- Department of Thyroid Surgery, The 1st Hospital of Jilin University, Changchun, China
Background: Clinical and ultrasonic risk factors for lateral lymph node metastasis (LLNM) in papillary thyroid microcarcinoma (PTMC) are not well-defined. Herein, a systematic review and meta-analysis was conducted to investigate clinicopathologic and ultrasonic risk features for LLNM in PTMC.
Methods: A systematic search of electronic databases (PubMed, Embase, Cochrane Library, and Web of Science) for studies published until April 2019 was performed. Case–control studies and randomized controlled trials that studied clinical and ultrasonic risk factors of LLNM in PTMC were included.
Results: Fourteen studies (all retrospective studies) involving 43,750 patients met final inclusion criteria. From the pooled analyses, younger age<45 (OR, 1.55; 95% CI, 1.16–2.07; P = 0.003), male patients (OR, 1.94; 95% CI, 1.55–2.42; P < 0.00), extrathyroidal extension (OR, 3.63; 95% CI, 2.28–5.77; P <0.00), tumor multifocality (OR, 2.24; 95% CI, 1.53–3.28; P <0.00), tumor > 0.5 cm (OR, 2.24; 95% CI, 1.53–3.28; P < 0.00), central lymph node metastasis (OR, 5.61; 95% CI, 4.64–6.79; P < 0.00), >25% tumor contact with thyroid capsule (OR, 6.66; 95% CI, 1.96–22.65; P = 0.002), tumor calcification (OR, 2.90; 95% CI, 1.71–4.93; P < 0.00), upper tumor (OR, 3.18; 95% CI, 2.23–4.55; P < 0.00) were significantly associated with increased risk of LLNM in PTMC, while Hashimoto's thyroiditis and other ultrasonic features (solid tumor, hypoechoic tumor, smooth margin, and taller than wide tumor) were not significantly associated with LLNM in PTMC.
Conclusions: Our analysis identified several clinicopathologic and ultrasonic factors associated with LLNM in PTMC. This finding highlights the need for a cautious and frequent postoperative surveillance of the lateral neck in high-risk PTMC patients. Moreover, high-risk ultrasonic features also need to be considered during selection of PTMC for active surveillance.
Introduction
The global incidence of papillary thyroid carcinoma (PTC) has increased substantially during past decade, with 4.4% annual percent increase in the United States from 1974 to 2013 (1–3). This has been driven largely by the rise in papillary thyroid microcarcinoma (PTMC), which is defined as PTC measuring ≤1 cm in greatest dimension (2, 3). Although the majority of PTMCs are indolent with <0.5% thyroid-cancer related death, some (1–5%) may have locoregional recurrence, which is still a major concern for patients and clinicians (4). Mounting studies reported that lateral lymph node metastasis (LLNM) was associated with locoregional recurrence for PTMC (4–6). The revised American Thyroid Association (ATA) guidelines in 2015 also considered around 20% PTC patients with LLNM would have structural recurrence in the future (7).
It is well-accepted that lateral neck dissection (LND) is only recommended for PTMC patients when LLNM is diagnosed by preoperative evaluation such as physical examination, radiological imaging, and/or fine needle aspiration (FNA) (7). Prophylactic LND is not recommended because no reliable clinicopathological features are identified which can differentiate the subset of high-risk PTMC, which are more likely accompanied with microscopic lymph nodes and the potential of progressing to clinical LLNM. But some researchers still believed early treatment for PTMC with LLNM might be appropriate as long as high-risk PTMC patients could be distinguished by clinical and/or ultrasonic risk factors (8).
Moreover, active surveillance could be recommended as the first-line treatment for low-risk PTC patients (7). But around 3.8% patients under active surveillance will have novel LLNM, which is also a major concern for patients and clinicians (9, 10). So it is necessary to identify patients at high-risk for LLNM, which helps the enrollment of low-risk PTMC in active surveillance protocols (9, 10).
Accordingly, several studies have published on the preoperative clinicopathologic risk factors of LLNM for PTMC (11, 12). However, there have been no consensus on this, and the subject remains debatable. Considering the low incidence of LLNM in PTMC, a single study with small patient number may draw an unreliable conclusion with a considerable bias. Therefore, we performed a systematic review to evaluate the clinicopathologic and ultrasonic predictive factors for LLNM in PTMC.
Methods
According to the guidelines proposed by the preferred reporting items for systematic reviews and meta-analyses (PRISMA in Supplementary Material) statement, we conducted this systematic review and meta-analysis.
Search Strategy
A systematic search of PubMed, Embase, Cochrane Library, and Web of Science was conducted for articles published until April 2019. The following terms were used in searching: “lateral lymph node metastasis” or “lateral lymph node dissection” combined with “papillary thyroid microcarcinoma” with language restriction “English.” All possible spelling and synonyms were also used for searching. Details of the search strategy are provided as shown in Supplementary File 1. The title, abstract or descriptors was reviewed independently by two authors (SX and PSW) to identify related studies for extensive review.
Study Selection
Studies returned from the search were checked by the following inclusion criteria: (1) original articles; (2) PTMC patients who received thyroidectomy as primary surgical procedure; (3) evaluation of clinicopathologic and/or ultrasonic risk factors for LLNM; and (4) study of the association between LLNM and relevant risk factors. Initially, titles, and abstracts were checked to include studies which fulfilled the inclusion criteria. After excluding studies which did not fulfill inclusion criteria, letters to the editor, abstracts, and meeting posters were also excluded. The same reviewers independently assessed eligibility after obtaining full text of candidate studies. In case of disagreement, a third investigator (ZH) was consulted. Discrepancies were resolved by discussion and consensus.
Data Extraction
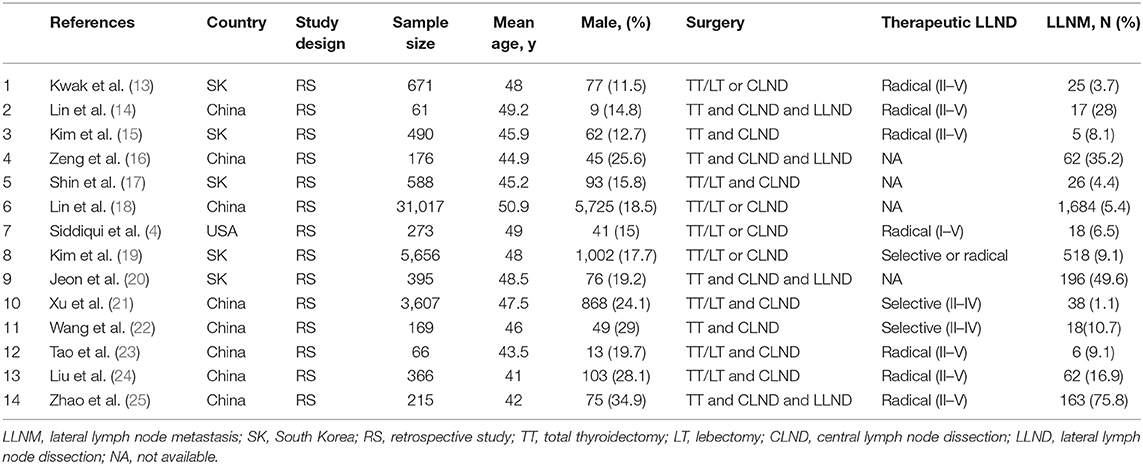
Two investigators (SX and PSW) independently summarized the studies meeting the inclusion criteria and performed data extraction. Disagreement was resolved by discussion and a third investigator (ZH) was consulted. We extracted the following data for each study: first author's last name, publication year, country, study design, sample size, patient characteristics (age and gender), surgical intervention (surgery type and therapeutic LND scope), and percentage of patients with LLNM. Following review by an expert panel (ZH and GC), we selected 14 factors that had been analyzed in at least three studies. These factors included clinicopathological factors like age, sex, extrathyroidal extension, multifocality, tumor size, Hashimoto's thyroiditis (HT), central lymph node metastases (CLNM), and ultrasonic characteristics of tumor like >25% contact with thyroid capsule, calcification, composition, echo, margin, shape, location.
Risk of Bias Analysis
The Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) was used in our study to evaluate the quality of non-randomized experimental studies. The six domains included in the RoBANS tool are the selection of participants, confounding variables, measurement of intervention, blinding for outcome assessment, incomplete outcome data, and selective outcome reporting were considered for assessment. Two reviewers (ZH and QYL) independently reviewed each term for every study, and disagreement was resolved by re-evaluation and a third investigator (GC) was consulted. The measurement of each item is categorized as low risk, high risk, or unclear risk. Review Manager 5.3 (Cochrane Collaboration, Oxford, UK) was used to report the result of this evaluation.
Statistical Analyses
Revman software (version 5.3; Cochrane Collaboration, Oxford, UK) and Stata software (version 12.0; Stata Corporation, College Station, TX, USA) were used for this meta and statistical analysis. We also calculated the odds ratios (ORs) and 95% confidence intervals (CIs) for estimating the association between binary factors and LLNM. Meta-analysis was performed using the random-effects model or the fixed-effect model according to the absence or presence of significant heterogeneity. The heterogeneity among studies was evaluated by Cochran's chi-squared statistics and with significance set at P < 0.10. The I2 statistic was used to quantify heterogeneity as followings: (1) exclude heterogeneity if I2 was from 0 to 40%; (2) moderate heterogeneity if I2 was from 30 to 60%. (3) Substantial heterogeneity if I2 was from 50 to 90%; (4) considerable heterogeneity if I2 was from 75 to 100%. If heterogeneity was present among the included studies, sensitivity analysis were performed to explore the origins of the heterogeneity.
The risk of publication bias was analyzed using Egger's test and was presented as a funnel plot. The statistical power of Egger's test was mainly dependent on the number of studies included in a meta-analysis. The P < 0.1 has been used as evidence of asymmetry for funnel plot because of limited number of studies in this study.
Results
Description of Studies
We identified a total of 561 studies during initial literature search. After evaluation of the titles and abstracts, 112 duplicate studies and another 433 studies were excluded. After scrutiny of the full text of the remaining 16 articles, a further 2 studies were excluded for various reasons as shown in Figure 1. Finally, 14 studies (with a total of 43,750 patients) were included in this meta-analysis, which all were retrospective studies (4, 13–25). Figure 1 and Table 1 show the study selection process and the characteristics of the included studies, respectively.
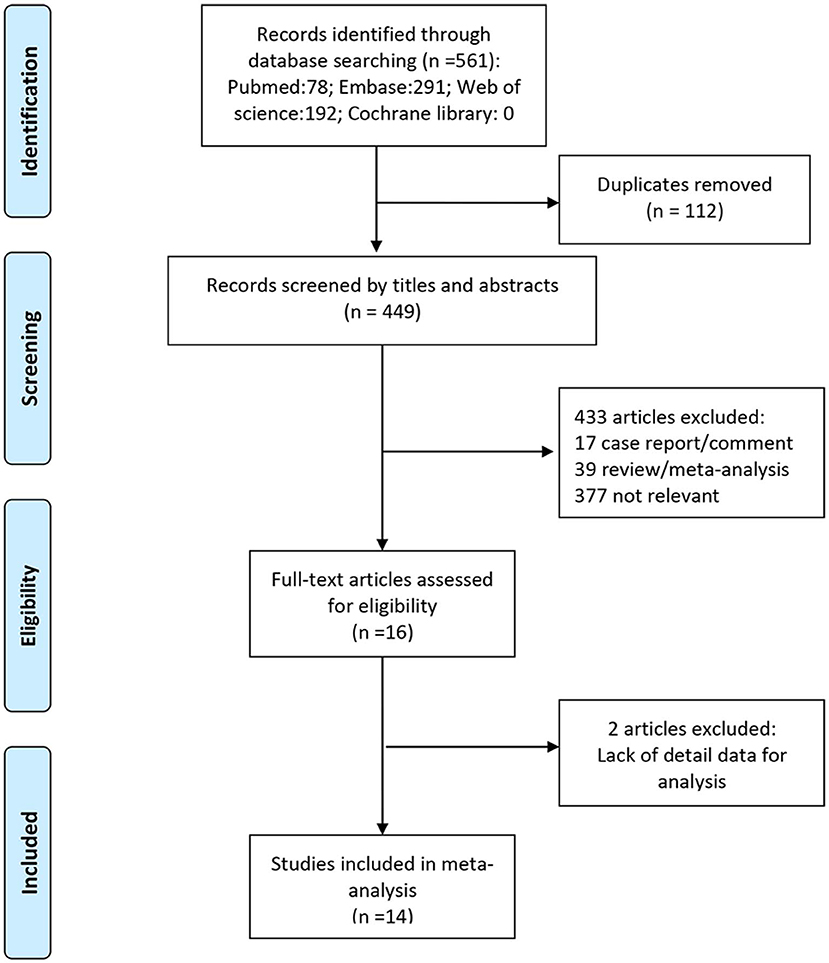
Figure 1. Preferred reporting items for systematic reviews and meta-analyses flow chart of the study selection process, showing the number of studies excluded at each step and the reasons for exclusion from the systematic review and meta-analysis.
Age and LLNM
The effect of age on the risk of LLNM was investigated in 7 studies (Figure 2A). A random-effects model was used due to the moderate heterogeneity (P = 0.05; I2 = 52%). On pooled analysis, the risk of LLNM was significantly higher in patients with younger age<45 (OR, 1.55; 95% CI, 1.16–2.07; P =0.003).
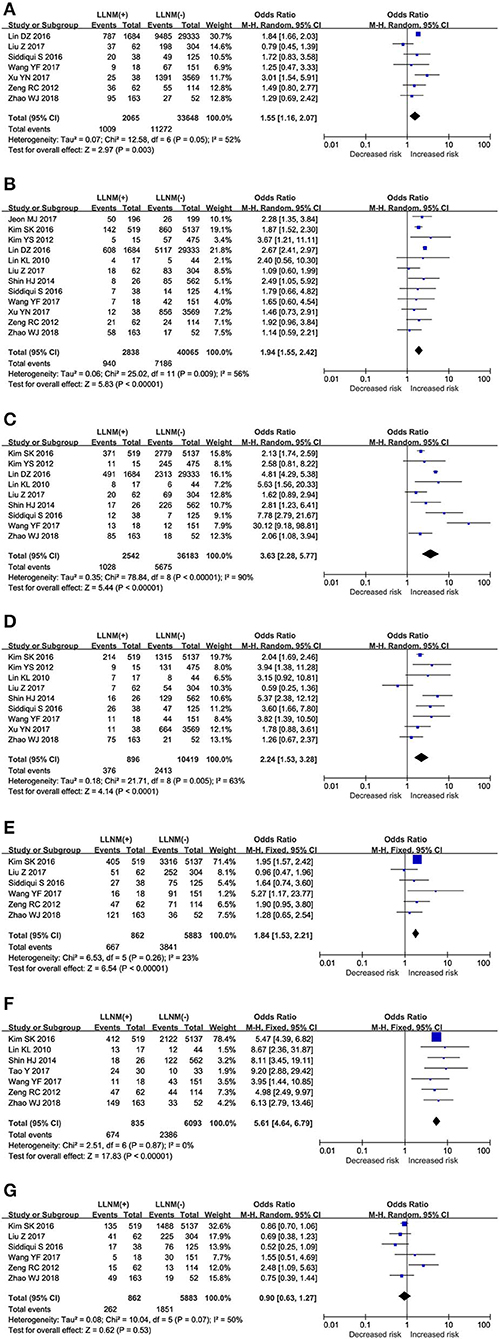
Figure 2. The role of clinical risk factors for LLNM in PTMC. Forest plots for the effects of (A) Age. (B) Sex. (C) ETE. (D) Multifocal. (E) Tumor size. (F) CLNM. (G) HT.
Sex and LLNM
The effect of sex on the risk of LLNM was investigated in 12 studies (Figure 2B). A random-effects model was applied due to the moderate heterogeneity (P = 0.009; I2 = 56%). On pooled analysis, the risk of LLNM was significantly higher in male patients (OR, 1.94; 95% CI, 1.55–2.42; P < 0.00).
ETE and LLNM
The effect of ETE on the risk of LLNM was investigated in 9 studies (Figure 2C). A random-effects model was applied due to the moderate heterogeneity (P < 0.00; I2 = 90%). On pooled analysis, the risk of LLNM was significantly higher in patients with ETE (OR, 3.63; 95% CI, 2.28–5.77; P < 0.00).
Multifocality and LLNM
The effect of multifocality on the risk of LLNM was investigated in 9 studies (Figure 2D). A random-effects model was applied due to the moderate heterogeneity (P = 0.009; I2 = 63%). On pooled analysis, the risk of LLNM was significantly higher in patients with multifocal tumor (OR, 2.24; 95% CI, 1.53–3.28; P < 0.00).
Tumor Size and LLNM
Assessment of tumor size as a risk factor for LLNM was conducted in 9 studies (Figure 2E). A fixed-effects model was applied due to low heterogeneity (P = 0.37; I2 = 23%). On pooled analysis, the risk of LLNM was significantly higher in patients with tumor larger than 0.5 cm. (OR, 2.24; 95% CI, 1.53–3.28; P < 0.00).
CLNM and LLNM
Assessment of CLNM as a risk factor for LLNM was conducted in 7 studies (Figure 2F). A fixed-effects model was applied due to low heterogeneity (P = 0.87; I2 = 0%). On pooled analysis, the risk of LLNM was significantly higher in patients with CLNM (OR, 5.61; 95% CI, 4.64–6.79; P < 0.00).
HT and LLNM
The effect of HT on the risk of LLNM was investigated in 6 studies (Figure 2G). A random-effects model was applied due to the moderate heterogeneity (P = 0.07; I2 = 50%). On pooled analysis, the risk of LLNM was not significantly lower in HT patients (OR, 0.90; 95% CI, 0.63–1.27; P < 0.53).
>25% Contact of Tumor With Thyroid Membrane and LLNM
The prediction of >25% contact of tumor with thyroid membrane on the risk of LLNM was investigated in 3 studies (Figure 3A). A random-effects model was applied due to the moderate heterogeneity (P = 0.02; I2 = 75%). On pooled analysis, the risk of LLNM was significantly higher in patients with tumor contact >25% thyroid membrane (OR, 6.66; 95% CI, 1.96–22.65; P = 0.002).
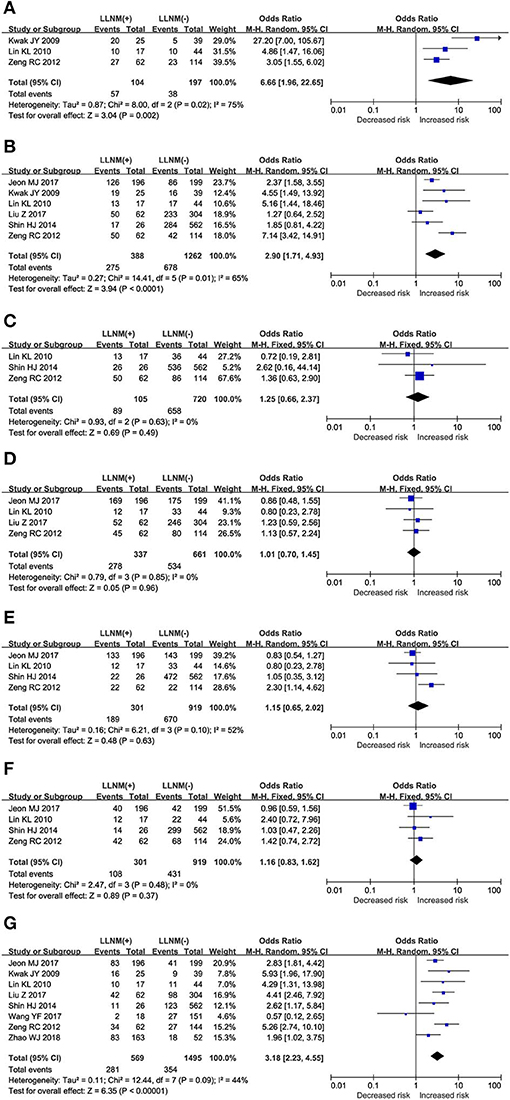
Figure 3. The role of ultrasonic risk factors for LLNM in PTMC. Forest plots for the effects of (A) Contact>25% thyroid membrane. (B) Calcification. (C) Composition. (D) Echo. (E) Margin. (F) Shape. (G) Location.
Calcification and LLNM
The prediction of calcification on the risk of LLNM was investigated in 6 studies (Figure 3B). A random-effects model was applied due to the moderate heterogeneity (P = 0.01; I2 = 65%). On pooled analysis, the risk of LLNM was significantly higher in patients with tumor calcification (OR, 2.90; 95% CI, 1.71–4.93; P < 0.00).
Composition and LLNM
Assessment of tumor composition as a risk factor for LLNM was conducted in 3 studies (Figure 3C). A fixed-effects model was applied because of low heterogeneity (P = 0.63; I2 = 0%). On pooled analysis, the risk of LLNM was not significantly higher in patients with solid tumor (OR, 1.25; 95% CI, 0.66–2.37; P < 0.00).
Echo and LLNM
Assessment of tumor echo as a risk factor for LLNM was conducted in 4 studies (Figure 3D). A fixed-effects model was applied because of low heterogeneity (P = 0.85; I2 = 0%). On pooled analysis, the risk of LLNM was not significantly higher in patients with hypoechoic tumor (OR, 1.01; 95% CI, 0.70–1.45; P = 0.96).
Margin and LLNM
The prediction of tumor margin on the risk of LLNM was investigated in a total of 4 studies (Figure 3E). A random-effects model was applied due to the moderate heterogeneity (P = 0.10; I2 = 52%). On pooled analysis, the risk of LLNM was not significantly higher in patients with smooth margin (OR, 1.15; 95% CI, 0.65–2.02; P < 0.63).
Shape and LLNM
Assessment of tumor shape as a risk factor for LLNM was conducted in a total of 4 studies (Figure 3F). A fixed-effects model was applied due to low heterogeneity (P = 0.48; I2 = 0%). On pooled analysis, the risk of LLNM was not significantly higher in patients with taller than wide tumor (OR, 1.16; 95% CI, 0.83–1.62; P < 0.37).
Location and LLNM
The prediction of tumor location on the risk of LLNM was investigated in a total of 8 studies (Figure 3G). A random-effects model was applied due to the moderate heterogeneity (P = 0.09; I2 = 44%). On pooled analysis, the risk of LLNM was significantly higher in patients with upper tumor (OR, 3.18; 95% CI, 2.23–4.55; P < 0.00).
Assessment of Study Quality and Bias
As shown in Figure 4, the risk for bias are summarized for 14 studies included in this meta-analysis by RoBANS tool. In the 14 studies included in the assessment, 100% were low risk for confounding variables and 92.9% were evaluated as low risk in the selection of participants. The unclear risk ratios of performance and detection biases were estimated to be 78.6 and 42.9%, respectively. The low risk ratios of attrition and reporting biases were 64.3 and 78.6%, respectively.
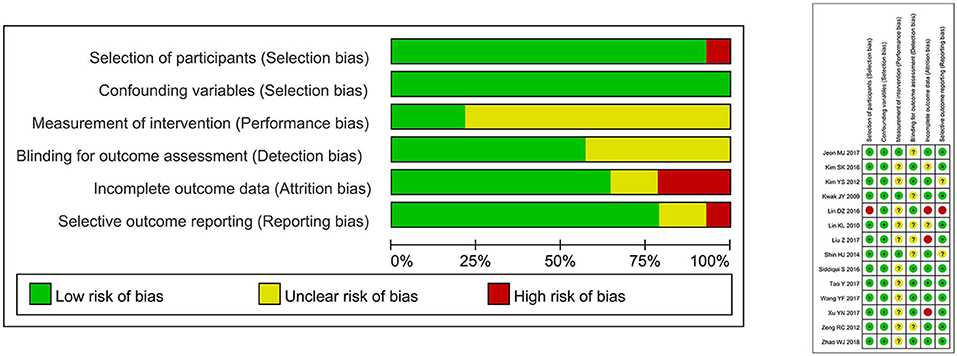
Figure 4. Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Sensitivity Analysis
Overall, leave-one-out meta-analysis revealed that age, sex, multifocal, HT, contact>75%, calcification and margin did not retain significance when single studies were excluded. For ETE and tumor location, no single studies were identified for the huge heterogeneity. The detailed results, including the forest plots, of the sensitivity analyses have been reported in Supplementary File 2.
Publication Bias
Publication bias was evaluated by Egger test and no bias existed in this meta-analysis, as shown in Supplementary File 3.
Discussion
To the best of our knowledge, this is the first meta-analysis to assess clinical and ultrasonic risk factors for LLNM in PTMC. It was found that younger age, male gender, ETE, multifocal tumors, larger tumor size, and positive CLNM were clinical risk factors for LLNM in PTMC. Additionally, >25% tumor contact with thyroid capsule, calcification, and upper location were ultrasonic risk features for LLNM. In contrast, HT and other US features (such as composition, echo, margin, shape) were not considered as risk factors of LLNM in these patients.
High heterogeneity with an I2 >50% was found in the analysis of age, sex, ETE, multifocality, HT, >25% contact with thyroid capsule, calcification as well as margin, and moderate heterogeneity with an I2 >30% was found when analyzing tumor location. Interestingly, after the removal of one study from the analysis, similar results were confirmed, but the heterogeneity was decreased remarkably in the analysis of age, sex, multifocal, HT, contact>25%, calcification, and margin. Possible reasons for high heterogeneity were presented as Supplementary File 2. In contrast, the I2 values for the analysis of ETE and tumor location were not changed significantly in the sensitivity analysis.
Younger age was identified to be associated with aggressiveness of PTC, such as vascular invasion and lymph node metastases (26). However, there is mounting evidence that younger PTC patients have a better disease-specific survival although they present high-risk clinical-pathological features (27–29). For PTMC under active surveillance, younger age was also associated with more probability of disease progression like tumor enlargement by ≥3 mm or novel appearance of nodal metastasis (30). The specific mechanism of age-dependent tumorigenesis and tumor progression has not been intensively investigated. Tumor surveillance by the immune system, which varies according to age, may be the reason for the difference in prognosis between younger and older patients (31).
Similarly, cervical lymph node metastases and ETE were more frequent in PTC patients with multifocality (32, 33). A meta-analysis from Australia summarized 21 papers and concluded the multifocality was an independent risk factor for recurrence, tumor progression, and aggressiveness (34). However, Wang et al. retrospectively reviewed 2,638 PTC patients from 11 medical centers from 6 countries and found that tumor multifocality has no independent risk prognostic value in clinical outcomes of PTC (35). This result was also validated on a series of 89,680 patients from SEER database (35). Recently, a study from Israel compared 690 PTC patients using propensity score matching analysis and demonstrated multifocality was not an independent prognostic factor for long-term outcomes of PTC (36). Multifocal PTC can represent either an intraglandular spread from a single primary tumor or multiple independent foci accompanied by intrathyroidal metastasis (37–39). These different clonal origins of multifocal tumor have distinct growth patterns, which may explain discrepancy between the above-discussed studies (40).
Furthermore, another novelty of this study is the first meta-analysis for ultrasonic features of LLNM in PTMC. A >25% tumor contact with thyroid capsule is related with the degree of ETE, which is also associated with LLNM of PTMC. Moreover, microcalcification(s) at US can also predict a diagnosis of malignancy, clinically cervical lymph node metastases, and prognosis, as reported previously (41, 42). In addition, an increasing number of studies have reported that upper tumor location was related with LLNM in PTC and PTMC no matter whether central neck had metastatic lymph node or not (11, 20, 43, 44). The presence of a direct lymphatic drainage from the upper third of thyroid to the neck may explain this finding (44, 45).
Strengths and Weaknesses
By meta-analyzing populations from different studies, the present study was able to evaluate the risk factors of LLNM for PTMC in a larger study sample and to adjust the results for the presence of some confounding factors. Except for ETE and tumor location, high heterogeneity of other risk factors was compensated by sensitivity analysis. The results of ETE and tumor location should be interpreted with caution because of moderate heterogeneity, and further study is needed to confirm the corresponding results.
This meta-analysis has some potential limitations. First, patients in the 14 studies were predominantly Asian. Whether ethnicity plays a role in LLNM remains unknown. Accordingly, risk factors of LLNM in PTMC from other races need further evaluation. Second, the limited number of studies hindered the implementation of subgroup and meta regression analysis. Third, the retrospective and non-randomized nature of all studies included in the analysis might be considered a source of bias. This provided associative, not causal, evidence, and mandates caution when interpreting these results. In future trials, randomized controlled trials of a higher methodological quality are needed to improve the quality of evidence.
Conclusion
Taken together, several risk factors for LLNM in PTMC patients which are readily available in clinical settings were identified in our systematic review and meta-analysis. The identification of high-risk patients is of utmost importance to plan a more cautious and frequent evaluation of lateral neck in the post-operative course. Moreover, high-risk ultrasonic features also need to be considered during selection of PTMC for active surveillance.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by Natural Science Foundation of Jilin Province (20180101138JC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00436/full#supplementary-material
References
1. Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. (2016) 12:646–53. doi: 10.1038/nrendo.2016.110
2. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. (2017) 317:1338–48. doi: 10.1001/jama.2017.2719
3. Hedinger C, Williams ED, Sobin LH. The WHO histological classification of thyroid tumors: a commentary on the second edition. Cancer. (1989) 63:908–11.
4. Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan EL, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. (2016) 26:807–15. doi: 10.1089/thy.2015.0429
5. Kim TY, Hong SJ, Kim JM, Kim WG, Gong G, Ryu JS, et al. Prognostic parameters for recurrence of papillary thyroid microcarcinoma. BMC Cancer. (2008) 8:296. doi: 10.1186/1471-2407-8-296
6. Pisanu A, Saba A, Podda M, Reccia I, Uccheddu A. Nodal metastasis and recurrence in papillary thyroid microcarcinoma. Endocrine. (2015) 48:575–81. doi: 10.1007/s12020-014-0350-7
7. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
8. Zhan S, Luo D, Ge W, Zhang B, Wang T. Clinicopathological predictors of occult lateral neck lymph node metastasis in papillary thyroid cancer: a meta-analysis. Head Neck. (2019) 41:2441–9. doi: 10.1002/hed.25762
9. Xue S, Wang P, Hurst ZA, Chang YS, Chen G. Active surveillance for papillary thyroid microcarcinoma: challenges and prospects. Front Endocrinol. (2018) 9:736. doi: 10.3389/fendo.2018.00736
10. Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endocrinol. (2016) 4:933–42. doi: 10.1016/S2213-8587(16)30180-2
11. Zhang L, Wei WJ, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. Risk factors for neck nodal metastasis in papillary thyroid microcarcinoma: a study of 1066 patients. J Clin Endocrinol Metab. (2012) 97:1250–7. doi: 10.1210/jc.2011-1546
12. Luo Y, Zhao Y, Chen K, Shen J, Shi J, Lu S, et al. Clinical analysis of cervical lymph node metastasis risk factors in patients with papillary thyroid microcarcinoma. J Endocrinol Invest. (2019) 42:227–36. doi: 10.1007/s40618-018-0908-y
13. Kwak JY, Kim EK, Kim MJ, Son EJ, Chung WY, Park CS, et al. Papillary microcarcinoma of the thyroid: predicting factors of lateral neck node metastasis. Ann Surg Oncol. (2009) 16:1348–55. doi: 10.1245/s10434-009-0384-x
14. Lin KL, Wang OC, Zhang XH, Dai XX, Hu XQ, Qu JM. The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol. (2010) 17:3294–300. doi: 10.1245/s10434-010-1129-6
15. Kim YS. Patterns and predictive factors of lateral lymph node metastasis in papillary thyroid microcarcinoma. Otolaryngol Head Neck Surg. (2012) 147:15–9. doi: 10.1177/0194599812439277
16. Zeng RC, Li Q, Lin KL, Zhang W, Gao EL, Huang GL, et al. Predicting the factors of lateral lymph node metastasis in papillary microcarcinoma of the thyroid in eastern China. Clin Transl Oncol. (2012) 14:842–7. doi: 10.1007/s12094-012-0875-2
17. Shin HJ, Kim EK, Moon HJ, Yoon JH, Han KH, Kwak JY. Can increased tumoral vascularity be a quantitative predicting factor of lymph node metastasis in papillary thyroid microcarcinoma? Endocrine. (2014) 47:273–82. doi: 10.1007/s12020-013-0131-8
18. Lin DZ, Qu N, Shi RL, Lu ZW, Ji QH, Wu WL. Risk prediction and clinical model building for lymph node metastasis in papillary thyroid microcarcinoma. Onco Targets Ther. (2016) 9:5307–16. doi: 10.2147/OTT.S107913
19. Kim SK, Park I, Woo JW, Lee JH, Choe JH, Kim JH, et al. Predictive factors for lymph node metastasis in papillary thyroid microcarcinoma. Ann Surg Oncol. (2016) 23:2866–73. doi: 10.1245/s10434-016-5225-0
20. Jeon MJ, Chung MS, Kwon H, Kim M, Park S, Baek JH, et al. Features of papillary thyroid microcarcinoma associated with lateral cervical lymph node metastasis. Clin Endocrinol. (2017) 86:845–51. doi: 10.1111/cen.13322
21. Xu Y, Xu L, Wang J. Clinical predictors of lymph node metastasis and survival rate in papillary thyroid microcarcinoma: analysis of 3607 patients at a single institution. J Surg Res. (2018) 221:128–34. doi: 10.1016/j.jss.2017.08.007
22. Wang Y. Risk factors analysis of cervical lymph node metastasis of papillary thyroid microcarcinoma. Biomed Res. (2017) 28:9571–8.
23. Tao Y, Wang C, Li L, Xing H, Bai Y, Han B, et al. Clinicopathological features for predicting central and lateral lymph node metastasis in papillary thyroid microcarcinoma: analysis of 66 cases that underwent central and lateral lymph node dissection. Mol Clin Oncol. (2017) 6:49–55. doi: 10.3892/mco.2016.1085
24. Liu Z, Lei J, Liu Y, Fan Y, Wang X, Lu X. Preoperative predictors of lateral neck lymph node metastasis in papillary thyroid microcarcinoma. Medicine. (2017) 96:e6240. doi: 10.1097/MD.0000000000006240
25. Zhao W, Chen S, Hou X, Liao Q, Chen G, Zhao Y. Predictive factors of lateral lymph node metastasis in papillary thyroid microcarcinoma. Pathol Oncol Res. (2018) 25:1245–51. doi: 10.1007/s12253-018-0511-8
26. Niemann AC, Reid AT, Smith J, Hammond J, DeBolle SA, Wei I, et al. Association of patient age with high-risk pathologic features in papillary thyroid cancer. J Surg Res. (2017) 211:228–32. doi: 10.1016/j.jss.2016.12.021
27. Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, et al. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid. (2015) 25:1106–14. doi: 10.1089/thy.2015.0104
28. Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the surveillance, epidemiology, and end results database. Thyroid. (2015) 25:125–32. doi: 10.1089/thy.2014.0116
29. Shah S, Boucai L. Effect of age on response to therapy and mortality in patients with thyroid cancer at high risk of recurrence. J Clin Endocrinol Metab. (2018) 103:689–97. doi: 10.1210/jc.2017-02255
30. Miyauchi A, Kudo T, Ito Y, Oda H, Sasai H, Higashiyama T, et al. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery. (2018) 163:48–52. doi: 10.1016/j.surg.2017.03.028
31. Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. (2014) 30:16–22. doi: 10.3109/09513590.2013.852531
32. Gur EO, Karaisli S, Haciyanli S, Kamer E, Genc H, Atahan K, et al. Multifocality related factors in papillary thyroid carcinoma. Asian J Surg. (2019) 42:297–302. doi: 10.1016/j.asjsur.2018.05.004
33. Al Afif A, Williams BA, Rigby MH, Bullock MJ, Taylor SM, Trites J, et al. Multifocal papillary thyroid cancer increases the risk of central lymph node metastasis. Thyroid. (2015) 25:1008–12. doi: 10.1089/thy.2015.0130
34. Joseph KR, Edirimanne S, Eslick GD. Multifocality as a prognostic factor in thyroid cancer: a meta-analysis. Int J Surg. (2018) 50:121–5. doi: 10.1016/j.ijsu.2017.12.035
35. Wang F, Yu X, Shen X, Zhu G, Huang Y, Liu R, et al. The prognostic value of tumor multifocality in clinical outcomes of papillary thyroid cancer. J Clin Endocrinol Metab. (2017) 102:3241–50. doi: 10.1210/jc.2017-00277
36. Geron Y, Benbassat C, Shteinshneider M, Or K, Markus E, Hirsch D, et al. Multifocality is not an independent prognostic factor in papillary thyroid cancer: a propensity score-matching analysis. Thyroid. (2019) 29:513–22. doi: 10.1089/thy.2018.0547
37. Giannini R, Ugolini C, Lupi C, Proietti A, Elisei R, Salvatore G, et al. The heterogeneous distribution of BRAF mutation supports the independent clonal origin of distinct tumor foci in multifocal papillary thyroid carcinoma. J Clin Endocrinol Metab. (2007) 92:3511–6. doi: 10.1210/jc.2007-0594
38. Jovanovic L, Delahunt B, McIver B, Eberhardt NL, Grebe SK. Most multifocal papillary thyroid carcinomas acquire genetic and morphotype diversity through subclonal evolution following the intra-glandular spread of the initial neoplastic clone. J Pathol. (2008) 215:145–54. doi: 10.1002/path.2342
39. Shattuck TM, Westra WH, Ladenson PW, Arnold A. Independent clonal origins of distinct tumor foci in multifocal papillary thyroid carcinoma. N Engl J Med. (2005) 352:2406–12. doi: 10.1056/NEJMoa044190
40. Lu Z, Sheng J, Zhang Y, Deng J, Li Y, Lu A, et al. Clonality analysis of multifocal papillary thyroid carcinoma by using genetic profiles. J Pathol. (2016) 239:72–83. doi: 10.1002/path.4696
41. Lu Z, Mu Y, Zhu H, Luo Y, Kong Q, Dou J, et al. Clinical value of using ultrasound to assess calcification patterns in thyroid nodules. World J Surg. (2011) 35:122–7. doi: 10.1007/s00268-010-0827-3
42. Bai Y, Zhou G, Nakamura M, Ozaki T, Mori I, Taniguchi E, et al. Survival impact of psammoma body, stromal calcification, and bone formation in papillary thyroid carcinoma. Mod Pathol. (2009) 22:887–94. doi: 10.1038/modpathol.2009.38
43. So YK, Kim MJ, Kim S, Son YI. Lateral lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg. (2018) 50:94–103. doi: 10.1016/j.ijsu.2017.12.029
44. Lee YS, Shin SC, Lim YS, Lee JC, Wang SG, Son SM, et al. Tumor location-dependent skip lateral cervical lymph node metastasis in papillary thyroid cancer. Head Neck. (2014) 36:887–91. doi: 10.1002/hed.23391
Keywords: risk factor, lateral lymph node metastasis, papillary thyroid microcarcinoma, meta-analysis, review
Citation: Xue S, Han Z, Lu Q, Wang P and Chen G (2020) Clinical and Ultrasonic Risk Factors for Lateral Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 10:436. doi: 10.3389/fonc.2020.00436
Received: 22 May 2019; Accepted: 11 March 2020;
Published: 03 April 2020.
Edited by:
Piero Nicolai, University of Padova, ItalyReviewed by:
Chandra Shekhar Dravid, Tata Memorial Hospital, IndiaGiuseppe Mercante, Humanitas University, Italy
Copyright © 2020 Xue, Han, Lu, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Chen, amlkYXlpeXVhbmp6eEBzaW5hLmNvbQ==
 Shuai Xue
Shuai Xue Guang Chen
Guang Chen