- 1Department of Pathology, Ajou University School of Medicine, Suwon, South Korea
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
- 3Asan Medical Center, Asan Institute for Life Science, University of Ulsan College of Medicine, Seoul, South Korea
- 4Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, South Korea
- 5Department of Biomedical Sciences, University of Ulsan College of Medicine, Asan Medical Center, Seoul, South Korea
- 6Convergence Medicine Research Center, Asan Medical Center, Seoul, South Korea
- 7Department of Biomedical Science, Ajou University Graduate School of Medicine, Suwon, South Korea
- 8Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
- 9Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, South Korea
The risk stratification of diffuse large B-cell lymphoma (DLBCL) is crucial. The International Prognostic Index, the most commonly used and the traditional risk stratification system, is composed of fixed and artificially dichotomized attributes. We aimed to develop a novel prognostic model that allows the incorporation of up-to-date attributes comprehensively without information loss. We analyzed 204 patients with primary DLBCL who were uniformly treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) from 2007 to 2012 at Asan Medical Center. Using the multivariable fractional polynomial (MFP) method and bootstrap resampling, we selected the variables of significance and the best fitted functional form in fractional polynomials. Age, serum β2-microglobulin, serum lactate dehydrogenase, and BCL2 expression were selected as significant variables in predicting overall survival (OS), while age was excluded in predicting 2-years event-free survival. The prognostic score calculated by the MFP model effectively classifies patients into four risk groups with 5-years OS of 89.91% (low risk), 81.21% (low-intermediate risk), 66.40% (high-intermediate risk), and 37.89% (high risk). We suggest a new prognostic model that is simple and flexible. By using the MFP method, we can incorporate various clinicopathologic factors into a risk stratification system without arbitrary dichotomization.
1. Introduction
Diffuse large B-cell lymphoma (DLBCL) is an aggressive and the most prevalent subtype of non-Hodgkin lymphoma (NHL) (1, 2). Despite the improvement of overall outcomes with up-front immunochemotherapy (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP]), ~40% of patients with DLBCL fail to achieve remission and ultimately succumb to death (3–5). One reason for treatment failure is the limitations of current prognostication systems for DLBCL. Since 1993, the International Prognostic Index (IPI) has been the most commonly used clinical tool for the risk stratification of patients with DLBCL (6). It considers age (>60 years), the Ann Arbor Stage (III/IV), serum lactate dehydrogenase (LDH) levels (>upper limit of normal), performance status [Eastern Cooperative Oncology Group (ECOG) >1], and the number of extranodal involvements (>1). However, the IPI does not completely predict the prognosis of patients and also narrows the outcome differences between the IPI risk groups (7). In addition, the risk stratification system solely based on clinical factors does not reflect the fundamental biological properties of the tumors. Recently, with the advancement of research on the pathophysiology of DLBCL, many biological and molecular features, such as the cell of origin (COO), BCL2 and MYC status, and genetic alteration, have been discovered to be associated with poor prognosis (8–13). Additionally, new therapeutic agents, including immune checkpoint blockers, are undergoing clinical trials to overcome the limitation of current R-CHOP therapy (14). Therefore, there is an impending unmet medical need to develop a risk stratification system that allows incorporation of the latest clinical and biological attributes comprehensively.
One limitation of the current IPI is that attributes that originally had continuous values are dichotomized by using the artificially assigned thresholds. To build prognostic models, most studies have included continuous predictors as categorized forms with their arbitrary optimal cut-off points. Although there has been constant criticism of this approach (15–17), categorization is widespread in clinical studies (18). One of the perceived advantages of categorization is that it is easy to apply to clinical practice that determines the diagnostic or therapeutic procedures. However, finding an “optimal” cut-off point is virtually infeasible; it has been reported that neither Kaplan–Meier nor the receiver operating characteristic (ROC) methods can be relied upon to represent a true biological threshold in prognosis (19). Fundamentally, categorization induces the inevitable loss of information and statistical power and may increase the probability of false-positive results (20–22).
To overcome these pitfalls of dichotomization, Royston et al. developed the multivariable fractional polynomial (MFP) approach to build models from multiple predictors with a combination of continuous and categorical variables (23, 24). In handling the continuous variable, a logistic regression model presumes a linear relationship between covariates and a response variable in the logit scale. However, various functional forms of covariates which incorporate non-linear relationships should also be considered (25). For that reason, the MFP model uses possible transformed predictors with various powers and performs closed test to select significant predictors. In brief, the MFP method can be explained in two main concepts: backward elimination among all possible predictors and selection of an fractional polynomial (FP) function to incorporate non-linear relationship of continuous variables (26). This allows us the determination of whether an explanatory variable is important and the return of its optimal functional form among the possible combinations of fractional polynomials (FP) (27). In addition, using the prognostic index which is the combination of selected variables with weights taken from the Cox model can explain the relative hazard of the patient with certain predictor values.
In this study, we developed a new risk stratification model for DLBCL using the MFP method. Among the various clinical and biological factors related to DLBCL, statistically significant variables were selected while retaining the properties of the continuous variables. We selected suitable FP functions among selected variables to build a parsimonious and medically consistent final model. The model stability was also investigated using the bootstrap assessment. The final survival model was formed by fitting a Cox model with finally selected covariates. Using this MFP method, we suggest a clinically feasible and flexible model that can comprehensively allow for continuously updating clinical and biological attributes.
2. Materials and Methods
2.1. Patients and Clinicopathologic Information
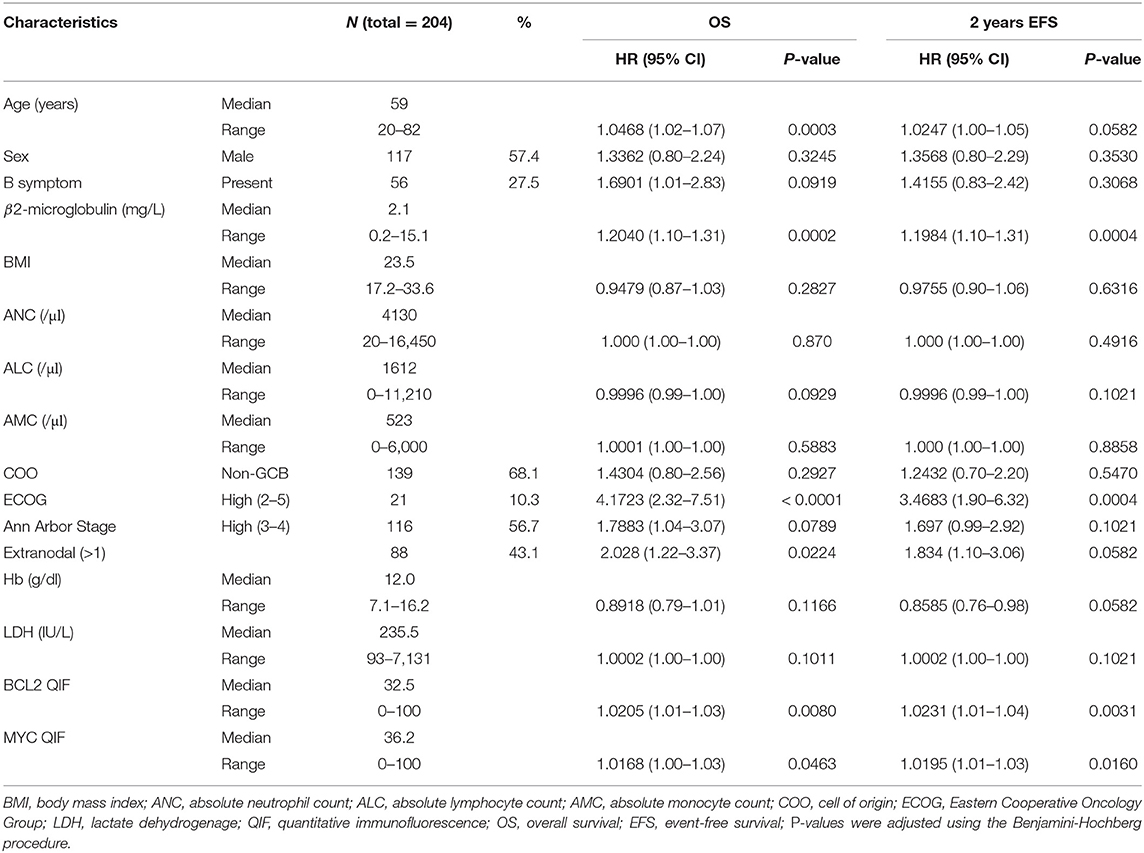
For the analysis, clinicopathologic information was retrospectively collected from patients with primary DLBCL diagnosed between 2007 and 2012 at Asan Medical Center. A total of 204 cases were comprehensively reviewed, and diagnoses were confirmed by two expert hematopathologists (RJ and CSP) according to the 2016 WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues (2). All patients underwent the standard staging procedures and were treated with R-CHOP. Patients with primary central nervous system lymphoma and patients who were initially treated with other treatments rather than R-CHOP were excluded. Clinicopathological information known to be associated with the prognosis of DLBCL was meticulously obtained from medical records including sex, age, body mass index (BMI) (28), status of concurrent hepatitis B virus infection (29), levels of serum LDH and B2M (30), hemoglobin (Hb) levels (31), and baseline peripheral absolute neutrophil, lymphocyte, and monocyte counts (32), Ann Arbor stage, ECOG performance status, presence of B symptoms, involvement of two or more extranodal sites, and COO. The COO was determined using immunohistochemistry (IHC) according to the Hans classification at the time of diagnosis (33).
Among the clinicopathological variables, B2M contained 11 missing values, which accounted for 5.39% of the total. These missing values were imputed by multiple imputations using the Amelia package in R which is known to be effective for handling large numbers of missing data (34). This study was approved by the institutional review board (No. 2015-0720).
2.2. Quantitative Analysis of Biomarker Expression
We selected BCL2 and MYC as candidates for predictors because numerous studies on DLBCL have reported their association with poor prognosis (9, 35, 36). Although the proportions of 40 and 50% were generally considered as cut-off points for positivity in MYC and BCL2 IHC, respectively (2), it was mostly dependent on visual observation, and there have been controversies regarding various proportional cut-off points (36, 37). Therefore, we employed a digital quantitative acquisition and analysis for the objective measurements of BCL2 and MYC expressions without dichotomization.
The quantitative analyses of BCL2 and MYC expressions were performed using multiplex immunofluorescence (IF) labeling and the Automated Quantitative Analysis (AQUA) scoring method to minimize interobserver variation. Multiplex IF labeling with tyramide signal amplification was performed using the Opal IHC Kit (NEL8100KT, PerkinElmer) according to the manufacturer's instructions. To analyze tumor-specific biomarker expression, tumor cells were selected using CD20 expression. The tumor-specific quantitative immunofluorescence (QIF) score representing protein expression on a cell was calculated using the AQUA scoring method. The QIF scores for BCL2 and MYC were calculated as the signal intensities of each biomarker in the target compartment divided by the pixel area of the target compartment (38) (Figure S1). Details on multiplex IF labeling and QIF scoring are provided in the Supplementary Material.
2.3. Modeling With Multivariable Fractional Polynomials
Using the MFP algorithm, which variables are significant and what functional form to take is determined through iterative fashion. At first, the complexity of the functional form for continuous variables and a nominal P-value for the inclusion of variables were determined. The maximum degree of FP for each continuous variable was set at two to prevent the formation of overly complex model, and the nominal significance level for testing variables and functions was set at the conventional 0.05 level (39). FP of a certain degree contains various terms, depending on the number of powers allowed. By convention, powers are selected from the collection (−2, −1, −0.5, 0, 0.5, 1, 2, 3), where 0 indicates the log transformation. Repeated power indicates powers of log(X). For example, an FP2 with powers (−1, −1) is of the form . All categorical variables are not subject to FP transformation and are modeled with one degree of freedom. Among these categorical variables and FP-transformed continuous variables, significant variables were selected by using backward elimination.
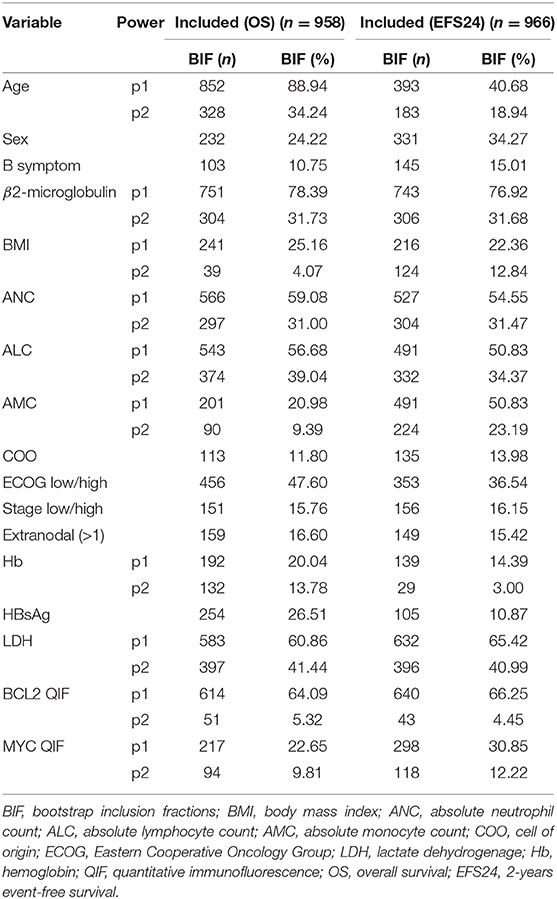
To reduce the risk of overfitting and find a stable multivariable model, bootstrap resampling was performed (40, 41). After one thousand bootstrap replications, variables for the final model were selected according to the resulting bootstrap inclusion fractions (BIF). BIF are defined as the proportion of bootstrap replications in which a given variable or type of function selected by MFP (41). To obtain a stable and interpretable model, variables with BIF >60% were selected for the final model. The final model in the survival analysis aimed to produce a prognostic index which is a weighted combination of the predictors with weights (regression coefficients) taken from the Cox model. The prognostic index value for a given individual summarizes the relative hazard of that person with respect to the control population.
2.4. Statistics
We verified the performance of the fitted Cox model using the resampling model calibration of the rms and time-dependent receiver operating characteristic (ROC) curve of the survivalROC packages, respectively. Survival curves were plotted using the Kaplan–Meier method and the log-rank test was used to analyze the statistical differences between survival curves. P-values in the univariate analysis were adjusted using the Benjamini-Hochberg procedure considering multiple comparison testing. The nomogram for predicting overall survival with the calculated prognostic score was drawn using the rms package (42). All statistical calculations including MFP modeling were conducted using R version 3.4.0. (R Foundation for Statistical Computing, https://www.R-project.org/).
2.5. Outcomes
Overall survival (OS) was defined as the time from diagnosis until death as a result of any cause. Event-free survival (EFS) was defined as the time from diagnosis until relapse or progression, unplanned re-treatment of lymphoma after initial immunochemotherapy, or death as a result of any cause. OS and EFS time were measured in months. Outcome indicators with given cut-off points (ie, EFS at 24 months [EFS24]) were defined based on the outcome status at each cut-off point from the date of the diagnosis (43).
3. Results
3.1. Patient Characteristics
For the 204 patients, OS60 and EFS24 were 69.5 and 71.5%, respectively, with a median follow up of 59 months. The clinicopathological characteristics of the patients are summarized in Table 1. Seven categorical (Sex, B symptom, COO, ECOG, Ann Arbor stage, presence of HBsAg, and extranodal involvement) and ten continuous (age, B2M, BMI, ANC, ALC, AMC, Hb, LDH, BCL2 QIF score, and MYC QIF score) variables were considered as candidates for selection. ECOG and Ann Arbor stage were classified into two groups: low (0–1)/high (2–5) ECOG and low (1–2)/high (3–4) Ann Arbor stage. Results of univariate analysis for OS and EFS24 were also described in Table 1. Fluorescence in situ hybridization (FISH) results for BCL2 and MYC were available in 50 (24.51%) patients. Of those 50 patients, only one patient (2.00%) showed rearrangements of both BCL2 and MYC. All potential explanatory covariates were applied to the initial cycle, because the MFP method uses backward elimination for variable selection. Initially, the Spearman correlation matrix was examined to investigate the dependence between variables (Figure S2). Moderately strong positive correlations were noted between the serum LDH and B2M levels (correlation coefficient, 0.57). The Ann Arbor Stage and extranodal involvement showed a strong positive correlation (correlation coefficient, 0.66). There were no significant correlations among other variables. These results were later considered in developing a parsimonious multivariable model.
3.2. Building an MFP Model and Bootstrap Resampling
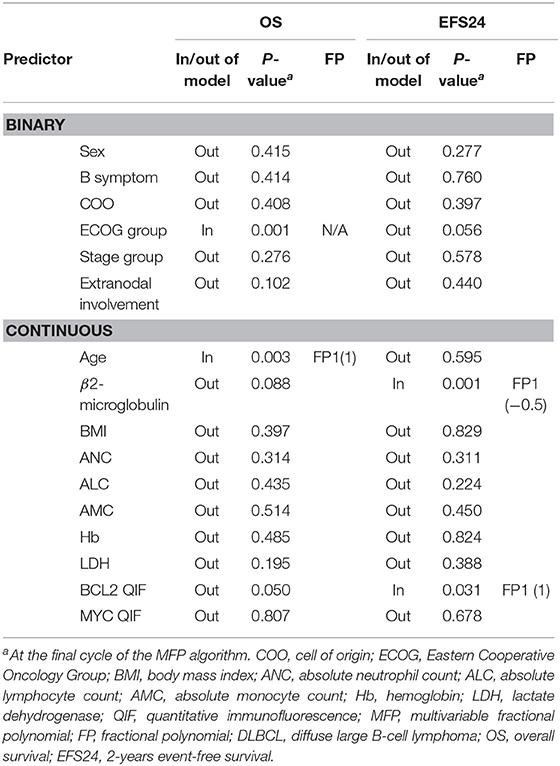
An initial MFP model was built for the entire cohort with variables described above at the conventional 5% of significance level (p <0.05) (39). For OS, age (p = 0.003) and ECOG group (p = 0.001) were selected as statistically significant variables in the model. For EFS24, B2M (p = 0.001) and BCL2 QIF score (p = 0.031) were selected in the model. Age and BCL2 QIF score were best fitted in the model when they were transformed as first-degree FP (FP1) with power 1. B2M was best fitted when it was transformed as FP1 with power −0.5 (Table 2). These results suggest that age, ECOG group, and BCL2 QIF score were the most important predictors in the initial MFP model. However, this model was difficult to consider as stable because the model targeted a single data set.
The stability of the established model was investigated by bootstrap resampling (Table 3). The same algorithm was applied to 1,000 bootstrap samples for OS and EFS24, respectively. Some algorithms built with bootstrap samples failed to converge. However, it was presumed that this did not affect the results because the model was successfully developed in over 90% of the replications. Overall, 958 and 966 MFP models with replications were built for OS and EFS24, respectively. BIF and frequencies for selected power are described in Table 3. Although the ECOG group was selected in the initial model, the inclusion proportions were only 47.60 and 36.54% in OS and EFS24, respectively. BIF for age was 88.94% in OS but only 40.68% in EFS24. In contrast, some of the variables that were not included in the initial model were frequently included in the replications. In addition, 60% of BIF was selected as a cut-off point for the importance of a variable considering the model complexity and clinical significance. The variables with more than 60% of BIF were as follows: age (88.94%), B2M (78.39%), LDH (60.86%), and BCL2 QIF score (64.09%) for OS; B2M (76.92%), LDH (65.42%), and BCL2 QIF score (66.25%) for EFS24. Between FP1 and FP2 transformations of each variable, a polynomial transformation was chosen that was selected more frequently in the model. Because second-degree FP consists of FP1 and additional polynomials, the BIF for FP1 contains that of FP2. Therefore, to determine the BIF of FP1 alone, the BIF of FP2 must be subtracted from that of FP1. Because the FP2 BIF for LDH was 41.44% out of 60.86% of the entire FP1 BIF, LDH was mainly selected with FP2 transformation in both OS and EFS24. The mainly selected polynomial and its power for each variable were as follows: age FP1 (1), LDH FP2 (3, 3), B2M FP1 (0), and BCL2 QIF score FP1 (1).
3.3. Final Model and Prognostic Index
To build a parsimonious model, it is necessary to review each selected variable and simplify unnecessary complex transformations. As a result of the preceding analysis, a non-monotonic second-degree FP was chosen for LDH with powers (3, 3). Full curves of the LDH-component of the log-hazard-ratio (HR) from both models with FP1 and FP2 were compared (Figure S3). The model with the first-degree FP for LDH was adjusted to 0 at 0.0008 which was 263.62 in raw data. In contrast, the second-degree FP for LDH was adjusted to 0 at −0.00611 which was 92.80 in raw data. Considering the general clinical knowledge regarding 250 IU/l as a cutoff value for increased serum LDH, the first-degree FP for serum LDH was selected for to the final model. In addition, dependence for inclusion between B2M and LDH was also analyzed because of their positive correlation (Figure S2). In all 958 replications, both LDH and B2M were selected in 463 replications (48.32%). When LDH was omitted, B2M was included in 288 replications (30.06%), and LDH was included in 120 replications (12.53%) when B2M was omitted (Table S1). This result supports that both variables of B2M and LDH should be included in the final model.
With the final model, prognostic scores for OS and EFS24 were calculated using the Cox model. The formulae for OS and EFS24 are as follows:
The performance of the fitted Cox model was assessed in terms of calibration and discrimination. In resampling model calibration, the mean absolute calibration error for OS and EFS were 5.9 and 2.9%, respectively (Figures S3A,B). For evaluating discrimination, time-dependent ROC curves were plotted (Figures S3C,D). After the last observed date for OS60 and EFS24, the area under the curve (AUC) stabilized at 0.68 for OS60 and 0.71 for EFS24, without decay of the performance.
Kaplan–Meier survival curves were depicted by applying temporary cutoff values to the prognostic score at the 25th, 50th, and 75th quantiles (Figures 1A,B). Patient groups were named as low, low-intermediate, high-intermediate, and high-risk groups from the low-scoring order. OS60 for low (n = 51), low-intermediate (n = 51), high-intermediate (n = 51), and high-risk (n = 51) patients were 89.81, 81.21, 66.40, and 37.89%, respectively. EFS24 for patients in each risk group were 89.96, 79.70, 68.26, and 48.33%, respectively. These survival curves classified patients as efficiently as those of conventional IPI (Figures 1C,D). Notably, log-HR values for OS and EFS24 using prognostic scores showed a continuously increasing tendency, while IPI showed non-continuous and inconsistent trends (Figure S4). Survival curves and log-HR values were also plotted with IPI risk group (Figure S6). IPI risk group was classified as low (IPI 0–1), low-intermediate (IPI 2), high-intermediate (IPI 3), and high (IPI 4–5). The results were similar to those with IPI. This promising result implies that the prognostic score calculated from the MFP model efficiently classifies the prognosis of patients with DLBCL.
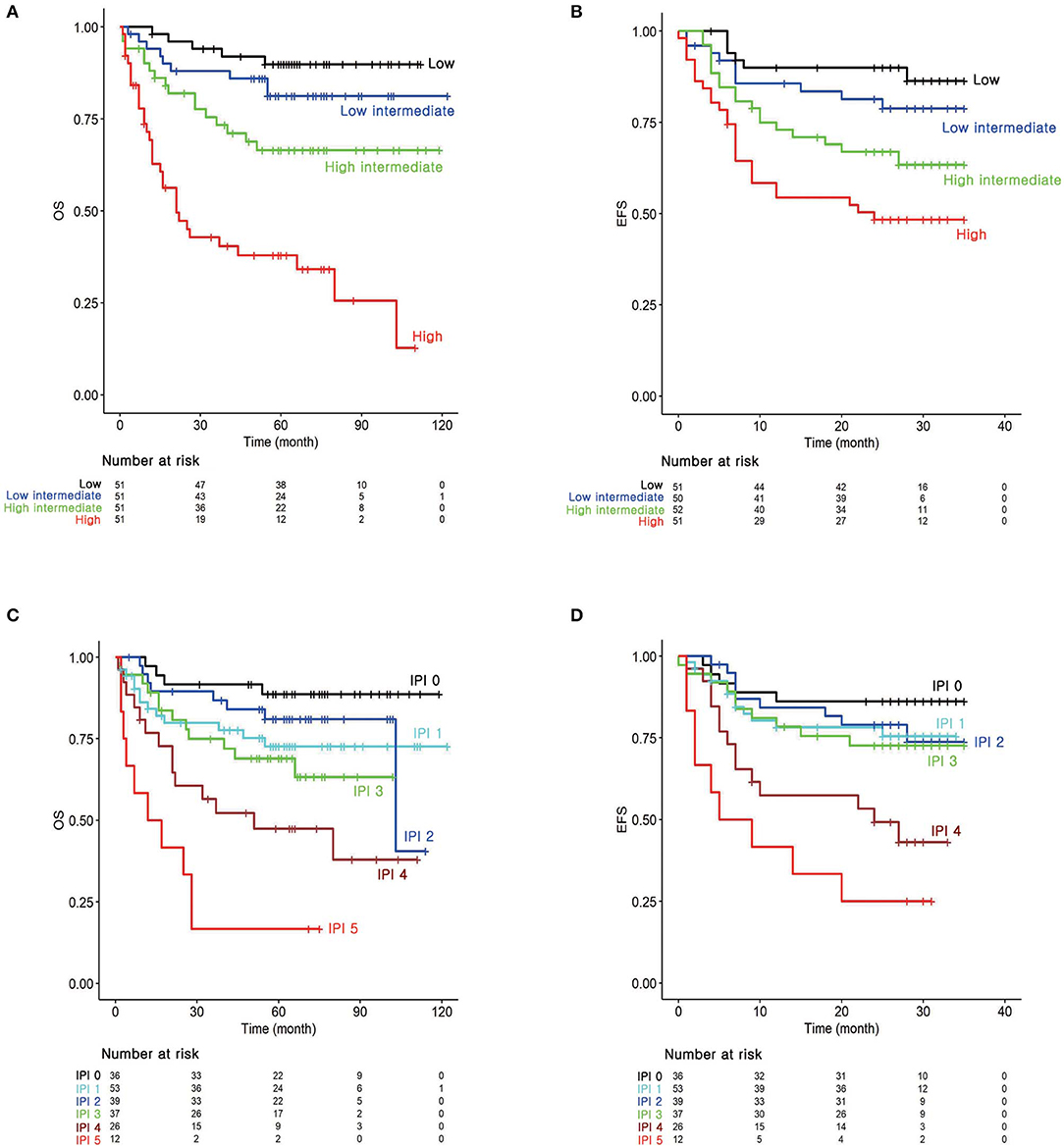
Figure 1. Survival analysis according to the risk groups. Kaplan–Meier survival curves for (A) overall survival (OS) and (B) 2-years event-free survival (EFS24) according to the prognostic index from the MFP model. (C) Kaplan–Meier survival curves for OS and (D) EFS24 according to the IPI.
4. Discussion
Recent advances in molecular pathobiology confirmed that DLBCL is highly heterogeneous both genetically and biologically (12, 44). Proper risk stratification and patient selection are required for both clinical practice and new drug development (45). The current risk stratification systems for DLBCL, such as IPI, have limitations in incorporating newly discovered tumor-intrinsic prognostic factors. Moreover, determining optimal cut-off points for these pathobiological prognostic factors is highly difficult as readouts of most biological molecules are continuous variables. Several studies have shown that the MFP approach provides an improved predictive ability in analyzing medical data that consists of various categorical and continuous variables (25, 46, 47). In this study, we developed a prognostic model using the MFP method in patients with DLBCL. By using this method, continuous variables could be used as they were without dichotomization, which minimized information loss. In addition, this model produced a prognostic score for each patient as a continuous variable, unlike that in the traditional methods. As a result, age, serum LDH, serum B2M, and BCL2 QIF score were selected to calculate prognostic score for OS. Serum LDH, serum B2M, and BCL2 QIF score were selected to calculate the prognostic score for EFS24. The higher the prognostic score of the patient, the worse was the prognosis of the patient. Risk groups based on the prognostic score from the MFP model were well-separated by their prognosis.
Attributes associated with the biological characteristics of tumors, such as serum LDH and B2M, were selected in the model for short-term outcomes (EFS24). Notably, age was additionally selected in the prognostic model for the long-term outcome (OS) compared to the prognostic model for the short-term outcome. These results imply the overall status of a patient may play an important role in their long-term prognosis. The serum LDH level is included in the current IPI and represents the tumor burden. In addition to serum LDH, serum B2M was also selected in the prognostic model for both OS and EFS24. The serum B2M levels in a patient with lymphoma, similar to the serum LDH levels, have been widely accepted as directly related to tumor burden (48). However, serum B2M levels also imply involvement of the immune reaction to the tumor because it is a component of major histocompatibility complex (MHC I) (49, 50). Based on these grounds, several studies have shown the poor prognostic effect of serum B2M in DLBCL (30, 51, 52). However, these results are controversial, and most of these studies have shown difficulties in determining the universal optimal cut-off point. The prognostic effect of serum B2M in DLBCL as a continuous value was confirmed without determining a specific cut-off point.
Using the current risk stratification system of hematologic malignancy, it is difficult to comprehensively predict the prognosis of patients with DLBCL. A major limitation of the IPI system is that it does not allow the incorporation of newly discovered prognostic factors, most notably BCL2 and MYC. BCL2 and/or MYC expression in DLBCL is associated with aggressive behavior and poor prognosis (9, 35, 36, 53). However, the proportional cut-off points of their expressions are still arbitrary, and a recent study has shown the importance of intensity of BCL2 expression as well as its extent (36). To overcome this limitation and produce an objective, the continuous variable for measuring protein expression, multiplex IF for BCL2 and MYC with tumor marker CD20 was exploited. Fluorescence is more linear and has a wider dynamic range compared to those of chromogenic immunohistochemistry (54), which allows object acquisition of the expression amount of each protein. As a result, the BCL2 QIF score was selected as a predictor of poor prognosis, which is consistent with previous findings. However, MYC expression was not selected in the current prognostic model. Of note, COO was not found to be an important predictor and this result is consistent with the existing controversies on the prognostic importance of COO (55). These results suggest that our prognostic model is flexible enough to incorporate biomarkers of DLBCL into a single risk stratification system and is consistent with the results of other previous studies. It can be also used to improve the prognostic ability of the existing prognostic model by adding continuously updating new information (56).
The limitation of this study is the relatively small sample size and event number. Also, the lack of external validation can be another limitation. To overcome these limitation and establish a stable prognostic model, we performed bootstrap resampling method. Although we verified the model using the internal validation, further external validation study may potentially provide better insight for performance of the model. Regarding the relatively small event number, the final number of variables for a Cox regression model was two or three. Considering the suggestions of previous simulation studies that minimum events per variable values of between 5 and 20 were needed for reliable results, the final number of covariates of this study seems to be reasonable (57).
In this study, we suggest a new prognostic model using the MFP method in DLBCL, which allows the flexible incorporation of variable clinicopathological factors into a single risk stratification system. It is a simple and interpretable model consisting of only objectively quantified measurements and incorporates non-linear relationships. The model also presents a continuous prognostic score in each patient that can provide enormous flexibility in classifying risk groups. In the clinical practice, this prognostic score can be used in the form of a nomogram predicting patients' survival (Figure S7). The MFP method has been studied in some solid cancers including breast cancer and renal cell carcinoma (39, 56, 58). Prognostic modeling using the MFP method with full information resulted in better performance in previous studies. Most notably, this is the first study to our knowledge to investigate the effectiveness of MFP methods in hematological malignancies. Further studies using a large patient population will increase the generalizability of this method.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Asan Medical Center (No. 2015-0720). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
C-SP and H-BO contributed to conceptualization, study design, manuscript preparation, and editing. JR contributed to drafting the manuscript and coordinating the data collection and analysis. YL contributed to data analysis. JJ, JC, JL, and RP contributed to the interpretation of the statistical results and manuscript editing. A-NL contributed to performing the immunofluorescence assay. H-KP and S-WK contributed to data collection. HC, DY, and JH contributed to the interpretation of clinicopathological data. All authors approved the final submitted manuscript.
Funding
This work was supported by the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (grant no. 2018-0527 to C-SP and grant No. 2016-0219 to H-BO) and the Ajou University Medical Center, Suwon, Korea (grant no. M-2018-C0460-00058) (JR).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2020.00329/full#supplementary-material
References
1. Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. (2019) 94:604–16. doi: 10.1002/ajh.25460
2. Gascoyne RD, Chan JKC, Campo E, Rosenwald A, Jaffe ES, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press (2017).
3. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. (2010) 116:2040–45. doi: 10.1182/blood-2010-03-276246
4. International Non-Hodgkin's Lymphoma Prognostic Factors P. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. (1993) 329:987–94. doi: 10.1056/NEJM199309303291402
5. Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. (2015) 125:22–32. doi: 10.1182/blood-2014-05-577189
6. Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. (2010) 28:2373–80. doi: 10.1200/JCO.2009.26.2493
7. Kanakry JA, Ambinder RF. Old variables, new value: a refined IPI for DLBCL. Blood. (2014) 123:800–1. doi: 10.1182/blood-2013-12-540914
8. Ennishi D, Jiang A, Boyle M, Collinge B, Grande BM, Ben-Neriah S. Double-Hit Gene expression signature defines a distinct subgroup of germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol. (2019) 37:190–201. doi: 10.1200/JCO.18.01583
9. Green TM, Young KH, Visco C, Xu-Monette ZY, Orazi A, Go RS, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. (2012) 30:3460–7. doi: 10.1200/JCO.2011.41.4342
10. Johnson NA, Savage KJ, Ludkovski O, Ben-Neriah S, Woods R, Steidl C, et al. Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. (2009) 114:2273–9. doi: 10.1182/blood-2009-03-212191
11. Rosenwald A, Wright G, Chan W, Connors J, Campo E, Fisher R, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. (2002) 346:1937–47. doi: 10.1056/NEJMoa012914
12. Schmitz R, Wright G, Huang D, Johnson C, Phelan J, Wang J, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. (2018) 378:1396–407. doi: 10.1056/NEJMoa1801445
13. Thieblemont C, Briere J, Mounier N, Voelker H, Cuccuini W, Hirchaud E, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol. (2011) 29:4079–87. doi: 10.1200/JCO.2011.35.4423
14. Zhang J, Medeiros L, Young K. Cancer Immunotherapy in diffuse large B-cell lymphoma. Front Oncol. (2018) 8:351. doi: 10.3389/fonc.2018.00351
15. Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. (2006) 25:127–41. doi: 10.1002/sim.2331
16. Royston P, Sauerbrei W. A new approach to modelling interactions between treatment and continuous covariates in clinical trials by using fractional polynomials. Stat Med. (2004) 23:2509–25. doi: 10.1002/sim.1815
17. Weinberg CR. How bad is categorization? Epidemiology. (1995) 6:345–7. doi: 10.1097/00001648-199507000-00002
18. Del Priore G, Zandieh P, Lee MJ. Treatment of continuous data as categoric variables in Obstetrics and Gynecology. Obstet Gynecol. (1997) 89:351–4. doi: 10.1016/S0029-7844(96)00504-2
19. Giannoni A, Baruah R, Leong T, Rehman MB, Pastormerlo LE, Harrell FE, et al. Do optimal prognostic thresholds in continuous physiological variables really exist? Analysis of origin of apparent thresholds, with systematic review for peak oxygen consumption, ejection fraction and BNP. PLoS ONE. (2014) 9:e81699. doi: 10.1371/journal.pone.0081699
20. Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using optimal cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. (1994) 86:829–35. doi: 10.1093/jnci/86.11.829
21. Austin PD, Brunner LJ. Inflation of the type I error rate when a continuous confounding variable is categorized in logistic regression analyses. Stat Med. (2004) 23:1159–78. doi: 10.1002/sim.1687
22. MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. (2002) 7:19–40. doi: 10.1037/1082-989X.7.1.19
23. Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. (1999) 28:964–74. doi: 10.1093/ije/28.5.964
24. Sauerbrei W, Royston P. Building multivariable prognostic and diagnostic models: transformation of the predictors by using fractional polynomials. J R Stat Soc Ser A. (1999) 162:71–94. doi: 10.1111/1467-985x.00122
25. Zhang Z, Chen K, Ni H, Fan H. Predictive value of lactate in unselected critically ill patients: an analysis using fractional polynomials. J Thorac Dis. (2014) 6:995–1003. doi: 10.3978/j.issn.2072-1439.2014.07.01
26. Sauerbrei W, Royston P. The Multivariable Fractional Polynomial Approach, With Thoughts About Opportunities and Challenges in Big Data. Big Data Clustering: Data Preprocessing, Variable Selection, and Dimension Reduction. WIAS Report No. 29 (2017). p. 36–54. doi: 10.20347/WIAS.REPORT.29
27. Zhang Z. Multivariable fractional polynomial method for regression model. Ann Transl Med. (2016) 4:174. doi: 10.21037/atm.2016.05.01
28. Kanemasa Y, Shimoyama T, Sasaki Y, Tamura M, Sawada T, Omuro Y, et al. Analysis of the prognostic value of BMI and the difference in its impact according to age and sex in DLBCL patients. Hematol Oncol. (2018) 36:76–83. doi: 10.1002/hon.2426
29. Yan X, Zhou M, Lou Z, Mu Q, Sheng L, Zhang P, et al. Diffuse large B-cell lymphoma with concurrent hepatitis B virus infection in the MabThera era: unique clinical features and worse outcomes. J Cancer Res Ther. (2018) 14:S248–53. doi: 10.4103/0973-1482.187285
30. Seo S, Hong JY, Yoon S, Yoo C, Park JH, Lee JB, et al. Prognostic significance of serum beta-2 microglobulin in patients with diffuse large B-cell lymphoma in the rituximab era. Oncotarget. (2016) 7:76934–43. doi: 10.18632/oncotarget.12734
31. Nakayama S, Matsuda M, Adachi T, Sueda S, Ohashi Y, Awaji S, et al. Novel prognostic index based on hemoglobin level and platelet count for diffuse large B-cell lymphoma, not otherwise specified in the R-CHOP era. Platelets. (2019) 30:637–45. doi: 10.1080/09537104.2018.1499889
32. Chen Y, Neelapu S, Feng L, Bi W, Yang TH, Wang M, et al. Prognostic significance of baseline peripheral absolute neutrophil, monocyte and serum beta2-microglobulin level in patients with diffuse large b-cell lymphoma: a new prognostic model. Br J Haematol. (2016) 175:290–9. doi: 10.1111/bjh.14237
33. Meyer PN, Fu K, Greiner TC, Smith LM, Delabie J, Gascoyne RD, et al. Immunohistochemical methods for predicting cell of origin and survival in patients with diffuse large B-cell lymphoma treated with rituximab. J Clin Oncol. (2011) 29:200–7. doi: 10.1200/JCO.2010.30.0368
34. Pedersen A, Mikkelsen E, Cronin-Fenton D, Kristensen N, Pham T, Pedersen L, et al. Missing data and multiple imputation in clinical epidemiological research. Clin Epidemiol. (2017) 9:157–66. doi: 10.2147/CLEP.S129785
35. Li L, Li Y, Que X, Gao X, Gao Q, Yu M, et al. Prognostic significances of overexpression MYC and/or BCL2 in R-CHOP-treated diffuse large B-cell lymphoma: a systematic review and meta-analysis. Sci Rep. (2018) 8:6267. doi: 10.1038/s41598-018-24631-5
36. Tsuyama N, Sakata S, Baba S, Mishima Y, Nishimura N, Ueda K, et al. BCL2 expression in DLBCL: reappraisal of immunohistochemistry with new criteria for therapeutic biomarker evaluation. Blood. (2017) 130:489–500. doi: 10.1182/blood-2016-12-759621
37. Ambrosio MR, Lazzi S, Bello GL, Santi R, Porro LD, de Santi MM, et al. MYC protein expression scoring and its impact on the prognosis of aggressive B-cell lymphoma patients. Haematologica. (2019) 104:e25–8. doi: 10.3324/haematol.2018.195958
38. Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nat Med. (2002) 8:1323–7. doi: 10.1038/nm791
39. Royston P, Reitz M, Atzpodien J. An approach to estimating prognosis using fractional polynomials in metastatic renal carcinoma. Br J Cancer. (2006) 94:1785–8. doi: 10.1038/sj.bjc.6603192
40. Royston P, Sauerbrei W. Stability of multivariable fractional polynomial models with selection of variables and transformations: a bootstrap investigation. Stat Med. (2003) 22:639–59. doi: 10.1002/sim.1310
41. Royston P, Sauerbrei W. Bootstrap assessment of the stability of multivariable models. Stata J. (2009) 9:547–70. doi: 10.1177/1536867X0900900403
42. Zhang Z, Kattan MW. Drawing Nomograms with R: applications to categorical outcome and survival data. Ann Transl Med. (2017) 5:211. doi: 10.21037/atm.2017.04.01
43. Maurer MJ, Ghesquieres H, Jais JP, Witzig TE, Haioun C, Thompson CA, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. (2014) 32:1066–73. doi: 10.1200/JCO.2013.51.5866
44. Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. (2018) 24:679–90. doi: 10.1038/s41591-018-0016-8
45. Gao H, Gong Z. Beyond international prognostic index: risk stratification in diffuse large B-cell lymphoma. Transl Cancer Res. (2017) 6:S210–6. doi: 10.21037/tcr.2017.02.42
46. Silke B, Kellett J, Rooney T, Bennett K, O'Riordan D. An improved medical admissions risk system using multivariable fractional polynomial logistic regression modelling. QJM. (2010) 103:23–32. doi: 10.1093/qjmed/hcp149
47. Wong E, Wang B, Garrison L, Alfonso-Cristancho R, Flum D, Arterburn D, et al. Examining the BMI-mortality relationship using fractional polynomials. BMC Med Res Methodol. (2011) 11:175. doi: 10.1186/1471-2288-11-175
48. Shi C, Zhu Y, Su Y, Chung LW, Cheng T. Beta2-microglobulin: emerging as a promising cancer therapeutic target. Drug Discov Today. (2009) 14:25–30. doi: 10.1016/j.drudis.2008.11.001
49. Li L, Dong M, Wang XG. The implication and significance of beta 2 microglobulin: a conservative multifunctional regulator. Chin Med J (Engl). (2016) 129:448–55. doi: 10.1007/978-3-658-11231-8
50. Bossard C, Bezieau S, Matysiak-Budnik T, Volteau C, Laboisse CL, Jotereau F, et al. HLA-E/beta2 microglobulin overexpression in colorectal cancer is associated with recruitment of inhibitory immune cells and tumor progression. Int J Cancer. (2012) 131:855–63. doi: 10.1002/ijc.26453
51. Kanemasa Y, Shimoyama T, Sasaki Y, Tamura M, Sawada T, Omuro Y, et al. Beta-2 microglobulin as a significant prognostic factor and a new risk model for patients with diffuse large B-cell lymphoma. Hematol Oncol. (2017) 35:440–6. doi: 10.1002/hon.2312
52. Miyashita K, Tomita N, Taguri M, Suzuki T, Ishiyama Y, Ishii Y, et al. Beta-2 microglobulin is a strong prognostic factor in patients with DLBCL receiving R-CHOP therapy. Leuk Res. (2015) 39: 1187–91. doi: 10.1016/j.leukres.2015.08.016
53. Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. (2012) 30:3452–9. doi: 10.1200/JCO.2011.41.0985
54. Gustavson MD, Bourke-Martin B, Reilly D, Cregger M, Williams C, Mayotte J, et al. Standardization of HER2 immunohistochemistry in breast cancer by automated quantitative analysis. Arch Pathol Lab Med. (2009) 133:1413–9. doi: 10.1043/1543-2165-133.9.1413
55. Staiger A, Ziepert M, Horn H, Scott D, Barth T, Bernd H, et al. Clinical impact of the cell-of-origin classification and the MYC/BCL2 dual expresser status in diffuse large B-cell lymphoma treated within prospective clinical trials of the German High-Grade Non-Hodgkin's Lymphoma Study Group. J Clin Oncol. (2017) 35:2515–26. doi: 10.1200/JCO.2016.70.3660
56. Winzer KJ, Buchholz A, Schumacher M, Sauerbrei W. Improving the prognostic ability through better use of standard clinical data–the Nottingham prognostic index as an example. PLoS ONE. (2016) 11:e0149977. doi: 10.1371/journal.pone.0149977
57. Ogundimu EO, Altman DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol. (2016) 76:175–82. doi: 10.1016/j.jclinepi.2016.02.031
Keywords: diffuse large B-cell lymphoma, prognosis, multivariable fractional polynomial, risk stratification, prognostic model
Citation: Roh J, Jung J, Lee Y, Kim S-W, Pak H-K, Lee A-N, Lee J, Cho J, Cho H, Yoon DH, Park RW, Huh J, Oh H-B and Park C-S (2020) Risk Stratification Using Multivariable Fractional Polynomials in Diffuse Large B-Cell Lymphoma. Front. Oncol. 10:329. doi: 10.3389/fonc.2020.00329
Received: 08 January 2020; Accepted: 25 February 2020;
Published: 11 March 2020.
Edited by:
Basem M. William, The Ohio State University, United StatesReviewed by:
Zhongheng Zhang, Zhejiang University, ChinaMichele Merli, University of Insubria, Italy
Copyright © 2020 Roh, Jung, Lee, Kim, Pak, Lee, Lee, Cho, Cho, Yoon, Park, Huh, Oh and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heung-Bum Oh, aGJvaCYjeDAwMDQwO2FtYy5zZW91bC5rcg==; Chan-Sik Park, Y3Npa3BhcmsmI3gwMDA0MDthbWMuc2VvdWwua3I=
 Jin Roh
Jin Roh Jiwon Jung2,3
Jiwon Jung2,3 Hyo-Kyung Pak
Hyo-Kyung Pak Chan-Sik Park
Chan-Sik Park