomy and semi-end-to-end anastomosis. Although the anastomosis technique has been continuously improved, there are still problems, such as difficulty in anvil implantation under the laparoscope, inaccurate esophageal cutting margins, and high price. In addition, related studies (14–17) have shown that intracorporeal hand-sewn esophagojejunostomy is safe, feasible and low-cost, but it is complicated and time-consuming and requires a high-suture technique. Therefore, it is particularly important to explore a simple, safe and feasible technique for hand-sewn esophagojejunostomy.
Materials and Methods
Patients
The clinical data of 32 patients with the esophageal suspension method for hand-sewn esophagojejunostomy (suspension group) after TLTG were collected from February 2018 to June 2019. During the same period, 32 patients with traditional hand-sewn esophagojejunostomy (traditional group) after TLTG were used as the control group. There were 32 patients in the suspension group, including 22 males and 10 females. The average age was 63.34 ± 9.86 years, and the average BMI was 21.80 ± 2.55 kg/m2. The tumor was located in the esophagogastric junction in 26 cases, including 10 cases of Siewert type II, 16 cases of Siewert type III, and in the middle of the stomach in six cases. The average follow-up time was 9.47 ± 2.83 months. There were 32 patients in the traditional group, including 24 males and eight females. The average age was 64.59 ± 10.90 years, and the average BMI was 22.42 ± 3.01 kg/m2. The tumor was located in the esophagogastric junction in 23 cases, including five cases of Siewert type II, 18 cases of Siewert type III, and in the middle of the stomach in nine cases. The average follow-up time was 9.34 ± 4.62 months (Table 1).
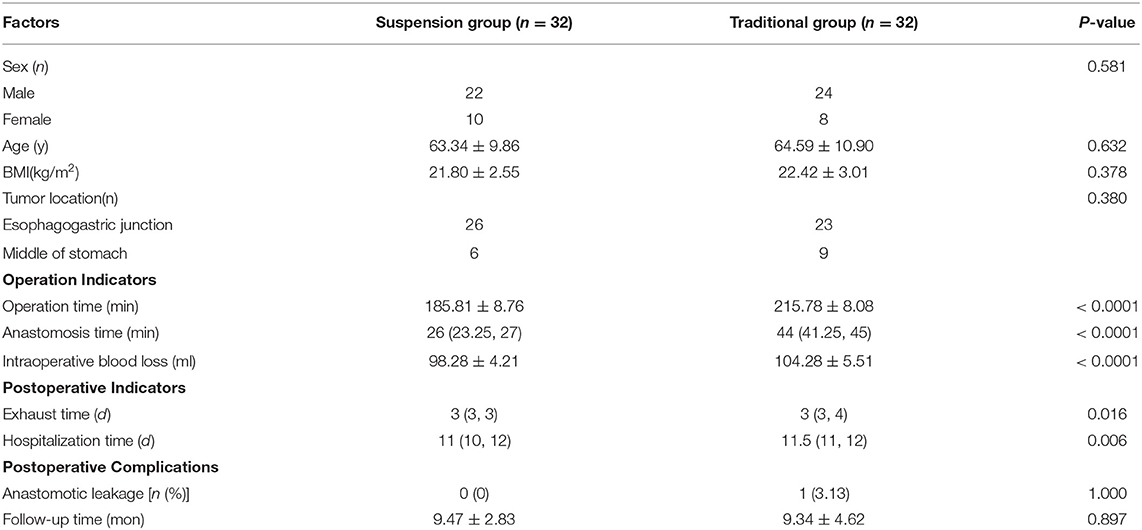
Table 1. Comparison of general characteristics and related indicators between the two groups of patients.
Inclusion and Exclusion Criteria
The inclusion criteria of this study were as follows: 1. preoperative diagnosis was made by gastroscopy and pathology; 2. preoperative CT staging was T1−2N0−1M0; 3. patients had no history of abdominal surgery; 4. patients did not have neoadjuvant radiotherapy or chemotherapy before surgery; 5. TLTG was performed by the same treatment group; 6. patients signed informed consent; and 7. it was approved by the ethics committee of our hospital. We excluded patients with severe heart, lung, kidney, and brain dysfunction, as well as coagulopathy and intolerance.
Operative Procedures
All patients underwent general anesthesia with tracheal intubation. A stomach tube and catheter were routinely inserted before operation. Patients were placed in the supine position with the two legs split. The operator stood on the left side of the patient, the first assistant stood on the right side of the patient, and the mirror holder stood between the patient's legs. The trocar was placed in a 5-hole method. After artificial pneumoperitoneum was established, a 10 mm trocar was placed under the umbilicus as the observation hole. The 5 mm trocars were placed in the left and right midline of the clavicle 2 cm above the umbilicus and 2 cm below the costal margin of the right anterior axillary line, respectively. The 12 mm trocar was placed 2 cm below the costal margin of the left anterior axillary line. The pneumoperitoneum pressure was maintained at 12–15 mmHg (1 mmHg = 0.133 kPa). Abdominal and pelvic conditions were routinely explored to rule out peritoneal implantation and distant metastasis. According to the requirements of radical gastrectomy, the perigastric vessels were isolated, and the corresponding lymph nodes were dissected with a harmonic scalpel. The duodenum and esophagus were cut with a linear cutting closure device, and the whole stomach specimen was taken through a semicircular incision around the umbilicus. The specimens were examined to ensure that the esophageal cutting edge was ~2 cm, and the upper and lower cutting edges were confirmed to be negative by frozen sections and reconstruction was started.
Digestive Tract Reconstruction
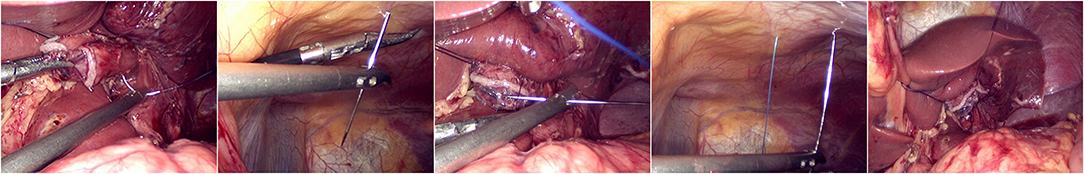
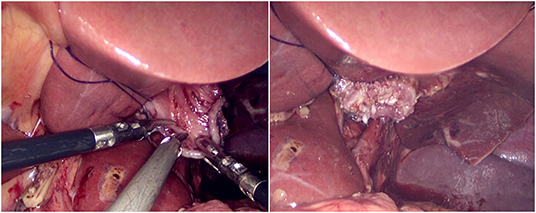
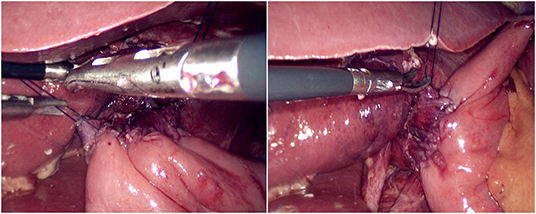
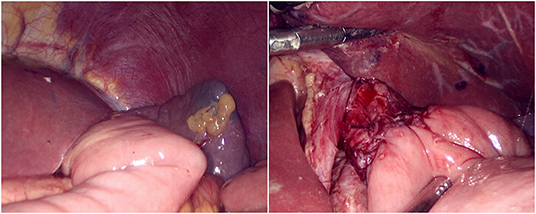
The jejunum was disconnected with a linear cutting closure device at a distance of 10–15 cm from the ligament of Treitz. The proximal jejunum and jejunum at a distance of 30–40 cm from the anastomosis of the esophageal jejunum was anastomosed end-to-side through the specimen incision. A small incision ~1.5–2 cm long was made at a distance of 3–5 cm from the distal jejunum to the stump and then put into the abdominal cavity. The pneumoperitoneum was established again. In the suspension group, the left and right sides of the proximal esophagus at a distance of 2 cm from the stump were suspended and fixed to the abdominal wall by the purse line or by the 3-0 Vichy line and purse line, respectively (Figures 1, 2). A harmonic scalpel was used to dissect the proximal esophageal stump (Figure 3). The distal jejunum was lifted posteriorly through the transverse colon, and the 3-0 barbed line was used to suture the posterior wall of the esophagus and jejunum with continuous full-layer suture from left to right (Figure 4) and to suture the anterior wall of the esophagus and jejunum with continuous full-layer inverting suture from right to left (Figure 5). After the anastomotic suture was completed, the suspension suture was cut off (Figure 6). The stomach tube was placed in the distal jejunum and esophagojejunostomy was completed (Figure 7). In the traditional group, hand-sewn esophagojejunostomy was performed directly without esophageal suspension.
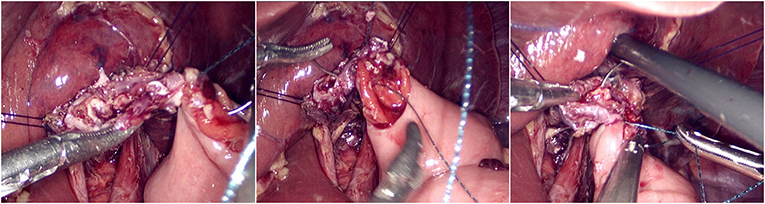
Figure 4. Continuous full-layer suture of the posterior wall of the esophagus and jejunum from left to right using 3-0 barbed line.
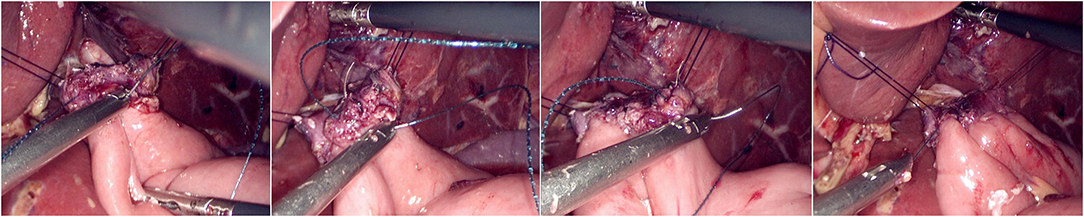
Figure 5. Continuous full-layer inverting suture of the anterior wall of the esophagus and jejunum from right to left using 3-0 barbed line.
Observation Indicators
Operation indicators included operative time (from the insertion of the first trocar to the closure of the abdomen), anastomosis time (from the first suture to the end of anastomosis), and intraoperative blood loss. Postoperative recovery indicators included postoperative exhaust time and postoperative hospital stay. Postoperative complications included duodenal stump leakage, anastomotic leakage, anastomotic bleeding, anastomotic stenosis, obstructive complications, and perioperative mortality.
Statistical Analysis
We used the single sample Kolmogorov-Smirnov test to test the normality of the data. If the quantitative data obeyed a normal distribution, it was described as the mean ± standard deviation; otherwise, it was described as the median and interquartile range. Quantitative data comparisons between the two groups were performed using the independent-samples t-test; otherwise, the Mann-Whitney U-test was used. Enumeration data comparisons between the two groups were performed using the chi-square test. A P < 0.05 was considered statistically significant. Data were analyzed using SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
There were no statistically significant differences in sex (P = 0.581), age (P = 0.632), BMI (P = 0.378), tumor location (P = 0.380), or follow-up time (P = 0.897) between the two groups; there were statistically significant differences in operation time (P < 0.0001), anastomosis time (P < 0.0001), intraoperative blood loss (P < 0.0001), exhaust time (P = 0.016), and hospitalization time (P = 0.006) between the two groups (Table 1). The results showed that the operative time, anastomosis time, exhaust time and hospitalization time of the suspension group were shorter than those of the traditional group, and the intraoperative blood loss in the suspension group was less than that in the traditional group. There were no postoperative complications associated with the suspension group. There was no statistically significant difference in postoperative complications between the two groups (P = 1.000). One case in the traditional group developed an anastomotic leakage and recovered after conservative treatment. There were no other postoperative complications associated with either group (Table 1).
Discussion
In recent years, LDG has been widely accepted and carried out in clinical practice as a safe and feasible method for the treatment of gastric cancer. Compared with laparotomy, LDG has advantages such as fast recovery of gastrointestinal function, short hospital stay, mild pain, small incision and fewer complications (18, 19). Meanwhile, the incidence of the adenocarcinoma of esophagogastric junction (AEG) is on the rise worldwide (20, 21). According to the Japanese gastric cancer treatment guidelines (22), except for early cancer, the retained stomach can be larger than 1/2, proximal gastric resection is used, and the rest should be considered for total gastrectomy. However, TLTG not only is a difficult operation but also has high technical requirements and a long learning curve. Therefore, it has not been widely carried out yet, and esophagojejunostomy is one of its difficulties. Laparoscopic total gastrectomy is currently performed mainly by laparoscopically assisted surgery, which is used to complete the reconstruction of the digestive tract through assisted small incision. However, for patients with a thick abdominal wall, large anterior and posterior diameter, narrow subcostal angle and left liver hypertrophy, it is difficult to complete anastomosis. If forced to complete the reconstruction, it is necessary to extend the incision and strengthen the traction, which not only loses the advantage of being minimally invasive but also increases the incidence of pain and anastomotic ischemia in patients (9, 10, 23). Therefore, to solve this problem, the development of TLTG is particularly important. Relevant studies (24, 25) reported that total laparoscopic distal gastrectomy (TLDG) has certain advantages compared with LADG, such as fast recovery of gastrointestinal function, short hospital stay, mild pain, and small incision. Previous studies (26–28) have also shown that total laparoscopic gastrectomy (TLG) and laparoscopic intracorporeal anastomosis were safe and feasible. In addition, TLG in intracorporeal anastomosis had the advantages of high safety, less adhesion, rapid recovery, and small scars (29, 30).
At present, the related literature (9–13) reports that esophagojejunostomy is mainly performed with circular and linear staplers. In fact, a variety of techniques for esophagojejunostomy have emerged in recent years, but none of them was considered as the standard technique (31). Although the anastomosis technique has been continuously improved, there are still problems, such as difficulty in anvil implantation under the laparoscope, inaccurate esophageal cutting margins, higher incidence of total anastomotic complications and high price (9, 32). Studies (33, 34) reported that the purse-string suture method was a safe and reliable technique. However, the long operation time, high operation difficulty and high price limit its application (35, 36). However, intracorporeal hand-sewn esophagojejunostomy under visualization can not only ensure the tension at the anastomosis but also make the anastomosis more reliable. More importantly, this method does not require a long esophageal stump and can significantly improve the R0 resection rate. Because the anastomosis was performed after the specimen was removed, a frozen section could be used to confirm the negative cutting edge for anastomosis. In addition, the hand-sewn esophagojejunostomy can reduce the use of the device, thereby reducing the cost of surgery. Related studies (14–17) have shown that intracorporeal hand-sewn esophagojejunostomy is safe, feasible and low-cost, but it is complicated and time-consuming and requires a high-suture technique. Facy et al. (37) believed that the incidence of anastomotic leakage, anastomotic bleeding, and anastomotic stenosis was low after laparoscopic hand-sewn esophagojejunostomy with a barbed line, so it was safe. In addition, the safety of barbed line sutures has also been demonstrated in large-scale trials (38, 39). This study used a 3-0 barbed line for esophagojejunostomy. Only one case developed an anastomotic leakage and recovered after conservative treatment. The esophageal suspension method for hand-sewn esophagojejunostomy can make suturing simple and easy. Relevant studies (40, 41) showed that increased operative time could significantly lengthen hospital stay for patients who underwent a primary laparoscopic Roux-en-Y gastric bypass. Meanwhile, Carter et al. (42) indicated that prolonged operating time can predict longer hospitalization after laparoscopic gastric bypass operation. In addition, short exhaust time makes patients eat early to accelerate the recovery of patients after surgery, thus shortening the hospital length of stay. This study showed that the operative time, anastomosis time, exhaust time and hospitalization time of the suspension group were shorter than those of the traditional group, and the intraoperative blood loss in the suspension group was less than that in the traditional group. There were no postoperative complications in the suspension group. Our experience was that, for Siewert type II AEJ, 3-0 Vichy line and purse line were used for esophageal suspension and fixation, while Siewert type III AEJ can be directly suspended and fixed by purse line. We believed that compared with the traditional hand-sewn method, the esophageal suspension method has the following advantages: 1. esophageal suspension and fixation on both sides can not only provide a stable suture field but also provide better exposure in the surgical field and reduce the secondary injury of tissues caused by repeated turnover of the esophagus and jejunum during operation; 2. the esophageal suspension method has lower requirements on the free length of the esophageal stump, which reduces the incidence of tissue bleeding; 3. the esophageal suspension method for hand-sewn esophagojejunostomy has relatively lower requirements for laparoscopic suture technique, which makes the learning curve relatively short and shortens the operation time. In addition, it should be noted that when the esophageal was suspended and fixed, the tearing of the esophageal wall caused by too shallow of an esophageal suture and excessive fixation force should be avoided. This study has some limitations. This study was a single-center, small-sample study, and therefore, a multicenter, large-scale prospective randomized controlled trial is necessary.
In conclusion, for those who have some experience in laparoscopic suture technique, the esophageal suspension method for hand-sewn esophagojejunostomy after TLTG is a simple, safe and feasible suture technique, which has an important reference value for the wide development of TLTG in the future.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Second Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CH and ZZ designed the study. CH, JZ, and ZL collected clinical data. CH and JH analyzed the data. CH wrote the manuscript with contribution from all authors. All authors read and approved the final version of the paper.
Funding
This present study was supported by the National Natural Science Foundation of China (Grant Number: 81560389) and Key Research and Development Program of Jiangxi Province (Grant Number: 20181BBG70015).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg laparosc Endosc. (1994) 4:146–8.
2. Kim H-H, Hyung WJ, Cho GS, Kim MC, Han S-U, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report—a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann surg. (2010) 251:417–20. doi: 10.1097/SLA.0b013e3181cc8f6b
3. Shi Y, Xu X, Zhao Y, Qian F, Tang B, Hao Y, et al. Short-term surgical outcomes of a randomized controlled trial comparing laparoscopic versus open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc. (2018) 32:2427–33. doi: 10.1007/s00464-017-5942-x
4. Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, et al. Effect of laparoscopic vs open distal gastrectomy on 3-Year disease-free survival in patients with locally advanced gastric cancer: the CLASS-01 randomized clinical trial. JAMA. (2019) 321:1983–92. doi: 10.1001/jama.2019.5359
5. Huscher CG, Mingoli A, Sgarzini G, Brachini G, Binda B, Di Paola M, et al. Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg. (2007) 194:839–44. doi: 10.1016/j.amjsurg.2007.08.037
6. Tanimura S, Higashino M, Fukunaga Y, Kishida S, Ogata A, Fujiwara Y, et al. Laparoscopic gastrectomy with regional lymph node dissection for upper gastric cancer. Br J Surg. (2007) 94:204–7. doi: 10.1002/bjs.5542
7. Kim HS, Kim BS, Lee IS, Lee S, Yook JH, Kim BS. Intracorporeal laparoscopic Roux-en-Y gastrojejunostomy after 95% gastrectomy for early gastric cancer in the upper third of the stomach: a report on 21 cases. J Laparoendosc Adv Surg Tech A. (2013) 23:250–7. doi: 10.1089/lap.2012.0371
8. Fein M, Fuchs K-H, Thalheimer A, Freys SM, Heimbucher J, Thiede A. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy: a randomized trial. Ann Surg. (2008) 247:759–65. doi: 10.1097/SLA.0b013e318167748c
9. Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil™) after laparoscopic total gastrectomy. Surg Endosc. (2009) 23:2624. doi: 10.1007/s00464-009-0461-z
10. Kim H-I, Cho I, Jang D-S, Hyung WJ. Intracorporeal esophagojejunostomy using a circular stapler with a new purse-string suture technique during laparoscopic total gastrectomy. J Am Coll Surg. (2013) 216:e11–6. doi: 10.1016/j.jamcollsurg.2012.10.008
11. Kwon IG, Son Y-G, Ryu SW. Novel intracorporeal esophagojejunostomy using linear staplers during laparoscopic total gastrectomy: π-shaped esophagojejunostomy, 3-in-1 technique. J Am Coll Surg. (2016) 223:e25–9. doi: 10.1016/j.jamcollsurg.2016.06.011
12. Duan W, Liu K, Fu X, Shen X, Chen J, Su C, et al. Semi-end-to-end esophagojejunostomy after laparoscopy-assisted total gastrectomy better reduces stricture and leakage than the conventional end-to-side procedure: a retrospective study. J Surg Oncol. (2017) 116:177–83. doi: 10.1002/jso.24637
13. Du J, Xue H, Zhao L, Hua J, Hu J, Zhang Z. Intracorporeal circular-stapled anastomosis after totally laparoscopic gastrectomy: a novel, simplest u-shaped parallel purse-string suture technique. J Surg Oncol. (2019) 120:501–7. doi: 10.1002/jso.25596
14. So KO, Park J-M. Totally laparoscopic total gastrectomy using intracorporeally hand-sewn esophagojejunostomy. J Gastric Cancer. (2011) 11:206–11. doi: 10.5230/jgc.2011.11.4.206
15. Chen K, Wu D, Pan Y, Cai J-Q, Yan J-F, Chen D-W, et al. Totally laparoscopic gastrectomy using intracorporeally stapler or hand-sewn anastomosis for gastric cancer: a single-center experience of 478 consecutive cases and outcomes. World J Surg Oncol. (2016) 14:115. doi: 10.1186/s12957-016-0868-7
16. Norero E, Muñoz R, Ceroni M, Manzor M, Crovari F, Gabrielli M. Two-layer hand-sewn esophagojejunostomy in totally laparoscopic total gastrectomy for gastric cancer. J Gastric Cancer. (2017) 17:267–76. doi: 10.5230/jgc.2017.17.e26
17. Xu X, Huang C, Mou Y, Zhang R, Pan Y, Chen K, et al. Intra-corporeal hand-sewn esophagojejunostomy is a safe and feasible procedure for totally laparoscopic total gastrectomy: short-term outcomes in 100 consecutive patients. Surg Endosc. (2018) 32:2689–95. doi: 10.1007/s00464-017-5964-4
18. Kim MC, Kim KH, Kim HH, Jung GJ. Comparison of laparoscopy-assisted by conventional open distal gastrectomy and extraperigastric lymph node dissection in early gastric cancer. J Surg Oncol. (2005) 91:90–4. doi: 10.1002/jso.20271
19. Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N, Group JLSS. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. (2007) 245:68. doi: 10.1097/01.sla.0000225364.03133.f8
20. Liu K, Yang K, Zhang W, Chen X, Chen X, Zhang B, et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988–2012: a single-institution, high-volume experience in China. Ann Surg. (2016) 263:88. doi: 10.1097/SLA.0000000000001148
21. Kauppila JH, Lagergren J. The surgical management of esophago-gastric junctional cancer. Surg Oncol. (2016) 25:394–400. doi: 10.1016/j.suronc.2016.09.004
22. Association JGC. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric cancer. (2011) 14: 113-23. doi: 10.1007/s10120-011-0042-4
23. Kim MG, Kim BS, Kim TH, Kim KC, Yook JH, Kim BS. The effects of laparoscopic assisted total gastrectomy on surgical outcomes in the treatment of gastric cancer. J Korean Surg Soc. (2011) 80:245–50. doi: 10.4174/jkss.2011.80.4.245
24. Chen K, Xu X, Mou Y, Pan Y, Zhang R, Zhou Y, et al. Totally laparoscopic distal gastrectomy with D2 lymphadenectomy and Billroth II gastrojejunostomy for gastric cancer: short-and medium-term results of 139 consecutive cases from a single institution. Int J Med Sci. (2013) 10:1462–70. doi: 10.7150/ijms.6632
25. Chen K, Mou Y-P, Xu X-W, Pan Y, Zhou Y-C, Cai J-Q, et al. Comparison of short-term surgical outcomes between totally laparoscopic and laparoscopic-assisted distal gastrectomy for gastric cancer: a 10-y single-center experience with meta-analysis. J Surg Res. (2015) 194:367–74. doi: 10.1016/j.jss.2014.10.020
26. Kanaya S, Gomi T, Momoi H, Tamaki N, Isobe H, Katayama T, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg. (2002) 195:284–7. doi: 10.1016/S1072-7515(02)01239-5
27. Kim J-J, Song KY, Chin HM, Kim W, Jeon HM, Park CH, et al. Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc. (2008) 22:436–42. doi: 10.1007/s00464-007-9446-y
28. Dulucq J-L, Wintringer P, Perissat J, Mahajna A. Completely laparoscopic total and partial gastrectomy for benign and malignant diseases: a single institute's prospective analysis. J Am Coll Surg. (2005) 200:191–7. doi: 10.1016/j.jamcollsurg.2004.10.004
29. Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, et al. Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc. (2009) 23:2374. doi: 10.1007/s00464-009-0360-3
30. Kim BS, Yook JH, Choi YB, Kim KC, Kim MG, Kim TH, et al. Comparison of early outcomes of intracorporeal and extracorporeal gastroduodenostomy after laparoscopic distal gastrectomy for gastric cancer. J Laparoendosc Adv Surg Tech. (2011) 21:387–91. doi: 10.1089/lap.2010.0515
31. LaFemina J, Viñuela E, Schattner M, Gerdes H, Strong V. Esophagojejunal reconstruction after total gastrectomy for gastric cancer using a transorally inserted anvil delivery system. Ann Surg Oncol. (2013) 20:2975–83. doi: 10.1245/s10434-013-2978-6
32. Shim JH, Yoo HM, Oh SI, Nam MJ, Jeon HM, Park CH, et al. Various types of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy for gastric cancer. Gastric Cancer. (2013) 16:420–7. doi: 10.1007/s10120-012-0207-9
33. Okuno K, Gokita K, Tanioka T, Ogawa N, Otsuki S, Inokuchi M, et al. Esophagojejunostomy using the purse-string suturing device after laparoscopic total or proximal gastrectomy for gastric cancer. World J Surg. (2017) 41:2605–10. doi: 10.1007/s00268-017-4033-4
34. Kinoshita T, Oshiro T, Ito K, Shibasaki H, Okazumi S, Katoh R. Intracorporeal circular-stapled esophagojejunostomy using hand-sewn purse-string suture after laparoscopic total gastrectomy. Surg Endosc. (2010) 24:2908–12. doi: 10.1007/s00464-010-1041-y
35. Huang C-M, Huang Z-N, Zheng C-H, Li P, Xie J-W, Wang J-B, et al. An isoperistaltic jejunum-later-cut overlap method for esophagojejunostomy anastomosis after totally laparoscopic total gastrectomy: a safe and feasible technique. Ann Surg Oncol. (2017) 24:1019–20. doi: 10.1245/s10434-016-5658-5
36. Lee T-G, Lee I-S, Yook J-H, Kim B-S. Totally laparoscopic total gastrectomy using the overlap method; early outcomes of 50 consecutive cases. Surg Endosc. (2017) 31:3186–90. doi: 10.1007/s00464-016-5343-6
37. Facy O, de Blasi V, Goergen M, Arru L, de Magistris L, Azagra J-S. Laparoscopic gastrointestinal anastomoses using knotless barbed sutures are safe and reproducible: a single-center experience with 201 patients. Surg Endosc. (2013) 27:3841–5. doi: 10.1007/s00464-013-2992-6
38. Milone M, Di Minno MND, Galloro G, Maietta P, Bianco P, Milone F, et al. Safety and efficacy of barbed suture for gastrointestinal suture: a prospective and randomized study on obese patients undergoing gastric bypass. J Laparoendosc Adv Surg Tech. (2013) 23:756–9. doi: 10.1089/lap.2013.0030
39. Costantino F, Dente M, Perrin P, Sarhan FA, Keller P. Barbed unidirectional V-Loc 180 suture in laparoscopic Roux-en-Y gastric bypass: a study comparing unidirectional barbed monofilament and multifilament absorbable suture. Surg Endosc. (2013) 27:3846–51. doi: 10.1007/s00464-013-2993-5
40. Ballantyne GH, Svahn J, Capella RF, Capella JF, Schmidt HJ, Wasielewski A, et al. Predictors of prolonged hospital stay following open and laparoscopic gastric bypass for morbid obesity: body mass index, length of surgery, sleep apnea, asthma and the metabolic syndrome. Obes Surg. (2004) 14:1042–50. doi: 10.1381/0960892041975460
41. Krell RW, Birkmeyer NJ, Reames BN, Carlin AM, Birkmeyer JD, Finks JF, et al. Effects of resident involvement on complication rates after laparoscopic gastric bypass. J Am Coll Surg. (2014) 218:253–60. doi: 10.1016/j.jamcollsurg.2013.10.014
Keywords: totally laparoscopic total gastrectomy, gastric cancer, esophageal suspension method, hand-sewn esophagojejunostomy, suture technique
Citation: Huang C, Zhao J, Liu Z, Huang J and Zhu Z (2020) Esophageal Suspension Method for Hand-Sewn Esophagojejunostomy After Totally Laparoscopic Total Gastrectomy: A Simple, Safe, and Feasible Suturing Technique. Front. Oncol. 10:575. doi: 10.3389/fonc.2020.00575
Received: 02 December 2019; Accepted: 30 March 2020;
Published: 21 April 2020.
Edited by:
Marco Scarpa, University Hospital of Padua, ItalyReviewed by:
Xian Jian Lin, Fujian Medical University, ChinaCesare Ruffolo, University Hospital of Padua, Italy
Copyright © 2020 Huang, Zhao, Liu, Huang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengming Zhu, enptODY1NEAxNjMuY29t
 Chao Huang
Chao Huang Jiefeng Zhao
Jiefeng Zhao Zhengming Zhu
Zhengming Zhu