- 1State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou, China
- 2Department of Thoracic Surgery, Sun Yat-sen University Cancer Center, Guangzhou, China
- 3Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, China
- 4Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
- 5Department of Molecular Pathology, Sun Yat-sen University Cancer Center, Guangzhou, China
- 6Department of Medical Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China
- 7Department of Anesthesiology, Sun Yat-sen University Cancer Center, Guangzhou, China
- 8Department of Thoracic Surgical Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 9Department of Colorectal Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Introduction: A certain number of small cell lung cancer (SCLC) patients become long-term survivors after treatment, and they are at high risk to develop a second primary malignancy, including non-small cell lung cancer. However, the optimal management of early-stage second primary non-small cell lung cancer (SPLC) after SCLC remains unknown. This study aims to evaluate the survival benefits of surgery in these patients.
Methods: Patients with early-stage SPLC after SCLC were identified from the Surveillance, Epidemiology, and End Results database. Patients were balanced with propensity score matching (PSM). Overall survival (OS) and lung cancer-specific survival (CSS) were compared between non-surgery group and surgery group with the Kaplan–Meier method and Cox multivariate regressions.
Results: A total of 228 patients with early-stage SPLC after SCLC were identified. Surgery was associated with significantly improved OS and CSS in the multivariate Cox regression analysis (OS, 5-year survival: 41.2 vs. 11.6%, HR: 0.42, 95% CI: 0.31–0.59, P < 0.01; CSS, 5-year survival: 46.8 vs. 24.3%, HR: 0.53, 95% CI: 0.37–0.75, P < 0.01). However, no statistically significant survival difference was found between sublobar resection and lobectomy (OS, 5-year survival: 41.0 vs. 45.3%, P = 0.73; CSS, 5-year survival: 43.5 vs. 54.1%, P = 0.49). After 1:1 PSM, 162 patients were selected for further analysis, and surgery continued to demonstrate superior survival (OS, 5-year survival: 44.2 vs. 7.2%, HR: 0.48, 95% CI: 0.33–0.70, P < 0.01; CSS, 5-year survival: 48.0 vs. 20.6%, HR: 0.44, 95% CI: 0.42–0.97, P = 0.03).
Conclusion: The resection of early-stage SPLC after SCLC led to significantly improved OS and CSS and therefore should be considered whenever possible. Nevertheless, further randomized controlled trials are warranted to investigate the safety and effect of surgery in these patients.
Introduction
Small cell lung cancer (SCLC) is an important type of lung cancer and accounts for ~13% of all lung cancer (1). SCLC is notorious for its aggressive behavior, and most SCLC is initially diagnosed as extensive-stage disease. Chemotherapy and radiotherapy are currently the predominant treatment modalities for SCLC due to its chemoradiosensitivity. The prognosis of SCLC patients is bleak with a 5-year survival rate of only 6% (2, 3). However, a certain number of SCLC patients become long-term survivors after radiotherapy and chemotherapy. Previous studies have demonstrated that these patients are at high risk for developing a second primary malignancy, including non-small cell lung cancer (NSCLC), possibly related to the secondary effect of chemoradiation (4, 5). In addition, It is well-established that different histology is one of the most robust criteria for the diagnosis of second primary lung cancer, which are endorsed by both International Association for the Study of Lung Cancer (IASLC) TNM staging proposal and American College of Chest Physicians (ACCP) guideline (6, 7). Therefore, it is reasonable to consider NSCLC diagnosed after SCLC as second primary lung cancer because these two cancers are different in histology.
Currently, no clinical guideline or consensus has addressed the optimal treatment strategy for early-stage second primary non-small cell lung cancer (SPLC) after SCLC. In addition, to the best of our knowledge, no previous study has evaluated the survival benefits of surgical resection for these patients.
In this study, we searched the Surveillance, Epidemiology, and End Results (SEER) database to analyze the characteristics of early-stage SPLC after SCLC, and we aimed to evaluate the survival benefits of surgery for these patients.
Materials and Methods
Study Population and Outcomes
Patients with early-stage SPLC after SCLC were identified from the SEER Multiple Primary Standardized Incidence Ratios (MP-SIR) session (April 2019 released, 2000–2016). A minimal interval of 6 months was required between initial SCLC and SPLC, considering the general treatment and surveillance protocol of SCLC.
Patients' clinicopathological characteristics, treatment information, and survival outcomes were also extracted from SEER. Tumor histology was classified with the International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) morphology codes according to the 2015 World Health Organization Classification of Lung Tumors (8). Specific inclusion criteria were listed as follows: (1) ICD-O-3 topographical codes: C340–C349 (lung and bronchus); (2) ICD-0-3 histology codes of the first primary lung cancer: 8002, 8041–8045 (small cell lung cancer); (3) age at diagnosis of SCLC: no less than 18 years old; (4) time of diagnosis of SPLC: 2000.1–2014.12. On the other hand, specific exclusion criteria were listed as follows: (1) ICD-O-3 histology site of the second primary lung cancer: 8002, 8041–8045 (small cell lung cancer as SPLC was excluded); (2) interval between the two cancers was less than 6 months; (3) cause of death or TNM staging information unknown; (4) N2/3 station lymph node involvement or distant metastasis. Surgery on SPLC included sublobar resection (wedge resection, segmentectomy, or other unspecified resections of less than one lobe), lobectomy, bilobectomy, pneumonectomy, and other non-specified local resection. Therefore, all selected SPLC patients with SEER Site-Specific Surgery of Primary Site Code between 10 and 90 were classified as surgery group, while Code 00 and 99 were classified as non-surgery group.
The primary and secondary outcomes in this study were overall survival (OS) and lung cancer-specific survival (CSS), respectively. The survival time was calculated from the time of SPLC diagnosis to the time of death or the last follow-up in months. All patients were followed up to December 31, 2016; patients who were alive at the last follow-up were censored. In addition, causes of death other than lung cancer were also censored in the CSS analysis.
Statistical Analysis
The differences between the non-surgery group and the surgery group were compared by Pearson chi-square tests or Fisher's exact tests. Multiple comparisons were adjusted by the Bonferroni correction. The Kaplan–Meier method and log-rank tests were applied in survival analysis. Potential statistically significant factors (P < 0.10) identified from the univariate survival analysis were further selected into the Cox proportional hazards regression model for the multivariate survival analysis and subgroup analysis. The Cox regression model was developed by forward stepwise selection (likelihood-ratio) with entry and removal probabilities of 0.05 and 0.10, respectively. For the subgroup analysis, variables deemed clinically significant or those that demonstrated a survival difference in the previous multivariate Cox regression were included. Surgery for SPLC served as the comparing factor while the other included variables served as subgroup variables in turn.
Propensity score matching (PSM) was performed between the non-surgery group and the surgery group to reduce potential bias in this retrospective study. Variables deemed clinically significant and variables significantly different between the non-surgery group and the surgery group were selected as the predictor covariates in PSM. The match tolerance (caliper) was set at 0.02 and 1:1 nearest neighbor matching was performed. In this study, PSM sampled without replacement and implemented logistic regression (9).
All statistical analysis and PSM were conducted with IBM SPSS Statistics Version 25, and the survival curves and forest plot were drawn by R version 3.6.1. A two-sided P-value < 0.05 was considered statistically significant.
Results
A total of 228 early-stage SPLC after SCLC patients were identified according to the selection criteria, among which 105 received surgery while 123 did not (selection flow, Figure 1). For the surgery group, the interval between the two cancers and the median follow-up time were 37 and 36 months, respectively; as for the non-surgery group, the interval between the two cancers and the median follow-up time were 47 and 21 months, respectively.
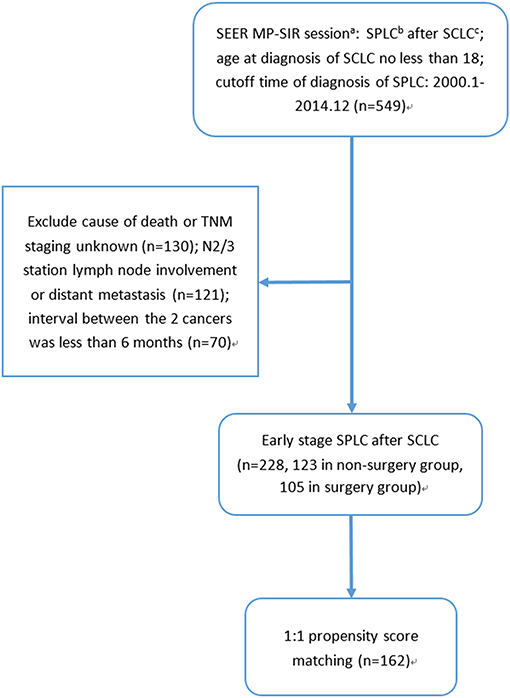
Figure 1. Selection flow: (a) SEER MP-SIR: Surveillance, Epidemiology, and End Results (SEER) Multiple Primary Standardized Incidence Ratios (MP-SIR) session specializes in conducting an analysis examining multiple subsequent cancers. (b) SPLC: second primary non-small cell lung cancer after small cell lung cancer. (c) SCLC: small cell lung cancer.
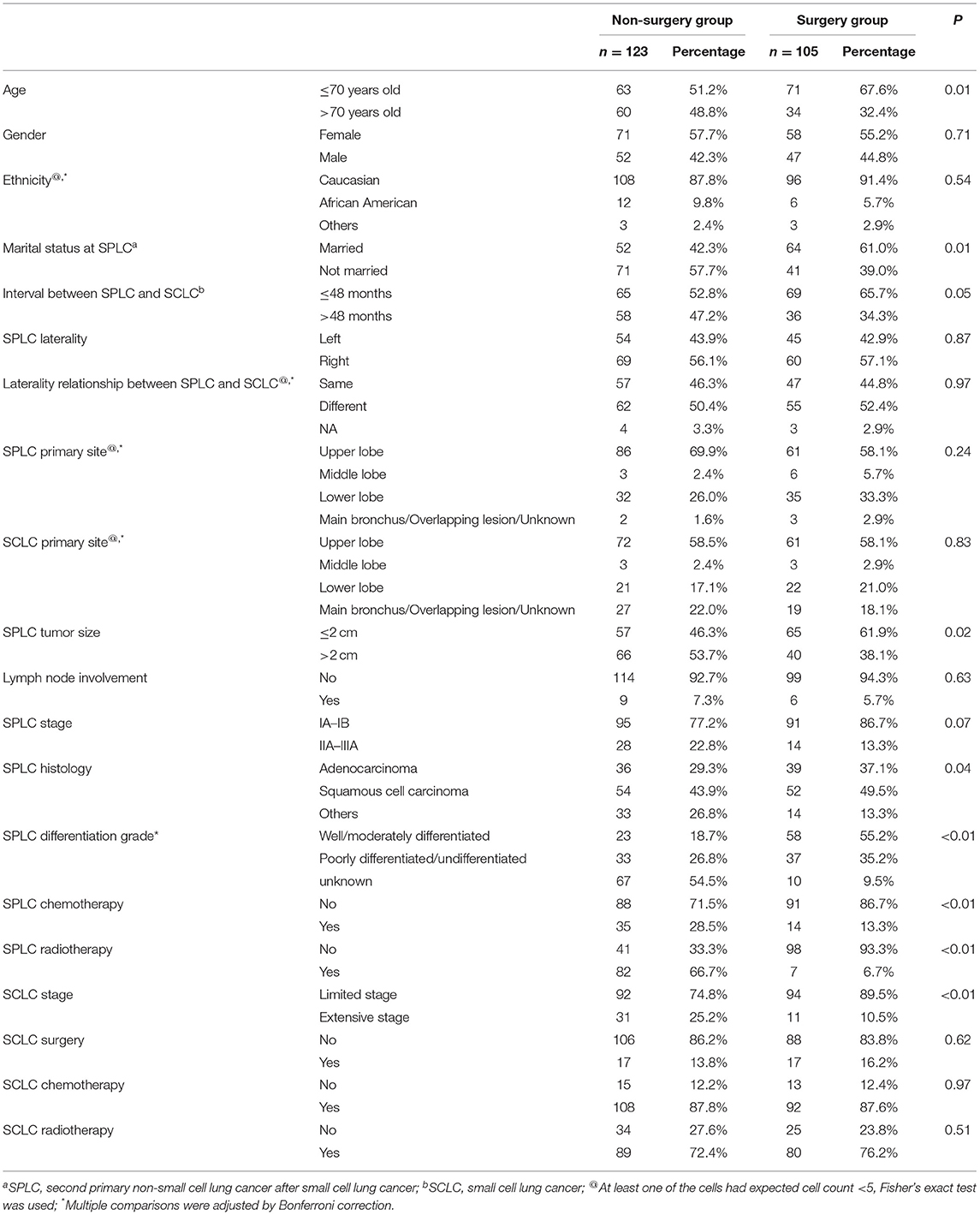
The pertinent clinicopathological factors were compared between the non-surgery group and the surgery group, and patients who underwent surgery were more likely to be younger and married. The surgery group was associated with limited stage SCLC, SPLC of smaller tumor size, and shorter interval between cancers. On the other hand, the histology and differentiation grade in the non-surgery group were more likely to be unclassified. Moreover, patients in the non-surgery group were more likely to receive chemotherapy and radiotherapy for SPLC. However, proportions of previous chemotherapy and radiotherapy for the initial SCLC were not significantly different between the two groups (Table 1).
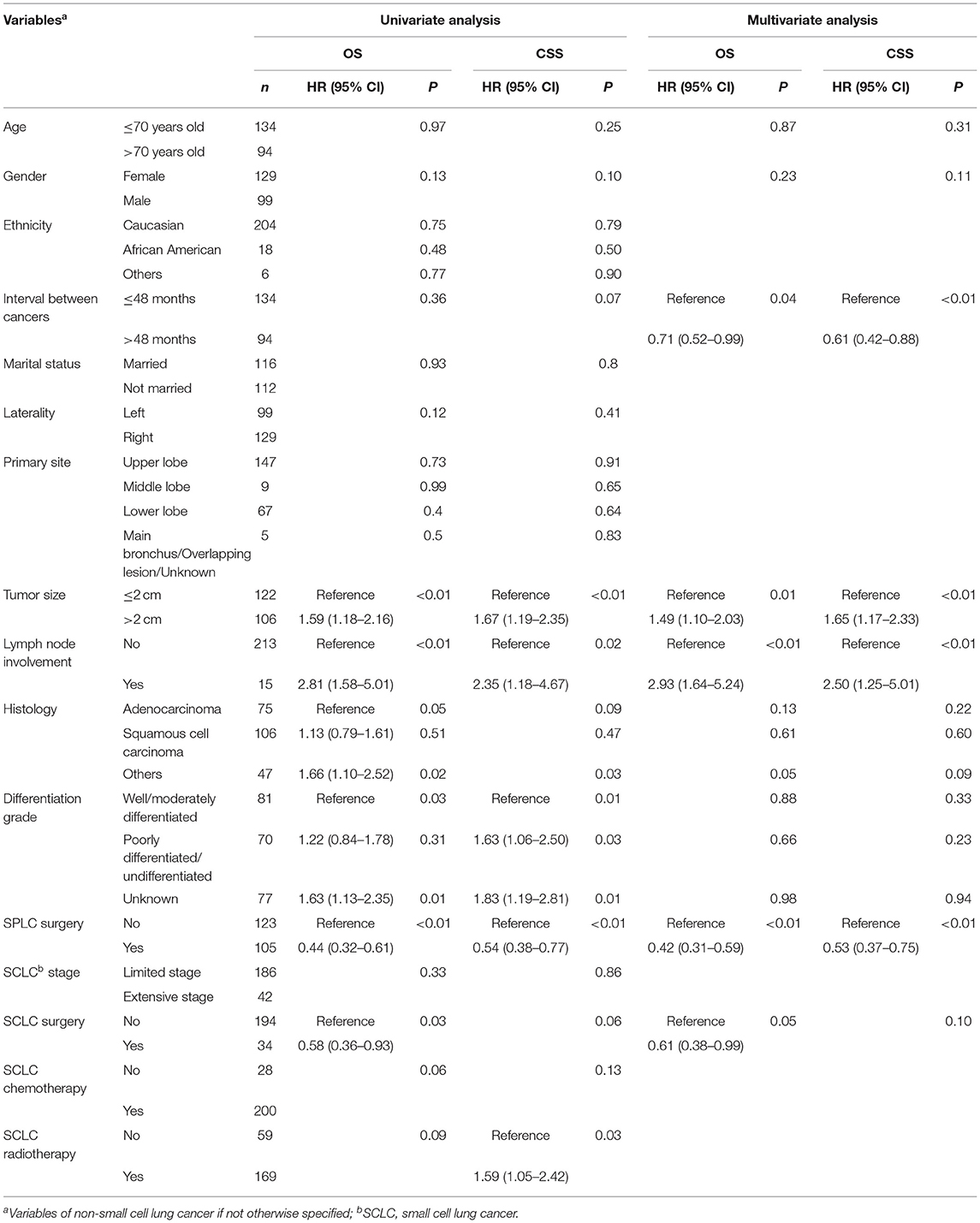
In the univariate survival analysis, patients with SPLC ≤ 2 cm, without N1 lymph node involvement, and who underwent surgery for SPLC were associated with both better OS and CSS. Surgery for SCLC demonstrated survival benefit in OS but not in CSS. Meanwhile, unclassified SPLC histology and differentiation grades were associated with worse OS and CSS. As for multivariate Cox regression, surgery for SPLC was associated with significantly improved survival (OS, 5-year rate: 41.2 vs. 11.6%, HR: 0.42, 95% CI: 0.31–0.59, P < 0.01; CSS, 5-year rate: 46.8 vs. 24.3%, HR: 0.53, 95% CI: 0.37–0.75, P < 0.01). In addition, a longer interval between cancers, SPLC ≤ 2 cm, SPLC without N1 lymph node involvement, and surgery for SCLC were also associated with significantly longer survival (Table 2).
Subgroup analysis by age, gender, interval between cancers, SPLC tumor size, SPLC differentiation grade, SCLC stage, SCLC surgery, SCLC chemotherapy, and SCLC radiotherapy demonstrated that surgery for SPLC was associated with improved survivals for most subgroups (forest plot, Figure 2).
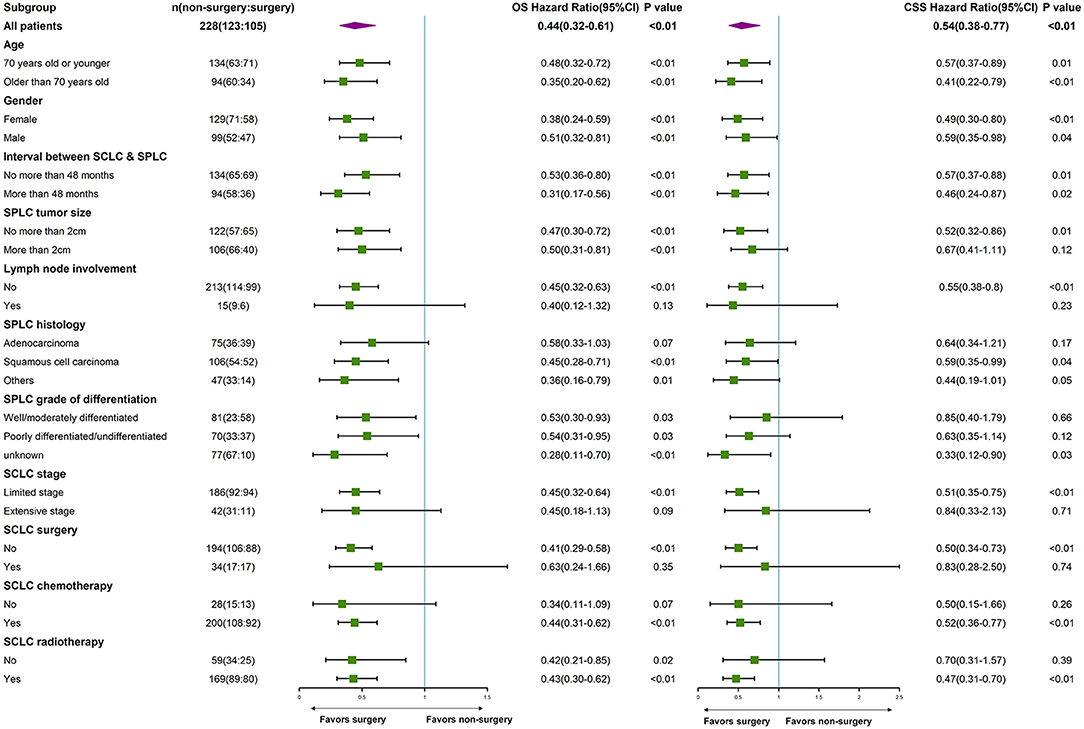
Figure 2. Subgroup analysis by age, gender, interval between cancers, SPLC tumor size, SPLC differentiation grade, SCLC stage, SCLC surgery, SCLC chemotherapy, and SCLC radiotherapy. (a) SPLC: second primary non-small cell lung cancer after SCLC. (b) SCLC: small cell lung cancer.
Among the 105 patients in the surgery group, 43.8% underwent sublobar resection and 51.4% underwent lobectomy, and no statistically significant survival difference was found between sublobar resection and lobectomy (OS, 5-year survival: 41.0 vs. 45.3%, P = 0.73; CSS, 5-year survival: 43.5 vs. 54.1%, P = 0.49). Regarding the lymph node evaluation during surgery for SPLC, the median number of evaluated lymph node during surgery was 2 and 69.5% patients had at least one lymph node examined during surgery. However, there is no statistically significant survival difference between patients with and without surgical lymph node evaluation (OS, 5-year survival: 30.3 vs. 45.8%, P = 0.38; CSS: 5-year survival: 33.1 vs. 52.6%, P = 0.29).
Predictor covariates used in PSM included age, gender, marital status, tumor size, lymph node involvement, surgery for SCLC, and interval between cancers. It should be noted that SPLC chemotherapy or radiotherapy was not chosen as predictor covariate in PSM, because they had strong correlation with SPLC surgery and might cause multicollinearity problem. After 1:1 PSM, 162 patients were selected for further analysis (selection flow, Figure 1). No statistically significant difference was found between two groups in predictor covariates or other clinically significant covariates after PSM, except for SPLC differentiation grade (Table 3). It should be noted that differentiation grade was considered a pathological variable and was best acquired from resected sample from surgery, which made the percentage of unknown differentiation grade significantly higher in the non-surgery group. We believed that the difference of differentiation grade of the two groups was mainly derived from surgery. Therefore, differentiation grade was not included as predictor covariates in PSM and did not compromise the comparison between the non-surgery group and the surgery group. Moreover, in the multivariate Cox regression, differentiation grade was not associated with any statistically significant survival difference.
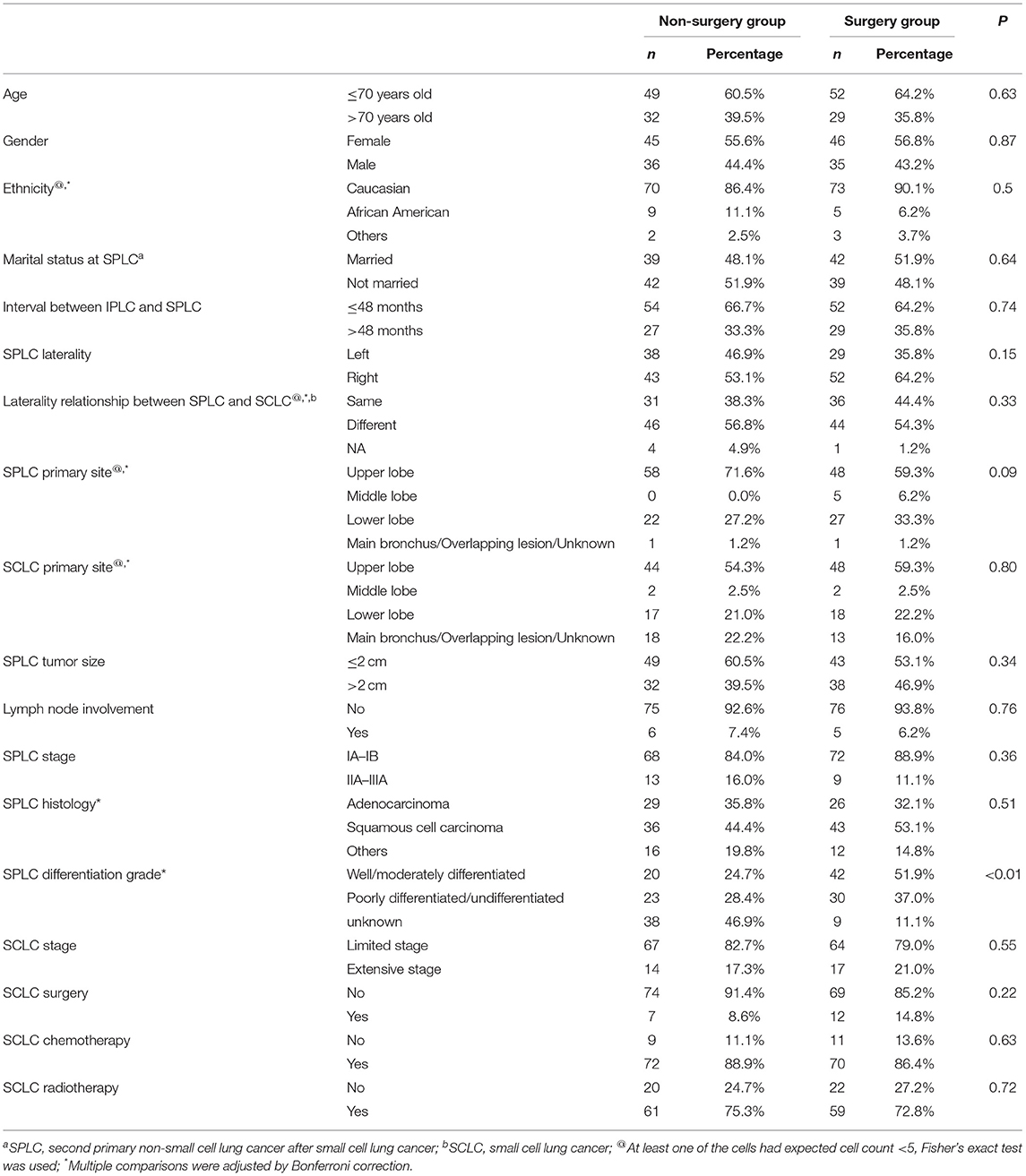
Table 3. Comparison of clinicopathological factors between the non-surgery group and surgery group after propensity score matching.
Notably, patients who underwent surgery demonstrated significantly improved survival both before (OS, 5-year rate: 41.2 vs. 11.6%, HR: 0.44, 95% CI: 0.32–0.61, P < 0.01; CSS, 5-year rate: 46.8 vs. 24.3%, HR: 0.54, 95% CI: 0.38–0.77, P < 0.01, Figures 3A,B) and after PSM (OS, 5-year rate: 44.2 vs. 7.2%, HR: 0.48, 95% CI: 0.33–0.70, P < 0.01; CSS, 5-year rate: 48.0 vs. 20.6%, HR: 0.44, 95% CI: 0.42–0.97, P = 0.03, Figures 3C,D) compared to patients without surgery for SPLC.
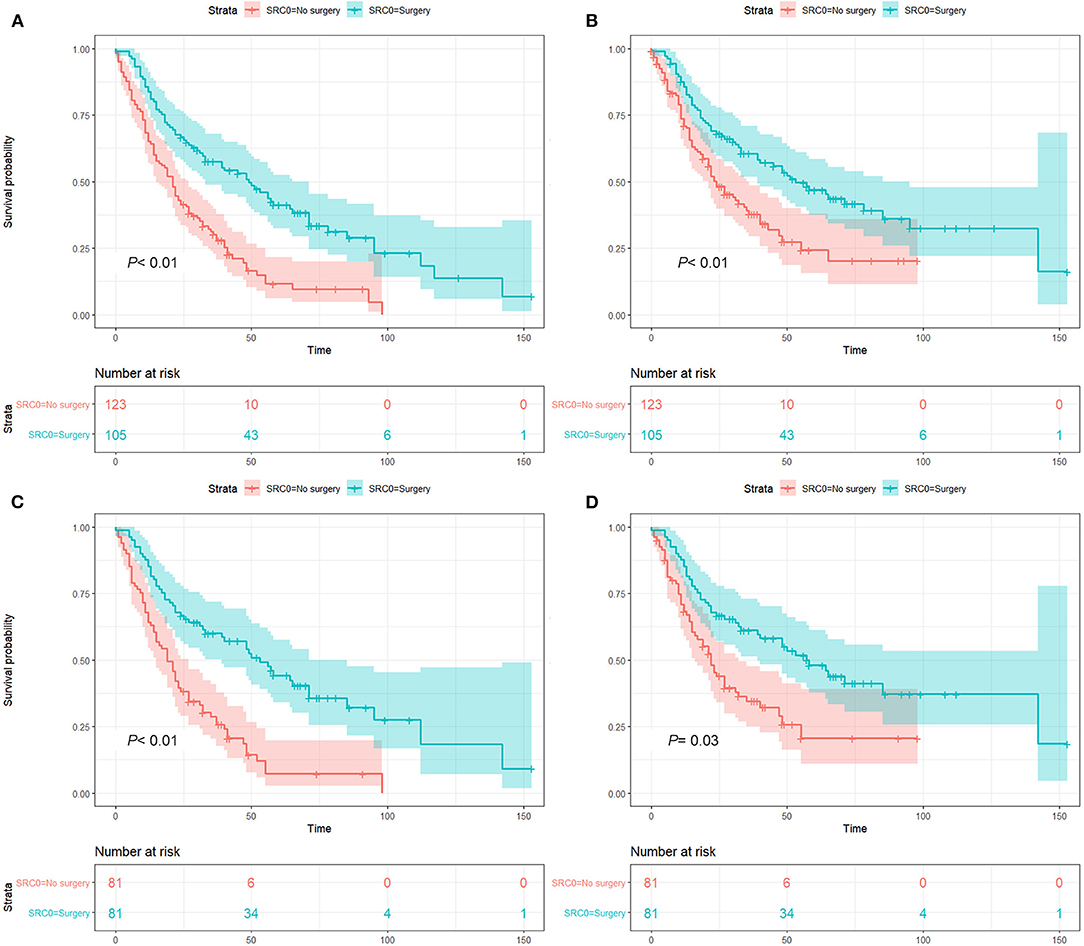
Figure 3. Survival analysis before and after propensity score matching (PSM): before PSM, overall survival (A) and lung cancer-specific survival (B) analysis; after PSM, overall survival (C) and lung cancer-specific survival (D) analysis. P value was calculated from log-rank test and pooled over strata, and the 95% confidence interval of the survival curves is depicted as a color band.
Discussion
Although the survival rate of SCLC remains dismal, certain number of patients survived long enough to develop second primary malignancies, including NSCLC (4, 5). However, the optimal management strategy for these patients remains unknown, and to the best of our knowledge, no previous study has evaluated the survival benefit of surgery in these patients. In this study, we utilized the SEER database to identify the largest cohort of early-stage SPLC after SCLC, and we found that surgery was associated with significantly better OS and CSS in these patients. To further confirm the survival benefits of surgery, we also conducted multivariate Cox regression, subgroup analysis, and PSM. As it turned out, surgery demonstrated consistent survival benefits in the multivariate Cox regression and most subgroups, indicating a wide range of application in early-stage SPLC after SCLC. Furthermore, surgery was also associated with better OS and CSS after PSM.
Not so many thoracic surgeons recommend surgery for early-stage SPLC after SCLC, especially after chemoradiation for the initial SCLC, as the dissection of pulmonary vessels can be challenging and technically demanding after definitive chemoradiation. Although studies on surgery after SCLC chemoradiation have been scarce, multiple studies have demonstrated that salvage surgery after failed Stereotactic Body Radiotherapy in early-stage NSCLC was safe and feasible and led to improved survival (10–12). A phase III randomized controlled trial also found that resection after induction chemoradiation was feasible and comparable to definitive chemoradiation in patients with locally advanced NSCLC (13). In our study, most patients received chemotherapy and radiotherapy for the initial SCLC. Similar to the studies described above, we believe that surgery for SPLC after SCLC can be safe, feasible, and beneficial in experienced hands.
The extent of resection and lymph node evaluation during surgery are two important aspects of NSCLC surgery. Previous studies have provided convincing evidence that recommended lobectomy over sublobar resection as standard of care for early-stage NSCLC (14–16), based on the associated longer survival and lower recurrence. On the other hand, the number of examined lymph node was also found to be an important prognostic factor (17, 18), and thorough lymph node evaluation may avoid downstaging. However, in our study, neither the extent of resection nor the number of examined lymph node was a prognostic factor within the surgery group. We believe that the decision on the extent of resection and lymph node evaluation should be individually based for patients with early-stage SPLC after SCLC. Multiple factors, including patients' remaining pulmonary function, life expectancy, operative risk, and surgeon's experience should be considered. Moreover, further randomized controlled trials are needed to determine the optimal extent of resection and lymph node evaluation in patients with early-stage SPLC after SCLC.
Several limitations in our study are worth mentioning. First, several important variables are not available in SEER, including cardiopulmonary function, specific protocols of chemotherapy and radiotherapy, SCLC treatment response, and perioperative morbidity and mortality. Second, potential bias is inevitable due to the retrospective nature of this study. However, it should be noted that multivariate Cox regression and PSM were implemented to minimize this potential bias.
In conclusion, this population-based study has demonstrated that surgery is associated with significantly improved survival. Therefore, surgery should be considered in patients with early-stage SPLC after SCLC whenever possible. Nevertheless, further randomized controlled trials are warranted to confirm the safety and effect of surgery in these patients.
Data Availability Statement
All data in this study are publicly available in the SEER database: Multiple Primary Standardized Incidence Ratios (MP-SIR) session (April 2019 released, 2000–2016).
Author Contributions
RZ, LC, YL, and LZ: conception and design. YW, NZ, FW, XZ, YL, and LZ: provision of study materials or patients. GW, ZH, XY, KX, LY, and DZ: collection and assembly of data. RZ and LC: data analysis and interpretation. All authors: manuscript writing and final approval of manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors sincerely thank all the staff of the SEER program for their important work and diligent effort.
References
1. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. (2006) 24:4539–44. doi: 10.1200/JCO.2005.04.4859
2. van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. (2011) 378:1741–55. doi: 10.1016/S0140-6736(11)60165-7
3. Noone A, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. SEER Cancer Statistics Review, 1975-2015. Bethesda, MD: National Cancer Institute (2018).
4. Sagman U, Lishner M, Maki E, Shepherd FA, Haddad R, Evans WK, et al. Second primary malignancies following diagnosis of small-cell lung cancer. J Clin Oncol. (1992) 10:1525–33. doi: 10.1200/JCO.1992.10.10.1525
5. Kono M, Allen PK, Lin SH, Wei X, Jeter MD, Welsh JW, et al. Incidence of second malignancy after successful treatment of limited-stage small-cell lung cancer and its effects on survival. J Thorac Oncol. (2017) 12:1696–703. doi: 10.1016/j.jtho.2017.07.030
6. Detterbeck FC, Franklin WA, Nicholson AG, Girard N, Arenberg DA, Travis WD, et al. The IASLC Lung Cancer Staging Project: background data and proposed criteria to distinguish separate primary lung cancers from metastatic foci in patients with two lung tumors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. (2016) 11:651–65. doi: 10.1016/j.jtho.2016.01.025
7. Kozower BD, Larner JM, Detterbeck FC, Jones DR. Special treatment issues in non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143(5 Suppl.):e369S–9S doi: 10.1378/chest.12-2362
8. Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin J, Beasley MB, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. (2015) 10:1243–60. doi: 10.1097/JTO.0000000000000630
9. Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. (2014) 33:1242–58. doi: 10.1002/sim.5984
10. Hamaji M, Chen F, Matsuo Y, Ueki N, Hiraoka M, Date H. Treatment and prognosis of isolated local relapse after stereotactic body radiotherapy for clinical stage I non-small-cell lung cancer: importance of salvage surgery. J Thorac Oncol. (2015) 10:1616–24. doi: 10.1097/JTO.0000000000000662
11. Allibhai Z, Cho BC, Taremi M, Atallah S, Hope A, Hwang D, et al. Surgical salvage following stereotactic body radiotherapy for early-stage NSCLC. Eur Respir J. (2012) 39:1039–42. doi: 10.1183/09031936.00075811
12. Neri S, Takahashi Y, Terashi T, Hamakawa H, Tomii K, Katakami N, et al. Surgical treatment of local recurrence after stereotactic body radiotherapy for primary and metastatic lung cancers. J Thorac Oncol. (2010) 5:2003–7. doi: 10.1097/JTO.0b013e3181f8a785
13. Albain KS, Swann RS, Rusch VW, Turrisi AR, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. (2009) 374:379–86. doi: 10.1016/S0140-6736(09)60737-6
14. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. (1995) 60:615–22; discussion 622–3. doi: 10.1016/0003-4975(95)00537-u
15. Dai C, Shen J, Ren Y, Zhong S, Zheng H, He J, et al. Choice of surgical procedure for patients with non-small-cell lung cancer ≤ 1 cm or > 1 to 2 cm among lobectomy, segmentectomy, and wedge resection: a population-based study. J Clin Oncol. (2016) 34:3175–82. doi: 10.1200/JCO.2015.64.6729
16. Khullar OV, Liu Y, Gillespie T, Higgins KA, Ramalingam S, Lipscomb J, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the national cancer data base. J Thorac Oncol. (2015) 10:1625–33. doi: 10.1097/JTO.0000000000000664
17. Ludwig MS, Goodman M, Miller DL, Johnstone PA. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. (2005) 128:1545–50. doi: 10.1378/chest.128.3.1545
18. Osarogiagbon RU, Decker PA, Ballman K, Wigle D, Allen MS, Darling GE. Survival implications of variation in the thoroughness of pathologic lymph node examination in American College of Surgeons Oncology Group Z0030 (Alliance). Ann Thorac Surg. (2016) 102:363–9. doi: 10.1016/j.athoracsur.2016.03.095
Keywords: small cell lung cancer, non-small cell lung cancer, second primary lung cancer, surgery, survival, SEER database
Citation: Zhang R, Cai L, Wang G, Wen Y, Wang F, Zhou N, Zhang X, Huang Z, Yu X, Xi K, Yang L, Zhao D, Lin Y and Zhang L (2020) Resection of Early-Stage Second Primary Non-small Cell Lung Cancer After Small Cell Lung Cancer: A Population-Based Study. Front. Oncol. 9:1552. doi: 10.3389/fonc.2019.01552
Received: 17 October 2019; Accepted: 23 December 2019;
Published: 05 February 2020.
Edited by:
Marco Lucchi, University of Pisa, ItalyReviewed by:
Bernard Joon Hahn Park, Memorial Sloan Kettering Cancer Center, United StatesLorenzo Spaggiari, European Institute of Oncology (IEO), Italy
Copyright © 2020 Zhang, Cai, Wang, Wen, Wang, Zhou, Zhang, Huang, Yu, Xi, Yang, Zhao, Lin and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongbin Lin, bGlueWJAc3lzdWNjLm9yZy5jbg==; Lanjun Zhang, emhhbmdsakBzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work
 Rusi Zhang
Rusi Zhang Ling Cai
Ling Cai Gongming Wang
Gongming Wang Yingsheng Wen1,2
Yingsheng Wen1,2 Fang Wang
Fang Wang Kexing Xi
Kexing Xi