
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 15 April 2020
Sec. Surgical Oncology
Volume 10 - 2020 | https://doi.org/10.3389/fonc.2020.00516
This article is part of the Research Topic Neoadjuvant Treatment in Pancreatic Cancer View all 16 articles
Evaluation of response to preoperative therapy for patients with pancreatic adenocarcinoma has been historically difficult. Therefore, preoperative regimens have generally been selected on the basis of baseline data such as radiographic stage and serum CA 19-9 level and then typically administered for a pre-specified duration as long as 6 months or more. The decision to proceed with resection following preoperative therapy likewise has rested upon the absence of disease progression rather than evidence for tumor response. This article reviews the basis for the evaluation of therapeutic response after preoperative therapy for pancreatic cancer in the existing scientific literature, and providing updates and new perspectives.
Pancreatic ductal adenocarcinoma (PDAC) is a extremely lethal disease, and is anticipated to be the second cause of cancer-related death in the US by 2020, surpassed only by lung cancer (1). Anatomically, localized PDAC is defined as resectable (R), borderline resectable (BR), and locally advanced (LA) based on evidence for venous and arterial involvement on cross-sectional imaging. Among all patients who present with PDAC in the USA, over 30% have a LA or BR disease and only 15% to 20% are eligible to undergo oncologic resection, the only potentially curative strategy (2).
Systemic therapy following resection improves survival outcomes relative to surgery alone (3), but with the advent of more effective chemotherapy regimens over recent years, efforts have focused on optimizing the administration of chemotherapy and/or (chemo)radiation prior to, instead of following, resection of the primary tumor. The goals of preoperative therapy are primarily to maximize the likelihood of a microscopically complete (R0) resection by reducing the size and/or anatomic extent of the tumor, to identify poor responders who progress on treatment preoperatively in order to spare them a futile operation, and to treat occult systemic disease in order to prolong survival. Practice guidelines now recognize the administration of preoperative therapy as the preferred strategy for patients with BR PDAC, while many high volume centers for pancreatic surgery are increasingly delivering it to patients with potentially R PDAC as well (4, 5).
Unfortunately, evaluation of therapeutic response to preoperative therapy has been historically difficult for pancreatic adenocarcinoma. Therefore, preoperative regimens have been typically administered, in the absence of radiographic or serologic evidence of disease progression, for a pre-specified duration as long as 6 months or more.
This article reviews the basis of tumor response evaluation after preoperative therapy for PDAC in the existing scientific literature and offers new perspectives to fuel the scientific debate on the important topic of surgical approach after preoperative therapy (Figure 1).
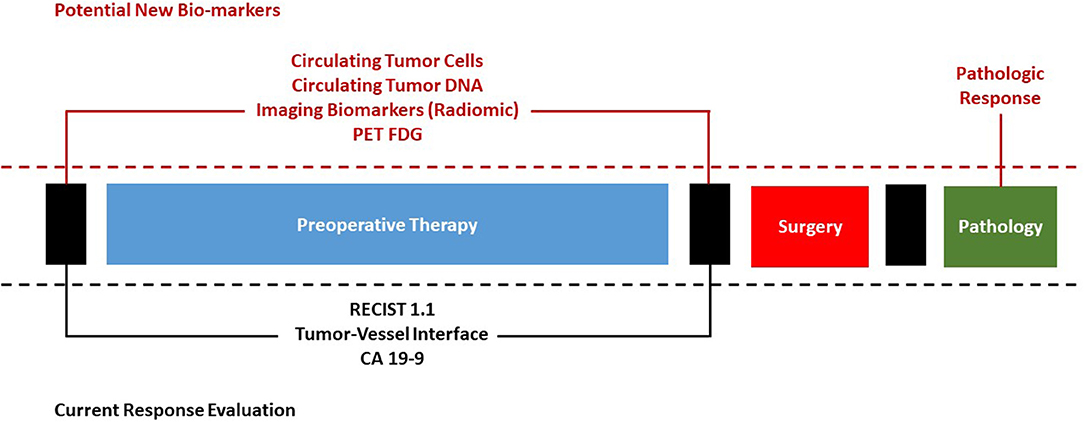
Figure 1. Current evaluation of response to preoperative therapy for pancreatic cancer and potential future bio-markers.
Anatomically, localized PDAC is often defined as resectable (R), borderline resectable (BR), and locally advanced (LA) according to the apparent involvement of mesenteric vasculature on cross-sectional imaging. CT and MRI studies with dedicated pancreatic-protocol are equally effective for staging, with CT being more commonly used in clinical practice (6). Vascular abutment or encasement by tumors seems to be associated with higher rates of microscopically non-radical resection, longer operative times, and higher perioperative morbidity (7, 8). Different criteria defining resectability have been proposed, all based on radiographic criteria on cross-sectional imaging. These include the expert consensus guidelines by AHPBA/SSAT/SSO/GSSC, the NCCN guidelines, and the International Study Group of Pancreatic Surgery (ISGPS) guidelines (4, 9, 10). Recognizing that patients with non-metastatic PDAC are a heterogeneous population not only anatomically, but also physiologically and oncologically, we have additionally categorized patients with PDAC on the basis of tumor anatomy (BR-A), cancer biology (BR-B), and patient comorbidities and condition (BR-C) (11).
Induction systemic chemotherapy, often followed by (chemo)radiation therapy, represents the standard of care for BR and LA PDAC, as recommended by both NCCN and ASCO guidelines (4, 5). ASCO guidelines also consider preoperative therapy as an acceptable option for patients with R disease, and specifically recommend it for patients with R tumors but radiographic findings suspicious (but not diagnostic) for extra-pancreatic disease, a performance status or comorbidity profile unfitting for a major abdominal surgery (but potentially reversible) or a CA 19-9 level (in absence of jaundice) suggestive of disseminated cancer. Today, the commonly used preoperative regimens in patients with good performance status are the combination regimens fluorouracil [5-FU], leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) and Gemcitabine-NabPaclitaxel (GA), given the efficacy of these regimens in the metastatic setting (12, 13). External beam radiation therapy with concurrent chemosensitizing 5-FU, capecitabine, or gemcitabine, or stereotactic body radiotherapy (SBRT) may also be delivered at some centers, typically following induction chemotherapy.
In the setting of LA disease, the primary goals of preoperative therapy are to reduce systemic disease burden, to reduce tumor-related symptoms, and to prolong overall survival, as only a relatively small percentage of patients with LA cancers are downstaged by preoperative therapy to an extent sufficient to make safe surgical resection realistic. The extent to which “preoperative” therapy is truly “preoperative” in such patients is therefore a matter of debate. On the other hand, the primary goal of preoperative therapy in the BR and R settings is to select patients with favorable “disease biology” who will not experience early systemic progression, as well as to improve the likelihood of complete macroscopic and microscopic resection. Together with tumor dimensions and lymph node status, in fact, R status is recognized as the most relevant determinant for prognosis in patients undergoing surgery (14–17).
The administration of preoperative therapy may also be a valuable strategy to deliver to patients with localized disease the maximum load of chemotherapy, since as high as 40% of patients will never be eligible for adjuvant treatments due to postoperative morbidity and/or failure to improve performance status following pancreatectomy (18, 19). Another purpose of primary systemic therapy is to treat occult micro-metastases at the time of diagnosis, thereby attacking the cancer foci accountable for early recurrence after resection and selecting for a “locally dominant phenotype” (20). The delivery of chemotherapy drugs prior to surgery may also allow them to better penetrate neoplastic cells since patients' tissues are still not altered by inflammation and fibrosis induced by any surgical procedure.
Preoperative therapy still presents some limitations. For example, most patients with BR PDAC have jaundice at the time of presentation. Neoadjuvant therapy necessitates the placement of biliary stents to decompress the biliary obstruction of patients with jaundice. The placement of biliary stents before surgery rises the infections risk in the perioperative period. Furthermore, many patients may require readmission or further procedures or may suffer significant complications such as pancreatitis, cholangitis and death (21, 22). Biliary stenting and preoperative therapy delay surgery, and it is not clear how the risk of progression of the disease to the point of becoming unresectable bay be increased by this delay.
No clinical trial has yet definitively clarified which therapeutic modalities are most effective as preoperative therapy for PDAC between chemotherapy, (chemo)radiation, or novel agents, and how and when each modality should be administered to maximize patient's survival and quality of life while minimizing morbidity. Therefore, current guidelines have predominantly relied upon relatively low-level data (4, 23).
Gaps in knowledge have only begun to be addressed in recent years by multicenter trials. For example, two recent trials from Korea (NCT01458717) and the Netherlands (PREOPANC-1) randomized patients with localized PDAC to either gemcitabine-based (chemo)radiation or surgery, to assess the efficacy of perioperative radiotherapy (24, 25). In both studies R0 resection rate and overall survival were more favorable in patients who received (chemo)radiation. However, although these trials suggest a possible role for (chemo)radiation in the preoperative setting, in both trial radiation was compared to surgery de novo and not with an alternate preoperative regimen, like systemic chemotherapy. The recently-completed Alliance for Clinical Trials in Oncology A021501 study randomized patients with BR PDAC to receive either 8 cycles of FOLFIRINOX or 7 cycles of FOLFIRINOX followed by stereotactic body radiotherapy (26). Although complete data are not yet available, it is known that the chemotherapy plus stereotactic body radiation therapy arm met the predetermined futility boundary for R0 resection and was closed prematurely. Nonetheless, local disease control or even survival for some patients with BR PDAC may still be well improved by radiation, and the role of effective local therapies can only increase if systemic therapies improve as well. After the positive results of PREOPANC-1, PREOPANC-2 (to be completed within 2022) was designed to recognize the best preoperative treatment for R and BR PDAC, randomizing patients to receive preoperative FOLFIRINOX (8 cycles) alone or preoperative gemcitabine-based chemo-radiotherapy (3 cycles) and subsequent adjuvant treatment. Finally, other phase II trials are comparing different preoperative chemotherapy regimens. SWOG S1505 for example is a randomized phase II study comparing modified FOLFIRINOX vs. GA as preoperative therapy for resectable PDAC (27). ESPAC-5F is a 4-arm phase II trial comparing upfront surgery vs. different options of preoperative strategies for patients with BR PDAC. Enrolled patients are randomized to undergo upfront resection or either preoperative gemcitabine plus capecitabine (8 weeks), preoperative FOLFIRINOX (8 weeks) or preoperative (chemo)radiation.
The optimal duration of preoperative treatment is not well understood. Therefore, the durations with which chemotherapy is administered in the preoperative setting have been somewhat arbitrary. While patients with progressive disease can be easily identified and spared ineffective surgery, the decision to proceed with surgical exploration following preoperative therapy usually rests on evidence of disease stability after a highly variable amount of chemotherapy with or without subsequent (chemo)radiation. We use to treat patients with BR PDAC with systemic chemotherapy for ~4 months, followed by radiation therapy, attempting to individualize patients' treatment also on the basis of clinical parameters such as their physiologic profile and serum carbohydrate antigen 19–9 level, instead of anatomy alone. However, a multimodal evaluation of the response to preoperative therapy that uses novel biomarkers and tools for response prediction is very much needed to ensure accurate selection of patients for surgery and inform treatment decisions like therapy sequencing and optimal chemotherapy regimens.
The accuracy of radiologic assessment of response to preoperative therapy has been historically challenged. Changes in tumor size on diagnostic imaging as assessed by RECIST 1.1 (Response Evaluation Criteria in Solid Tumors version 1.1) (28), although reflective of therapeutic efficacy (or lack thereof) in other cancer treatment settings, have been felt to be insufficient in predicting response—and in particular respectability—in the setting of preoperative therapy for PDAC. The problem was identified clearly as early as 2001, with a study that suggested that CT after preoperative therapy seemed to underestimate the possibility of resecting a tumor to negative margins (29). The peculiar nature of the PDAC extensive and dense fibrous stroma, which after chemotherapy is often associated with a persistence of fibrosis tissue that prevents the tumor from shrinking on imaging—even after the destruction of cancer cells—seems to be the main responsible for this perceived lack of accuracy (30).
In 2012, for example, we reported that among 129 patients treated with preoperative gemcitabine-based therapy for BR PDAC from 2005 to 2010, only 15 (12%) experienced RECIST PR and the tumor of only 1 (0.8%) patient was downstaged to R (31). However, 85 (66%) of patients were still able to undergo resection, 81 of whom with negative margins (R0). A 2013 study showed diminished performance of CT scan accuracy predicting R0 resectability or unresectability after preoperative therapy with gemcitabine or 5-Flourouracil when compared to patients treated with first-line surgery (58% vs 83% and 52 vs 88%, respectively) (32). A study from the same year, despite being carried out in a small number of selected patients, concluded that neither changes in tumor CT attenuation nor changes in vascular involvement contributed to the prediction of resectability, and its findings were partially confirmed by a later study performed on a larger cohort, showing that only a partial decrease in tumor-vessel contact or even small decrease in tumor size was associated with R0 resection, in contrast to changes in tumor attenuation (33, 34). Focusing on 40 BR or LAPC PDAC preoperatively treated with FOLFIRINOX, a 2015 study concluded that after preoperative therapy images no longer predict unresectability, as 70% of them were re-classified as BR or LAPC after therapy although an R0 resection was achieved in 92% of them (35). Similar results were achieved in another multicenter retrospective study with 36 patients treated with FOLFIRINOX, where despite a significant tumor shrinkage after therapy, preoperative CT failed to accurately predict resectability (36).
In a recent study by Truty and colleagues, among 194 patients with BR or LAPC PDAC treated with “total neoadjuvant therapy” with FOLFIRINOX or GA, 28% had radiographic downstaging (37). A similar rate of radiographic downstaging, strikingly higher than that reported in the past, maybe due to a higher efficacy of modern chemotherapy regimens, but it may also simply reflect artifact of study conduct (such as, in this case, the exclusion of patients who did not undergo surgery). Regardless, in this study, radiological downstaging was not associated with overall and recurrence free survival.
It is interesting to notice how the majority of these studies focused on radiologic prediction of resectability, or rates of radiographic downstaging (based on vascular anatomy) in surgical series, rather than trying to assess possible radiographic predictors of tumor response to chemotherapy and survival. True response metrics would be important to determine if and how well a regimen was working preoperatively, limiting the possibility that a patient with otherwise R disease could be treated with an ineffective regimen for an inappropriate length of time. RECIST is an important, standardized and reproducible classification system, used to report and compare response rate to therapy in most prospective clinical trials, despite historically not being widely used in this clinical setting. However, it remains limited as it relies upon 2-dimensional measurement of maximum tumor diameter and uses a fixed cutoff of 30% to discriminate between a stable disease and a partial response. More objective metrics of tumor response to therapy (based, for example, on tridimensional tumor volume) may be useful to inform the delivery of induction therapy and to better assess the actual systemic response to preoperative chemotherapy, guaranteeing a more individualized approach to treatment that has higher resolution than simple dichotomous determination of resectable/not resectable.
Cancer antigen 19-9 (CA 19-9) measurement is the only biomarker for monitoring the response to therapy in PDAC approved by the US Food and Drug Administration, and it has been incorporated into the clinical staging and treatment algorithms of patients with both localized and metastatic disease. However, despite being the most commonly used marker to track response or recurrence in this setting, its use is limited to patients with a Sialyl-LewisA-positive genotype (~90% of patients). Furthermore, proper interpretation of CA 19-9 measurements requires a normal bilirubin level, and elevated CA 19-9 may also be associated with inflammatory processes such as radiation therapy (38).
With the above-mentioned limitations, the change in serum CA 19-9 that happens during the administration of preoperative therapy can be clinically used as a surrogate of response to treatment and as a marker of long-term prognosis, as CA 19-9 normalization is a favorable clinical indicator associated with prolonged overall survival. In 2010, we showed how normal pretreatment and posttreatment CA 19-9 levels had high positive predictive values for respectively completing preoperative therapy and undergoing resection, despite low negative predicting values compromising their clinical utility (39). In a later study, we found that CA 19-9 normalization was associated with longer survival among both resected and non resected patients with BR disease (40).
The largest study investigating CA 19-9 as a predictor of response included 454 metastatic patients treated with gemcitabine with or without nab-paclitaxel. Ninety-six percent of radiographic responders in this study showed a decrease also in CA 19-9, compared to 78% of patients who were radiographically stable after chemotherapy, and a decrease in CA 19-9 levels predicted a survival benefit (41). The decrease of CA 19-9 after chemotherapy was also investigated as a part of the ACCORD/PRODIGE4 trial, comparing FOLFIRINOX to gemcitabine in 160 metastatic patients. Patients with a CA 19-9 ≥20% had an overall response rate significantly higher than patients with CA 19-9 <20% (44 vs. 22.9%) (42).
Recently CA19-9 normalization, rather than the magnitude of change, has been confirmed to be the strongest prognostic marker for long-term survival in a retrospective series of 131 patients with elevated (>35U/dl) CA19-9 at diagnosis who underwent preoperative therapy and resection (43). Furthermore our institution recently showed how a major pathologic response is really unlikely in patients who have elevated CA 19-9 after preoperative therapy. In this study, among 28 patients having a major pathologic response to preoperative therapy, 27 (96%) had a normal posttreatment CA 19-9 despite an elevated pretreatment CA 19-9 in 75% of the cases (see below, “Pathologic response”) (44).
The measurement of serum CA 19-9 both prior to and following the administration of preoperative therapy for PDAC is supported by these findings, and its role is emphasized as an easily assayed marker that provides insight into the biology of each patient's tumor and the patient's likely long-term outcome following completion of multimodal therapy.
Tumor response to preoperative therapies may be measured histologically by the extent of residual viable cancer in the resected specimen. Although preoperative therapy is currently widely used, the tumor regression grading system for PDAC following preoperative therapy is not standardized. Six different systems are being used in the evaluation of PDAC following preoperative therapy, from Evans et al. to Chatterjee et al. (MD Anderson) and College of American Pathologists (CAP) 2017 (29, 45–49). Most of the systems are based on the evaluation of the destruction of viable cancer cells and/or the extent of fibrosis induced by the treatment. This metric has important prognostic implications for patients who have undergone resection of PDAC, as we previously demonstrated. Patients who experience either pathologic complete response (pCR, no viable cancer cells) or pathologic major response (pMR, <5% of residual viable cancer cells) to preoperative therapy live significantly longer than patients who have 5 to 100% viable residual cancer cells in their specimen (48, 50, 51). Unfortunately, a pCR or even a pMR in PDAC is rather rare compared to other solid tumors like breast cancer and colorectal cancer and is seen in only 3 to 11% of resected specimen treated with preoperative therapy. An accurate, standardized, and repeatable method for pathology examination of the residual cancer tissue following preoperative therapy is needed to better compare publications on this topic and to establish pathways for the diagnostic and therapeutic management of these patients.
Only few, retrospective studies have related pathologic response after preoperative therapy for PDAC to possible clinical, radiographic and serologic predictors. In 2017, a study from MD Anderson showed that baseline factors including young age, pretreatment CA 19-9 level, and use of gemcitabine as a radio-sensitizer were associated with pMR. Two other studies correlated positron emission tomography (PET) complete metabolic response and CA 19-9 response to pCR (37, 52, 53).
In a recently published study, we sought to identify potential radiographic and serologic predictors of pMR, occurring in only 28 (10%) patients, even after modern preoperative regimens. We found that posttreatment CA 19-9, RECIST Partial Response and % of tumor volume decrease were independent predictors of pMR, and a volume loss of at least 55% of the baseline tumor volume after treatment had a sensitivity of 78% and a specificity of 75% in predicting pMR (44). More importantly, pMR was extraordinarily unlikely in the absence of a posttreatment CA 19-9 within the normal range or a reduction in tumor volume with therapy. In this study, patients experiencing a pMR were confirmed to have a strikingly higher median overall survival compared to non-pMR patients (not reached vs 38 months; P < 0.01).
Novel radiomic and serologic predictors of response are actively being investigated, and it is very likely that not far in the future functional imaging or quantitative bio-imaging will provide fundamental advances.
At our institution, Amer et al. recently evaluated 4 cohorts of patients and showed that in each, the change in the radiographic interface between tumor and adjacent pancreatic parenchyma that often occurred in association with (chemo)radiation was associated with outcome. Moreover, in one of the cohorts, patients who met criteria for a radiomic response had a greater likelihood of achieving a pMR or pCR (21 vs. 0%, P = 0.01) (54). Our group has also identified an imaging biomarker that can be assessed using routine computer tomographic images and may be used to stratify patient's tumors into distinctive biophysical subtypes (55).
Preoperative therapy may also affect a patient's muscle mass and adipose tissue. We studied anthropometric changes occurring in patients during preoperative therapy for PDAC (56). We showed that up to 52% of patients met anthropometric criteria for sarcopenia prior to surgery, and how further depletion of skeletal muscle, as well as adipose tissue, occurred during preoperative therapy. These changes did not, however, preclude resection. Conversely, a recent multicenter study showed that adipose tissue significantly decreased during preoperative treatment, while muscle mass slightly increased (57). Resected patients experienced a higher increase in muscular tissue during preoperative treatment compared with unresected patients. Although these anthropometric measures are not direct metrics of therapeutic effect, they are important and could be supplemental measures that could potentially provide additional information to help clinicians in the re-evaluation of patients after preoperative therapy.
Systemic inflammation ratios reflect the antitumor inflammation capacity of the host and have prognostic value in patients with PDAC (58). Recently, Kawai and colleagues showed how a low lymphocyte-to-monocyte ratio after preoperative therapy is associated with poorer survival outcomes. Tumor-infiltrating lymphocytes have a crucial role in enhancement of antitumor immune response, and low lymphocyte-to-monocyte ratio may have a potential role in stratification of treatment strategies (59).
Liquid biopsy has been used in numerous recent studies to detect tumor-associated biomarkers in different extractable body fluids and is hopeful to monitor response to treatment and disease progression, and to even predict patient outcome (60). Circulating cell-free tumor DNA (ctDNA) is a tumor-associated biomarker released in the bloodstream as a result of tumor cells death and represents the molecular signature of cancer cells. Blood samples is much less invasive compared to tumor biopsies a can represent cancer heterogeneity to a larger extent. In case of chemotherapy response, therapy-induced tumor cell death should lead in theory to an increase of ctDNA levels, but in practice ctDNA levels will eventually become undetectable. Increasing ctDNA levels in the long term could indicate disease progression as a result of increasing tumor burden. Bernard et al. calculated the fraction of mutant KRAS in circulating exosomal DNA and found that an increase was associated with disease progression, suggesting how longitudinal monitoring using liquid biopsy samples through exoDNA and ctDNA provides both predictive and prognostic information relevant to therapeutic stratification (61).
Circulating tumor cells (CTCs) fluctuations were also reported to be used to monitor disease progression and clinical response to therapy in patients with PDAC (62, 63). A recent study by the Johns Hopkins University found a significantly lower number of total CTCs in patients who received preoperative chemotherapy, compared to patients who did not and preoperative number of CTCs was the only predictor of early recurrence within 12 months from surgery. In the future, CTCs dynamic may serve, at least to some degree, also as a readout of pathologic response, helping to inform the delivery of preoperative therapy and to better assess the actual systemic response to preoperative chemotherapy, guaranteeing a more individualized approach to treatment.
Current radiographic and serologic evaluation of tumor response to preoperative therapy is limited for PDAC, and therapeutic decisions are typically made on the basis of absence of progression rather than evidence of tumor response. Novel biomarkers reveal the high potential for longitudinal monitoring and the use of real-time radiographic or circulating biomarkers to direct therapy. Prospective studies with a complete set of information including liquid biopsies and pathology are needed to give us a whole picture of the status of patient response to preoperative therapy. Eventually, clinical characteristics, pathology features and biomarker expression before and after preoperative therapy should be all combined to enhance the clinical significance of these biomarkers in the context of precision medicine and give a comprehensive, personalized evaluation of tumor regression.
GP devised the structure and focus of the article and wrote the first draft of it. LP wrote portions of it and edited the first draft. MK managed the manuscript through its composition, final revision and publication processes. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, (2019). CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
3. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul J-L, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. (2018) 379:2395–406. doi: 10.1056/NEJMoa1809775
4. Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, nCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN. (2017) 15:1028–61. doi: 10.6004/jnccn.2017.0131
5. Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, et al. Potentially curable pancreatic cancer: american society of clinical oncology clinical practice guideline. J Clin Oncol Off J Am Soc Clin Oncol. (2016) 34:2541–56. doi: 10.1200/JCO.2016.67.5553
6. Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Radiology. (2014) 270:248–60. doi: 10.1148/radiol.13131184
7. Giovinazzo F, Turri G, Katz MH, Heaton N, Ahmed I. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg. (2016) 103:179–91. doi: 10.1002/bjs.9969
8. Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Büchler MW, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg. (2011) 254:882–93. doi: 10.1097/SLA.0b013e31823ac299
9. Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. (2009) 16:1727–33. doi: 10.1245/s10434-009-0408-6
10. Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. Borderline resectable pancreatic cancer: a consensus statement by the international study group of pancreatic surgery (ISGPS). Surgery. (2014) 155:977–88. doi: 10.1016/j.surg.2014.02.001
11. Katz MHG, Pisters PWT, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. (2008) 206:833–46; discussion 846–848. doi: 10.1016/j.jamcollsurg.2007.12.020
12. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. (2011) 364:1817–25. doi: 10.1056/NEJMoa1011923
13. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-Paclitaxel plus gemcitabine. N Engl J Med. (2013) 369:1691–703. doi: 10.1056/NEJMoa1304369
14. Strobel O, Hank T, Hinz U, Bergmann F, Schneider L, Springfeld C, et al. Pancreatic cancer surgery: the new r-status counts. Ann Surg. (2017) 265:565–73. doi: 10.1097/SLA.0000000000001731
15. Rau BM, Moritz K, Schuschan S, Alsfasser G, Prall F, Klar E. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery. (2012) 152(3 Suppl 1):S103–111. doi: 10.1016/j.surg.2012.05.015
16. Liu Z-Q, Xiao Z-W, Luo G-P, Liu L, Liu C, Xu J, et al. Effect of the number of positive lymph nodes and lymph node ratio on prognosis of patients after resection of pancreatic adenocarcinoma. Hepatobiliary Pancreat Dis Int HBPD INT. (2014) 13:634–41. doi: 10.1016/S1499-3872(14)60264-2
17. Malleo G, Maggino L, Capelli P, Gulino F, Segattini S, Scarpa A, et al. Reappraisal of nodal staging and study of lymph node station involvement in pancreaticoduodenectomy with the standard international study group of pancreatic surgery definition of lymphadenectomy for cancer. J Am Coll Surg. (2015) 221:367–79.e4. doi: 10.1016/j.jamcollsurg.2015.02.019
18. Tzeng C-WD, Tran Cao HS, Lee JE, Pisters PWT, Varadhachary GR, Wolff RA, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg Off J Soc Surg Aliment Tract. (2014) 18:16–24; discussion 24–25. doi: 10.1007/s11605-013-2412-1
19. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the cONKO-001 randomized trial. JAMA. (2013) 310:1473–81. doi: 10.1001/jama.2013.279201
20. Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol Off J Am Soc Clin Oncol. (2009) 27:1806–13. doi: 10.1200/JCO.2008.17.7188
21. van der Gaag NA, Rauws EAJ, van Eijck CHJ, Bruno MJ, van der Harst E, Kubben FJGM, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. (2010) 362:129–37. doi: 10.1056/NEJMoa0903230
22. Andriulli A, Loperfido S, Napolitano G, Niro G, Valvano MR, Spirito F, et al. Incidence rates of post-ERCP complications: a systematic survey of prospective studies. Am J Gastroenterol. (2007) 102:1781–8. doi: 10.1111/j.1572-0241.2007.01279.x
23. Neuzillet C, Gaujoux S, Williet N, Bachet J-B, Bauguion L, Colson Durand L, et al. Pancreatic cancer: french clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, fFCD, gERCOR, uNICANCER, sFCD, sFED, sFRO, aCHBT, aFC). Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. (2018) 50:1257–71. doi: 10.1016/j.dld.2018.08.008
24. Jang J-Y, Han Y, Lee H, Kim S-W, Kwon W, Lee K-H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a Prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. (2018) 268:215–22. doi: 10.1097/SLA.0000000000002705
25. Versteijne E, van Eijck CHJ, Punt CJA, Suker M, Zwinderman AH, Dohmen MAC, et al. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials. (2016) 17:127. doi: 10.1186/s13063-016-1262-z
26. Katz MHG, Ou F-S, Herman JM, Ahmad SA, Wolpin B, Marsh R, et al. Alliance for clinical trials in oncology (ALLIANCE) trial a021501: preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer. (2017) 17:505. doi: 10.1186/s12885-017-3441-z
27. Sohal D, McDonough SL, Ahmad SA, Gandhi N, Beg MS, Wang-Gillam A, et al. SWOG s1505: a randomized phase iI study of perioperative mFOLFIRINOX vs. gemcitabine/nab-paclitaxel as therapy for resectable pancreatic adenocarcinom. J Clin Oncol. (2017) 35:TPS508. doi: 10.1200/JCO.2017.35.4_suppl.TPS508
28. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised rECIST guideline (version 1.1). Eur J Cancer Oxf Engl. (2009). 45:228–47. doi: 10.1016/j.ejca.2008.10.026
29. White RR, Paulson EK, Freed KS, Keogan MT, Hurwitz HI, Lee C, et al. Staging of pancreatic cancer before and after neoadjuvant chemoradiation. J Gastrointest Surg Off J Soc Surg Aliment Tract. (2001) 5:626–33. doi: 10.1016/S1091-255X(01)80105-0
30. Zins M, Matos C, Cassinotto C. Pancreatic adenocarcinoma staging in the era of preoperative chemotherapy and radiation therapy. Radiology. (2018) 287:374–90. doi: 10.1148/radiol.2018171670
31. Katz MHG, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. (2012) 118:5749–56. doi: 10.1002/cncr.27636
32. Cassinotto C, Cortade J, Belleannée G, Lapuyade B, Terrebonne E, Vendrely V, et al. An evaluation of the accuracy of cT when determining resectability of pancreatic head adenocarcinoma after neoadjuvant treatment. Eur J Radiol. (2013) 82:589–93. doi: 10.1016/j.ejrad.2012.12.002
33. Dudeja V, Greeno EW, Walker SP, Jensen EH. Neoadjuvant chemoradiotherapy for locally advanced pancreas cancer rarely leads to radiological evidence of tumour regression. HPB. (2013) 15:661–7. doi: 10.1111/hpb.12015
34. Cassinotto C, Mouries A, Lafourcade J-P, Terrebonne E, Belleannée G, Blanc J-F, et al. Locally advanced pancreatic adenocarcinoma: reassessment of response with cT after neoadjuvant chemotherapy and radiation therapy. Radiology. (2014) 273:108–16. doi: 10.1148/radiol.14132914
35. Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, et al. Radiological and surgical implications of neoadjuvant treatment with fOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. (2015) 261:12–7. doi: 10.1097/SLA.0000000000000867
36. Wagner M, Antunes C, Pietrasz D, Cassinotto C, Zappa M, Sa Cunha A, et al. CT evaluation after neoadjuvant fOLFIRINOX chemotherapy for borderline and locally advanced pancreatic adenocarcinoma. Eur Radiol. (2017) 27:3104–16. doi: 10.1007/s00330-016-4632-8
37. Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/Locally advanced pancreatic cancer. Ann Surg. (2019) doi: 10.1097/SLA.0000000000003284
38. Mann DV, Edwards R, Ho S, Lau WY, Glazer G. Elevated tumour marker cA19-9: clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. (2000) 26:474–9. doi: 10.1053/ejso.1999.0925
39. Katz MHG, Varadhachary GR, Fleming JB, Wolff RA, Lee JE, Pisters PWT, et al. Serum cA 19-9 as a marker of resectability and survival in patients with potentially resectable pancreatic cancer treated with neoadjuvant chemoradiation. Ann Surg Oncol. (2010) 17:1794–801. doi: 10.1245/s10434-010-0943-1
40. Tzeng C-WD, Balachandran A, Ahmad M, Lee JE, Krishnan S, Wang H, et al. Serum carbohydrate antigen 19-9 represents a marker of response to neoadjuvant therapy in patients with borderline resectable pancreatic cancer. HPB. (2014) 16:430–8. doi: 10.1111/hpb.12154
41. Chiorean EG, Von Hoff DD, Reni M, Arena FP, Infante JR, Bathini VG, et al. CA19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase iII trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. J Eur Soc Med Oncol. (2016) 27:654–60. doi: 10.1093/annonc/mdw006
42. Robert M, Jarlier M, Gourgou S, Desseigne F, Ychou M, Bouché O, et al. Retrospective analysis of cA19-9 decrease in patients with metastatic pancreatic carcinoma treated with fOLFIRINOX or gemcitabine in a randomized phase iII study (ACCORD11/PRODIGE4). Oncology. (2017) 93:367–76. doi: 10.1159/000477850
43. Tsai S, George B, Wittmann D, Ritch PS, Krepline AN, Aldakkak M, et al. Importance of normalization of cA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg. (2020) 271:740–7. doi: 10.1097/SLA.0000000000003049
44. Perri G, Prakash L, Wang H, Bhosale P, Varadhachary GR, Wolff R, et al. Radiographic and serologic predictors of pathologic major response to preoperative therapy for pancreatic cancer. Ann Surg. (2019) doi: 10.1097/SLA.0000000000003442
45. Ishikawa O, Ohhigashi H, Teshima T, Chatani M, Inoue T, Tanaka S, et al. Clinical and histopathological appraisal of preoperative irradiation for adenocarcinoma of the pancreatoduodenal region. J Surg Oncol. (1989) 40:143–51. doi: 10.1002/jso.2930400303
46. Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. (1992) 127:1335–9. doi: 10.1001/archsurg.1992.01420110083017
47. Le Scodan R, Mornex F, Partensky C, Mercier C, Valette P-J, Ychou M, et al. Histopathological response to preoperative chemoradiation for resectable pancreatic adenocarcinoma: the french phase iI fFCD 9704-SFRO trial. Am J Clin Oncol. (2008) 31:545–52. doi: 10.1097/COC.0b013e318172d5c5
48. Chatterjee D, Katz MH, Rashid A, Varadhachary GR, Wolff RA, Wang H, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer. (2012) 118:3182–90. doi: 10.1002/cncr.26651
49. Washington K, Berlin J, Branton P. Protocol for the examination of specimens from patients with carcinoma of the pancreas. Coll Am Pathol. (2017).
50. Chuong MD, Frakes JM, Figura N, Hoffe SE, Shridhar R, Mellon EA, et al. Histopathologic tumor response after induction chemotherapy and stereotactic body radiation therapy for borderline resectable pancreatic cancer. J Gastrointest Oncol. (2016) 7:221–7. doi: 10.3978/j.issn.2078-6891.2015.075
51. Zhao Q, Rashid A, Gong Y, Katz MH, Lee JE, Wolf R, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol. (2012) 16:29–37. doi: 10.1016/j.anndiagpath.2012.04.002
52. Cloyd JM, Wang H, Egger ME, Tzeng C-WD, Prakash LR, Maitra A, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. (2017) 152:1048–56. doi: 10.1001/jamasurg.2017.2227
53. Mellon EA, Jin WH, Frakes JM, Centeno BA, Strom TJ, Springett GM, et al. Predictors and survival for pathologic tumor response grade in borderline resectable and locally advanced pancreatic cancer treated with induction chemotherapy and neoadjuvant stereotactic body radiotherapy. Acta Oncol Stockh Swed. (2017) 56:391–7. doi: 10.1080/0284186X.2016.1256497
54. Amer AM, Zaid M, Chaudhury B, Elganainy D, Lee Y, Wilke CT, et al. Imaging-based biomarkers: changes in the tumor interface of pancreatic ductal adenocarcinoma on computed tomography scans indicate response to cytotoxic therapy. Cancer. (2018) 124:1701–9. doi: 10.1002/cncr.31251
55. Koay EJ, Lee Y, Cristini V, Lowengrub JS, Kang Y, Lucas FAS, et al. A visually apparent and quantifiable cT imaging feature identifies biophysical subtypes of pancreatic ductal adenocarcinoma. J Am Assoc Cancer Res. (2018) 24:5883–94. doi: 10.1158/1078-0432.CCR-17-3668
56. Cooper AB, Slack R, Fogelman D, Holmes HM, Petzel M, Parker N, et al. Characterization of anthropometric changes that occur during neoadjuvant therapy for potentially resectable pancreatic cancer. Ann Surg Oncol. (2015) 22:2416–23. doi: 10.1245/s10434-014-4285-2
57. Sandini M, Patino M, Ferrone CR, Alvarez-Pérez CA, Honselmann KC, Paiella S, et al. Association between changes in body composition and neoadjuvant treatment for pancreatic cancer. JAMA Surg. (2018) 153:809–15. doi: 10.1001/jamasurg.2018.0979
58. Aziz MH, Sideras K, Aziz NA, Mauff K, Haen R, Roos D, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: a Retrospective multicenter cohort study. Ann Surg. (2019) 270:139–46. doi: 10.1097/SLA.0000000000002660
59. Kawai M, Hirono S, Okada K-I, Miyazawa M, Shimizu A, Kitahata Y, et al. Low lymphocyte monocyte ratio after neoadjuvant therapy predicts poor survival after pancreatectomy in patients with borderline resectable pancreatic cancer. Surgery. (2019) doi: 10.1016/j.surg.2018.12.015
60. Samandari M, Julia MG, Rice A, Chronopoulos A, Del Rio Hernandez AE. Liquid biopsies for management of pancreatic cancer. Transl Res J Lab Clin Med. (2018) 201:98–127. doi: 10.1016/j.trsl.2018.07.008
61. Bernard V, Kim DU, San Lucas FA, Castillo J, Allenson K, Mulu FC, et al. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology. (2019) 156:108–118.e4. doi: 10.1053/j.gastro.2018.09.022
62. Xu Y, Qin T, Li J, Wang X, Gao C, Xu C, et al. Detection of circulating tumor cells using negative enrichment immunofluorescence and an in situ hybridization system in pancreatic cancer. Int J Mol Sci. (2017) 18:4. doi: 10.3390/ijms18040622
Keywords: pancreatic cancer, preoperative therapy, tumor response, pathologic response, radiographic response
Citation: Perri G, Prakash LR and Katz MHG (2020) Response to Preoperative Therapy in Localized Pancreatic Cancer. Front. Oncol. 10:516. doi: 10.3389/fonc.2020.00516
Received: 09 September 2019; Accepted: 23 March 2020;
Published: 15 April 2020.
Edited by:
Francesco Giovinazzo, Queen Elizabeth Hospital Birmingham, United KingdomReviewed by:
Alessandro Zerbi, Humanitas University, ItalyCopyright © 2020 Perri, Prakash and Katz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew H. G. Katz, bWhna2F0ekBtZGFuZGVyc29uLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.