
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 17 April 2020
Sec. Genitourinary Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00558
Objectives: The association of body composition with survival and the efficacy of first-line treatment was investigated in patients with castration-resistant prostate cancer (CRPC).
Methods: The records of CRPC patients treated with docetaxel or androgen receptor signaling inhibitors (ARSi) between 2005 and 2018 were reviewed. Skeletal muscle index (SMI), visceral fat index, and subcutaneous fat index were evaluated using pretreatment computed tomography images.
Results: Of 230 eligible patients, 144 received docetaxel, and 86 received ARSi as the first-line treatment for CRPC. The SMIhi (based on median values) group had higher prostate-specific antigen (PSA) progression-free survival (median 13.5 vs. 8.3 months, p = 0.030), radiologic progression-free survival (14.9 vs. 9.1 months, p < 0.001), and overall survival (24.1 vs. 16.9 months, p = 0.015) than the SMIlo group. In docetaxel-treated patients, the SMIhi group had higher PSA progression-free survival (13.5 vs. 5.9 months, p = 0.016) and radiologic progression-free survival (14.6 vs. 6.7 months, p < 0.001) than the SMIlo group. However, PSA progression-free survival and radiologic progression-free survival were comparable between the two groups in ARSi-treated patients. SMI was independently associated with the risk of radiologic progression in patients treated with docetaxel but not in those treated with ARSi.
Conclusions: High skeletal muscle mass may be associated with reduced risk of disease progression and mortality in patients with CRPC. However, the significance of these relationships is limited in patients treated with docetaxel. These results suggest that assessing skeletal muscle mass may be worthwhile when selecting treatments for CRPC; however, further prospective validation and large-scale studies are needed.
The treatment of castration-resistant prostate cancer (CRPC) remains a major challenge despite the availability of several therapeutic options including docetaxel-based chemotherapy and androgen signaling inhibitors (ARSi) (1). The optimal treatment strategy remains to be fully determined, underscoring the importance of predicting the response to treatment in patients with CRPC (2).
The clinical implications of individual body composition in patients with cancer have been investigated extensively, and a relationship between cancer patient survival and muscle mass or fat mass has been identified (3, 4). In addition, body composition is associated with disease progression and treatment response in cancer patients (5–7).
Studies evaluating CRPC patients who underwent chemotherapy reported that muscle mass and subcutaneous fat mass are associated with overall survival (8–10). However, the survival outcomes according to body composition in CRPC patients who received first-line ARSi were not clearly determined. Moreover, the relationship between body composition and disease progression or treatment efficacy remains to be investigated. Here, we investigated the association between body composition and disease progression, mortality, and efficacy of first-line treatment in patients with CRPC.
The records of 314 men diagnosed with CRPC who underwent first-line therapy at the National Cancer Center Hospital between January 2005 and June 2018 were reviewed. Patients who did not undergo adequate abdominopelvic computed tomography (CT) scanning before starting first-line treatment (n = 75), those treated for <2 months (n = 5), and those followed-up for <6 months (n = 4) were excluded. The study protocol was approved by the Institutional Review Board of the National Cancer Center (IRB No. NCC2018-0123). The study design followed all relevant principles of the Declaration of Helsinki.
Demographic characteristics, clinical characteristics, laboratory findings, radiologic findings, pathologic features, and survival outcomes were evaluated retrospectively. The following parameters were examined: age, treatment type, pretreatment prostate-specific antigen (PSA) level, metastatic disease status, presence of pain, secondary treatment, diabetes, performance status, biopsy tumor grade, alkaline phosphatase, lactate dehydrogenase, hemoglobin levels, and body composition indices. All patients underwent androgen deprivation therapy before castration-resistant disease progression. Before starting first-line treatments for CRPC, all patients underwent height and weight measurements, blood tests, and abdominopelvic CT. Patients were not randomly assigned to docetaxel or ARSi arms. Treatment selection was determined by the treating physician and patients. The chemotherapy treatment protocol comprised docetaxel (75 mg/m2 once every 3 weeks) and prednisolone (5 mg twice daily). Enzalutamide was administered orally at a dose of 160 mg per day, and abiraterone was administered orally at a dose of 1,000 mg per day along with prednisolone (5 mg twice daily).
According to the follow-up protocol, the serum concentration of PSA was measured monthly, and abdominopelvic CT and bone scanning were performed every 3–6 months. Docetaxel or ARSi treatments were continued until the emergence of evidence of PSA progression, radiologic progression, or severe treatment-related adverse events.
The primary endpoint was radiologic progression-free survival. Secondary endpoints were PSA progression-free survival and overall survival. PSA progression and radiologic progression were determined by serum PSA tests and imaging studies in accordance with Prostate Cancer Working Group 2 (PCWG2) criteria (11).
Muscle mass and subcutaneous and visceral fat mass were evaluated using pretreatment CT images. Skeletal muscle index (SMI; cm2/m2), subcutaneous fat index (SFI; cm2/m2), and visceral fat index (VFI; cm2/m2) were calculated based on the height and cross-sectional area of the skeletal muscle, subcutaneous fat, and visceral fat at the 3rd lumber vertebra (12). The cross-sectional CT images of each patient were extracted from a picture archiving and communication system (PACS) and analyzed using AWS 2 (GE Healthcare, Chicago, IL, USA) by two readers. SMI, SFI, and VFI were dichotomized at median values.
Clinicopathological data were presented as means or medians for continuous variables and as frequencies for categorical variables. Between-group differences in categorical variables were assessed with the Chi-square test, and differences in continuous variables were assessed with the Student's t-test or the Wilcoxon rank sum test. PSA progression-free survival, radiologic progression-free survival, and overall survival were estimated using the Kaplan–Meier method, and survival curves between groups were compared using the log-rank test. The association between variables and outcomes was assessed by multivariable analysis using a Cox proportional hazards model. Covariates, chosen based on previous studies, were adjusted to group variables. Optimal cut-off levels for SMI, VFI, and SFI were defined using the median values of each index, and the Contal and O'Quigley method (Table S1). Because the results of two methods showed similar cut-off points, each index was dichotomized into high and low indices based on median values.
Propensity score matching was used to ensure a similar distribution of covariates between groups. The effect of group on prognosis was then evaluated in the dichotomized subset based on median values.
All statistical tests were two-tailed, and statistical significance was defined as p < 0.05. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R project software 3.5.1.
Of 230 eligible patients who were diagnosed with CRPC, 144 received docetaxel chemotherapy and 86 received ARSi (enzalutamide: 61; abiraterone: 25) as first-line treatment for CRPC (Table 1). The median follow-up was 21.3 months. Baseline characteristics were different between docetaxel-treated and ARSi-treated patients except tumor grade, primary treatment, and metastatic status, which were similar between the two groups. The median time on therapy was shorter for patients treated with docetaxel than for patients treated with ARSi (4.6 vs. 11.0 months, respectively). Median overall survival was 17.7 months in patients treated with docetaxel and 25.0 months in those treated with ARSi. Secondary treatments were administered to 61.8 and 61.6% of docetaxel-treated and ARSi-treated patients, respectively.
The median SMI, VFI, and SFI were 49.9, 59.4, and 48.2, respectively. Recurrence and survival outcomes based on SMI are shown in (Figure 1), and outcomes based on VFI and SFI are shown in (Figures S1, S2). The SMIhi group had higher PSA progression-free survival (median, 13.5 vs. 8.3 months, p = 0.030; Figure 1A), radiologic progression-free survival (14.9 vs. 9.1 months, p < 0.001; Figure 1B), and overall survival (24.1 vs. 16.9 months, p = 0.015; Figure 1C) than the SMIlo group, whereas the type of treatment was similar between the two groups (use of docetaxel: 60.0% vs. 65.2%, p = 0.414). In the multivariable analysis (Table S2), SMI was independently associated with risk of PSA progression [hazard ratio (HR) = 0.68; 95% confidence interval (CI), 0.50–0.93; p = 0.017], radiologic progression (HR = 0.54; 95% CI, 0.39–0.75; p = 0.001), and overall survival (HR = 0.72; 95% CI, 0.52–0.98; p = 0.037) regardless of BMI. Detailed results of the multivariable analyses for each measured outcome are shown in (Tables S2). To examine the robustness of the results, we performed sensitivity analyses. The results revealed that high SMI was associated with progression-free survival when patients were categorized into three tertiles based on SMI (Figure S3).
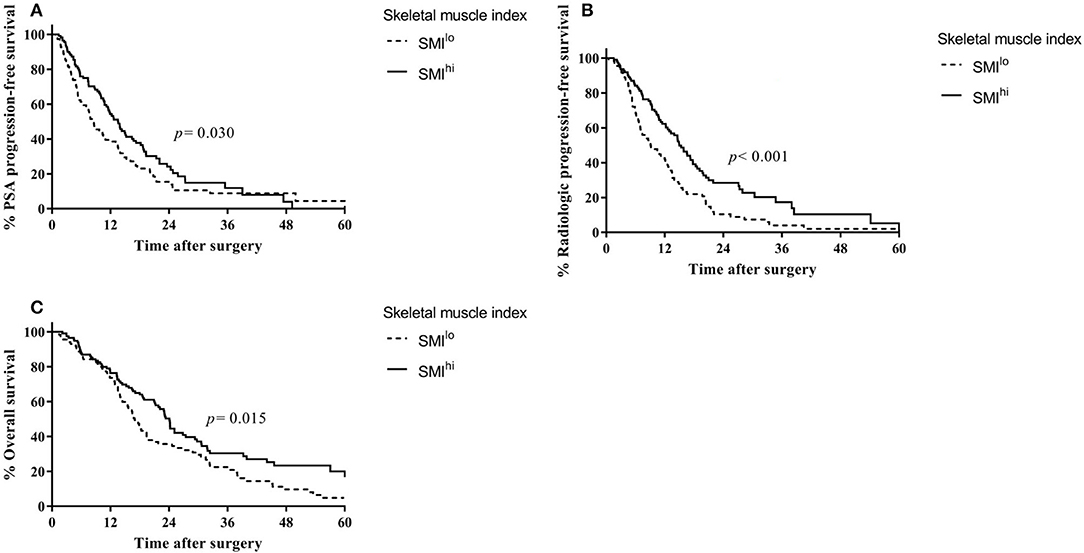
Figure 1. Outcomes based on skeletal muscle index in overall patients with castration-resistant prostate cancer from the time of diagnosis. (A) Prostate-specific antigen progression-free survival. (B) Radiologic progression-free survival. (C) Overall survival.
Overall, 144 patients were treated with docetaxel chemotherapy, with a median of six treatment cycles. In docetaxel-treated patients, PSA progression-free survival (13.5 vs. 5.9 months, p = 0.016; Figure 2A) and radiologic progression-free survival (14.6 vs. 6.7 months, p < 0.001; Figure 2B) were higher in the SMIhi than in the SMIlo group. Overall survival was also higher in patients with SMIhi than in those with SMIlo with near statistical significance (23.3 vs. 15.6, p = 0.051; Figure 2C). Time on therapy was shorter for the SMIlo group than for the SMIhi group, although the difference was not statistically significant (median, 4.1 vs. 5.2 months, respectively; p = 0.191). Overall, six patients discontinued chemotherapy due to toxicity (three patients in the SMIlo group and three in the SMIhi group). SMIhi patients experienced fewer grade 3 and 4 adverse events than SMIlo patients (44.6% vs. 63.5%, p = 0.044). The incidence of neutropenic fever was similar between patients with SMIhi and SMIlo (10.5% vs. 7.8%, p = 0.754).
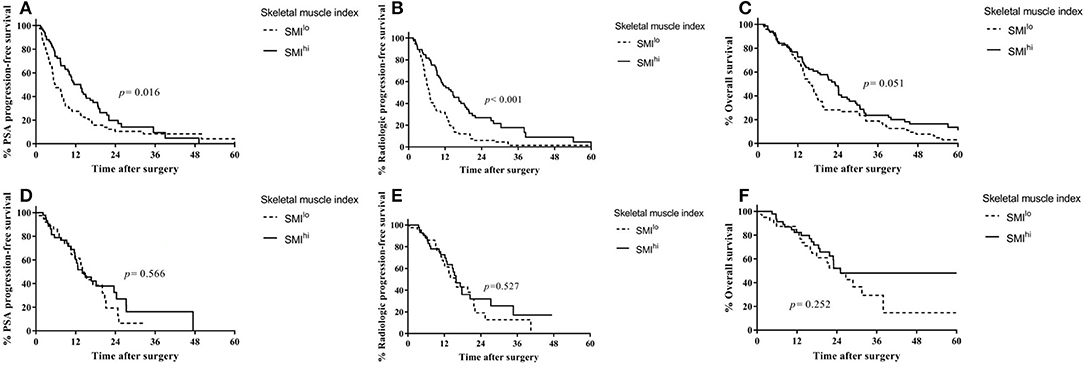
Figure 2. Outcomes according to first-line treatment based on skeletal muscle index in patients with castration-resistant prostate cancer. (A) Prostate-specific antigen progression-free survival in patients treated with docetaxel. (B) Radiologic progression-free survival in patients treated with docetaxel. (C) Overall survival in patients treated with docetaxel. (D) Prostate-specific antigen progression-free survival in patients treated with ARSi. (E) Radiologic progression-free survival in patients treated with ARSi. (F) Overall survival in patients treated with ARSi.
In ARSi-treated patients, PSA progression-free survival (14.5 vs. 14.0 months, p = 0.566; Figure 2D), radiologic progression-free survival (15.9 vs. 15.6 months, p = 0.527; Figure 2E), and overall survival (25.1 vs. 25.0, p = 0.252; Figure 2F) were similar between the SMIhi and SMIlo groups.
After adjusting for covariates (Table S2), SMIhi was associated independently with decreased risk of radiologic progression in patients treated with docetaxel (HR = 0.44; 95% CI, 0.30–0.65; p < 0.001) but not in those treated with ARSi (HR = 0.84; 95% CI, 0.46–1.54; p = 0.579). In ARSi-treated patients, SFIhi was associated with decreased risk of radiologic progression (HR = 0.48; 95% CI, 0.24–0.94; p = 0.033).
To adjust clinical parameters prior to first-line treatment between patients in the docetaxel and ARSi groups, propensity score-based matching was performed (Table S3). HR values for radiologic progression derived from Cox models suggested that patients with SMIlo are more likely to benefit from ARSi than docetaxel (Figure S4).
In this study, we found that skeletal muscle mass was associated with disease progression and survival after first-line therapy in patients with CRPC regardless of BMI. In addition, skeletal muscle mass was associated with the efficacy of docetaxel but not that of ARSi. These results suggest that assessment of muscle mass may be useful for first-line treatment selection in patients with CRPC.
Although several treatment options for CRPC have demonstrated a survival benefit and have been approved for clinical use, the optimal treatment strategy remains to be determined. Docetaxel and ARSi are the most widely used first-line treatments in patients with CRPC; however, randomized trial results have not been reported to date (13). Therefore, predictive markers of survival and treatment response in CRPC are critically needed.
The clinical implications of body habitus based on anthropometric measures have been intensively studied in various cancers (14–17). However, anthropometric measures such as BMI, waist circumference, or waist:hip ratio have limitations, and do not fully reflect muscle and fat mass (4). Recently, body composition has emerged as an alternative to traditional indicators. Body composition can be estimated easily and accurately using CT and software programs (12). Many studies report a significant association between body composition and survival of patients with cancer (3, 4). The relationship between body composition and prostate cancer was also investigated in those with localized and metastatic disease (8–10, 18, 19).
Subcutaneous adiposity has protective effects in cancer patients; potential explanations for this phenomenon include energy metabolism, adipose tissue signaling, and increased frequency of medical care (20). Previous studies report that subcutaneous fat mass is related to survival in CRPC patients treated with docetaxel (10) or ARSi (21). In this study, we found that a high subcutaneous fat mass was associated with better survival outcomes for ARSi-treated patients, which is consistent with the study by Antoun et al. (21).
In this study, we focused on the skeletal muscle mass. High skeletal muscle mass was significantly associated with a decreased risk of PSA progression, radiologic progression, and overall mortality in patients with CRPC. These relationships between SMI and survival outcomes remained significant in patients treated with docetaxel but not in those treated with ARSi. Cushen et al. examined body composition in CRPC patients treated with docetaxel and reported that low skeletal muscle mass was associated with an increased risk of not only chemotherapy toxicity, but also overall mortality (8). Ohtaka et al. also reported that sarcopenia is an independent factor that raises the risk of mortality (9). A recent study by Stangl-Kremser et al. demonstrated that sarcopenia is associated with tumor progression in CRPC patients (22). In addition, one study reported an association between muscle mass and survival in patients with non-metastatic prostate cancer (19). Although the underlying mechanisms remain unclear, skeletal muscle mass may be associated with insulin growth factor signaling, host immunity, systemic inflammation, and hormonal activity, which are related to the aggressiveness of prostate cancer (19). Moreover, accumulating evidence suggests that low muscle mass is associated with higher toxicity and poor tolerance to chemotherapy (7–10). Several studies reported that low muscle mass also contributes to decreased response to chemotherapy in patients with solid malignancies, including breast, colorectal, lung, and ovary cancer (7), which supports the present data.
ARSi drives changes in metabolic profiles and body composition. Previous studies demonstrate relationships between ARSi therapy and lipid metabolism and statin use (23–25). Both abiraterone and enzalutamide can induce increases in body fat mass and decreases in lean body mass (26, 27). However, the relationship between body composition and the response to ARSi in CRPC patients is largely unknown. One study reported that subcutaneous adiposity is associated with overall survival in metastatic CRPC patients treated with ARSi after chemotherapy (21). The present data indicate that the efficacy of first-line ARSi in CRPC patients is unrelated to skeletal muscle mass, unlike that of docetaxel. These results indicate that skeletal muscle mass may be helpful for first-line treatment selection and for predicting treatment responses in patients initially diagnosed with CRPC. Based on the results of this study, CRPC patients with low skeletal muscle mass should be considered for first-line ARSi therapy rather than docetaxel.
The present study has several limitations. First, the retrospective design may have caused inevitable inherent bias. The relatively short duration of follow-up and the lack of a standardized protocol for first-line and second-line treatment selection may be potential significant confounders and preclude analysis of overall survival. Second, the results of this study may not be applicable to other ethnic groups, such as Caucasians, because of ethnic and racial variations in body composition (4). Third, we did not analyze body composition changes during the treatment period. Individual changes in body composition may influence the efficacy of treatment (28). Although muscle or fat mass may be associated with prognosis or treatment response in those with CRPC, the mechanisms underlying this phenomenon and any causal relationships remain unclear; therefore, caution should be exercised when attempting to draw definitive conclusions from this study.
Despite these limitations, we believe that the present study provides valuable information to support the management of patients with CRPC. To the best of our knowledge, this study is the first to demonstrate differences in the treatment response to docetaxel or ARSi according to individual muscle mass and fat mass.
In conclusion, skeletal muscle mass was associated with PSA progression, radiologic progression, and mortality in patients initially diagnosed with CRPC. These relationships remained significant in patients treated with docetaxel but not in those treated with ARSi. These results suggest that treatment responses may differ according to muscle mass, and that assessing the SMI may be worthwhile when selecting first-line treatment and predicting responses to therapy. Large-scale prospective validation is warranted.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
This study protocol was approved by the Institutional Review Board of the National Cancer Center, Goyang, Korea. Written informed consent was not required.
SP designed the study, performed data analysis, and drafted the manuscript. MK performed data analysis. EP performed statistical analysis. SK and KL participated in data acquisition. JJ designed the study. All authors read and approved the final manuscript.
This work was supported by a research grant from the National Cancer Center (No. 1810021), Republic of Korea.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00558/full#supplementary-material
Figure S1. Outcomes based on visceral fat index in overall patients with castration-resistant prostate cancer from the time of diagnosis. (A) Prostate-specific antigen progression-free survival. (B) Radiologic progression-free survival. (C) Overall survival.
Figure S2. Outcomes based on subcutaneous fat index in overall patients with castration-resistant prostate cancer from the time of diagnosis. (A) Prostate-specific antigen progression-free survival. (B) Radiologic progression-free survival. (C) Overall survival.
Figure S3. Outcomes based on skeletal muscle index tertiles in overall patients with castration-resistant prostate cancer from the time of diagnosis. (A) Prostate-specific antigen progression-free survival. (B) Radiologic progression-free survival. (C) Overall survival. 1st tertile: 26.73–46.92, 2nd tertile: 46.99–53.07, 3rd tertile: 53.09–77.24.
Figure S4. Treatment-specific hazards of radiologic progression.
Table S1. Cut-off points of body composition indices based on median values and the Contal and O'Quigley method.
Table S2. Multivariable analysis based on selected covariates.
Table S3. Characteristics of propensity score-matched patients.
1. Lowrance WT, Murad MH, Oh WK, Jarrard DF, Resnick MJ, Cookson MS. Castration-resistant prostate cancer: AUA Guideline Amendment 2018. J Urol. (2018) 200:1264–72. doi: 10.1016/j.juro.2018.07.090
2. Crawford ED, Higano CS, Shore ND, Hussain M, Petrylak DP. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol. (2015) 194:1537–47. doi: 10.1016/j.juro.2015.06.106
3. Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. (2016) 57:58–67. doi: 10.1016/j.ejca.2015.12.030
4. Shachar SS, Williams GR. The obesity paradox in cancer-moving beyond BMI. AACR Cancer Epidemiol Biomarkers Prev. (2017) 26:13–6. doi: 10.1158/1055-9965.EPI-16-0439
5. Caan BJ, Feliciano EMC, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. (2018) 4:798–804. doi: 10.1001/jamaoncol.2018.0137
6. Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of systemic inflammation and sarcopenia with survival in nonmetastatic colorectal cancer: results from the C SCANS study. JAMA Oncol. (2017) 3:e172319. doi: 10.1001/jamaoncol.2017.2319
7. Bozzetti F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. (2017) 28:2107–18. doi: 10.1093/annonc/mdx271
8. Cushen SJ, Power DG, Murphy KP, McDermott R, Griffin BT, Lim M, et al. Impact of body composition parameters on clinical outcomes in patients with metastatic castrate-resistant prostate cancer treated with docetaxel. Clin Nutr ESPEN. (2016) 13:e39–e45. doi: 10.1016/j.clnesp.2016.04.001
9. Ohtaka A, Aoki H, Nagata M, Kanayama M, Shimizu F, Ide H, et al. Sarcopenia is a poor prognostic factor of castration-resistant prostate cancer treated with docetaxel therapy. Prost Int. (2019) 7:9–14. doi: 10.1016/j.prnil.2018.04.002
10. Lee JS, Lee HS, Ha JS, Han KS, Rha KH, Hong SJ, et al. Subcutaneous fat distribution is a prognostic biomarker for men with castration resistant prostate cancer. J Urol. (2018) 200:114–20. doi: 10.1016/j.juro.2018.01.069
11. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. (2008) 26:1148. doi: 10.1200/JCO.2007.12.4487
12. van Vugt JL, Levolger S, Gharbharan A, Koek M, Niessen WJ, Burger JW, Willemsen SP, et al. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cach Sarc Muscle. (2017) 8:285–97. doi: 10.1002/jcsm.12158
13. Sonpavde G, Huang A, Wang L, Baser O, Miao R. Taxane chemotherapy vs antiandrogen agents as first-line therapy for metastatic castration-resistant prostate cancer. BJU Int. (2018) 121:871–9. doi: 10.1111/bju.14152
14. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. (2008) 371:569–78. doi: 10.1016/S0140-6736(08)60269-X
15. De Ridder J, Julián-Almárcegui C, Mullee A, Rinaldi S, Van Herck K, Vicente-Rodríguez G, Huybrechts I. Comparison of anthropometric measurements of adiposity in relation to cancer risk: a systematic review of prospective studies. Cancer Causes Control. (2016) 27:291–300. doi: 10.1007/s10552-015-0709-y
16. Psaltopoulou T, Sergentanis TN, Ntanasis-Stathopoulos I, Tzanninis IG, Riza E, Dimopoulos MA. Anthropometric characteristics, physical activity and risk of hematological malignancies: a systematic review and meta-analysis of cohort studies. Int J Cancer. (2019) 145:347–59. doi: 10.1002/ijc.32109
17. Farris MS, Courneya KS, Kopciuk KA, McGregor SE, Friedenreich CM. Anthropometric measurements and survival after a prostate cancer diagnosis. Br J Cancer. (2018) 118:607. doi: 10.1038/bjc.2017.440
18. Mason RJ, Boorjian SA, Bhindi B, Rangel L, Frank I, Karnes RJ, et al. The association between sarcopenia and oncologic outcomes after radical prostatectomy. Clin Genit Cancer. (2018) 16:e629–36. doi: 10.1016/j.clgc.2017.11.003
19. Pak S, Park SY, Shin TJ, You D, Jeong IG, Hong JH, et al. Association of muscle mass with survival after radical prostatectomy in patients with prostate cancer. J Urol. (2019) 202:525–32. doi: 10.1097/JU.0000000000000249
20. Ebadi M, Martin L, Ghosh S, Field CJ, Lehner R, Baracos VE, et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer. (2017) 117:148. doi: 10.1038/bjc.2017.149
21. Antoun S, Bayar A, Ileana E, Laplanche AK, Fizazi, di Palma M, et al. High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur J Cancer. (2015) 51:2570–7. doi: 10.1016/j.ejca.2015.07.042
22. Stangl-Kremser J, Suarez-Ibarrola R, D'Andrea D, Korn SM, Pones M, Kramer G, et al. Assessment of body composition in the advanced stage of castration-resistant prostate cancer: special focus on sarcopenia. Prost Cancer Prost Dis. (2019) 1–7. doi: 10.1038/s41391-019-0186-6
23. Gordon JA, Buonerba C, Pond G, Crona D, Gillessen S, Lucarelli G, et al. Statin use and survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone or enzalutamide after docetaxel failure: the international retrospective observational STABEN study. Oncotarget. (2018) 9:19861. doi: 10.18632/oncotarget.24888
24. Di Lorenzo G, Sonpavde G, Pond G, Lucarelli G, Rossetti S, Facchini G, Scagliarini S, et al. Statin use and survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate. Eur UrolFocus. (2018) 4:874–9. doi: 10.1016/j.euf.2017.03.015
25. Yang H, Pang L, Hu X, Wang W, Xu B, Zhang X, et al. The effect of statins on advanced prostate cancer patients with androgen deprivation therapy or abiraterone/enzalutamide: a systematic review and meta-analysis. J Clin Pharm Therap. (2020). doi: 10.1111/jcpt.13092. [Epub ahead of print].
26. Attard G, Merseburger AS, Arlt W, Sternberg CN, Feyerabend S, Berruti A, et al. Assessment of the safety of glucocorticoid regimens in combination with abiraterone acetate: a randomized, open-label phase 2 study. JAMA Oncol. (2019). doi: 10.1001/jamaoncol.2019.1011. [Epub ahead of print].
27. Tombal B, Borre M, Rathenborg P, Werbrouck P, Van Poppel H, Heidenreich A, et al. Long-term antitumor activity and safety of enzalutamide monotherapy in hormone naive prostate cancer: 3-year open label followup results. J Urol. (2018) 199:459–64. doi: 10.1016/j.juro.2017.08.103
Keywords: castration-resistant prostate cancer, body composition, survival, treatment efficacy, disease progression
Citation: Pak S, Kim MS, Park EY, Kim SH, Lee KH and Joung JY (2020) Association of Body Composition With Survival and Treatment Efficacy in Castration-Resistant Prostate Cancer. Front. Oncol. 10:558. doi: 10.3389/fonc.2020.00558
Received: 10 December 2019; Accepted: 27 March 2020;
Published: 17 April 2020.
Edited by:
Fabio Grizzi, Humanitas Research Hospital, ItalyReviewed by:
Giuseppe Di Lorenzo, Azienda Sanitaria Locale Salerno, ItalyCopyright © 2020 Pak, Kim, Park, Kim, Lee and Joung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae Young Joung, dXJvanlAbmNjLnJlLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.