- 1College of Life Science, Northwest University, Xi'an, China
- 2Department of Hepatobiliary Surgery, Institute of Advanced Surgical Technology and Engineering, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Hepatocellular carcinoma (HCC) is still one of the malignant tumors with high morbidity and mortality in China and worldwide. Although alpha-fetoprotein (AFP) as well as core fucosylated AFP-L3 have been widely used as important biomarkers for HCC diagnosis and evaluation, the AFP level shows a huge variation among HCC patient populations. In addition, the AFP level has also been proved to be associated with pathological grade, progression, and survival of HCC patients. Understanding the intrinsic heterogeneities of HCC associated with AFP levels is essential for the molecular mechanism studies of HCC with different AFP levels as well as for the potential early diagnosis and personalized treatment of HCC with AFP negative. In this study, an integrated N-glycoproteomic and proteomic analysis of low and high AFP levels of HCC tumors was performed to investigate the intrinsic heterogeneities of site-specific glycosylation associated with different AFP levels of HCC. By large-scale profiling and quantifying more than 4,700 intact N-glycopeptides from 20 HCC and 20 paired paracancer samples, we identified many commonly altered site-specific N-glycans from HCC tumors regardless of AFP levels, including decreased modifications by oligo-mannose and sialylated bi-antennary glycans, and increased modifications by bisecting glycans. By relative quantifying the intact N-glycopeptides between low and high AFP tumor groups, the great heterogeneities of site-specific N-glycans between two groups of HCC tumors were also uncovered. We found that several sialylated but not core fucosylated tri-antennary glycans were uniquely increased in low AFP level of HCC tumors, while many core fucosylated bi-antennary or hybrid glycans as well as bisecting glycans were uniquely increased in high AFP tumors. The data provide a valuable resource for future HCC studies regarding the mechanism, heterogeneities and new biomarker discovery.
Introduction
Hepatocellular carcinoma (HCC), accounting for 90% of primary liver cancer, is the fifth malignant cancer in the world (1). Due to its high morbidity and mortality, HCC is the third leading cause of deaths among cancers worldwide, including ~383,000 deaths each year from HCC in China (2). The main risk factors for HCC include alcoholic liver disease, nonalcoholic steatohepatitis (NASH), chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infection (1). Although liver surgical resection and transplantation can treat HCC validly, the 5-year overall survival rate is still at ~70% (3).
Early diagnosis and accurate prognosis of HCC are crucial for their effective treatment. Although advances in analytical techniques have produced many novel potential biomarkers, alpha-fetoprotein (AFP) is still the most widely used biomarker for HCC diagnosis and evaluation (4). Unfortunately, the concentration of serum AFP varies dramatically with patients. It is known that 30–40% of HCC patients have a AFP level below the detection level of 20 ng/mL in serum, and remain AFP negative until the later-stage (5–7). Meanwhile, many other studies have also revealed that the high concentration of AFP in patients' serum was associated with high risk of tumor recurrence/progression and reduced survival at different stages of the diseases (8–10). For example, Koji Okuda and co-authors revealed that the level of AFP > 200 ng/mL in serum was the most independently significant factor among several conventional prognostic factors for predicting survival after surgery (11). Bai and co-authors revealed that the AFP level at diagnosis was an independent risk factor of tumor differentiation, TNM stage, tumor size, and survival of HCC patients through 78,743 HCC patients. The study also showed that AFP-positive was associated with less differentiated tumors, more advanced TNM stage, larger tumor sizes, and inferior survival compared with AFP-negative (12). These studies remind us that the AFP levels in HCC patients' sera might be associated with the intrinsic heterogeneities of the HCC tumors. Understanding the molecular mechanism of those heterogeneities associated with AFP levels will be beneficial for the clinical treatment of HCC with different levels of AFP as well as for the new biomarker discovery of HCC patients with AFP negative.
Glycosylation has been recognized as one of the most important post-translational modifications (PTMs) of protein (13, 14). The attached oligosaccharides on proteins are involved in diverse biological processes, such as protein-protein recognition, receptor interaction, cell communication, adhesion, fertilization, embryonic development, immune defense, and inflammation. Abnormal glycosylation has been recognized as an essential feature of tumorigenesis and development including HCC (15). AFP-L3 has been proved by the U.S. Food and Drug Administration (FDA) to be used as another important serum biomarker for HCC diagnosis. It has been confirmed that AFP-L3 has the identical protein sequence as normal AFP but N-linked glycans attached at the glycosite Asn-251 of AFP-L3 were core fucosylated (16). The elevated AFP-L3 could further exclude the possibility of other liver diseases such as hepatitis and hepatocirrhosis, and therefore could reduce the false positive results of HCC diagnosed by sole AFP (17). The example of AFP-L3 for HCC diagnosis inspires us to explore the important site-specific glycosylation changes in HCC tumors with various characteristics including different levels of serum AFP.
In this study, we performed integrated glycoproteomic and global proteomic analysis of HCC tumors with low (< 20 ng/mL) and high AFP (> 1,000 ng/mL) in patients' sera to investigate the differences in site-specific glycosylation and proteins between HCC tumors with low and high levels of AFP. The paired paracancer tissues were also included as controls to determine the site-specific glycosylation changes in HCC tumors. The alteration extents in global proteome, site-specific glycosylation and glycosylation occupancy in different HCC samples were systematically compared, and altered site-specific N-glycans that commonly or uniquely identified from HCC tumors with low and high AFP were analyzed based on their glycan features and involved Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. The data will enhance our understanding of HCC heterogeneities that might be associated with AFP concentrations in patients' sera.
Experimental Section
Tissue Samples From HCC Patients
Tissue samples were prospectively collected from patient resection with pathological diagnosis of HBV-related hepatocellular carcinoma (HCC) at the First Affiliated Hospital of Xi'an Jiaotong University, China, from 2011 to 2013. The study was approved by Human Ethics Committee at the First Affiliated Hospital of Xi'an Jiaotong University, and written informed consents were obtained from all participants. Based on the AFP concentration, ten tissue samples each with low (<20 ng/mL) and high (>1,000 ng/mL) levels of AFP were selected for this study. The selection of 1,000 ng/mL of AFP as a cutoff was determined based on the Milan criteria of AFP level > 1,000 ng/mL as an exclusion criterion for liver transplantation in patients with HCC (18). The serum AFP concentrations of all patients were measured before surgery, and these patients did not receive any neoadjuvant chemotherapy treatments. The age range of the participants was between 31 and 76 years old, with the average age of 54 ± 11.3. The corresponding paracancer tissues (20 samples) were also collected as control samples. The detailed information of 40 samples (20 pairs) was summarized in (Table S1). Tissue samples were stored at−80 °C until use.
Protein Digestion
Liver tissues (~ 50mg) were washed twice with PBS (precooled at 4°C) to remove serum. Then tissues were denatured in 8 M urea/1 M NH4HCO3 buffer, homogenized with a tissue homogenizer (60Hz, Shanghai Jing Xin, China) and sonicated by Ultrasonic Cell Distribution System until the upper solution was clear. The proteins were then reduced by 5mM dithiothreitol (DTT) at 37°C for 1 h and alkylated by 15 mM iodoacetamide at room temperature (RT) in the dark for 30 min. Another 2.5 mM DTT was added and incubated for 10 min at RT. Protein pellets solution was digested by sequencing grade trypsin (protein: enzyme, 100:1; Promega, USA) overnight at 37°C in less than 2M urea/0.25M NH4HCO3 buffer. The samples were acidified to pH < 2 with trifluoroacetic acid (TFA) and centrifuged at 13,000 rpm for 15 min to remove any particulate matter. The digested peptides were desalted with HLB column (Waters, USA) and eluted with 1 mL solution of 60% (v/v) acetonitrile (ACN) and 0.1% (v/v) TFA. The peptide concentrations were measured by BCA reagent (Beyotime, China).
Tandem Mass Tag (TMT) Labeling
Equal amounts of tryptic peptides from HCC tumors with low and high AFP, as well as their corresponding paracancerous tissues, were pooled into four samples (10 patients per sample). And they were labeled by four channels of 10-plex TMT reagents (an NH2-reactive N-terminal labeling reagent for relative quantitation of intact glycopeptides and protein expressions among samples) according to the manufacturer's protocols (Thermo Fisher Scientific, USA) for protein quantitation (TMT channels: HCC with low AFP and related paracancer: TMT 127N and 126, respectively; HCC with high AFP and related paracancer: TMT 129N and 128C, respectively). The TMT labeled samples were pooled and purified by HLB columns.
Enrichment of N-Linked Glycopeptides Using Mixed Anion-exchange Extraction Cartridges
The intact glycopeptides were enriched from pooled peptide samples with TMT labels using Mixed Anion-Exchange (MAX) columns. The tryptic peptides mixtures eluted from HLB column (in 60% ACN/0.1% TFA) were diluted by 100% ACN/1% TFA to a final solvent composition of 95%ACN/1%TFA. Peptides were loaded onto MAX extraction cartridges twice and the column was washed three times with 95% ACN/1% TFA. Enriched glycopeptides were eluted in 400 μL of 50% ACN/0.1% FA solution. The samples were dried by vacuum and resuspended in 50 μL of 0.1% FA solution for LC-MS/MS analysis.
LC-MS/MS Analysis
The TMT-labeled peptide and intact glycopeptide samples were separated by an Easy-nLC™ 1200 system (Thermo Fisher Scientific, Germany) with the use of Acclaim PepMap100 pre-column (2 cm, 75 μm i.d., 3 μm) and Acclaim PepMap100 separating column (50 cm, 75 μm i.d., 3 μm). The mobile phase flow rate was 300 nL/min and consisted of 0.1% FA in water (A) and 0.1% FA in 80% ACN (B). A complete run of 120 min was set as follows: 3–7% B for 1 min, 7-35% B for 90 min, 35–68% B for 19 min, 68–100% B for 1 min and equilibrated in 100% B for 9 min. MS analysis was performed using an Orbitrap Fusion Lumos mass spectrometer (Thermo Fisher Scientific, Germany). The spray voltage was set at 2.4 kV. Orbitrap MS1 spectra (AGC 4 x 105) were collected from 350 to 2000 m/z at a resolution of 60 K followed by data-dependent HCD MS/MS (resolution 50 K, collision energy 37%, activation time 0.1 ms) of the 20 most abundant ions using an isolation width of 1.6 Da. Peptides with charge states from 2 to 8 were selected for MS/MS acquisition. A dynamic exclusion time of 25 s was used to discriminate against previously selected ions.
Intact Glycopeptide and Protein Identifications
The LC-MS/MS data of global protein from tissue were searched against UniProt human protein databases (downloaded from http://www.uniprot.org on June, 2019) by Sequest in Proteome Discoverer software (Thermo Fisher Scientific, version 2.3). The search parameters for global proteins were set as follows: up to two missed cleavage were allowed for trypsin digestion, 10 ppm and 0.02 Da mass tolerance were set for precursor and MS/MS ions, respectively; Carbamidomethylation (C, +57.021464Da) and 10-plex TMT (N-termini of peptides, +229.162932Da) were set as static modifications; oxidation (M, +15.9949 Da), N-termini acetylation (+42.010565Da), and 10-plex TMT (K at the C-termini of peptides, +229.162932Da) were set as dynamic modifications. All results were filtered with 1% false-discovery rate (FDR). The quantification results from TMT tag were normalized using the normalization factor obtained in proteome data.
The LC-MS/MS data of intact glycopeptides were searched using GPQuest 2.0 (19, 20), with the same parameters as mentioned above for proteomic data using the human UniProt database. For intact glycopeptide identification, the intact N-glycopeptide MS data were first converted to “mzML” format using Trans-Proteome Pipline (TPP) and searched against by GPQuest 2.0. The search parameters were set as follows: at least two oxonium ions out of the top 10 fragment ions in the MS/MS spectra were used for extraction of intact glycopeptide MS/MS spectra. The mass tolerances of 10 ppm and 20 ppm were allowed for precursors and fragmentation ions. The FDR of identified intact glycopeptides was estimated by the decoy peptide method and 1% FDR was allowed for intact glycopeptide identification.
Intact Glycopeptides Quantitation
The quantification information of intact glycopeptides were extracted from their identified MS/MS spectra based on intensities of their TMT reporter ions. The glycopeptide ratios among samples were normalized using the normalization factors obtained from the global proteomic results. After filtering the results by ≥ 5 peptide-spectrum matches (PSMs) per glycopeptide, the medium ratio of each glycopeptide was used for its quantitation.
Gene Ontology and KEGG Pathway Analyses
Gene ontology (GO) terms enrichment and KEGG pathway analysis were performed using differentially expressed glycoproteins in HCC tumor samples with low and high AFP using STRING: functional protein association networks (https://string-db.org) (21). The thresholds of count > 2 and p-value < 0.05 were applied as filters on GO and KEGG pathway analyses of altered proteins.
Statistical Analysis
Statistical analyses of intact glycopeptide and proteins intensities were performed using SPSS version 22.0. Mean, median, standard deviation and range were calculated for each group of patients. The Mann-Whitney U-test was used to calculate p-values between AFP-Low and AFP-High tumor diameters. P-values less than 0.05 were considered as statistically significant differences and p-values less than 0.01 as highly significant difference. Multivariate statistic Principal Component Analysis (PCA) was performed on four pooled samples (low and high AFP tumors, as well as their paracancer tissues, 10 samples per pool) based on their intact glycopeptide expression profiles. PCA of the four pooled samples was created in R 3.2.2 using the FactoMineR (22) (http://CRAN.R-project.org/), which relied on singular value decomposition, and the original feature (intact glycopeptide) space was orthogonally transformed into a set of linearly uncorrelated variables (principal components) (23). Hierarchical clustering analysis of intact glycopeptide, protein and glycosylation occupancy among four pooled samples were performed using QCanvas (http://compbio.sookmyung.ac.kr/~qcanvas) (24).
Results
Study Design and the Workflow
In order to explore the heterogeneity of glycosylation in hepatocellular carcinoma (HCC) tissues with different levels of alpha-fetoprotein (AFP) in patient serum, an integrated glycoproteomics and proteomics analysis was performed on two paired groups of HCC-related tissues: ten paired tumor and paracancer tissues with low AFP concentration (< 20 ng/mL) in patients' sera, and ten paired tumor and paracancer tissues with high AFP concentration [> 1,000 ng/mL, an exclusion criterion in Milan Criteria for liver transplantation in patients with HCC (18)] (Figure 1 and Table S1). The whole proteins were extracted from each of 40 tumors/paracancer tissues. The proteins were then digested and pooled into four samples using equal amounts of peptides per sample within each group. Each pooled peptide sample was labeled with one channel of 10-plex TMT reagents. Four TMT-labeled peptide samples were mixed and then separated into two aliquots: one aliquot was used for intact glycopeptides enrichment using mixed anion-exchange (MAX) cartridges for site-specific glycosylation analysis, and the other aliquot was directly analyzed by mass spectrometry for global proteomic analysis. After triplicate LC-MS/MS analyses per sample, intact glycopeptides and proteins were identified and quantified using GPQuest 2.0 and Proteome Discoverer v2.3, respectively. The integrated analyses of glycoproteome and proteome data were then performed to investigate the alterations of site-specific glycans as well as their occupancies. (Figure 1).
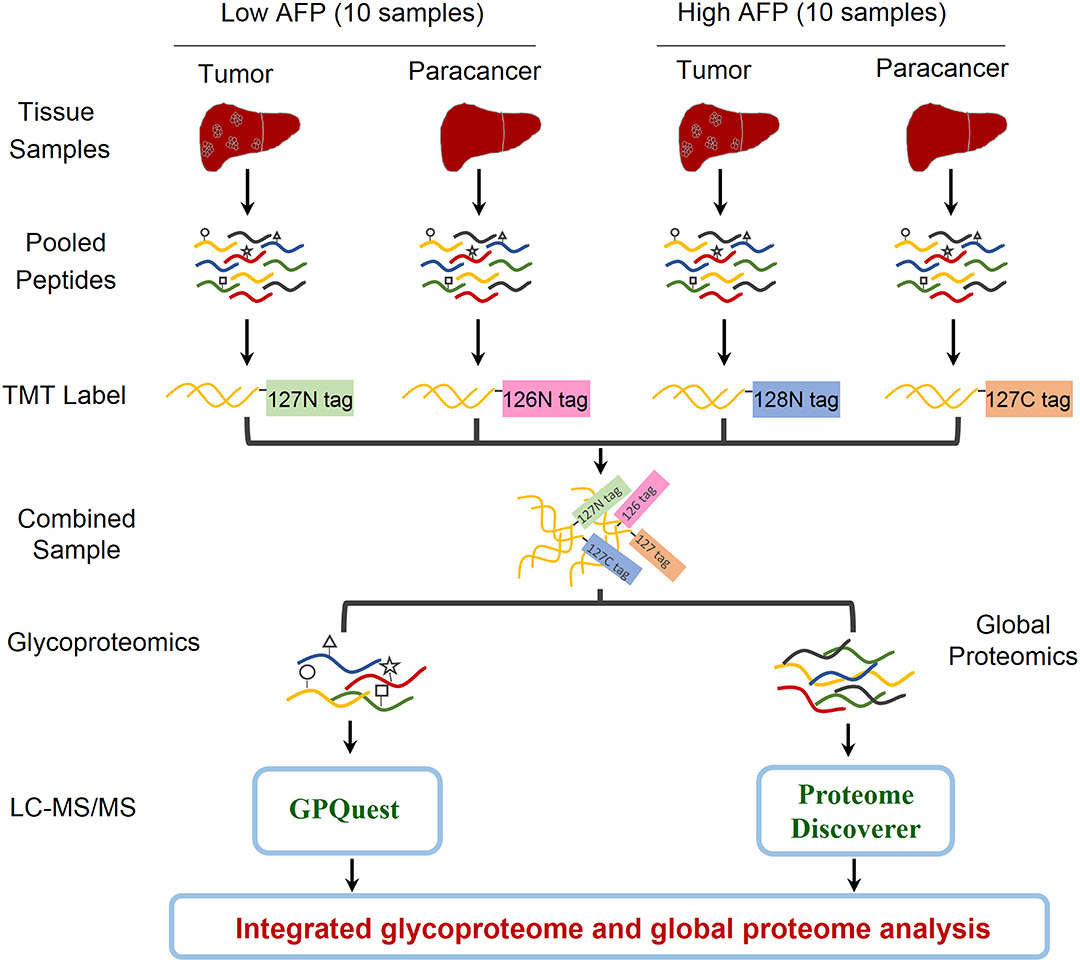
Figure 1. Workflow of integrated glycoproteome and quantitative proteome analysis of hepatocellular carcinoma (HCC) tissues with low and high AFP levels. Proteins were extracted from 20 HCC tumors and 20 paired paracancer tissue samples followed by tryptic digestion. The peptide samples were then pooled into four samples including HCC tumors and paracancer tissues with low and high AFP levels (2 × 2 = 4). After labeling the peptides using the NH2-reactive N-terminal label reagents (10-plex TMT reagents), the peptides were combined and separated into two aliquots. One aliquot was used for intact glycopeptide enrichment and the other aliquot was used for direct proteomic analysis. Both intact glycopeptides and peptides were analyzed by LC-MS/MS and quantitatively identified using GPQuest and Proteome Discoverer for site-specific glycosylation and proteomic analysis, respectively.
Site-Specific Glycosylation Profiling of HCC Tumors
By using the above workflow, 4,741 unique N-linked intact glycopeptides were identified from TMT-labeled HCC samples as well as their paracancer tissues within 1% false discovery rate (FDR) (Table S2), which were comprised of 221 N-glycan compositions and 1,184 glycosylation sites from 894 glycoproteins (Figure 2A). A representative MS/MS spectrum for intact glycopeptide identification with a peptide YKN#NSDISSTR modified by a core fucosylated glycan HexNAc4Hex5Fuc1Sia1 (N4H5F1S1) is shown in (Figure S1). Among 221 N-glycans, 17 were oligo-mannose glycans with seven containing core fucose, 26 were hybrid glycans, and 178 were complex glycans (Figure 2A). As for peptide backbone of the intact glycopeptides, the majority of the glycosites were solely occupied by one kind of glycan subtypes, with 63.5% of which were solely occupied by complex glycans. By calculating the peptide-spectrum matches (PSMs) of intact glycopeptides, we found that the majority of glycosites were occupied by complex glycans (2,108 PSMs, 59.8%), followed by oligo-mannose (1,027 PSMs, 29.1%) and hybrid (390 PSMs, 11.1%) glycans (Figure 2B). Further analysis of complex glycans showed that about 2/3 of complex glycans were biantennary glycans and the other 1/3 were tri/tetra-antennary glycans. In addition, nearly half of the glycosylation sites were modified by glycans containing one to three sialic acids. One third of all glycosites were fucosylated, with 85% of which containing one fucose and the other 15% containing two or more fucoses (Figure 2B).
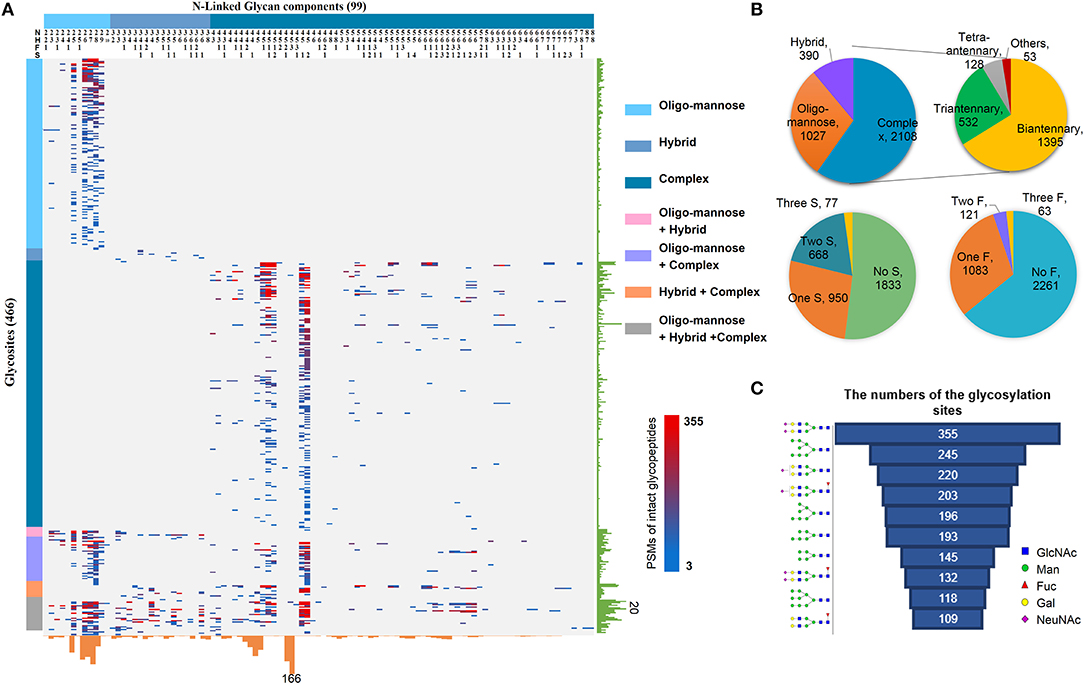
Figure 2. Large-scale profiling of intact glycopeptides in HCC tumors and paired paracancer tissues. (A) Profiling of N-linked glycans at individual glycosylation sites identified from all HCC tumor and paracancer samples. The peptide-spectrum-matches (PSMs) of each out of 4,741 intact glycopeptides, comprising of 221N-glycans (upper) and 894 glycosites (left), were exhibited in the heat map with different colors. The numbers of glycosites modified by each glycan and of glycans at each glycosite were summarized at the bottom and right parts of the figure, respectively. N: HexNAc; H: Hex; F: Fucose; S: Sialic acid. (B) The PSM percentages of different glycans based on glycan subtypes, antennary numbers, as well as the numbers of sialic acid and fucose modifications. (C) Top ten glycans detected from all HCC tumor and paracancer tissue samples based on the numbers of their modified glycosylation sites.
Based on the numbers of modified glycosylation sites, the top ten glycans from all tumor and paracancer tissue samples were mainly bi-antennary glycans (N4H5S2, N4H5S1, N4H5F1S1, N4H5F1S2, N4H5F1) and oligo-mannose glycans (N2H8, N2H7, N2H6, N2H5, N2H9) (Figure 2C). Except the oligo-mannose glycans that are usually located in the endomembrane system of the cells, the five complex glycans among these top ten glycans are quite similar with the top glycans that were identified from human serum (25), in which biantennary glycans (with 0-1 core fucose and 0-2 sialic acids) were modified on 3/4 of identified glycosites. This is quite reasonable as the serum proteins are mainly synthesized by liver and these complex glycans are normally located on the cell surface and secret glycoproteins.
Quantitation of Site-Specific Glycosylation in Low and High AFP Level of HCC Tumors
The identified intact glycopeptides were then quantified among four groups of samples, including liver tumors and paracancer tissues with low or high level of AFP. To ensure the quantitation accuracy by using the TMT-labeling approach, only intact glycopeptides with at least five MS/MS spectral assignments (1110 glycopeptides) were considered for the quantitation analysis (Table S3). In addition, as almost all glycopeptides (99.9%) were varied within two-fold between two groups of paracancer tissues, we used two-fold change as the cutoff to determine the differentially expressed glycopeptides among samples. Compared with paired paracancer samples, 11.4 and 17.1% of glycopeptides changed in tumors with low and high AFP, respectively (Figure 3A). By further compared the glycopeptide ratios of tumor/paracancer between low and high AFP groups, we identified 82 differently expressed glycopeptides (7.4%) between low and high AFP of HCC tumors.
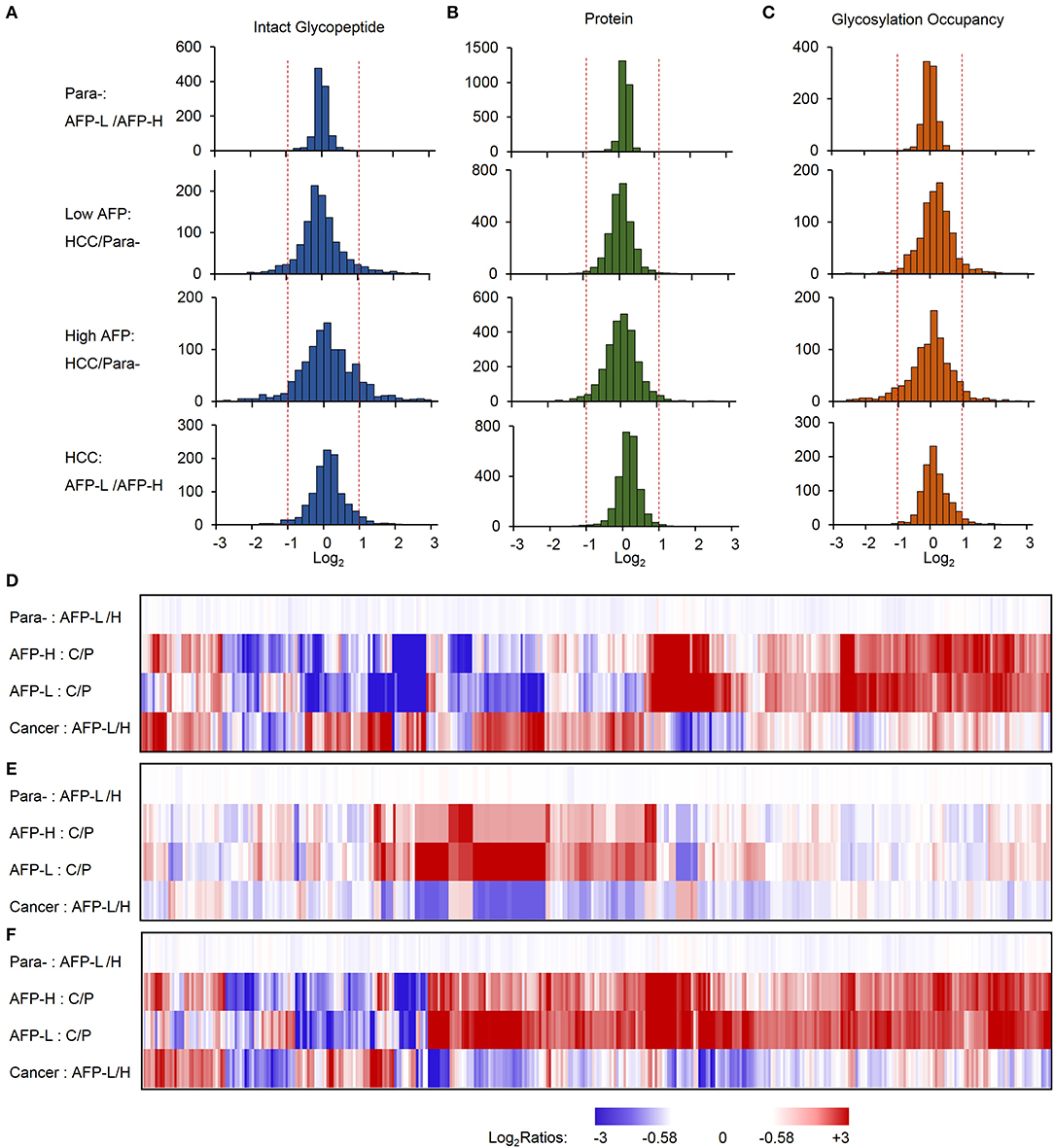
Figure 3. Relative quantification of intact glycopeptides, proteins, and glycosylation occupancies among different sample groups. (A–C) The frequencies of intact glycopeptides (A), proteins (B), and glycosylation occupancies (C) were compared between paracancer tissues with low and high AFP (upper), low AFP HCC tumors and their paired paracancer tissues, high AFP HCC tumors and their paired paracancer tissues, as well as HCC tumors with low and high AFP (lower). (D–F) Heat map shows the fold changes of altered intact glycopeptides (D), corresponding proteins (E) and glycosylation occupancies (F) among four sample groups. AFP-L: low AFP; AFP-H: high AFP; Para-: paracancer tissue; C/P: cancer/paired paracancer.
To further investigate whether these changes occurred at the protein expression level or at the glycosylation occupancies level, we performed integrated analyses of glycoproteome with their corresponding proteome data. At the global proteome level, among 3063 proteins identified from TMT-labeled samples, 99.8% of proteins were varied within two-fold between low and high AFP groups of paracancerous samples, and 98.2 and 95.5% of proteins were changed within 2-fold in low and high AFP tumor samples when compared with their corresponding paracancerous samples, respectively (Figure 3B). The comparison between low and high AFP of tumor samples also showed that 98.7% of proteins varied within 2-fold. These results indicated that the protein expression differences were not as high as the differences of site-specific glycosylation between tumors and paracancerous tissues, as well as between low and high AFP tumors. Next, we calculated the glycosylation occupancy changes in addition to the protein changes by comparing intact glycopeptide ratios with their protein ratios. Using the approach, we found that the glycosylation occupancies on 99.3% of glycopeptides varied within 2-fold between low and high AFP groups of paracancerous samples, but only 90.1 and 82.4% of glycopeptides were stabilized within 2-fold in low and high AFP tumor samples when compared with their corresponding paracancerous samples, respectively (Figure 3C). The comparison between low and high AFP of tumor samples showed that 3.5% of intact glycopeptides changed at glycosylation occupancies. This result was consistent with the difference in glycopeptide levels, clearly indicating that the site-specific glycosylation changes existed not only between HCC tumors and their paired paracancer tissues, but also between low and high AFP tumors, and these differences were much larger at the glycosylation level than that at the protein expression level. This observation was further confirmed by the Principal Component Analysis (PCA) of intact glycopeptides among four groups, which indicated that paracancer tissues with both low and high AFP, low AFP tumors, and high AFP tumors are located in three distinct regions (Figure S2). In addition, a relatively larger difference was observed between high AFP tumors and their paracancer tissues tumors than that in low AFP tumors.
By clustering the intact glycopeptides ratios among four groups of samples, a clear distinction was observed between low and high AFP tumors but no significant difference was observed between two groups of corresponding paracancer samples (Figure 3D and Figure S3), which demonstrated the high heterogeneities of HCC tumors at the site-specific glycosylation level. Comparably, a smaller difference was observed at the overall protein level not only between tumors and paracancer tissues but also between low and high AFP groups of HCC tumors (Figure 3E). Furthermore, the differences among low and high AFP of HCC tumors as well as paracancer tissues were also shown in (Figure 3F), which revealed that many site-specific glycosylation differences among different sample groups occurred at the glycosylation occupancy level instead of the protein expression level.
Commonly Altered Site-Specific Glycosylation in HCC Tumors
We first focused on the investigation of intact glycopeptides that were commonly changed in HCC tumors with both low and high AFP levels. These changes largely represented the site-specific glycosylation alterations in HCC tumors regardless of the AFP concentration in patients' sera. The quantification information of intact glycopeptides were extracted from their identified MS/MS spectra based on intensities of their TMT reporter ions. The glycopeptide ratios among samples were normalized using the normalization factors obtained from the global proteomic results. After filtering the results by ≥5 PSMs per glycopeptide, the medium ratio of each glycopeptide was used for its quantitation. From both low and high AFP-related tumors, we totally identified 54 glycopeptides that had at least 2-fold changes compared with their paracancers, including nine increased and 45 decreased glycopeptides (Figure 4A and Table S4). These glycopeptides were comprised of 17 N-glycans and 36 glycosites from 17 glycoproteins. It should be noted that since each glycosite can be modified by multiple glycans, the intact glycopeptide changes only represented the site-specific glycan alterations instead of glycoprotein expression changes. By classifying these changed site-specific glycans based on their attached glycan structures, we found that 25 intact glycopeptides modified by oligo-mannose glycans (Man4–Man9) were decreased in HCC tumors (Figure 4B). These glycopeptides were modified by six oligo-mannose glycans on 15 glycosites from 12 glycoproteins. Eleven of these glycoproteins were located in endomembrane system including nine located in ER (Figure 4C), and five of which were involved in the complement and coagulation cascades pathway (Figure 4D and Table S5A). In addition, a close interaction was observed among eight out of these glycoproteins (Figure 4E). The decrease of oligo-mannose glycan modifications on given glycosites of these glycoproteins might have been involved in tumorigenesis and/or progressions of HCC. Other decreased glycopeptides in HCC tumors included 20 glycopeptides modified by bi-antennary glycans with 0-2 sialic acids, with four of which also containing core fucose (Figure 4B), and two modified by two hybrid glycans. These glycopeptides were identified from 18 glycoproteins, which were mainly involved in complement and coagulation cascades, ECM-receptor interaction, focal adhesion, PI3K-Akt signaling pathway, and phagosome pathways (Figure 4F and Table S5B).
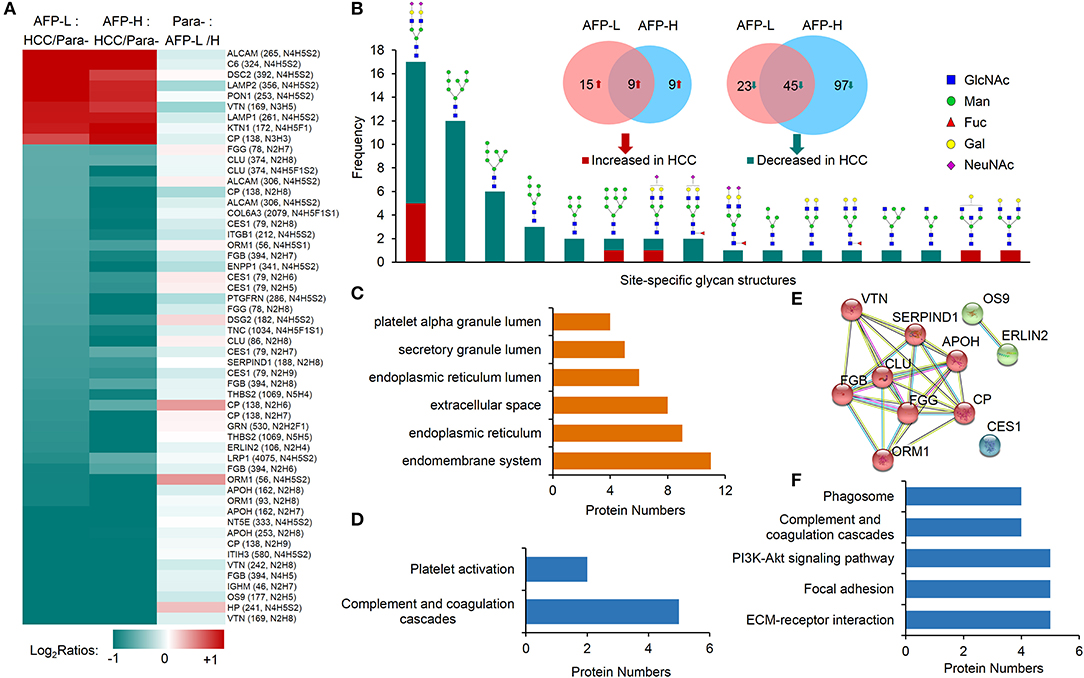
Figure 4. Commonly altered glycopeptides in both low and high AFP of HCC tumors compared with paracancer tissues. (A) Profiling of nine increased glycopeptides and 45 decreased glycopeptides in HCC tumors (both low and high AFP). AFP-L: low AFP; AFP-H: high AFP; Para-: paracancer tissues. (B) Frequencies of commonly changed site-specific glycans in HCC tumors. (C) The cellular components, (D) involved pathways, and (E) interactions of glycoproteins with decreased oligo-mannose glycan modification in HCC tumors. (F) KEGG pathways that involved by glycoproteins with decreased glycosylation in HCC tumors.
Among nine commonly increased glycopeptides in HCC tumors, six of them were also modified by bi-antennary glycans with 1-2 sialic acids, other three were modified by two bisecting glycans and one Man-9 glycan. These increased glycopeptides were identified from nine glycoproteins, and they were involved in the tuberculosis, phagosome, lysosome, autophagy, and complement and coagulation cascades pathways (Figure S4).
Heterogeneities of Site-Specific Glycosylation in HCC Tumors With Low and High AFP Concentrations
We next studied on the intact glycopeptides that show differential expressions in low and high AFP related HCC tumors but didn't show significant differences in their paired paracancer tissues. During the step, the glycosylation occupancy data were used to exclude the effect of possible changes occurred at the protein expression level (related to Figures 3C,F). To get better statistic results, we also reduced our quantification cutoff to a 1.5-fold difference. Using this strategy, we found that 96.9% of intact glycopeptides were varied within the cutoff range between low and high AFP of paracancer samples (Figure 3C). By comparing low and high AFP HCC tumors with each other, we finally identified 67 and 52 increased glycopeptides that belonged to 24 and 39 glycoproteins in low and high AFP of HCC tumors, respectively (Figure 5A and Table S6). These intact glycopeptides didn't show any significant changes in low and high AFP paracancer tissues at either intact glycopeptides, proteins or glycosylation occupancy levels, and therefore should be specific in HCC tumors and associated with AFP concentration in patients' serum.
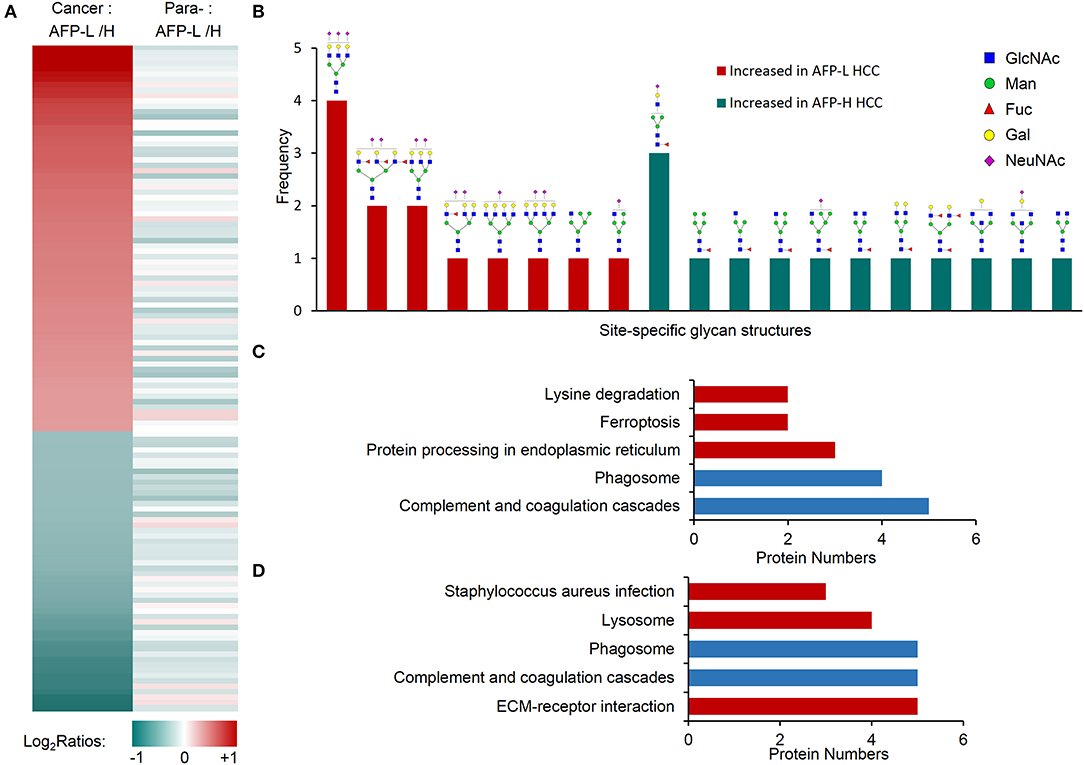
Figure 5. Glycopeptides with increased glycosylation occupancy in either low or high AFP level of HCC tumors. (A) Profiling of glycopeptides uniquely increased in low or high AFP of HCC tumors. There were 73 and 53 increased glycopeptides in low and high AFP of HCC tumors, respectively. AFP-L: low AFP; AFP-H: high AFP; Para-: paracancer tissues. (B) Frequencies of site-specific glycans that uniquely increased in low or high AFP of HCC tumors. (C) KEGG pathways involved by glycoproteins with increased glycosylation in low AFP of HCC tumors. (D) KEGG pathways involved by glycoproteins with increased glycosylation in high AFP of HCC tumors. Blue bars represent the common pathways, while red bars represent unique pathways involved by increased glycopeptides in low or high AFP tumors.
Some tumor specific glycan subtypes were commonly increased in both low and high AFP tumors compared with their paracancers (Figure S5), which were mainly high-mannose (54/126, 42.8%) and bi-antennary glycans with 1-2 sialic acids (35/126, 27.8%). More interestingly, we found that the glycans uniquely over-expressed in low AFP tumors were mainly tri-antennary with 1-3 sialic acids, two of which also contained one or three antennary fucosylation but no core fucosylation (Figure 5B). The glycans that were uniquely over-expressed in high AFP tumors were mainly core fucosylated glycans (7/10) or bisecting glycans (2/10), most of which were hybrid or bi-antennary complex glycans. These results indicated that the sialylated tri-antennary glycans were uniquely increased in low AFP HCC tumors, while the relatively simple hybrid or bi-antennary glycans with core fucose or bisected HexNAc are uniquely increased in high AFP HCC tumors.
The KEGG pathway enrichment analysis showed that the increased glycoproteins in both low and high AFP tumors were enriched in complement and coagulation cascades, and phagosome pathways (Figures 5C,D, and Table S7). Although these two pathways were also enriched by commonly changed glycoproteins in HCC tumors (related to Figure 4F and Figure S4), these involved glycoproteins were different in different tumor groups (Figure S6), implying that the same pathway could be regulated by different site-specific glycans in different HCC tumor groups. The other pathways that the glycoproteins uniquely increased in low AFP tumors included the “protein processing in endoplasmic reticulum,” “ferroptosis,” and “lysine degradation,” the glycoproteins uniquely increased in high AFP tumors involved included “ECM-receptor interaction,” “lysosome,” and “staphylococcus aureus infection” (Figures 5C,D).
Discussions
HCC is one of the malignant tumors with high morbidity and mortality, which places a heavy burden on society. AFP is a widely used serum biomarker for detecting liver cancer worldwide. The N-glycosylated isoform of AFP (AFP-L3), which contains core fucosylation on its N-linked glycans, only exists in HCC but is absent in other liver diseases and therefore can further enhance the specificity of HCC diagnosis. Unfortunately, a quite large proportion of HCC patients have an AFP negative until the later-stage (5, 6). In addition, the AFP level has also been associated with pathological grade, progression, and survival HCC patients. Understanding the intrinsic heterogeneities of HCC associated with AFP levels will be beneficial for the diagnosis and clinical treatment of HCC patients with AFP negative.
Due to the important roles of protein glycosylation on the regulation of protein structures and functions, glycoproteomic analysis of HCC has been reported in several previous studies (26–29). In these studies, the commonly used analytical strategy was to separate the glycans from the glycosylated peptides by enzymatic or chemical methods, and then separately performed mass spectrometry analysis on the released glycan or deglycosylated peptide. The overall expression and variation of glycans and/or glycoproteins were analyzed in the samples. This strategy has been well applied in liver cancer related research, but due to the heterogeneity of glycosylation of proteins, complete information about glycosylation sites and their corresponding glycans couldn't be obtained using this strategy (30). With the rapid development of mass spectrometry and the advancement of related analytical methods, directly intact glycopeptide analysis has been achieved in recent years (25, 31, 32). These methods and tools give us additional opportunities for heterogeneity analysis of site-specific glycosylation in HCC.
In this study, we first profiled site-specific glycans from low and high AFP levels of HCC tumors and non-tumor tissues. Overall, 4,741 unique N-linked intact glycopeptides were identified and quantified, containing 221 N-glycan compositions modified on 1,184 glycosylation sites from 894 glycoproteins. Nearly half of these glycosylation sites were modified by sialylated glycans, which indicated that sialylation in liver cancer tissues was relatively common. Sialylation is an important modification in cellular glycosylation, as sialylated carbohydrates have an important role in cellular recognition, cell adhesion and cell signaling (33). Especially the increased level of α2,6- and α2,3- linked sialylation is closely related to cancer (34). In addition, 1/3 of the complex glycan modifications are tri/tetra-antennary or more branched glycans. Increased number of branches on N-linked glycan have been shown to be associated with liver cancers (17, 35). Unlike the previously reported studies of serum glycosylation where sialylated biantennary glycans were the main glycans, high mannose glycans also accounted for a large proportion of N-glycans in tissues. This is due to the fact that during the synthesis of N-glycans, high mannose is mainly in the Golgi and endoplasmic reticulum (intracellular), while complex glycans are mainly extracellular (36).
The investigation of intrinsic heterogeneities of the HCC tumors with different AFP levels is the first step toward the molecular mechanism exploration of AFP level differences in HCC tumors. In previous studies, glycomics and immunohistochemical staining approaches have been applied in the quantitative analysis of released glycans from glycoproteins in HCC serum (37–40). Many altered glycans, including sialylated, bisecting, and core fucosylated glycans, had been identified in HCC serum, but few study focused on the glycan heterogeneity analysis of HCC tumors with different AFP levels. In a recent study, Jiang and co-authors have reported the great differences of proteomes between low and high AFP of HCC tumors (41), which inspired us to explore the site-specific glycosylation differences in HCC tumors with different levels of serum AFP by using our recently developed approach for intact glycopeptide analysis (42). In this study, by integrating quantitative information from the proteome and glycoproteome, large numbers of glycopeptides that specifically elevated in low or high AFP level of HCC tumors were identified. By comparing the quantification data between glycoproteome with global proteome, we found that the differences between low and high AFP of HCC tumors were much greater at the site-specific glycosylation level than at the protein expression level. In addition, by further analyzing the glycan structures on these glycopeptides, we interestingly found for the first time that several sialylated but not core fucosylated tri-antennary glycans were uniquely increased in low AFP tumors, while a large numbers of core fucosylated bi-antennary or hybrid glycans or bisecting glycans were uniquely increased in high AFP tumors. These data revealed the high heterogeneities of site-specific glycans between low and high AFP level of HCC tumors, even though their functional implications still need further investigations.
In summary, an integrated glycoproteomic and proteomic analysis of low and high AFP level of HCC tumors was performed in this study to explore the intrinsic heterogeneities of site-specific glycosylation associated with different AFP levels of HCC. In addition to the profiling the site-specific glycosylation in HCC tumors, we found that the differences between low and high AFP of HCC tumors were much greater at the site-specific glycosylation level than the protein expression level. By relative quantifying the intact glycopeptides among four sample groups, we uncovered the great heterogeneities of site-specific N-glycans between HCC tumors and non-tumors as well as between low and high AFP level of HCC tumors. The data provide a valuable resource for various HCC researches including new biomarker discovery of HCC with AFP-negative, the personalized treatment of HCC, as well as the carcinogenesis of HCC.
Data Availability Statement
The mass spectrometry data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the dataset identifier PXD016406.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Ethics Committee at the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
TZ, LJ, and SS designed the experiments. YC, QL and LH collected the tissue samples and clinical information. TZ performed experiments with help from LD, BZ, YZ, RL, and ZH. TZ performed MS analysis. TZ analyzed data with the help from JL, CM, JW, JS, PL, and YX. TZ, JL, and SS wrote and edited the manuscript, all the authors contributed to manuscript review.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81773180, 91853123, 21705127 and 81800655), Natural Science Foundation of Shaanxi Province (Grant No: 2018JM7086074), and China Postdoctoral Science Foundation (Grant No. 2019TQ0260, 2019M653715, and 2019M663798).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00496/full#supplementary-material
Abbreviations
HCC, Hepatocellular Carcinoma; AFP, Alpha-fetoprotein; NASH, Nonalcoholic Steatohepatitis; HBV, Chronic Hepatitis B Virus; HCV, Chronic Hepatitis C Virus; PSM, Peptide-spectrum Match; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PCA, Principal Component Analysis; FDR, False Discover Rate.
References
1. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. (2011) 365:1118–27. doi: 10.1056/NEJMra1001683
2. Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of china. Hepatology. (2014) 60:2099–108. doi: 10.1002/hep.27406
3. Mokdad AA, Singal AG, Yopp AC. JAMA pATIENT pAGE. Treatment of Liver Cancer. JAMA. (2016) 315:100. doi: 10.1001/jama.2015.15431
4. Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. (2009) 137:110–8. doi: 10.1053/j.gastro.2009.04.005
5. Ye X, Li C, Zu X, Lin M, Liu Q, Liu J, et al. A large-Scale multicenter study validates aldo-Keto reductase family 1 member b10 as a prevalent serum marker for detection of hepatocellular carcinoma. Hepatology. (2019) 69:2489–501. doi: 10.1002/hep.30519
6. Forner A, Bruix J. Biomarkers for early diagnosis of hepatocellular carcinoma. Lancet Oncol. (2012) 13:750–1. doi: 10.1016/S0168-8278(00)00053-2
7. Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the scientific registry of transplant recipients database. Hepatology. (2009) 49:832–8. doi: 10.1002/hep.22693
8. Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of milan criteria. Gastroenterology. (2012) 143:986–94 e3; quiz e14–5. doi: 10.1053/j.gastro.2012.08.011
9. European Association. For the study of the LR European organisation for, and treatment of c. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2012) 56:908–43. doi: 10.1016/j.jhep.2011.12.001
10. Okuda K, Tanaka M, Kanazawa N, Nagashima J, Satomura S, Kinoshita H, et al. Evaluation of curability and prediction of prognosis after surgical treatment for hepatocellular carcinoma by lens culinaris agglutinin-reactive alpha-fetoprotein. Int J Oncol. (1999) 14:265–71. doi: 10.3892/ijo.14.2.265
11. Bai DS, Zhang C, Chen P, Jin SJ, Jiang GQ. The prognostic correlation of aFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. (2017) 7:12870. doi: 10.1038/s41598-017-12834-1
12. Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the sWISS-PROT database. Biochim Biophys Acta. (1999) 1473:4–8. doi: 10.1016/S0304-4165(99)00165-8
13. Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. (2017) 27:R1147–R151. doi: 10.1016/j.cub.2017.09.019
14. Chen R, Jiang X, Sun D, Han G, Wang F, Ye M, et al. Glycoproteomics analysis of human liver tissue by combination of multiple enzyme digestion and hydrazide chemistry. J Proteome Res. (2009) 8:651–61. doi: 10.1021/pr8008012
15. Dela Rosa MA, Chen WC, Chen YJ, Obena RP, Chang CH, Capangpangan RY, et al. One-Pot two-Nanoprobe assay uncovers targeted glycoprotein biosignature. Anal Chem. (2017) 89:3973–80. doi: 10.1021/acs.analchem.6b04396
16. Li D, Mallory T, Satomura S. AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta. (2001) 313:15–9. doi: 10.1016/S0009-8981(01)00644-1
17. Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the milan criteria. Liver Transpl. (2014) 20:945–51. doi: 10.1002/lt.23904
18. Toghi Eshghi S, Shah P, Yang W, Li X, Zhang H. GPQuest: a Spectral library matching algorithm for site-Specific assignment of tandem mass spectra to intact n-glycopeptides. Anal Chem. (2015) 87:5181–8. doi: 10.1021/acs.analchem.5b00024
19. Hu Y, Shah P, Clark DJ, Ao M, Zhang H. Reanalysis of global proteomic and phosphoproteomic data identified a large number of glycopeptides. Anal Chem. (2018) 90:8065–71. doi: 10.1021/acs.analchem.8b01137
20. Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. (2015) 43:D447–52. doi: 10.1093/nar/gku1003
21. Le S, Josse J, Husson F. FactoMineR: an r package for multivariate analysis. J Stat Softw. (2008) 25:1–18. doi: 10.18637/jss.v025.i01
22. Doll S, Dreßen M, Geyer PE, Itzhak DN, Braun C, Doppler SA, et al. Region and cell-type resolved quantitative proteomic map of the human heart. Nat Commun. (2017) 8:2. doi: 10.1038/s41467-017-01747-2
23. Kim N, Park H, He N, Lee HY, Yoon S. QCanvas: an advanced tool for data clustering and visualization of genomics data. Genomics Inform. (2012) 10:263–5. doi: 10.5808/GI.2012.10.4.263
24. Sun S, Hu Y, Jia L, Eshghi ST, Liu Y, Shah P. Site-Specific profiling of serum glycoproteins using n-Linked glycan and glycosite analysis revealing atypical n-Glycosylation sites on albumin and alpha-1B-Glycoprotein. Anal Chem. (2018) 90:6292–99. doi: 10.1021/acs.analchem.8b01051
25. Kaji H, Saito H, Yamauchi Y, Shinkawa T, Taoka M, Hirabayashi J, et al. Lectin affinity capture, isotope-coded tagging and mass spectrometry to identify n-linked glycoproteins. Nat Biotechnol. (2003) 21:667–72. doi: 10.1038/nbt829
26. Cao Q, Zhao X, Zhao Q, Lv X, Ma C, Li X, et al. Strategy integrating stepped fragmentation and glycan diagnostic ion-based spectrum refinement for the identification of core fucosylated glycoproteome using mass spectrometry. Anal Chem. (2014) 86:6804–11. doi: 10.1021/ac501154a
27. Zhang H, Li XJ, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat Biotechnol. (2003) 21:660–6. doi: 10.1038/nbt827
28. Zhu J, Sun Z, Cheng K, Chen R, Ye M, Xu B, et al. Comprehensive mapping of protein n-glycosylation in human liver by combining hydrophilic interaction chromatography and hydrazide chemistry. J Proteome Res. (2014) 13:1713–21. doi: 10.1021/pr401200h
29. Parker BL, Thaysen-Andersen M, Solis N, Scott NE, Larsen MR, Graham ME, et al. Site-specific glycan-peptide analysis for determination of n-glycoproteome heterogeneity. J Proteome Res. (2013) 12:5791–800. doi: 10.1021/pr400783j
30. Woo CM, Iavarone AT, Spiciarich DR, Palaniappan KK, Bertozzi CR. Isotope-targeted glycoproteomics (IsoTaG): a mass-independent platform for intact n- and o-glycopeptide discovery and analysis. Nat Methods. (2015) 12:561–7. doi: 10.1038/nmeth.3366
31. Pai PJ, Hu Y, Lam H. Direct glycan structure determination of intact n-linked glycopeptides by low-energy collision-induced dissociation tandem mass spectrometry and predicted spectral library searching. Anal Chim Acta. (2016) 934:152–62. doi: 10.1016/j.aca.2016.05.049
32. Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. (2015) 15:540–55. doi: 10.1038/nrc3982
33. Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J. (1997) 14:569–76. doi: 10.1023/A:1018580324971
34. Mehta A, Norton P, Liang H, Comunale MA, Wang M, Rodemich-Betesh L, et al. Increased levels of tetra-antennary n-linked glycan but not core fucosylation are associated with hepatocellular carcinoma tissue. Cancer Epidemiol Biomarkers Prev. (2012) 21:925–33. doi: 10.1158/1055-9965.EPI-11-1183
35. Chung CY, Majewska NI, Wang Q, Paul JT, Betenbaugh MJ. SnapShot: n-Glycosylation processing pathways across kingdoms. Cell. (2017) 171:258–8 e1. doi: 10.1016/j.cell.2017.09.014
36. Yang L, Du X, Peng Y, Cai Y, Wei L, Zhang Y, et al. Integrated pipeline of isotopic labeling and selective enriching for quantitative analysis of n-Glycome by mass spectrometry. Anal Chem. (2019) 91:1486–1493. doi: 10.1021/acs.analchem.8b04525
37. Cao Y, Karsten U, Otto G, Bannasch P. Expression of mUC1, thomsen-Friedenreich antigen. Tn, sialosyl-Tn, and α2,6-linked sialic acid in hepatocellular carcinomas and preneoplastic hepatocellular lesions. Virchows Archiv. (1999) 434:503–9. doi: 10.1007/s004280050375
38. Kamiyama T, Yokoo H, Furukawa J, Kurogochi M, Togashi T, Miura N, et al. Identification of novel serum biomarkers of hepatocellular carcinoma using glycomic analysis. Hepatology. (2013) 57:2314–25. doi: 10.1002/hep.26262
39. Goldman R, Ressom HW, Varghese RS, Goldman L, Bascug G, Loffredo CA, et al. Detection of hepatocellular carcinoma using glycomic analysis. Clin Cancer Res. (2009) 15:1808–13. doi: 10.1158/1078-0432.CCR-07-5261
40. Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. (2019) 567:257–61. doi: 10.1038/s41586-019-0987-8
41. Sun S, Shah P, Eshghi ST, Yang W, Trikannad N, Yang S, et al. Comprehensive analysis of protein glycosylation by solid-phase extraction of n-linked glycans and glycosite-containing peptides. Nat Biotech. (2016) 34:84–88. doi: 10.1038/nbt.3403
Keywords: hepatocellular carcinoma, alpha-fetoprotein, glycoproteome, intact glycopeptide, site-specific glycosylation, mass spectrometry
Citation: Zhao T, Jia L, Li J, Ma C, Wu J, Shen J, Dang L, Zhu B, Li P, Zhi Y, Lan R, Xu Y, Hao Z, Chai Y, Li Q, Hu L and Sun S (2020) Heterogeneities of Site-Specific N-Glycosylation in HCC Tumors With Low and High AFP Concentrations. Front. Oncol. 10:496. doi: 10.3389/fonc.2020.00496
Received: 30 November 2019; Accepted: 19 March 2020;
Published: 21 April 2020.
Edited by:
Dapeng Zhou, Tongji University, ChinaReviewed by:
Sumit Sahni, Royal North Shore Hospital, AustraliaStephen John Ralph, Griffith University, Australia
Copyright © 2020 Zhao, Jia, Li, Ma, Wu, Shen, Dang, Zhu, Li, Zhi, Lan, Xu, Hao, Chai, Li, Hu and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shisheng Sun, c3Vuc0Bud3UuZWR1LmNu; Liangshuo Hu, aHVsaWFuZ3NodW8xOTgzQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Ting Zhao1†
Ting Zhao1† Liuyi Dang
Liuyi Dang Shisheng Sun
Shisheng Sun