- 1Mayo Clinic Florida, Jacksonville, FL, United States
- 2Mayo Clinic, Rochester, MN, United States
Radiation therapy is one of the most widely used therapies for malignancies. The therapeutic use of heavy ions, such as carbon, has gained significant interest due to advantageous physical and radiobiologic properties compared to photon based therapy. By taking advantage of these unique properties, carbon ion radiotherapy may allow dose escalation to tumors while reducing radiation dose to adjacent normal tissues. There are currently 13 centers treating with carbon ion radiotherapy, with many of these centers publishing promising safety and efficacy data from the first cohorts of patients treated. To date, carbon ion radiotherapy has been studied for almost every type of malignancy, including intracranial malignancies, head and neck malignancies, primary and metastatic lung cancers, tumors of the gastrointestinal tract, prostate and genitourinary cancers, sarcomas, cutaneous malignancies, breast cancer, gynecologic malignancies, and pediatric cancers. Additionally, carbon ion radiotherapy has been studied extensively in the setting of recurrent disease. We aim to provide a comprehensive review of the studies of each of these disease sites, with a focus on the current trials using carbon ion radiotherapy.
Introduction
The therapeutic advantages of particle radiotherapy were first recognized by Robert Wilson in the 1940s (1). Since that time, particle therapy has enjoyed a rapid growth, with centers across the world treating with protons and other heavy ions, including carbon ions. The National Institute of Radiologic Sciences (NIRS) opened the first heavy ion accelerator for clinical use in Chiba, Japan, in 1994 (2). Since that time, over 20,000 patients have been treated with carbon ion radiation therapy (CIRT) (3). Today, there are five countries and a total of 13 centers treating with CIRT (1, 4). At NIRS, 22% of patients treated have had localized prostate cancer, with other common sites including bone and soft tissue (13%) and head and neck (11%) (5).
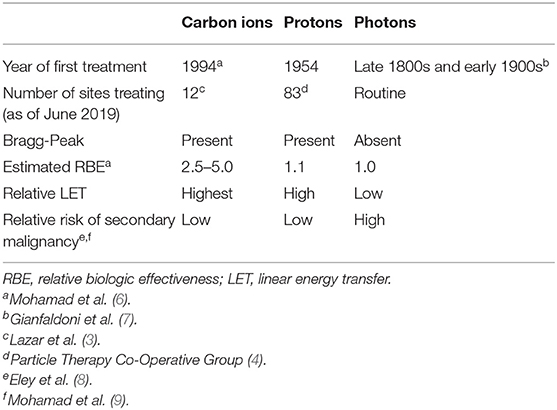
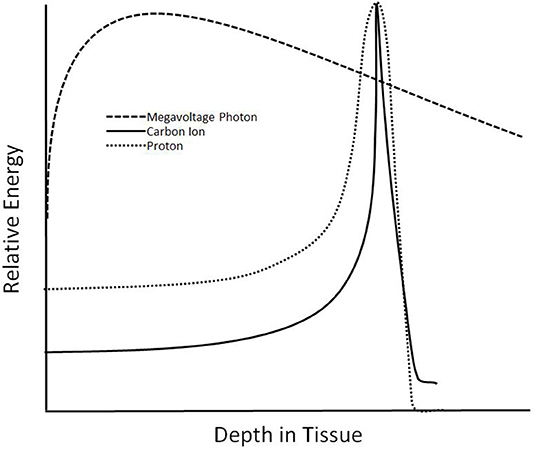
Treatment with carbon ions provides several unique physical and radiobiologic properties (Table 1). Carbon ions exhibit a characteristic energy distribution in depth, known as the “Bragg Peak,” where low levels of energy are deposited in tissues proximal to the target, and the majority of energy is released in the target (Figure 1). Distal tissues receive little energy, although, unlike protons, there is energy deposited distally due to nuclear fragmentation (10). Additionally, a steeper lateral dose penumbra is observed at greater depths than with heavy ions, such as carbon, than with photons or protons (1, 6). Furthermore, carbon exhibit a higher linear energy transfer (LET) than photons and protons. This leads to a higher relative biological effectiveness (RBE), where damage caused by carbon ions is clustered in the DNA, overwhelming the cellular repair systems (6). With a higher LET than other methods of radiation and the characteristics of the Bragg Peak, CIRT provides a promising treatment choice for providing higher doses to targets while reducing irradiation to organs at risk (OARs).
Centers treating with CIRT can take advantage of two types of treatment techniques to help conform the dose distribution to minimize dose to OARs. Similar to proton therapy, CIRT can be delivered either through passive scattering (using a collimator to shape the beam in the lateral direction and a range compensator to shape the beam distally) or active scanning (using a narrow “pencil beam” that avoids the use of a collimator or compensator) (11). Notably, use of active scanning, along with the physical properties of the Bragg Peak and a slower treatment time, may lead to higher uncertainty due to physiologic motion. Mitigating the dose uncertainty due to motion is an active area of research.
Due to the size and expense associated with CIRT, the majority of centers are treating with fixed-beam gantries, limiting the treatment positions available and requiring changes in patient setup prior to irradiation with multiple beams. Currently, there are two centers with rotating gantries, thus with the ability to irradiate from all angles (1). The reader is referred to the text Carbon Ion Radiotherapy: Principles, Practices, and Treatment Planning by Tsujii et al. for a more thorough discussion regarding the technical aspects of treatment (11).
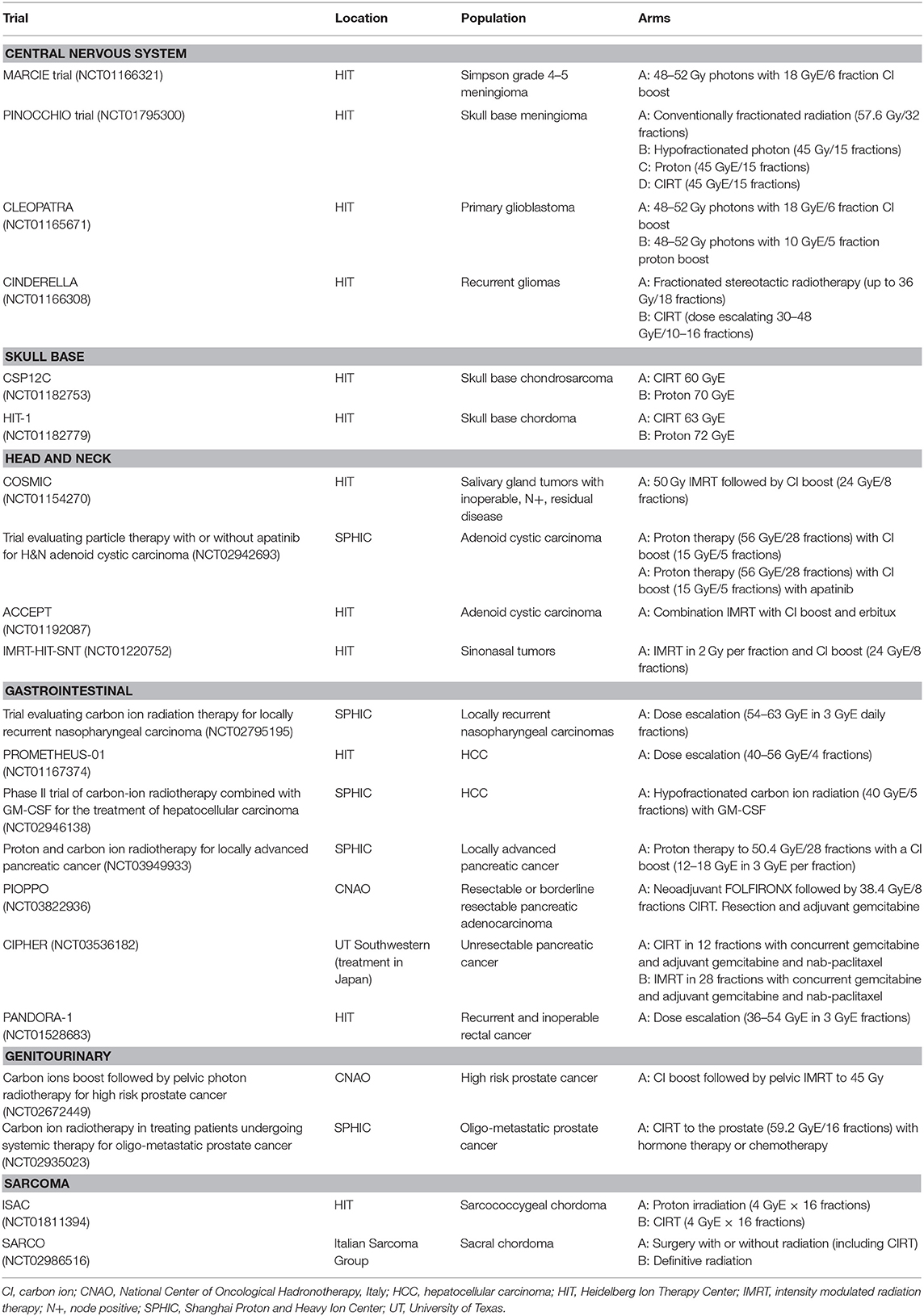
To date, there have been multiple textbooks and review articles summarizing the current radiobiological, physics, and clinical knowledge of CIRT (1–3, 5, 6, 11–20). In this review, our aim is to provide a comprehensive and updated summary of the current clinical literature for patients treated with carbon ion radiotherapy, with an emphasis currently accruing studies. An overview of the current trials is provided in Table 2.
Intracranial Tumors
Meningioma
Multiple studies have confirmed the safety and favorable toxicity profile of carbon radiotherapy for intracranial malignancies, with early use focusing on delivering a carbon ion boost following conventional photon or proton therapy (18, 21–24). A preliminary study at the Heidelberg Ion Therapy Center (HIT) evaluated 10 patients with high-risk meningioma treated with photon based radiotherapy and a carbon ion boost to a dose of 18 GyE, with local control (LC) of 72% at 7 years (25, 26).
The phase II MARCIE trial (NCT01166321) is further investigating the use of carbon ion boost in patients with residual disease following surgical resection (Simpson Grade 4–5). Enrolled patients receive 48–52 Gy of photon therapy followed by an 18 GyE carbon ion boost given in 6 fractions (27). The PINOCCHIO study is a four-arm trial investigating conventionally fractionated photon therapy, hypofractionated photon therapy, proton therapy, and CIRT for the treatment of skull base meningioma (NCT01795300).
High Grade Glioma
High grade gliomas are typically radioresistant, with a poor prognosis despite aggressive treatment (26, 28, 29). Because of this, there has been significant interest in using CIRT as a novel treatment strategy for patients with glioma. Mizoe et al. reported on 48 patients treated with 50 Gy of photons followed by a carbon ion boost of 16.8–24.8 GyE in 8 fractions with nimustine hydrochloride. Overall survival was improved in patients receiving a higher carbon boost dose, with a median progression free survival (PFS) of 26 months. Notably, there were few grade 3 or higher acute events and no late grade 3 toxicities (24, 30). Stemming from data collected in this trial, simulated survival curves were generated for patients treated with both temozolomide and CIRT, with a potential benefit to concurrent therapy identified (31). This is currently being investigated as part of the CLEOPATRA trial.
The CLEOPATRA trial is a phase II study at HIT evaluating the use of a carbon ion boost or proton boost after concurrent photon therapy and temozolomide for primary glioblastoma. Patients are treated with 48–52 Gy of photons followed by a carbon boost of 18 GyE in 6 fractions or 10 GyE in 5 fractions in the proton group (32).
In the setting of disease recurrence, the CINDERELLA trial is a randomized, phase I/II study comparing carbon and fractionated radiation therapy for progressive or recurrent gliomas. This trial, which has recently completed accrual, treated patients with escalating dose from 30 GyE in 10 fractions to 48 GyE in 16 fractions and 36 Gy in 18 fractions in the photon group (33).
Skull Base Chordoma/Chondrosarcoma
Skull base tumors present a challenge for treatment given their proximities to OARs. CIRT thus provides a theoretical advantage for treatment. Mizoe et al. reported on three protocols from NIRS, where a dose of 60.8 GyE in 15 fractions was given over 4 weeks. The 5-year LC was 100% without excessive toxicity (34). Further, with a median dose of 60 GyE in 20 fractions, HIT reported on 96 patients with a 70% 5-year LC. Late grade 3 optic neuropathy was seen in 4.1% of patients, and temporal lobe injury in 7.2% (35). In nine patients treated with resection and adjuvant CIRT, the 7-year overall survival (OS) was 85.7% and the 3-year and recurrence free survival (RFS) rate was 70.0% (36).
For skull base chondrosarcoma, a LC of 96.2% at 3 years and 89.8% at 4 years for patients with low and intermediate grade disease was seen following treatment to a dose of 60 GyE. One patient developed acute grade 3 mucositis with no other acute grade 3 toxicity. One patient had late grade 3 toxicity (37).
In order to compare clinical outcomes following proton and carbon ion therapy in the treatment of low grade skull base chondrosarcomas, HIT opened a randomized trial comparing 60 GyE in 20 fractions of CIRT and 70 GyE in 2 GyE per fraction of proton therapy (NCT01182753) (38). A similar trial is open comparing proton therapy (72 GyE in 2 GyE per fraction) to CIRT (63 GyE in 3 GyE per fraction) for the treatment of skull base chordoma (NCT01182779).
Uveal Melanoma
Tsuji et al. reported on 59 patients with locally advanced or unfavorably located choroidal melanoma treated at NIRS from 2001 to 2006. Patients were treated with a single anterior field to doses between 60 GyE and 85 GyE, each given in five fractions, with a 3-year LC of 97.4%. Overall, 40% of patients developed neovascular glaucoma, mostly in the high dose group, with three requiring enucleation (5% of all patients) (39).
Orbital Tumors
HIT evaluated 24 patients with radioresistant malignant lacrimal gland tumors treated with active raster scanning technique. The median local control was 24 months, with no grade 4 or higher toxicity (40). NIRS reported on 33 patients with lacrimal gland tumors with extraorbital extension treated to either 57.6 GyE or 64 GyE in 16 fractions. Although there was an 86% ipsilateral eye preservation rate, 36.4% of patients developed grade 4 optic nerve disorders (41).
Head and Neck Tumors
Adenoid Cystic Carcinoma
Early results from HIT reported a 3-year LC rate of 62% for the 21 patients with unfavorable adenoid cystic carcinomas treated with combination photon and CIRT. No grade 3 or 4 toxicities were observed (17). Sixteen patients with locally advanced adenoid cystic carcinoma were enrolled in a phase I/II trial photon and CIRT boost to a dose of 72 GyE. Three-year LC was 64.6% with no patient developing grade 3 or higher complications (42).
A subanalysis of J-CROS 1402 HN patients found a 3-year LC and OS of 81 and 94% with a median dose of 64 GyE in 16 fractions. Two patients experienced late grade 3 toxicity of dysphagia and brain abscess (43). A prospective study analyzing 35 patients at Gunma University Heavy Ion Medical Center for non-squamous cell carcinomas of the head and neck showed promising results with patients receiving 64 GyE and 57.6 GyE in 16 fractions, with 3-year LC and OS rates of 93 and 88%, respectively (44).
The COSMIC trial is a phase II trial evaluating combined IMRT to a dose of 50 Gy followed by carbon ion boost to 24 GyE over 8 fractions for patents with salivary gland tumors with inoperable, node positive, or residual disease (NCT01154270). Apatinib is also being investigated with proton therapy followed by a carbon ion boost for adenoid cystic carcinomas (NCT02942693). The ACCEPT study is a phase II trial of combination IMRT followed by carbon ion boost with cetuximab for adenoid cystic carcinoma, and is currently open at HIT (NCT01192087).
Parotid Gland Tumors
Patients treated for locally advanced parotid gland tumors with CIRT at NIRS showed a 5-year local control of 74.5% and overall survival of 70.1%. Of the 30 patients without facial nerve deficits prior to radiation, 25 continued to have no evidence of radiation induced facial nerve damage (45).
Nasopharyngeal Cancer
In particular, nasopharyngeal carcinomas are among the most accepted indications and may benefit the most from particle therapy, as they often abut critical OARs like the brainstem, optic apparatus, and temporal lobes. HIT retrospectively analyzed 26 patients with high risk nasopharyngeal cancer treated with IMRT and carbon ion boost for a cumulative dose of 74 Gy RBE. With a median follow up of 40 months, 60% had a complete response, with 20% demonstrating partial response and 12% stable disease. The 2-year OS, LC, and distant progression-free survival (PFS) rates were 100, 95, and 93%, respectively. Acute grade 3 toxicity was seen in 20% of patients, with 16% developing late grade 3 toxicity. There were no grade 4 or 5 toxicities (46).
Akbaba et al. described 59 patients with adenoid cystic carcinoma of the nasopharynx treated at the Heidelberg Ion-Beam Therapy Centers with combination photon and carbon ion boost radiation therapy. Patients were treated to a dose of 50–56 Gy IMRT followed by 18–24 GyE boost with carbon. The 2-year OS and LC were 87 and 83%, with 12% acute and 8% late grade 3 toxicity (47).
In the setting of recurrent disease, CIRT to a dose of 50–66 GyE with varying fractionation schedules (between 2 and 3 GyE per fraction) delivered by raster scanning has shown promising results, with a 1-year PFS and OS were both 98%. Grade 3 and 4 late toxicities included mucosal necrosis (9%), xerostomia (1%), and temporal lobe necrosis (1%) (48). Additionally, the Shanghai Proton and Heavy Ion Center (SPHIC) is evaluating the maximum tolerated dose of retreatment using raster scanning CIRT with concurrent cisplatin (49).
Sinonasal Cancer
Koto et al. investigated 22 patients with sinonasal adenocarcinoma treated with CIRT either as definitive therapy or following surgery or chemotherapy, with 14 patients receiving 57.6 GyE in 16 fractions and 8 receiving 64.0 GyE in 16 fractions. With a median follow up of 43 months, the 3-year LC and OS were 76.9 and 59.1%, respectively. Notably, five patients experienced lateral vision loss. Symptomatic brain necrosis and mucosal ulceration were observed in one patient each, respectively (50).
HIT is currently investigating IMRT followed by a 24 GyE in 8 fraction boost to inoperable or residual disease (NCT01220752). A dose escalation study of CIRT for recurrent nasopharyngeal carcinoma is currently open at the SPHIC, with doses from 55 to 65 GyE at 2.5 GyE per day (NCT02795195).
Otic Cancer
Primary otic tumors are extremely rare with a poor prognosis, with surgery the mainstay of treatment. JCROS evaluated 31 patients treated for external auditory canal or middle ear carcinomas, with a median dose of 64 GyE in 15 fractions. Three-year OS was 58.7% with similar LC rates. Grade 3 dermatitis was seen in 9.7% of patients, with central nervous system (CNS) necrosis in 6.5%. There were no grade 4 or 5 toxicities (51). Further, NIRS reported on 13 patients with a 3-year LC of 56.4% and OS of 41.6%. Two patients had severe temporal bone necrosis, and four patients developed grade 1–2 localized brain necrosis (52).
Oral Cancer
In a large retrospective series, Ikawa et al. analyzed 76 patients with non-squamous oral cavity cancers treated across four institutions in Japan from 2004 and 2014. Forty-six patients had salivary gland carcinoma and 27 had mucosal melanoma. With a median follow up of 31.1 months, the 3-year LC, PFS, and OS were 86.6, 63.1, and 78.4%, respectively. Thirteen patients had late grade 3 or higher toxicity, with 9 patients having grade 3 osteoradionecrosis. There were no grade 5 toxicities. The authors conclude that carbon ion radiotherapy is effective with “acceptable” toxicity for oral cavity cancers (53).
Recurrent Disease
Re-irradiation with CIRT appears to be a reasonable treatment option in patients who developed recurrent disease after primary CIRT. SPHIC reported on 19 patients with recurrent or radiation induced sarcoma of the head and neck treated with CIRT to a median dose of 60 GyE. The 12 month survival was 86.5%, with two grade 4 toxicities (acute hemorrhage from the sphenopalatine artery). There were no grade 5 toxicities (54).
NIRS investigated 48 patients with locoregional failure previously treated with a mean dose of 57.6 GyE in 12 fractions using CIRT. With a median dose of 54 GyE, 10.4% of patients developed grade 3 acute toxicity and 37.5% of patients developed grade 3 or higher late toxicity. There was one grade 5 toxicity. The 2-year LC and OS rates were 40.5 and 59.6% (55). Further, SPHIC reported on 19 patients with recurrent or radiation induced disease treated to 60 GyE. No grade 5 toxicities were seen (54).
Lung Tumors
Non-small Cell Lung Cancer (NSCLC)
Dosimetric studies have shown lower OAR doses and a more homogenous target dose for NSCLC with CIRT compared to photon, potentially allowing for hypofractionation without increasing toxicity (56). In localized disease, Miyamoto et al. described 47 patients in a dose escalation trial with doses from 59.4 to 95.4 GyE. Grade 3 radiation pneumonitis occurred in three patients, although this was not dose limiting as defined by the protocol (57). Hypofractionation was then attempted to a dose of 72 GyE in 9 fractions, with a 94.7% LC rate and no grade 4–5 toxicity (58). In a second hypofractionation study, patients with stage IA were treated to 42.8 GyE and stage IB to 60.0 GyE in four fractions. The local control was 98% for T1 and 80% for T2 tumors. No grade 4 or 5 lung toxicities were seen (59). Single fraction CIRT appears to be feasible for early stage NSCLC with LC of 95% at 5 years with doses above 48 GyE (60).
For locally advanced NSCLC, hypofractionated dose escalation above 76 GyE resulted in unacceptable toxicity, and the recommended dose was 72 GyE in 16 fractions (61). Further, a phase I study from the Gunma University Heavy Ion Medical Center treated unresectable stage III NSCLC with a hypofractionated region of 54 GyE in 4 GyE daily fractions. Of the 6 patients that were treated, the overall response rate was 100% with no dose limiting toxicity (62). One study using 72 GyE in 16 fractions had seven (out of 141 patients) cases of grade 4 toxicity, including mediastinal hemorrhage, radiation pneumonitis, or bronchial fistulas (63).
A diagnosis of interstitial lung disease presents a challenge to radiation oncologists, as radiation can cause an exacerbation of the underlying lung disease. Notably, CIRT was found to be low risk in patients with lung disease, with only two of 29 patients (6.9%) experiencing an exacerbation (64).
Gastrointestinal Tumors
Esophageal Cancer
CIRT has been used successfully in the treatment of squamous cell carcinoma of the esophagus. In a phase I/II trial, 31 patients with resectable disease received between 28.8 and 36.8 GyE in 8 fractions given 4 fractions per week. A 38.7% pathologic complete response rate and 41.9% clinical partial response rate were seen (65).
Hepatocellular Carcinoma (HCC)
In Japan, two protocols (9603 and 0004) attempted hypofractionated treatment for HCC. 52.8 GyE in 4 fractions was the recommended dose, with a 5-year LC of 90% (66). Recent studies have attempted further dose-escalation, with 48.0–60 GyE given in 4 treatments. All doses had favorable LC and survival rates. Notably, 5.7% had grade 3–4 toxicity with 1.7% developing radiation induced liver disease (67). Given the safety of the four fraction regimens, these were applied to 21 patients with HCC lesions >3 cm, with similar LC and toxicity rates (68). Further, treatment with 15 fractions in patients with cirrhosis did not increase the Child-Pugh score by more than 2 points (69). Patients treated with 52.8 GyE in 4 fractions had no difference in OS or LC based on if the tumor was within 2 cm of the porta hepatis (70). There was also no difference in toxicity. Given this, the hypofractionated course is felt to be safe for treating in close proximity to the portal system.
In a propensity score matched review, 477 patients were treated with either CIRT or transarterial chemoembolization for treatment naive, single tumor hepatocelluar carcinoma. Doses of 52.8–60 Gy in 4 fractions were used, with 60.0 Gy in 12 fractions close to the GI tract. Treatment with CIRT showed improved OS (88% vs. 58%), LC (80% vs. 26%), and PFS (51% vs. 15%) compared to TACE for single tumor HCC (71).
The PROMETHEUS-01 trial (NCT01167374) is a phase I study evaluating CIRT in advanced HCC without evidence of extrahepatic disease. Patients will be treated at increasing doses from 40 GyE in 4 fractions to 56 GyE in 4 fractions (72). A current phase II study at SPHIC is investigating the use of hypofractionated CIRT to a dose of 40 GyE in 5 fractions with GM-CSF in the treatment of HCC (NCT02946138).
Liver Metastases
Makishima et al. found a 3-year LC rate of 82% for single fraction treatment for colorectal cancer liver metastasis at doses above 53 GyE, compared to 28% at lower doses. In contrast to the above study, there were two cases of grade 3 liver toxicity at 53 GyE, with both cases occurring near the hepatic portal region. The authors conclude that single fraction therapy is safe up to 58 GyE if the central hepatic portal region can be avoided (73).
Cholangiocarcinoma
The Japan Carbon Ion Radiation Oncology Study Group (J-CROS) investigated the role of CIRT for 56 patients with intrahepatic (27 patients) and perihilar (29 patients) cholangiocarcinoma. No patients underwent resection. Most patients were treated to a dose of 76 GyE in 20 fractions, with a median survival of 23.8 months for intrahepatic cholangiocarcinoma and 12.6 months in perihilar disease. Notably, there was one case of grade 5 liver injury and one grade 3 bile duct stenosis (74).
Pancreatic Cancer
Shinoto et al. evaluated the safety and efficacy of short course, neoadjuvant irradiation with CIRT for potentially resectable pancreatic cancer. The dose given was escalated from 30 to 36.8 GyE in 8 fractions, with surgery performed up to 4 weeks after completion of radiation. Sixty-five percentage of the 28 patients developed distant disease, with no patients experiencing local recurrence. One patient developing acute grade 3 liver toxicity and one patient developing late grade 4 portal vein stenosis (75).
The J-CROS Study 1403 retrospectively analyzed 72 patients from three institutions with locally advanced pancreatic adenocarcinoma and treated with CIRT to a dose of 52.8 GyE or 55.2 GyE in 12 fractions. The median OS was 21.5 months. The 2-year local recurrence rate was 24%. Twenty-six percentage of patients experienced grade 3 or four hematologic toxicities with 3% grade 3 anorexia (76).
The PHOENIX-1 trial was a phase I study at HIT evaluating the CIRT using raster scanning in combination with weekly gemcitabine and adjuvant gemcitabine in the treatment of locally advanced pancreatic cancer, although the trial is currently on hold due to “administrative barriers.” The dose escalation study aimed to determine the safety of CIRT ranging from 42 GyE in 14 fractions to 54 GyE in 18 fractions (77).
A phase I trial at SPHIC is investigating the use of 50.4 GyE in 28 fractions followed by a carbon ion boost of doses from 12 to 18 GyE in 3 GyE per fraction (NCT03949933). The PIOPPO study, a phase II study of neoadjuvant FOLFIRINOX followed by 38.4 GyE in 8 fractions over 2 weeks followed by resection and adjuvant gemcitabine for patients with resectable or borderline resectable pancreatic cancer, is currently accruing (NCT03822936).
In Japan, 64 patients with unresectable pancreatic cancer were treated with 55.2 GyE in 12 fractions, with a median survival of 25.1 months and 2-year LC of 82%. Four patients had acute grade 3 toxicity (78).
The CIPHER study, sponsored by the University of Texas Southwestern Medical Center, is a phase III trial comparing IMRT with CIRT for unresectable pancreatic cancer. Both arms receive concurrent gemcitabine followed by adjuvant gemcitabine and nab-paclitaxel. Patients randomized to the CIRT are flown to centers with CIRT for treatment (NCT03536182).
Rectal Cancer
Patients with a rectal cancer recurrence following primary curative intent therapy were treated with a dose of 73.6 Gy RBE in 16 fractions as part of the GUNMA 0801 prospective study (79). The 3-year OS, LC, and PFS for the 28 patients were 92, 86, and 31%, respectively. Similarly, the JCROS experience found that patients treated to 70.4 GyE or 73.6 GyE in 16 fractions for recurrent rectal cancer had 5-year OS rates of 51% and LC of 88%. Three patients had grade 3 toxicity with no grade 4 or 5 toxicities (80).
The PANODRA-01 phase I-II study at HIT (NCT01528683) is a dose escalation trial in the setting of recurrent and inoperable rectal cancer. Increasing doses from 36 to 54 GyE given in 3 GyE fractions will be given. Patients previously received 20–60 Gy of photon radiotherapy as part of their primary treatment (81).
Genitourinary Tumors
Prostate Cancer
J-CROS 1501 PR was the first multi-institutional observation study (NIRS, Gunma, and the Ion Beam Therapy Center in Saga, Japan) analyzing outcomes of prostate cancer patients treated with CIRT. Fifty-six percentage were high risk, 31% intermediate risk, and 12% were low risk. The 5-year biochemical relapse free survival (bRFS) rates were 99, 100, and 100%, respectively (82). SPHIC investigated 64 patients with localized prostate cancer treated to a dose between 59.2 and 66 GyE in 16–24 fractions without nodal irradiation. Urinary irritation declined temporarily, with quality of life scores returning to baseline at 1-year. The rates of acute grade 1 and 2 genitourinary (GU) toxicity were 20.3 and 10.9%, respectively. Late grade 1 and grade 2 GU toxicity were 3.1 and 1.6%, respectively. Notably, there was a 0% rate of late GI toxicity (83).
Similarly, patients with high risk or very high risk prostate cancer who received CIRT with long term androgen deprivation therapy had a 10-year prostate cancer specific mortality rate of 4.3%. The 10-year incidence of grade 2 GU toxicity was 11.7%, with grade 3 GU toxicity occurring in 0.5% of patients (84).
In a recent paper analyzing patients from NIRS and the Osaka registry, Mohamad et al. determined the risk of secondary malignancy was lower for patients treated with CIRT compared to conventional photon therapy (HR 0.81) or surgery (HR 0.80) for localized prostate cancer (9). A phase II trial evaluating 45 Gy IMRT to the pelvic lymph nodes, prostate, and seminal vesicles followed by a carbon ion boost is currently opened in 2016 in Italy (NCT02672449). Carbon ion therapy is currently being investigated in a phase II trial in the setting of oligometastatic prostate cancer (NCT02935023).
Renal Cell Carcinoma
CIRT has been used with good efficacy and safety for the treatment of primary renal cell carcinoma. The NIRS experience reported on 19 patients treated with 12 or 16 fractions CIRT, with 5-year cancer specific survival (100%) and LC rates (94.1%) (85). A non-randomized phase I/II study determined that 72 GyE was well-tolerated, with no dose limiting toxicity. Cancer specific survival rates and LC were 100% with a median follow up of 43.1 months (86).
Sarcoma
Osteosarcoma
Osteosarcomas of the trunk are traditionally challenging to treat, as surgical resection can lead to significant morbidity. Given this, Matsunobu et al. retrospectively analyzed 78 patients with medically inoperable osteosarcoma of the trunk treated with CIRT to a median dose of 70.4 GyE in 16 fractions over 4 weeks. The 5-year LC and OS were 62 and 33%, respectively. Three patients required skin grafts, although there were no other severe late toxicities. Eight out of nine patients (89%) who were disease free for 5 years were able to ambulate with or without a walker (87).
Extremity Sarcoma
CIRT has also been studied in localized primary sarcomas of the extremities. A phase I/II study enrolled 17 patients with either primary or recurrent disease and were treated to 52.8, 64, or 70.4 GyE in 13–16 fractions. LC and OS at 5 years was 76 and 56%, respectively. One patient had a femoral fracture, with no other grade 3 or higher late reactions (88). In a dose escalation study, Kamada et al. found a high rate of grade 3 acute skin reactions (7/17 patients) with 73.6 GyE, and dose escalation was stopped at that time (89).
Sacral Chordoma
A total of 95 patients with medically inoperable sacral chordoma were treated in Japan between 1996 and 2007. The carbon ion dose ranged from 52.8 to 73.6 GyE, with a median dose of 70.4 GyE over 16 fractions. The OS at 5 years was 86% with a LC rate of 88%. Ninety-one percentage of patients were able to ambulate without aide. Of note, two patients required skin grafts and 15 had severe sciatic nerve complications requiring indefinite medications (90).
A phase II trial at HIT is currently investigating the use of hypofractionated irradiation with protons or CIRT after R2 resection for patients with sacrococcygeal chordoma (NCT01811394). The SACRO study is a phase III randomized trial investigating the use of surgery or definitive radiation therapy, including carbon ion radiotherapy, in the treatment of sacral chordoma (NCT02986516).
Cutaneous Tumors
Skin Cancer
In a Chinese series, 45 patients with squamous cell carcinoma (16 patients), basal cell carcinoma (12 patients), melanoma (8 patients), Bowen's disease (8 patients), or Paget's disease (2 patients) were treated with various dosages. Non-melanoma skin cancers were treated with 60–70 GyE, melanoma 61–75 GyE, Bowen's disease 60 GyE, and Paget's 42.5 GyE over 6–11 fractions. CIRT had favorable local control rates at 1 year, ranging from 80 to 90% for all histologies (91).
Keloids
CIRT has been successfully used as adjuvant therapy in the treatment of keloids. In a case series from China, 16 patients with keloids were treated postoperatively with 16 GyE in 8 fractions. A 95% success rate was achieved with a mean follow up of 29.7 months. There was no grade 3 or higher toxicities (92).
Breast Cancer
There is currently a lack of clinical data investigating the use of CIRT in breast cancer. To date, there are no large clinical trials using CIBT. In a phase I dose escalation study, Karasawa et al. analyzed 7 patients who underwent CIBT who then underwent tumor excision for pathologic evaluation. Three patients received 48 GyE, three received 52.8 GyE, and one received 60.0 GyE. All patients were treated in four fractions and were treated supine with cast and thermoplastic immobilization. Four patients had acute grade 1 skin toxicity, with no other reported acute toxicity. At 3 months, most patients experienced some pathologic response to treatment, although the authors concluded that 3 months was not sufficient to fully evaluate treatment response (93).
Dosimetrically, there is unlikely a significant difference in dose distribution between passive and active scanning CIRT, although there may be a slight advantage to passive scanning in some patients with unfavorable anatomy (94).
Gynecologic Cancer
Cervical Cancer
In a systematic review of eight clinical studies from NIRS, Wang et al. concluded that carbon ion radiotherapy is safe and effective in the treatment for gynecologic cancers. The authors found lower rates of local recurrence at doses higher than 70 GyE for cervical cancer (95).
A pooled analysis of protocols 9403 and 9702 evaluated 44 patients with stage IIIB and IVA disease between 1995 and 2000 receiving CIRT for locally advanced cervical carcinoma. Patients received 16 fractions to the whole pelvis followed by a boost of 8 fractions to a dose up to 72.0 GyE. The most severe GI toxicity occurred at doses above 60 GyE, and the authors concluded that the dose to the intestines should be limited to 60 GyE (96). In the NIRS protocol 9902, no patient treated with 72.0 GyE failed locally (97).
The NIRS protocol 0508 is a phase I/II study of prophylactic extended field (involving pelvic nodes, para-aortic nodes, ovaries, and uterus) CIRT for locally advanced squamous cell carcinoma of the cervix. Twenty-one of the 26 patients had acute grade 1 or 2 toxicity with no late grade 3 or higher toxicities reported (98). Given this, prophylactic extended field radiation was considered feasible and safe.
Protocol 1001 is a phase I/II study evaluating concurrent chemoradiation for locally advanced cervical carcinoma. Patients received weekly cisplatin at 40 mg/m2 and a CIRT to a dose of 74.4 GyE. Treatment was well-tolerated, with two patients developing grade 3–4 GI toxicities. In patients treated with the recommended dose of 74.4 Gy, the 2-year LC, PFS, and OS were 71, 56, and 88%, respectively (99).
CIRT has also been used in combination with a brachytherapy boost for the treatment of locally advanced cervical cancer, with no dose limiting toxicities. All patients received 36 GyE in 12 fractions to the whole pelvis followed by a local boost of 19.2 GyE in 4 fractions (100).
Endometrial Cancer
In a pooled analysis of protocols 9704 and 9404 from NIRS, the authors found that the 5-year LC and OS were 86 and 68%, respectively. Of note, inclusion criteria included inoperable and previously untreated stage I–III endometrial carcinoma without para-aortic nodal metastasis. Radiation was given to the whole pelvis with CIRT to a dose of 36.0 GyE in 12 fractions followed by a CIRT boost to a total dose of 62.4–74.4 GyE in 20 fractions with no brachytherapy boost. Notably, the GI tract dose was limited to no more than 60 GyE. No patients experienced grade 3 or higher acute or late toxicity (101).
Pediatric Cancer
Out of a group of 394 patients treated with CIRT in Germany between 1997 and 2007 for skull base tumors, 17 patients were under the age of 21. The primary tumor was treated in 14 patients, and three patients had recurrent tumors. Patients were treated to a dose of 60 GyE using the raster scan technique. With a median follow up of 49 months, there were no severe side effects. Only one patient experienced tumor progression from a chordoma after 5 years (21, 102).
NIRS retrospectively reviewed 26 patients from 11 to 20 years old with inoperable osteosarcoma of the trunk. A median of 70.4 GyE in 16 fractions was delivered. A promising LC was also reported, at 62.9% at 5 years, with four patients developing grade 3 or higher late toxicities. Only one patient was unable to ambulate after treatment (103).
CIRT may provide a particularly important benefit in the pediatric population. As noted in the setting of prostate cancer, CIRT appears to have a lower rate of second malignancies compared to photons (9). Furthermore, the measured dose ambient dose equivalent for passive scatter carbon beams is lower than passive scattering beams for protons (104). This property, in combination with a lower neutron dose in active scanning beams compared with IMRT or passive scattering CIRT, may contribute to a lower secondary malignancy rate with CIRT (104–106).
Discussion
CIRT represents a promising new treatment technique, with early data suggesting that it is both safe and effective for a variety of tumors. Caution should be taken in interpreting the data; however, as there is a high degree of heterogeneity with treatments in the trials, especially compared to photon therapy. For instance, in glioblastoma, the standard of care is typically radiation therapy followed by temozolomide as per the Stupp trial, with a median survival of 14.6 months (28). There is no direct comparison for CIRT, where one trial used CIRT as a boost following 50 Gy with photons with concurrent nimustine, and the second trial retrospectively reviewed patients receiving CIRT alone, with a median survival of 18 months (24, 30, 31). This makes direct comparison difficult, although more recent trials are attempting to address these deficiencies.
Compounding this issue, many of the dose regimens are hypofractionated or unconventional, leading to a difficult direct comparison. As many of the treatment centers use only fixed-beam gantries with long treatment times, using hypofractionated regimens helps overcome logistical challenges present to delivery of radiation. An example of this is in esophageal cancer, with the Japanese treating to a dose of 52.8–55.2 GyE in 12 fractions, compared to 41.4 Gy in 23 fractions as part of the CROSS trial (76, 107). This pattern of hypofractionation is seen across disease sites, and makes direct comparison of fractionation schemes challenging.
One of the major concerns regarding CIRT, and heavy ion radiotherapy in general, is dose uncertainty. With the Bragg-Peak and sharp lateral penumbra, there is a higher susceptibility to intrafraction motion compared to photon-based therapy, where the effect of motion can be mitigated due to the “dose bath.” Further, there is higher likelihood that the Bragg-Peak be in normal tissues with intrafraction motion (108). Additionally, CIRT exhibits a fragmentation tail, where nuclear fragments contribute to dose distal to the target. This creates uncertainty to tissues distal to the target to a greater degree than proton therapy, which will be an important dosimetric principle to consider.
The primary goal of CIRT is to increase the therapeutic index and reduce normal tissue toxicity while dose escalating to the tumor. The exact radiobiologic effects on normal tissues remain unclear. Additionally, there are not well-established dose constraints for normal tissues, leading to uncertainty regarding the toxicity rates at a given dose. Further preclinical study is needed to establish and standardize normal tissue dose constraints prior to widespread use.
Uncertainty remains with the precise calculation of dose from RBE. Currently, there are three models for calculating RBE, with the local effect model being used in Europe, and the remaining two models (Microdosimetric Kinetic Model and mixed-beam model) used in Japan (109). Given this, there are no current standard doses for CIRT, as is the case of the CIPHER trial, where there are two fractionation regimens as part of the study based on if the patient is treated in Japan or Europe. A consensus on the definition and calculation of RBE for CIRT is necessary prior to more widespread adoption.
Another potential benefit of CIRT may be in combination with immunotherapy. High LET radiation has been shown to have an increased immunogenicity of radiation-induced cell death compared to photon radiation through a variety of mechanisms, thus leading to a hypothesized advantage in the setting of combined immunotherapy (110). High LET radiation, such as CIRT, has been shown to induce immune cell death at lower doses compared to photon irradiation (111). In addition, higher doses per fraction have been shown to increase the immune response following radiation, and most patients treated with CIRT are treated with a hypofractionated regimen (110, 112). The reader is referred to the review articles by Helm et al. and Durante et al. for a more detailed discussion of the exact mechanisms of heavy ion therapy and combination immunotherapy (110, 113). Although, there is currently a lack of clinical data regarding CIRT in combination with immunotherapies, this will likely be an important frontier, as more patients are treated with immunotherapy. Further pre-clinical and clinical trials will be necessary to elicit the potential benefit of CIRT in combination with immunotherapy.
Despite the promising early results, the cost of developing and maintaining a heavy-ion center has been prohibitive for adoption in United States. For instance, Pompos et al. estimates that the cost of developing a center with capacity of 1,000 patients per year is roughly twice as expensive as a proton center of the same size (108). The estimated cost for multiple room centers treating with multiple ions is ~$200 million (20). Much of this cost comes from the complexity of the system, such as the need for a synchrotron and additional shielding (108). Additionally, the cost-effectiveness of this expensive treatment remains unknown, especially in the United States current payment model. CIRT has been found to be a highly cost-effective option in Germany, specifically for the treatment of skull base chordoma (114, 115). Although performing a cost-effectiveness analysis is not currently possible using US data, the data from Germany can be extrapolated, and CIRT will likely be a cost-effective treatment option for many disease sites in the US.
Currently, there are not centers in the United States currently treating with CIRT, although interest in CIRT is increasing. Internationally, there are currently five centers planned or under construction (China, France, Japan, South Korea, and Taiwan) (4). In the US, UT Southwestern has recently opened the CIPHER trial, with one of the randomizations receiving CIRT, although patients are transported overseas for treatment. Furthermore, although not extensively studied, there is likely a high medical need for CIRT in the United States, similar to in Korea or Iran (116, 117). Given the promising early clinical data as well as the advantages of heavy ion therapy, our institution recently announced the intent to construct a heavy ion facility in Jacksonville, Florida. By opening the heavy ion facility, we hope to further research the potential benefits of CIRT.
Conclusions
Taking advantage of the unique radiobiological and physical properties, CIRT is a promising treatment technique in the treatment of a variety of malignancies. Extensive further prospective trials are needed to define the role of carbon ion therapy in clinical practice.
Author Contributions
TM and DT: conception and design. TM, DT, and AM: manuscript preparation. TM, DT, AM, SK, CB, and DS: editing and review.
Funding
This publication was made possible through the support of the Eveleigh Family Career Development Award for Cancer Research at Mayo Clinic in Florida.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rackwitz T, Debus J. Clinical applications of proton and carbon ion therapy. Semin Oncol. (2019) 46:226–32. doi: 10.1053/j.seminoncol.2019.07.005
2. Kamada T. Twenty years of carbon ion radiation therapy at the national institute of radiological sciences: accomplishments and prospects. Int J Particle Ther. (2015) 2:459–63. doi: 10.14338/IJPT-15-00030.1
3. Lazar AA, Schulte R, Faddegon B, Blakely EA, Roach M III. Clinical trials involving carbon-ion radiation therapy and the path forward. Cancer. (2018) 124:4467–76. doi: 10.1002/cncr.31662
4. Particle Therapy Co-Operative Group. Particle Therapy Facilities in Clinical Operation. (2019). Available online at: https://www.ptcog.ch/index.php/facilities-in-operation (accessed January 25, 2020)
5. Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M, et al. Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol. (2015) 16:e93–100. doi: 10.1016/S1470-2045(14)70412-7
6. Mohamad O, Sishc BJ, Saha J, Pompos A, Rahimi A, Story MD, et al. Carbon Ion radiotherapy: a review of clinical experiences and preclinical research, with an emphasis on DNA damage/repair. Cancers. (2017) 9:E66. doi: 10.3390/cancers9060066
7. Gianfaldoni S, Gianfaldoni R, Wollina U, Lotti J, Tchernev G, Lotti T. An overview on radiotherapy: from its history to its current applications in dermatology. Open Access Maced J Med Sci. (2017) 5:521–5. doi: 10.3889/oamjms.2017.122
8. Eley JG, Friedrich T, Homann KL, Howell RM, Scholz M, Durante M, et al. Comparative risk predictions of second cancers after carbon-ion therapy versus proton therapy. Int J Radiat Oncol Biol Phys. (2016) 95:279–86. doi: 10.1016/j.ijrobp.2016.02.032
9. Mohamad O, Tabuchi T, Nitta Y, Nomoto A, Sato A, Kasuya G, et al. Risk of subsequent primary cancers after carbon ion radiotherapy, photon radiotherapy, or surgery for localised prostate cancer: a propensity score-weighted, retrospective, cohort study. Lancet Oncol. (2019) 20:674–85. doi: 10.1016/S1470-2045(18)30931-8
10. Durante M, Debus J. Heavy charged particles: does improved precision and higher biological effectiveness translate to better outcome in patients? Semin Radiat Oncol. (2018) 28:160–7. doi: 10.1016/j.semradonc.2017.11.004
11. Tsujii H, Kamada T, Noda Kj, Tsuji H, Karasawa K. Carbon-Ion Radiotherapy: Principles, Practices, and Treatment Planning. Vol. xii. Tokyo: Springer (2014). p. 312. doi: 10.1007/978-4-431-54457-9
12. Schlaff CD, Krauze A, Belard A, O'Connell JJ, Camphausen KA. Bringing the heavy: carbon ion therapy in the radiobiological and clinical context. Radiat Oncol. (2014) 9:88. doi: 10.1186/1748-717X-9-88
13. Combs SE, Ellerbrock M, Haberer T, Habermehl D, Hoess A, Jakel O, et al. Heidelberg ion therapy center (HIT): initial clinical experience in the first 80 patients. Acta Oncol. (2010) 49:1132–40. doi: 10.3109/0284186X.2010.498432
14. Combs SE, Kessel KA, Herfarth K, Jensen A, Oertel S, Blattmann C, et al. Treatment of pediatric patients and young adults with particle therapy at the Heidelberg Ion Therapy Center (HIT): establishment of workflow and initial clinical data. Radiat Oncol. (2012) 7:170. doi: 10.1186/1748-717X-7-170
15. Goetz G, Mitic M, Mittermayr T, Wild C. Health technology assessment of carbon-ion beam radiotherapy: a systematic review of clinical effectiveness and safety for 54 oncological indications in 12 tumour regions. Anticancer Res. (2019) 39:1635–50. doi: 10.21873/anticanres.13269
16. Mohamad O, Makishima H, Kamada T. Evolution of carbon ion radiotherapy at the national institute of radiological sciences in Japan. Cancers. (2018) 10:E66. doi: 10.3390/cancers10030066
17. Schulz-Ertner D, Nikoghosyan A, Thilmann C, Haberer T, Jakel O, Karger C, et al. Results of carbon ion radiotherapy in 152 patients. Int J Radiat Oncol Biol Phys. (2004) 58:631–40. doi: 10.1016/j.ijrobp.2003.09.041
18. Wang X, Zhang Q, Zhang H, Gao J, Ran J, Li Q., editors. The Preliminary Results of Carbon Ion Radiotherapy in 60 Patients. ESTRO 35. Turin: Radiotherapy and Oncology (2016).
19. Combs SE, Debus J. Treatment with heavy charged particles: systematic review of clinical data and current clinical (comparative) trials. Acta Oncol. (2013) 52:1272–86. doi: 10.3109/0284186X.2013.818254
20. Durante M, Orecchia R, Loeffler JS. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol. (2017) 14:483–95. doi: 10.1038/nrclinonc.2017.30
21. Combs SE, Kessel K, Habermehl D, Haberer T, Jakel O, Debus J. Proton and carbon ion radiotherapy for primary brain tumors and tumors of the skull base. Acta Oncol. (2013) 52:1504–9. doi: 10.3109/0284186X.2013.818255
22. Rieken S, Habermehl D, Haberer T, Jaekel O, Debus J, Combs SE. Proton and carbon ion radiotherapy for primary brain tumors delivered with active raster scanning at the Heidelberg Ion Therapy Center (HIT): early treatment results and study concepts. Radiat Oncol. (2012) 7:41. doi: 10.1186/1748-717X-7-41
23. Rieken S, Habermehl D, Nikoghosyan A, Jensen A, Haberer T, Jakel O, et al. Assessment of early toxicity and response in patients treated with proton and carbon ion therapy at the Heidelberg ion therapy center using the raster scanning technique. Int J Radiat Oncol Biol Phys. (2011) 81:e793–801. doi: 10.1016/j.ijrobp.2010.12.018
24. Mizoe JE, Tsujii H, Hasegawa A, Yanagi T, Takagi R, Kamada T, et al. Phase I/II clinical trial of carbon ion radiotherapy for malignant gliomas: combined X-ray radiotherapy, chemotherapy, and carbon ion radiotherapy. Int J Radiat Oncol Biol Phys. (2007) 69:390–6. doi: 10.1016/j.ijrobp.2007.03.003
25. Combs SE, Hartmann C, Nikoghosyan A, Jakel O, Karger CP, Haberer T, et al. Carbon ion radiation therapy for high-risk meningiomas. Radiother Oncol. (2010) 95:54–9. doi: 10.1016/j.radonc.2009.12.029
26. Adeberg S, Harrabi SB, Verma V, Bernhardt D, Grau N, Debus J, et al. Treatment of meningioma and glioma with protons and carbon ions. Radiat Oncol. (2017) 12:193. doi: 10.1186/s13014-017-0924-7
27. Combs SE, Edler L, Burkholder I, Rieken S, Habermehl D, Jakel O, et al. Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE trial. BMC Cancer. (2010) 10:615. doi: 10.1186/1471-2407-10-615
28. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. (2005) 352:987–96. doi: 10.1056/NEJMoa043330
29. Maucort-Boulch D, Baron MH, Pommier P, Weber DC, Mizoe JE, Rochat J, et al. Rationale for carbon ion therapy in high-grade glioma based on a review and a meta-analysis of neutron beam trials. Cancer Radiother. (2010) 14:34–41. doi: 10.1016/j.canrad.2009.08.141
30. Hadziahmetovic M, Shirai K, Chakravarti A. Recent advancements in multimodality treatment of gliomas. Future Oncol. (2011) 7:1169–83. doi: 10.2217/fon.11.102
31. Combs SE, Bruckner T, Mizoe JE, Kamada T, Tsujii H, Kieser M, et al. Comparison of carbon ion radiotherapy to photon radiation alone or in combination with temozolomide in patients with high-grade gliomas: explorative hypothesis-generating retrospective analysis. Radiother Oncol. (2013) 108:132–5. doi: 10.1016/j.radonc.2013.06.026
32. Combs SE, Kieser M, Rieken S, Habermehl D, Jakel O, Haberer T, et al. Randomized phase II study evaluating a carbon ion boost applied after combined radiochemotherapy with temozolomide versus a proton boost after radiochemotherapy with temozolomide in patients with primary glioblastoma: the CLEOPATRA trial. BMC Cancer. (2010) 10:478. doi: 10.1186/1471-2407-10-478
33. Combs SE, Burkholder I, Edler L, Rieken S, Habermehl D, Jakel O, et al. Randomised phase I/II study to evaluate carbon ion radiotherapy versus fractionated stereotactic radiotherapy in patients with recurrent or progressive gliomas: the CINDERELLA trial. BMC Cancer. (2010) 10:533. doi: 10.1186/1471-2407-10-533
34. Mizoe JE, Hasegawa A, Takagi R, Bessho H, Onda T, Tsujii H. Carbon ion radiotherapy for skull base chordoma. Skull Base. (2009) 19:219–24. doi: 10.1055/s-0028-1114295
35. Schulz-Ertner D, Karger CP, Feuerhake A, Nikoghosyan A, Combs SE, Jakel O, et al. Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys. (2007) 68:449–57. doi: 10.1016/j.ijrobp.2006.12.059
36. Takahashi S, Kawase T, Yoshida K, Hasegawa A, Mizoe JE. Skull base chordomas: efficacy of surgery followed by carbon ion radiotherapy. Acta Neurochir. (2009) 151:759–69. doi: 10.1007/s00701-009-0383-5
37. Schulz-Ertner D, Nikoghosyan A, Hof H, Didinger B, Combs SE, Jakel O, et al. Carbon ion radiotherapy of skull base chondrosarcomas. Int J Radiat Oncol Biol Phys. (2007) 67:171–7. doi: 10.1016/j.ijrobp.2006.08.027
38. Nikoghosyan AV, Rauch G, Munter MW, Jensen AD, Combs SE, Kieser M, et al. Randomised trial of proton vs. carbon ion radiation therapy in patients with low and intermediate grade chondrosarcoma of the skull base, clinical phase III study. BMC Cancer. (2010) 10:606. doi: 10.1186/1471-2407-10-606
39. Tsuji H, Ishikawa H, Yanagi T, Hirasawa N, Kamada T, Mizoe JE, et al. Carbon-ion radiotherapy for locally advanced or unfavorably located choroidal melanoma: a Phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys. (2007) 67:857–62. doi: 10.1016/j.ijrobp.2006.09.022
40. Akbaba S, Lang K, Held T, Herfarth K, Rieber J, Plinkert P, et al. Carbon-ion radiotherapy in accelerated hypofractionated active raster-scanning technique for malignant lacrimal gland tumors: feasibility and safety. Cancer Manag Res. (2019) 11:1155–66. doi: 10.2147/CMAR.S190051
41. Hayashi K, Koto M, Ikawa H, Ogawa K, Kamada T. Efficacy and safety of carbon-ion radiotherapy for lacrimal gland carcinomas with extraorbital extension: a retrospective cohort study. Oncotarget. (2018) 9:12932–40. doi: 10.18632/oncotarget.24390
42. Schulz-Ertner D, Nikoghosyan A, Jakel O, Haberer T, Kraft G, Scholz M, et al. Feasibility and toxicity of combined photon and carbon ion radiotherapy for locally advanced adenoid cystic carcinomas. Int J Radiat Oncol Biol Phys. (2003) 56:391–8. doi: 10.1016/S0360-3016(02)04511-X
43. Hayashi K, Koto M, Demizu Y, Saitoh JI, Suefuji H, Okimoto T, et al. A retrospective multicenter study of carbon-ion radiotherapy for major salivary gland carcinomas: subanalysis of J-CROS 1402 HN. Cancer Sci. (2018) 109:1576–82. doi: 10.1111/cas.13558
44. Shirai K, Saitoh JI, Musha A, Abe T, Kobayashi D, Takahashi T, et al. Prospective observational study of carbon-ion radiotherapy for non-squamous cell carcinoma of the head and neck. Cancer Sci. (2017) 108:2039–44. doi: 10.1111/cas.13325
45. Koto M, Hasegawa A, Takagi R, Ikawa H, Naganawa K, Mizoe JE, et al. Definitive carbon-ion radiotherapy for locally advanced parotid gland carcinomas. Head Neck. (2017) 39:724–9. doi: 10.1002/hed.24671
46. Akbaba S, Held T, Lang K, Forster T, Federspil P, Herfarth K, et al. Bimodal radiotherapy with active raster-scanning carbon ion radiotherapy and intensity-modulated radiotherapy in high-risk nasopharyngeal carcinoma results in excellent local control. Cancers. (2019) 11:E379. doi: 10.3390/cancers11030379
47. Akbaba S, Ahmed D, Lang K, Held T, Mattke M, Hoerner-Rieber J, et al. Results of a combination treatment with intensity modulated radiotherapy and active raster-scanning carbon ion boost for adenoid cystic carcinoma of the minor salivary glands of the nasopharynx. Oral Oncol. (2019) 91:39–46. doi: 10.1016/j.oraloncology.2019.02.019
48. Hu J, Bao C, Gao J, Guan X, Hu W, Yang J, et al. Salvage treatment using carbon ion radiation in patients with locoregionally recurrent nasopharyngeal carcinoma: initial results. Cancer. (2018) 124:2427–37. doi: 10.1002/cncr.31318
49. Kong L, Gao J, Hu J, Hu W, Guan X, Lu R, et al. Phase I/II trial evaluating concurrent carbon-ion radiotherapy plus chemotherapy for salvage treatment of locally recurrent nasopharyngeal carcinoma. Chin J Cancer. (2016) 35:101. doi: 10.1186/s40880-016-0164-5
50. Koto M, Hasegawa A, Takagi R, Sasahara G, Ikawa H, Mizoe JE, et al. Feasibility of carbon ion radiotherapy for locally advanced sinonasal adenocarcinoma. Radiother Oncol. (2014) 113:60–5. doi: 10.1016/j.radonc.2014.09.009
51. Hayashi K, Koto M, Demizu Y, Saitoh JI, Suefuji H, Okimoto T, et al. A retrospective multicenter study of carbon-ion radiotherapy for external auditory canal and middle ear carcinomas. Cancer Med. (2019) 8:51–7. doi: 10.1002/cam4.1830
52. Koto M, Hasegawa A, Takagi R, Sasahara G, Ikawa H, Mizoe JE, et al. Carbon ion radiotherapy for locally advanced squamous cell carcinoma of the external auditory canal and middle ear. Head Neck. (2016) 38:512–6. doi: 10.1002/hed.23905
53. Ikawa H, Koto M, Demizu Y, Saitoh JI, Suefuji H, Okimoto T, et al. Multicenter study of carbon-ion radiation therapy for nonsquamous cell carcinomas of the oral cavity. Cancer Med. (2019) 8:5482–91. doi: 10.1002/cam4.2408
54. Yang J, Gao J, Wu X, Hu J, Hu W, Kong L, et al. Salvage carbon ion radiation therapy for locally recurrent or radiation-induced second primary sarcoma of the head and neck. J Cancer. (2018) 9:2215–23. doi: 10.7150/jca.24313
55. Hayashi K, Koto M, Ikawa H, Hagiwara Y, Tsuji H, Ogawa K, et al. Feasibility of Re-irradiation using carbon ions for recurrent head and neck malignancies after carbon-ion radiotherapy. Radiother Oncol. (2019) 136:148–53. doi: 10.1016/j.radonc.2019.04.007
56. Kubo N, Saitoh JI, Shimada H, Shirai K, Kawamura H, Ohno T, et al. Dosimetric comparison of carbon ion and X-ray radiotherapy for Stage IIIA non-small cell lung cancer. J Radiat Res. (2016) 57:548–54. doi: 10.1093/jrr/rrw041
57. Miyamoto T, Yamamoto N, Nishimura H, Koto M, Tsujii H, Mizoe JE, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer. Radiother Oncol. (2003) 66:127–40. doi: 10.1016/S0167-8140(02)00367-5
58. Miyamoto T, Baba M, Yamamoto N, Koto M, Sugawara T, Yashiro T, et al. Curative treatment of Stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys. (2007) 67:750–8. doi: 10.1016/j.ijrobp.2006.10.006
59. Miyamoto T, Baba M, Sugane T, Nakajima M, Yashiro T, Kagei K, et al. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol. (2007) 2:916–26. doi: 10.1097/JTO.0b013e3181560a68
60. Yamamoto N, Miyamoto T, Nakajima M, Karube M, Hayashi K, Tsuji H, et al. A dose escalation clinical trial of single-fraction carbon ion radiotherapy for peripheral stage i non-small cell lung cancer. J Thorac Oncol. (2017) 12:673–80. doi: 10.1016/j.jtho.2016.12.012
61. Takahashi W, Nakajima M, Yamamoto N, Yamashita H, Nakagawa K, Miyamoto T, et al. A prospective nonrandomized phase I/II study of carbon ion radiotherapy in a favorable subset of locally advanced non-small cell lung cancer (NSCLC). Cancer. (2015) 121:1321–7. doi: 10.1002/cncr.29195
62. Saitoh JI, Shirai K, Abe T, Kubo N, Ebara T, Ohno T, et al. A phase i study of hypofractionated carbon-ion radiotherapy for stage III non-small cell lung cancer. Anticancer Res. (2018) 38:885–91. doi: 10.21873/anticanres.12298
63. Hayashi K, Yamamoto N, Nakajima M, Nomoto A, Tsuji H, Ogawa K, et al. Clinical outcomes of carbon-ion radiotherapy for locally advanced non-small-cell lung cancer. Cancer Sci. (2019) 110:734–41. doi: 10.1111/cas.13890
64. Nakajima M, Yamamoto N, Hayashi K, Karube M, Ebner DK, Takahashi W, et al. Carbon-ion radiotherapy for non-small cell lung cancer with interstitial lung disease: a retrospective analysis. Radiat Oncol. (2017) 12:144. doi: 10.1186/s13014-017-0881-1
65. Akutsu Y, Yasuda S, Nagata M, Izumi Y, Okazumi S, Shimada H, et al. A phase I/II clinical trial of preoperative short-course carbon-ion radiotherapy for patients with squamous cell carcinoma of the esophagus. J Surg Oncol. (2012) 105:750–5. doi: 10.1002/jso.22127
66. Kasuya G, Kato H, Yasuda S, Tsuji H, Yamada S, Haruyama Y, et al. Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: combined analyses of 2 prospective trials. Cancer. (2017) 123:3955–65. doi: 10.1002/cncr.30816
67. Shibuya K, Ohno T, Terashima K, Toyama S, Yasuda S, Tsuji H, et al. Short-course carbon-ion radiotherapy for hepatocellular carcinoma: a multi-institutional retrospective study. Liver Int. (2018) 38:2239–47. doi: 10.1111/liv.13969
68. Shibuya K, Ohno T, Katoh H, Okamoto M, Shiba S, Koyama Y, et al. A feasibility study of high-dose hypofractionated carbon ion radiation therapy using four fractions for localized hepatocellular carcinoma measuring 3 cm or larger. Radiother Oncol. (2019) 132:230–5. doi: 10.1016/j.radonc.2018.10.009
69. Kato H, Tsujii H, Miyamoto T, Mizoe JE, Kamada T, Tsuji H, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. (2004) 59:1468–76. doi: 10.1016/j.ijrobp.2004.01.032
70. Imada H, Kato H, Yasuda S, Yamada S, Yanagi T, Kishimoto R, et al. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother Oncol. (2010) 96:231–5. doi: 10.1016/j.radonc.2010.05.019
71. Shiba S, Shibuya K, Katoh H, Kaminuma T, Miyazaki M, Kakizaki S, et al. A comparison of carbon ion radiotherapy and transarterial chemoembolization treatment outcomes for single hepatocellular carcinoma: a propensity score matching study. Radiat Oncol. (2019) 14:137. doi: 10.1186/s13014-019-1347-4
72. Combs SE, Habermehl D, Ganten T, Schmidt J, Edler L, Burkholder I, et al. Phase i study evaluating the treatment of patients with hepatocellular carcinoma (HCC) with carbon ion radiotherapy: the PROMETHEUS-01 trial. BMC Cancer. (2011) 11:67. doi: 10.1186/1471-2407-11-67
73. Makishima H, Yasuda S, Isozaki Y, Kasuya G, Okada N, Miyazaki M, et al. Single fraction carbon ion radiotherapy for colorectal cancer liver metastasis: a dose escalation study. Cancer Sci. (2019) 110:303–9. doi: 10.1111/cas.13872
74. Kasuya G, Terashima K, Shibuya K, Toyama S, Ebner DK, Tsuji H, et al. Carbon-ion radiotherapy for cholangiocarcinoma: a multi-institutional study by and the Japan carbon-ion radiation oncology study group (J-CROS). Oncotarget. (2019) 10:4369–79. doi: 10.18632/oncotarget.27028
75. Shinoto M, Yamada S, Yasuda S, Imada H, Shioyama Y, Honda H, et al. Phase 1 trial of preoperative, short-course carbon-ion radiotherapy for patients with resectable pancreatic cancer. Cancer. (2013) 119:45–51. doi: 10.1002/cncr.27723
76. Kawashiro S, Yamada S, Okamoto M, Ohno T, Nakano T, Shinoto M, et al. Multi-institutional Study of carbon-ion radiotherapy for locally advanced pancreatic cancer: japan carbon-ion radiation oncology study group (J-CROS) study 1403 pancreas. Int J Radiat Oncol Biol Phys. (2018) 101:1212–21. doi: 10.1016/j.ijrobp.2018.04.057
77. Combs SE, Habermehl D, Kieser M, Dreher C, Werner J, Haselmann R, et al. Phase I study evaluating the treatment of patients with locally advanced pancreatic cancer with carbon ion radiotherapy: the PHOENIX-01 trial. BMC Cancer. (2013) 13:419. doi: 10.1186/1471-2407-13-419
78. Shinoto M, Terashima K, Suefuji H, Matsunobu A, Toyama S, Fukunishi K, et al. A single institutional experience of combined carbon-ion radiotherapy and chemotherapy for unresectable locally advanced pancreatic cancer. Radiother Oncol. (2018) 129:333–9. doi: 10.1016/j.radonc.2018.08.026
79. Shiba S, Okamoto M, Kiyohara H, Ohno T, Kaminuma T, Asao T, et al. Prospective observational study of high-dose carbon-ion radiotherapy for pelvic recurrence of rectal cancer (GUNMA 0801). Front Oncol. (2019) 9:702. doi: 10.3389/fonc.2019.00702
80. Shinoto M, Yamada S, Okamoto M, Shioyama Y, Ohno T, Nakano T, et al. Carbon-ion radiotherapy for locally recurrent rectal cancer: Japan carbon-ion radiation oncology study group (J-CROS) study 1404 rectum. Radiother Oncol. (2019) 132:236–40. doi: 10.1016/j.radonc.2018.10.007
81. Combs SE, Kieser M, Habermehl D, Weitz J, Jager D, Fossati P, et al. Phase I/II trial evaluating carbon ion radiotherapy for the treatment of recurrent rectal cancer: the PANDORA-01 trial. BMC Cancer. (2012) 12:137. doi: 10.1186/1471-2407-12-137
82. Nomiya T, Tsuji H, Kawamura H, Ohno T, Toyama S, Shioyama Y, et al. A multi-institutional analysis of prospective studies of carbon ion radiotherapy for prostate cancer: a report from the Japan carbon ion radiation oncology study group (J-CROS). Radiother Oncol. (2016) 121:288–93. doi: 10.1016/j.radonc.2016.10.009
83. Zhang Y, Li P, Yu Q, Wu S, Chen X, Zhang Q, et al. Preliminary exploration of clinical factors affecting acute toxicity and quality of life after carbon ion therapy for prostate cancer. Radiat Oncol. (2019) 14:94. doi: 10.1186/s13014-019-1303-3
84. Kasuya G, Ishikawa H, Tsuji H, Haruyama Y, Kobashi G, Ebner DK, et al. Cancer-specific mortality of high-risk prostate cancer after carbon-ion radiotherapy plus long-term androgen deprivation therapy. Cancer Sci. (2017) 108:2422–9. doi: 10.1111/cas.13402
85. Kasuya G, Tsuji H, Nomiya T, Makishima H, Haruyama Y, Kobashi G, et al. Updated long-term outcomes after carbon-ion radiotherapy for primary renal cell carcinoma. Cancer Sci. (2018) 109:2873–80. doi: 10.1111/cas.13727
86. Kasuya G, Tsuji H, Nomiya T, Makishima H, Haruyama Y, Kobashi G, et al. Prospective clinical trial of 12-fraction carbon-ion radiotherapy for primary renal cell carcinoma. Oncotarget. (2019) 10:76–81. doi: 10.18632/oncotarget.26539
87. Matsunobu A, Imai R, Kamada T, Imaizumi T, Tsuji H, Tsujii H, et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer. (2012) 118:4555–63. doi: 10.1002/cncr.27451
88. Sugahara S, Kamada T, Imai R, Tsuji H, Kameda N, Okada T, et al. Carbon ion radiotherapy for localized primary sarcoma of the extremities: results of a phase I/II trial. Radiother Oncol. (2012) 105:226–31. doi: 10.1016/j.radonc.2012.09.010
89. Kamada T, Tsujii H, Tsuji H, Yanagi T, Mizoe JE, Miyamoto T, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. (2002) 20:4466–71. doi: 10.1200/JCO.2002.10.050
90. Imai R, Kamada T, Sugahara S, Tsuji H, Tsujii H. Carbon ion radiotherapy for sacral chordoma. Br J Radiol. (2011) 84 Spec No 1:S48–54. doi: 10.1259/bjr/13783281
91. Zhang H, Li S, Wang XH, Li Q, Wei SH, Gao LY, et al. Results of carbon ion radiotherapy for skin carcinomas in 45 patients. Br J Dermatol. (2012) 166:1100–6. doi: 10.1111/j.1365-2133.2011.10764.x
92. Chen Y, Dong F, Wang X, Xue J, Zhang H, Gao L, et al. Postoperative carbon ion radiotherapy for keloids: a preliminary report of 16 cases and review of the literature. Wounds. (2014) 26:264–72.
93. Karasawa K, Omatsu T, Arakawa A, Yamamoto N, Ishikawa T, Saito M, et al. A Phase I clinical trial of carbon ion radiotherapy for Stage I breast cancer: clinical and pathological evaluation. J Radiat Res. (2019) 60:342–7. doi: 10.1093/jrr/rry113
94. Matsubara H, Karasawa K, Furuichi W, Wakaisami M, Shiba S, Wakatsuki M, et al. Comparison of passive and scanning irradiation methods for carbon-ion radiotherapy for breast cancer. J Radiat Res. (2018) 59:625–31. doi: 10.1093/jrr/rry052
95. Wang L, Wang X, Zhang Q, Ran J, Geng Y, Feng S, et al. Is there a role for carbon therapy in the treatment of gynecological carcinomas? A systematic review. Future Oncol. (2019). 15:3081–95. doi: 10.2217/fon-2019-0187
96. Kato S, Ohno T, Tsujii H, Nakano T, Mizoe JE, Kamada T, et al. Dose escalation study of carbon ion radiotherapy for locally advanced carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. (2006) 65:388–97. doi: 10.1016/j.ijrobp.2005.12.050
97. Wakatsuki M, Kato S, Ohno T, Karasawa K, Ando K, Kiyohara H, et al. Dose-escalation study of carbon ion radiotherapy for locally advanced squamous cell carcinoma of the uterine cervix (9902). Gynecol Oncol. (2014) 132:87–92. doi: 10.1016/j.ygyno.2013.10.021
98. Wakatsuki M, Kato S, Kiyohara H, Ohno T, Karasawa K, Tamaki T, et al. Clinical trial of prophylactic extended-field carbon-ion radiotherapy for locally advanced uterine cervical cancer (protocol 0508). PLoS ONE. (2015) 10:e0127587. doi: 10.1371/journal.pone.0127587
99. Okonogi N, Wakatsuki M, Kato S, Karasawa K, Kiyohara H, Shiba S, et al. Clinical outcomes of carbon ion radiotherapy with concurrent chemotherapy for locally advanced uterine cervical adenocarcinoma in a phase 1/2 clinical trial (Protocol 1001). Cancer Med. (2018) 7:351–9. doi: 10.1002/cam4.1305
100. Ohno T, Noda SE, Murata K, Yoshimoto Y, Okonogi N, Ando K, et al. Phase I study of carbon ion radiotherapy and image-guided brachytherapy for locally advanced cervical cancer. Cancers. (2018) 10:338. doi: 10.3390/cancers10090338
101. Irie D, Okonogi N, Wakatsuki M, Kato S, Ohno T, Karasawa K, et al. Carbon-ion radiotherapy for inoperable endometrial carcinoma. J Radiat Res. (2018) 59:309–15. doi: 10.1093/jrr/rry003
102. Combs SE, Nikoghosyan A, Jaekel O, Karger CP, Haberer T, Munter MW, et al. Carbon ion radiotherapy for pediatric patients and young adults treated for tumors of the skull base. Cancer. (2009) 115:1348–55. doi: 10.1002/cncr.24153
103. Mohamad O, Imai R, Kamada T, Nitta Y, Araki N, Working Group for Bone and Soft Tissue Sarcoma. Carbon ion radiotherapy for inoperable pediatric osteosarcoma. Oncotarget. (2018) 9:22976–85. doi: 10.18632/oncotarget.25165
104. Yonai S, Matsufuji N, Akahane K. Monte Carlo study of out-of-field exposure in carbon-ion radiotherapy with a passive beam: organ doses in prostate cancer treatment. Phys Med. (2018) 51:48–55. doi: 10.1016/j.ejmp.2018.04.391
105. Yonai S, Matsufuji N, Kanai T, Matsui Y, Matsushita K, Yamashita H, et al. Measurement of neutron ambient dose equivalent in passive carbon-ion and proton radiotherapies. Med Phys. (2008) 35:4782–92. doi: 10.1118/1.2989019
106. Ohno T, Okamoto M. Carbon ion radiotherapy as a treatment modality for paediatric cancers. Lancet Child Dolesc Health. (2019) 3:371–2. doi: 10.1016/S2352-4642(19)30106-3
107. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–8. doi: 10.1016/S1470-2045(15)00040-6
108. Pompos A, Durante M, Choy H. Heavy ions in cancer therapy. JAMA Oncol. (2016) 2:1539–40. doi: 10.1001/jamaoncol.2016.2646
109. Fossati P, Matsufuji N, Kamada T, Karger CP. Radiobiological issues in prospective carbon ion therapy trials. Med Phys. (2018) 45:e1096–110. doi: 10.1002/mp.12506
110. Helm A, Ebner DK, Tinganelli W, Simoniello P, Bisio A, Marchesano V, et al. Combining heavy-ion therapy with immunotherapy: an update on recent developments. Int J Part Ther. (2018) 5:84–93. doi: 10.14338/IJPT-18-00024.1
111. Ando K, Fujita H, Hosoi A, Ma L, Wakatsuki M, Seino KI, et al. Intravenous dendritic cell administration enhances suppression of lung metastasis induced by carbon-ion irradiation. J Radiat Res. (2017) 58:446–55. doi: 10.1093/jrr/rrx005
112. Durante M. New challenges in high-energy particle radiobiology. Br J Radiol. (2014) 87:20130626. doi: 10.1259/bjr.20130626
113. Durante M, Formenti S. Harnessing radiation to improve immunotherapy: better with particles? Br J Radiol. (2019) 192:20190224. doi: 10.1259/bjr.20190224
114. Sprave T, Verma V, Sterzing F, Bruckner T, Hees K, Land B, et al. Cost-effectiveness of carbon ion radiation therapy for skull base chordoma utilizing long-term (10-Year) outcome data. Anticancer Res. (2018) 38:4853–8. doi: 10.21873/anticanres.12797
115. Jakel O, Land B, Combs SE, Schulz-Ertner D, Debus J. On the cost-effectiveness of Carbon ion radiation therapy for skull base chordoma. Radiother Oncol. (2007) 83:133–8. doi: 10.1016/j.radonc.2007.03.010
116. Cho I, Seo YS, Jung W, Kim MS. Estimation of the medical need for carbon-ion radiotherapy in Korea. J Radiat Res. (2018) 59:588–92. doi: 10.1093/jrr/rry046
Keywords: carbon, heavy ion, particle, radiation therapy, high LET radiation
Citation: Malouff TD, Mahajan A, Krishnan S, Beltran C, Seneviratne DS and Trifiletti DM (2020) Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front. Oncol. 10:82. doi: 10.3389/fonc.2020.00082
Received: 11 September 2019; Accepted: 16 January 2020;
Published: 04 February 2020.
Edited by:
Timothy James Kinsella, Warren Alpert Medical School of Brown University, United StatesReviewed by:
Roberto Pacelli, University of Naples Federico II, ItalyMarco Durante, GSI Helmholtzzentrum für Schwerionenforschung, Helmholtz-Gemeinschaft Deutscher Forschungszentren (HZ), Germany
Copyright © 2020 Malouff, Mahajan, Krishnan, Beltran, Seneviratne and Trifiletti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Michael Trifiletti, dHJpZmlsZXR0aS5kYW5pZWxAbWF5by5lZHU=
 Timothy D. Malouff
Timothy D. Malouff Anita Mahajan2
Anita Mahajan2 Sunil Krishnan
Sunil Krishnan Daniel Michael Trifiletti
Daniel Michael Trifiletti