
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 January 2020
Sec. Cancer Immunity and Immunotherapy
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.01497
This article is part of the Research Topic Understanding the Immuno-Oncological Mechanism of Cancer Using Systems Immunology Approaches View all 43 articles
The tumor environment is of vital importance for the incidence and development of colorectal cancer. Increasing evidence in recent years has elaborated the vital role of the tumor environment in cancer subtype classification and patient prognosis, but a comprehensive understanding of the colorectal tumor environment that is purely dependent on the stromal compartment is lacking. To decipher the tumor environment in colorectal cancer and explore the role of its immune context in cancer classification, we performed a gene expression microarray on the stromal compartment of colorectal cancer and adjacent normal tissues. Through the integrated analysis of our data with public gene expression microarray data of stromal and epithelial colorectal cancer tissues processed through laser capture microdissection, we identified four highly connected gene modules representing the biological features of four tissue compartments by applying a weighted gene coexpression network analysis algorithm and classified colorectal cancers into three immune subtypes by adopting a nearest template prediction algorithm. A systematic analysis of the four identified modules mainly reflected the close interplay between the biological changes of intrinsic and extrinsic characteristics at the initiation of colorectal cancer. Colorectal cancers were stratified into three immune subtypes based on gene templates identified from representative gene modules of the stromal compartment: active immune, active stroma, and mixed type. These immune subtypes differed by the immune cell infiltration pattern, expression of immune checkpoint inhibitors, mutation landscape, extent of mutation burden, extent of copy number burden, prognosis and chemotherapeutic sensitivity. Further analysis indicated that activation of the NF-kB signaling pathway was the major mechanism causing the no immune infiltration milieu in the active stroma subtype and that inhibitors of the NF-kB signaling pathway could be candidate drugs for treating patients with an active stroma. Overall, these results suggest that characterizing colorectal cancer by the tumor environment is of vital importance in predicting patients' clinical outcomes and helping guide precision and personalized treatment.
Colorectal cancer is the third most common cancer and ranks second in terms of cancer-related mortality (1). Most colorectal cancer patients die because of a late diagnosis, recurrence after surgical excision, or resistance to chemotherapy or radiotherapy. Patients with the same American Joint Committee on Cancer (AJCC) stage and pathomorphological features are given consistent treatment regimens and often have distinct prognoses and treatment responses. The current treatment dilemma underscores the critical need to improve colorectal cancer classification with distinct molecular features and survival outcomes for reasonable clinical treatment decisions.
The tumor epithelium and surrounding microenvironment closely interact through the extracellular matrix or secreted factors such as exosomes, cytokines, and angiogenic factors (2). The depiction of a transcriptome map of the altered biological processes in the epithelial and stromal compartments will not only allow investigators to comprehensively understand the mechanism of cancer initiation and the complex coevolving relationships between the intrinsic and extrinsic factors of tumors (3) but also help in the detection of druggable epithelial–stromal crosstalk targets (4). Nishida previously used a laser capture microdissection (LCM)-processed miRNA and gene expression microarray to reveal two miRNA clusters with high expression in the cancer stroma (5). However, the major changes in the biological features of the epithelial and stromal compartments between colorectal cancer and adjacent normal tissues remain poorly understood. A systematic analysis of the different compartments of colorectal tissues is needed to better understand the mechanisms of tumor initiation.
The tumor microenvironment (TME), which includes blood vessels, lymph vessels, immune cells and mesenchymal cells, is a complex ecosystem of stromal cells and plays a critical role during tumorigenesis and progression. Previously identified transcriptome subtypes of colorectal cancer associated with a poor prognosis, including the stem (6), serrated (7), and mesenchymal (8) subtypes, are enriched with genes derived from the activated stromal compartment. The differentially expressed genes of preoperative chemoradiotherapy-treated rectal carcinomas between responders and non-responders are mainly contributed by the stroma and not tumor glands (9). The TME is a major contributing factor for patient outcomes and chemoradiotherapy treatment responses (10, 11). Additionally, recent studies have indicated that TME characteristics are closely associated with the response to immune checkpoint blockade (ICB) treatment (12, 13). For example, epithelial-mesenchymal transition (EMT)-, stroma- and angiogenesis-related signatures are significant contributors to ICB treatment resistance (14, 15), while the high infiltration of cytotoxic T cells can elicit an effective immune response to attack tumor cells (16). Thus, the surrounding tumor environment can shape the biological behavior and the reaction of tumor cells to a drug regimen.
Despite increasing evidence proving the crucial role of the immune context in determining immunological treatment reactions and prognoses, most studies have focused on bulk tumor transcriptomes, with mixed data from the tumor epithelium and stroma. Few studies have focused on the changes in immunological responses purely modified by the surrounding immune context. In this study, we first described the major biological process changes in the epithelium and stroma of colorectal cancers and adjacent normal tissues and defined three diverse colorectal immune subtypes, namely, the “active immune,” “active stroma” and “mixed type” subtypes, based on the top 40 most connected genes from the identified network module by adopting weighted gene coexpression network analysis (WGCNA). These subtypes had distinct immune environments, genomic contexts, and ICB treatment and chemotherapy response tendencies. Drugs targeting the NF-kB signaling pathway could convert cold tumors into hot tumors. Overall, this work proposes a new colorectal cancer classification system that is purely based on the tumor environment and has the potential to guide treatment decisions.
Tissues from six patients with colorectal cancer and 6 adjacent normal tissue samples (located more than 5 cm away from the tumor edge) were obtained during surgery. Four patients had paired tumor and adjacent normal samples, and the other four samples were obtained from distinct patients. The obtained tissues were independently morphologically reviewed by two experienced pathologists to confirm the diagnostic accuracy. Representative histopathological images are showed in Figure S1. Fresh tissues were cleaned with normal saline solution and frozen at -80°C within 30 min before RNA preparation. No chemotherapy or radiotherapy was administered to the patients prior to therapeutic resection. All patients underwent resection of the primary tumor at the Department of Peritoneal Cancer Surgery, Beijing Shijitan Hospital, Capital Medical University (Beijing, China) between February 2016 and December 2016. Written informed consent was obtained from all patients, and the study protocol was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
The stromal compartment of the cancer and normal samples was obtained manually by microdissection. All colorectal tissues were embedded in OCT (Thermo Fisher) and cut into 10-μm slices with a freezing microtome. The frozen tissue slices were then placed in hematoxylin for 2-5 s. Next, stromal tissues were isolated with a needle under a microscope and collected in Eppendorf tubes. Schematic diagram of the marked stromal compartment processed through microdissection are illustrated in Figure S1. Total RNA extracted from the stromal compartment of the cancer and normal samples was labeled and hybridized to Agilent 8*60K Whole Human Genome Oligo Microarrays (G4851B) according to the manufacturer's protocol. All RNA integrity numbers (RINs) of the microdissected sample compartments were greater than 7.0, and RNA integrity was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). The raw and processed data are publicly available at the Gene Expression Omnibus (GEO) website under accession number GSE136735. A previously published dataset, GSE35602, which contains epithelial and stromal regions of colorectal cancer and normal tissues through LCM, was integrated to identify specific modules of different colorectal compartments (5). The background correction and normalization of raw data were processed by the R package “limma.” The Combat algorithm was used to eliminate technological bias caused by different microarray platforms between the two datasets described above (17). Since the stromal compartment in our microarray profile was obtained manually by microdissection and the GSE35602 dataset was processed through LCM, we used the term “microdissection” to generalize the two methods in our integrated data in this study. Basic clinical characteristics of the enrolled samples in the two datasets are demonstrated in Table S1.
In this study, we used the GSE39582 dataset, which is the largest microarray cohort with complete survival information among published colorectal cancer expression spectra, and the TCGA-COADREAD (TCGA-COAD and TCGA-READ) cohort. The R packages “GEOquery” (18) and “TCGAbiolinks” (19) were implemented to download the processed expression matrix and clinical data of the GEO datasets and the TCGA-COADREAD cohort, respectively, in March 2018. Available TCGA “level 3” gene expression data of the TCGA-COADREAD cohort were downloaded. Log2 (transcripts per kilobase million (TPM) + 1)-transformed normalized values were applied for immune cell infiltration pattern estimation and SubMap analysis. The survival information data of the TCGA cohort, including overall survival and relapse-free survival, were downloaded from the UCSC Xena browser, while other clinical data, such as age, sex, and microsatellite information, were obtained by the R package “TCGAbiolinks.” For genes with multiple probe sets, the mean expression levels were used as the gene expression values.
Four pretreatment tumor expression profiles of ICB cohorts were included in this study to assess the similarity between the identified subtypes and the ICB treatment response. The data of melanoma patients treated with anti-PD-1 (accession number: GSE78220) (15), metastatic melanoma patients treated with MAGE-A3 immunotherapy (accession number: GSE35640) (20), and mice AB1-HA tumors treated with anti-CTLA-4 (accession number: GSE63557) (21) were obtained from the National Center for Biotechnology Information (NCBI) GEO database (http://www.ncbi.nlm.nih.gov/geo/). The data of patients with metastatic urothelial tumors from the IMvigor210 cohort (22) treated with anti-PD-L1 were obtained from http://research-pub.gene.com/IMvigor210CoreBiologies/. Processed fragments per kilobase million (FPKM) data of the GSE78220 cohort were transferred into TPM data. Gene expression in the IMvigor210 cohort was normalized by implementing the “voom” function in the “limma” package. The processed normalized data of the remaining two microarray cohorts were obtained by the “GEOquery” package.
To illustrate the biological changes in the epithelial and stromal compartments between colorectal cancer and normal tissues, we applied WGCNA to identify the representative transcriptional network modules of the different compartments. Genes with a low dynamic range were excluded, and only the top 8000 genes with the highest standard deviation were evaluated to construct the coexpression network. The freely available statistical analysis software (“WGCNA” R package) and R tutorials for constructing the weighted gene coexpression network have been described previously (23).
To clarify the impact of the surrounding environment of the tumor on colorectal cancer, nearest template prediction (NTP) (24) was applied to assign patients into three transcriptomic subtypes. NTP performed class prediction using predefined gene markers and returned the significance level of each sample prediction with a nominal P-value. We set the Benjamini-Hochberg (BH)-corrected false discovery rate (FDR) to 0.2 as the prediction threshold for the significant classification of a sample according to a previous report (6). The tumor purity information of the TCGA-COADREAD and GSE39582 cohorts was extracted from previous TCGA research (25) using the ABSOLUTE method (26) and estimated using the R package “estimate” (27).
The Subclass Mapping (SubMap) method (28) was used to evaluate the similarity between the identified subtypes and the immunotherapy-treated patients. The SubMap algorithm uses the Gene Set Enrichment Analysis (GSEA) function to evaluate the extent of commonality of the different subtypes in independent datasets. P-values were used to evaluate the similarity, and the lower the P-values were, the higher the similarity. Recommended default parameters, including the number of marker genes (100), random permutations for the enrichment score (100), and random permutations for Fisher's statistics (1000), were used. The R package “complexHeatmap” (29) was implemented to visualize the results of the SubMap analysis.
Significantly mutated genes were generated by MutSigCV_1.41 for the TCGA-COADREAD cohort accessed from the mutation annotation file (https://gdc.cancer.gov/about-data/publications/panimmune).
MutSigCV (30) identifies significantly mutated genes more than expected by chance. The tumor mutation burden (TMB) of each patient was calculated as the total number of non-synonymous mutations per megabase. Fisher's exact test was applied to detect different mutated genes between the active immune and active stroma compartments. For copy number analysis, we applied GISTIC_2.0 to identify significantly amplified or deleted genomes (31). The burden of copy number loss or gain was calculated as the total number of genes with copy number changes at the focal and arm levels. NTP, SubMap, MutSigCV_1.41 and GISTIC_2.0 are freely available on GenePattern (https://cloud.genepattern.org).
The R package “clusterProfiler” (32) was applied for the Gene Ontology (GO) analysis of four core transcriptional modules inferred from the WGCNA. GSEA was applied to enrich hallmark gene sets downloaded from the Molecular Signatures Database (MSigDB). Input genes were ranked in descending order according to the log2FC values. Enrichment significance was estimated using default settings and 1000 permutations. Benjamini–Hochberg-adjusted P-values less than 0.05 were considered significantly enriched.
To estimate the immune and stromal cell infiltration patterns in colorectal cancer, the Microenvironment Cell Populations-counter (MCP-counter) method (33) using colorectal cancer gene expression profiles was applied. MCP-counter is a robust and highly informative method that quantifies eight types of immune cells and two types of stromal cells based on marker genes.
To further illustrate the molecular mechanism underlying the difference in immunogenicity between active immune and active stroma compartments and identify potentially useful drugs, we performed connectivity map analysis (34) using the 150 genes with the most significant fold changes (up- and downregulated). In total, we submitted 300 genes to the CMap website (https://clue.io/). All 300 genes were significantly different under the criterion of FDR-adjusted P < 0.05 in the TCGA-COADREAD cohort.
The R package “pRRophetic” (35)was applied to estimate the chemotherapeutic response of 5-fluorouracil and cisplatin in the TCGA-COADREAD and GSE39582 cohort. Cell lines originating from the digestive system and the “cgp2016” dataset were applied when implementing the “pRRopheticPredict” function. This methodology fitted the ridge regression model based on baseline gene expression and drug sensitivity of the cell line, thus allowing the prediction of the clinical chemotherapeutic response using only patients' baseline gene expression data (36). Drug sensitivity was measured by the concentration required for 50% of cellular growth inhibition (IC50).
All statistical analyses were performed using R (https://www.r-project.org/). The Wilcoxon rank-sum test was adopted to compare differences between two groups. We used the Kruskal–Wallis test to evaluate significant differences when comparing more than two groups. The “edgeR” pipeline was adopted for the differential expression analysis. Survival probabilities were estimated with the Kaplan-Meier method, and the log-rank (Mantel-Cox) test was used to compare the survival distributions between two groups. A log-rank P < 0.05 was considered statistically significant.
To systematically characterize the expression patterns of the epithelial and stromal compartments during colorectal carcinogenesis, we performed WGCNA on the 8000 most variable genes (Table S2) and identified 12 transcriptional modules with gene numbers ranging from 47 to 1874 genes (Figure 1A). In relating these modules to tissue compartment information by correlating the eigengenes of each module with compartment traits, four modules with the most significant correlations to the tumor epithelium (blue module), tumor stroma (yellow module), normal epithelium (red module), and normal stroma (brown module) were identified (Figure 1B). The eigenvalue of these selected modules was the highest within the most closely related samples relative to the samples in the remaining three groups, which also confirmed their representation (Figure 1C). The heat map in Figure 1D shows the expression levels of all modules, and these four modules have remarkably high expression levels with their most correlated samples. Given the representativeness of these four modules, biological process enrichment analysis was applied to investigate the related properties of tissue glands and the stroma in the process of tumorigenesis (Figure 1E, Table S3). The yellow module, which had a markedly high expression level in the colorectal tumor stroma, was characterized by the overexpression of genes involved in extracellular matrix organization, the cellular response to transforming growth factor-β stimulus and the collagen metabolic process. Immune-related pathways such as T cell activation, B cell activation and lymphocyte differentiation were enriched in the brown module, whose expression level was the highest in the normal stroma. Different GO biological processes between the normal stroma and tumor stroma in the colorectum consisted of the transformation of immune-infiltrating cells (from B lineage cells and T cells to fibroblasts) (Figures S2A,B). Cancer-associated fibroblast-secreted cytokines such as IL-6 influence the phenotype of neoplastic cells, including proliferation, migration, and angiogenesis (37). Consistent with this finding, the overexpression of CDK1, EREG and ANLN along with several biological processes related to cell proliferation, such as nuclear division, DNA replication and regulation of the mitotic cell cycle phase transition, were enriched in the blue module. The red module was characterized by genes involved in metabolic pathways, including lipid catabolic processes and steroid metabolic processes (Figure 1E). The systematic analysis of stromal and epithelial tissues between colorectal cancer and adjacent normal tissues indicated that the tumor stroma might provide a suitable niche prompting tumor cell proliferation and invasion, while a normal environment with abundant immune cells helps maintain the function of the normal colorectum.
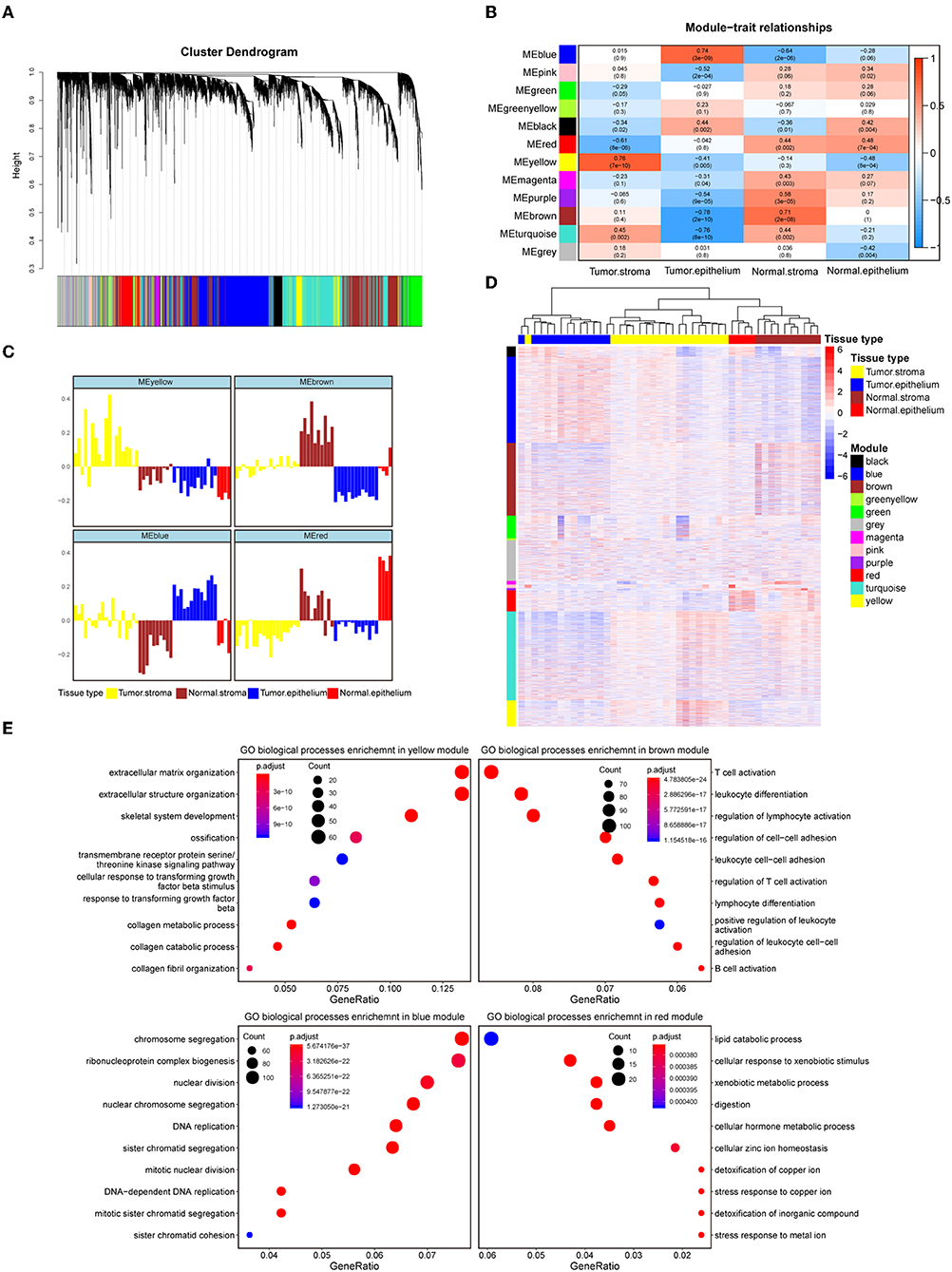
Figure 1. Identification of modules representative of diverse colorectal compartments. (A) Hierarchical cluster dendrogram of the top 8000 genes with the highest standard deviation. The identified modules underneath the tree are color coded. (B) Heat map of module-trait associations; rows represent the module eigengene, and columns represent clinical traits. The Spearman correlation and significance level enclosed in brackets are labeled in each cell. The color intensity of the cell corresponds to the correlation coefficient. (C) Eigengene bar plot of the yellow, brown, blue and red modules. Samples are ordered by the tumor stroma, normal stroma, tumor epithelium, and normal epithelium and are labeled in yellow, brown, blue and red, respectively. The module eigengene is defined as the first principal component of the module's expression matrix. (D) Hierarchical clustering heat map of the top 8000 genes with the highest standard deviation. Genes are ordered by the modules, ranging from the black module to the yellow module. The samples' corresponding tissue compartments are annotated in the column annotation panel on the top side of the heat map. The color intensity indicates the relative expression level of the genes. (E) Dot plot of the biological process enrichment results. The top 10 GO terms with the highest enrichment levels are shown; the dot size and color represent the gene count and enrichment level, respectively.
The TME plays a crucial role in colorectal tumorigenesis and progression. Considering the vital importance of the tumor environment, we classified colorectal cancer patients into distinct tumor types based on the context of the tumor environment for further analysis. The top 20 genes with the highest network degree in the yellow module and brown module were selected as the gene templates (Table S4). The gene templates and genes in the whole module were highly correlated in both the yellow (Spearman's correlation coefficient = 0.86, P < 0.001, Figure 2A, left) and brown (Spearman's correlation coefficient = 0.73, P < 0.001, Figure 2A, right) modules. We assigned patients into three immune subtypes, namely, the “active stroma,” “active immune” and “mixed type” subtypes by applying NTP analysis using these curated gene templates (Figure 2B). NTP, a well-acknowledged signature-based disease classification method, uses only a list of gene signatures to assess the possibility of each single sample belonging to a specific classification. As shown in Figure 2B, 33.7% (217/644) of the colorectal cancer patients from the TCGA-COADREAD cohort were predicted as having an active stroma compartment, 29.8% (192/644) were predicted as having an active immune compartment, and the remaining samples that failed to be classified into these categories (with an FDR above 0.2) fell into the third cluster, termed the mixed type (Table S6). The GSEA of the hallmark gene sets (Table S5) showed that the presence of the active stroma subtype was associated with angiogenesis, EMT, and myogenesis (Figure 2C), and gene sets enriched in immune activation, such as the interferon alpha response, the gamma response and allograft rejection, were observed in the active immune subgroup (Figure 2D). The active immune subgroup exhibited a trend toward better recurrence-free survival than the other two subgroups (TCGA-COADREAD: cohort log-rank P = 0.036, Figure 2E; GSE39582 cohort: log-rank P = 0.021, Figure 2F), while no significant difference in overall survival was observed between these identified subtypes (data not shown).
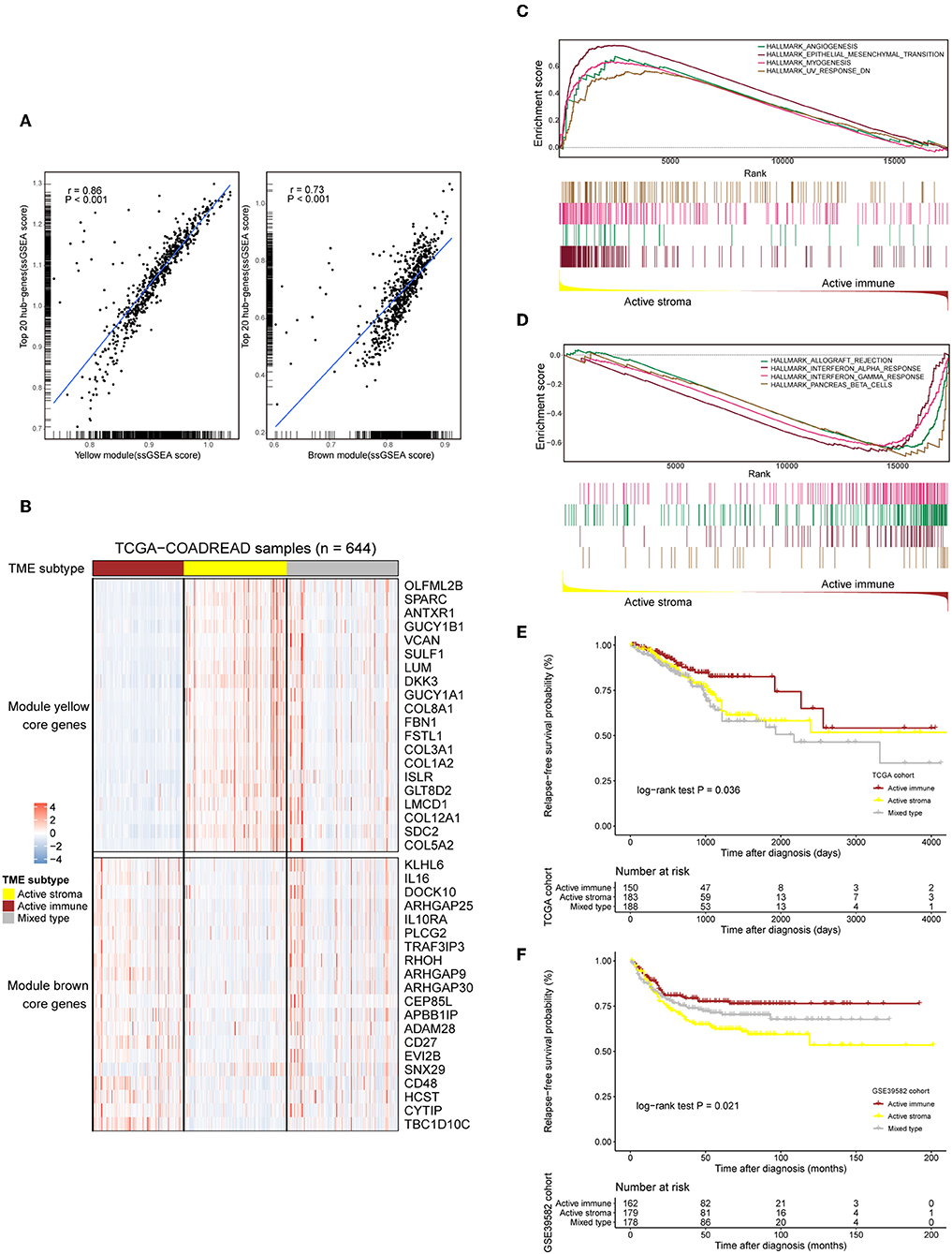
Figure 2. Selected gene template-based NTP reveals subtypes characterized by a distinct immune context and is associated with survival outcomes. (A) Correlation scatter plot of the 20 selected hub genes and genes in the whole module. The enrichment score of each sample in a given gene set was calculated through the ssGSEA algorithm. The correlation coefficient and the significance level of the test are annotated at the top left of the figure. Left: yellow module. Right: brown module. (B) Heat map showing the expression patterns of the selected hub genes in the TCGA-COADREAD cohort based on the identified immune subtypes. Genes annotated on the right side of the heat map are the selected core genes separated by the module to which they belong. The immune subtype classification of each sample is annotated at the top side of the heat map. Color intensities indicate the expression level of the genes. (C,D) GSEA plot of the enriched hallmark gene sets derived from the Molecular Signatures Database (MSigDB). The running score and preranked list are placed at the top and bottom of the GSEA plot. The middle indicates whether members of gene sets appear in the ranked list of genes. Multiple gene set enrichment results indicated by corresponding colors are shown on the same figure. (E,F) Kaplan–Meier curves for relapse-free survival (RFS) of the three identified immune subtypes in the TCGA-COADREAD and GSE39582 cohorts.
IFNG produced by immune cells in the tumor environment plays an important role in recruiting CD8 T cells and in NK cell infiltration (38). Cancers that feature high levels of angiogenesis factors usually exhibit an immunosuppressive phenotype, with high infiltration of regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) (39). Overall, the active immune subtype exhibits favorable immune conditions against tumor initiation and progression, while the active stroma subtype exhibits adverse conditions.
The distribution of sex, age at diagnosis and BMI were not different between these identified subtypes in TCGA-COADREAD cohort. Microsatellite instability-high (MSI-H) and -low (MSI-L) subtypes, CMS1 and CMS3, and early-stage tumors (stage I and stage II) were dominant in the active immune subtype, while the microsatellite stable (MSS) subtype, CMS4 and late-stage tumors (stage IV) were predominant in the active stroma subtype (Table 1).
Immunotherapy can induce the durable remission of metastatic melanoma and non-small cell lung cancer (NSCLC), yet only a small subset of patients obtain a clinical response. Thus, detecting patients with high sensitivity to immunotherapy before implementing treatment is of vital importance. To comprehensively depict the immune landscape of the identified subtypes, published functional gene sets (Table S11) were adopted to perform immune context annotation. Unexpectedly, the active immune group showed enriched T cells, cytotoxic lymphocytes, a high expression of immune checkpoints (PD1, PD-L1, and CTLA4) and some active immune response factors, including T cell cytotoxicity factors (GZMA, GZMB, and IFNG), and B cell markers (CD86 and CD80). In contrast, the active stroma group was enriched with endothelial cells, fibroblasts, EMT features and TGF-β signature genes (Figure 3A). Given the significant correlation between the expression of immune checkpoints (PD-1, PD-L1, and CTLA-4), the infiltration numbers of cytotoxic T cells and the immunosuppressive microenvironment with ICB responses (40, 41), we further explored the potential immunotherapy treatment benefit of the active immune group. SubMap analysis showed that the active stroma group shares high similarity with anti-PD-1 resistance in melanoma patients (Figure 3B, top left) and metastatic urothelial tumors treated with PD-L1 checkpoint inhibitors (Figure 3B, top right). The active immune class shares high similarity with melanoma patients who responded to treatment with MAGE-A3 (Figure 3B, bottom left) and BALB/c mice who responded to treatment with anti-CTLA-4 (Figure 3B, bottom right). Patients with a clinical response status to immunotherapy, including a complete response (CR) or partial response (PR), were considered immunotherapy responders, while those with stable disease (SD) or progressive disease (PD) were considered immunotherapy non-responders. Distinct immunotherapeutic regimens exhibited non-conformity with the identified immune subtypes, which might be due to the distinct resistance and reactive mechanisms of cancer cells adopted under different regimens and cancers (15, 42, 43). SubMap analysis on another colorectal cancer cohort, GSE39582 (Figure S3B), also achieved similar results, further confirming the non-conformity between the identified colorectal subtypes and the immunotherapy-treated cohorts that was mainly caused by distinct immunotherapy regimens and adopted tumor types. In addition, the expression of eight biomarkers established in the POPLAR trial (44), except for GZMB, was significantly higher in the active immune group than in the active stroma group (Figure 3C). By applying the identical analysis to the GSE39582 cohort, we obtained similar results (Figures S3A,B,D), which served as independent cross-validation. No difference was observed in tumor purity between these identified subtypes (Figure S3C, Table S9), thus eliminating potential bias elicited by differences in purity. Thus, our identified subtypes characterized by distinct immune contexts and their relationship with the immunotherapy treatment response may provide valuable information for clinical treatment decisions.
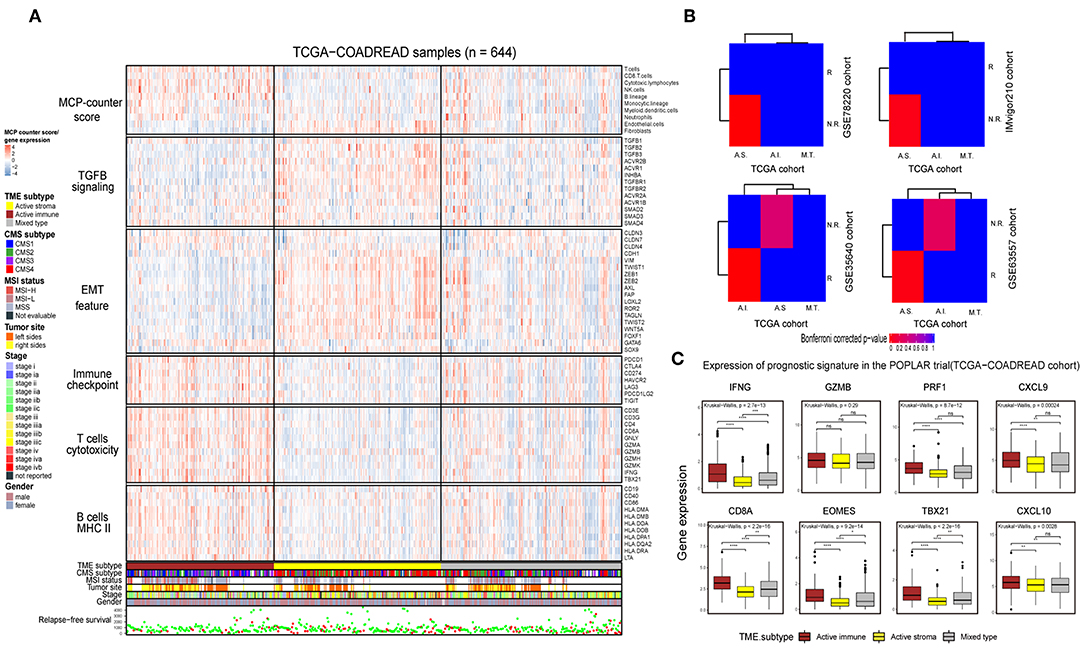
Figure 3. Identification of immune subtypes with distinct immune contexts and immunotherapeutic responses. (A) Complex heat map of the functional gene signature and immune cell infiltration score across immune subtypes in the TCGA-COADREAD cohort. The immune cell infiltration score was generated by MCP-counter. Corresponding feature names of the gene signatures are shown on the left side of the heat map. The TME subtype, CMS subtype, MSI status, tumor site, stage, sex and RFS are annotated in the lower panel. Color intensities indicate the expression level of the genes or the infiltration score of immune cells. (B) SubMap analysis of the TCGA-COADREAD cohort and four independent preimmunotherapeutic treatment datasets. The active stroma subtype shares high similarity with the immunotherapeutic resistance class in the GSE78220 and IMvigor210 cohorts, while the active immune subtype shares high similarity with the immunotherapeutic response class in the GSE35640 and GSE63557 cohorts, and the mixed type is not associated with either responders or non-responders. The colors labeled in each cell reflect the P-values of each subclass association. A.I., A.S., and M.T. represent the active immune, active stroma and mixed type, respectively. (C) Box plot of prognostic genes in the POPLAR study, with expression profiling among the immune classes. The gene expression level was normalized by log2 (TPM+1) transformation. The statistical significance of pairwise comparisons is annotated with symbols in which ns, *, **, and *** and **** represent not significant (P > 0.05), P ≤ 0.05, P ≤ 0.01, P ≤ 0.001, and P ≤ 0.0001, respectively. The Wilcoxon rank-sum test was used for comparisons between two groups, and the Kruskal–Wallis test was used for comparisons between more than two groups.
Recent analyses have linked the tumor genomic landscape with tumor cytolytic activity, indicating that a high TMB and specific somatic mutations are associated with antitumor immunity (45). The associated genomic data available in the TCGA database allowed us to investigate the underlying genomic mechanisms. In terms of TMB, patients in the active immune group and the mixed type group showed a higher TMB than those in the active stroma group (Table S10: active immune vs. active stroma: Wilcoxon rank-sum test P = 0.00016; mixed type vs. active stroma: Wilcoxon rank-sum test P = 0.00019). Notably, no significant difference was found between the active immune and mixed type groups (Table S10: active immune vs. mixed type: Wilcoxon rank-sum test P = 0.62), and the mixed type group did not exhibit particular immunologic characteristics or immunotherapeutic benefits compared with the active immune group (Figure 3B), which indicates that our immune subtype classification can identify patients with inflammatory milieu under similar TMB backgrounds. TMB of each sample in the TCGA-COADREAD cohort can be found in Table S8. To systematically elucidate the underlying genomic mechanism, we focused on 43 significantly mutated genes (SMGs) identified through MutSigCV_1.41 under a stringent threshold (q < 0.05) and identified 14 different mutated genes between the active stroma and active immune groups with a Benjamini-Hochberg-adjusted P < 0.05 (Figure 4B, Table S7). The identified mutated genes involved in antigen presentation (B2M) (45), cell cycle regulation (FBXW7) (46), the MAP kinase/ERK signaling pathway (BRAF) and the extrinsic apoptosis pathway (CASP8) (25)have been previously reported to be positively associated with immune cytolytic activity and the expression of costimulatory genes frequently mutated in the active immune group, while tumor suppressor genes including TP53 and APC were more frequently mutated in the active stroma group. TP53 (47) controls the expression of hundreds of genes involved in immunity, and the activation of the Wnt/beta-catenin pathway leads to a non-inflammatory milieu (48). These data suggest that tumor cells might adapt distinct escape mechanisms, primarily by impairing the extrinsic apoptosis pathway and antigen presentation in the active immune group and through the exclusion of immune effector cells in the active stroma group.
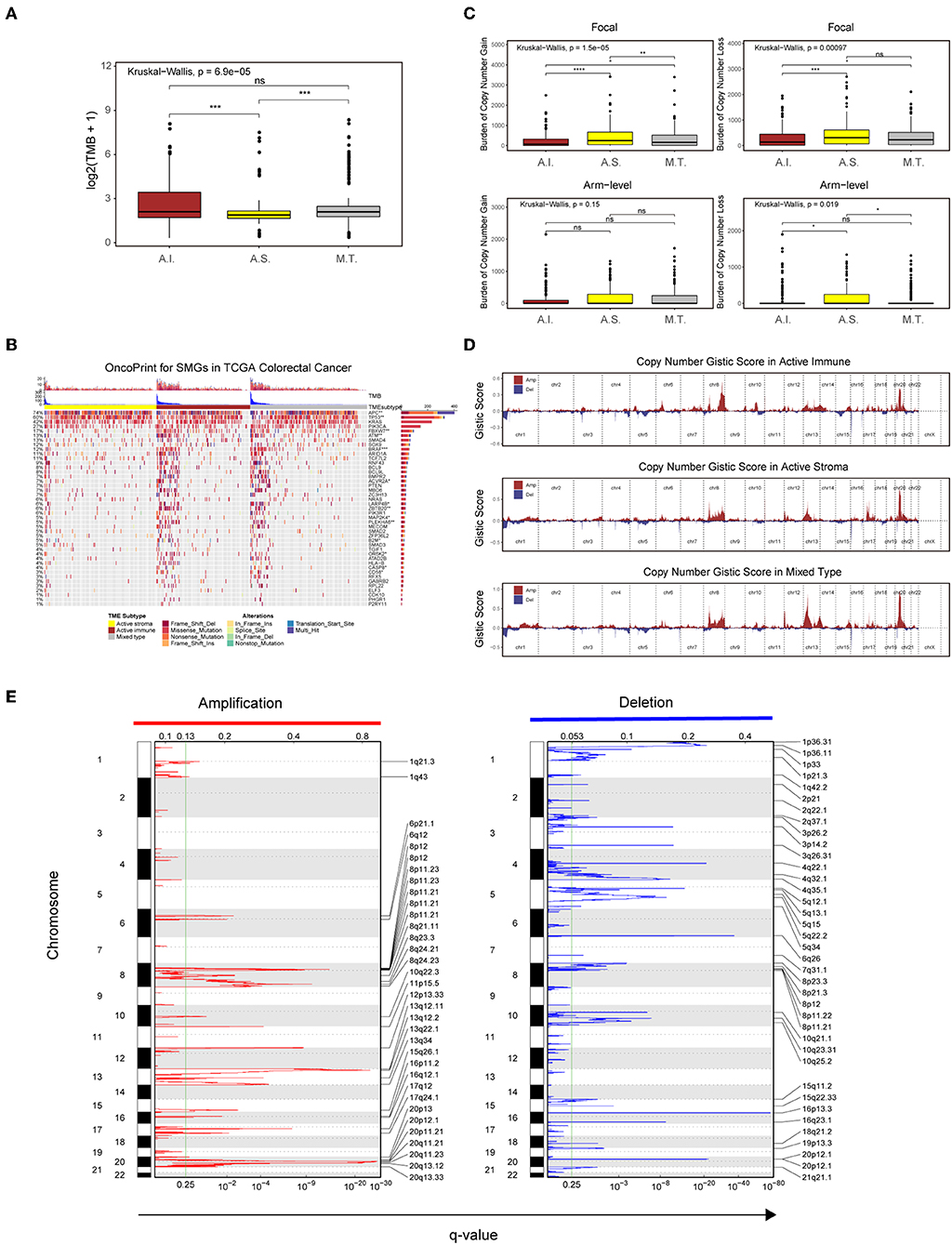
Figure 4. Genetic and copy number alterations across immune subtypes. (A,C) Distribution of TMB (A) and focal and broad copy number alterations (C) among the TME subtypes. The statistical significance of pairwise comparisons is annotated with symbols in which ns and *** represent not significant (P > 0.05) and P ≤ 0.001, respectively. A.I., A.S., and M.T. represent the active immune, active stroma and mixed type, respectively. (B) Mutation landscape of SMGs in the TCGA-COADREAD cohort. Genes are ordered by decreasing mutation frequency, and samples are sorted by the TMB in each subgroup. OncoPrint bar plot annotation; TMB and the TME subtype are annotated in the upper panel. Genes with a significant difference between the active immune and active stroma subtypes are annotated with symbols in which *, **, and *** represent P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively. (D) Copy number profiles for the active stroma, active immune and mixed subtypes, with gains in dark red and losses in midnight blue. Gene segments are placed according to their location on chromosomes, ranging from chromosome 1 to chromosome 22. (E) Detailed cytoband with focal amplification (left) and focal deletion (right) in the active stroma group generated with GISTIC_2.0 software. The q value of each locus is plotted horizontally.
Given the recently reported evidence that a high burden of copy number loss is positively related to anti-CTLA-4 blockade resistance (49, 50), we next explored copy number alterations between these distinct immune groups. Similar to the findings of previous reports, patients within the active immune group showed a lower burden of gain and loss at the focal level and a lower burden of gain at the arm level compared with those in the active stroma group (Table S10: active immune vs. active stroma on focal-level gain burden: P = 0.0000023; focal-level loss burden: P = 0.00025; and arm-level gain burden: P = 0.053). Copy number burden of each sample in the TCGA-COADREAD cohort can be found in Table S8. Figure 4D shows the distribution of the G-score across all chromosomes in these subtypes. Focal amplifications (13q34, 20p11.21, and 20q13.33) and deletions (4q32.1, 5q15, and 5q34) within chromosomal regions were detected in the active stroma group (Figure 4E). The focal alterations of the active immune and mixed type groups are shown in Figure S4. The somatic copy number alteration (SCNA) level correlated with reduced cytotoxic immune infiltration, while the increased total mutation number correlated with high immune infiltration in colorectal cancer. The burden of copy number gain and loss in the mixed type group fell between those of the active immune and active stroma groups at the focal level (Figure 4C). It appears that focal copy number alterations strongly contribute to the difference in immune infiltration in colorectal cancer.
Platinum and 5-fluorouracil are widely used in the treatment of advanced colorectal cancer. Considering that chemotherapy is the most widely used strategy in the treatment of colorectal cancer, we used the “pRRophetic” package to predict the treatment response to 5-fluorouracil and cisplatin. The active immune subtype was more sensitive to 5-fluorouracil, while the active stroma subtype was more sensitive to cisplatin (Figure 5A) in TCGA-COADREAD cohort, strong concordance between chemotherapeutic treatment sensitive and TME-based subtypes was also seen in GSE39582 cohort (Figure S3F). This result can be used to guide the personalized treatment of colorectal cancer patients.
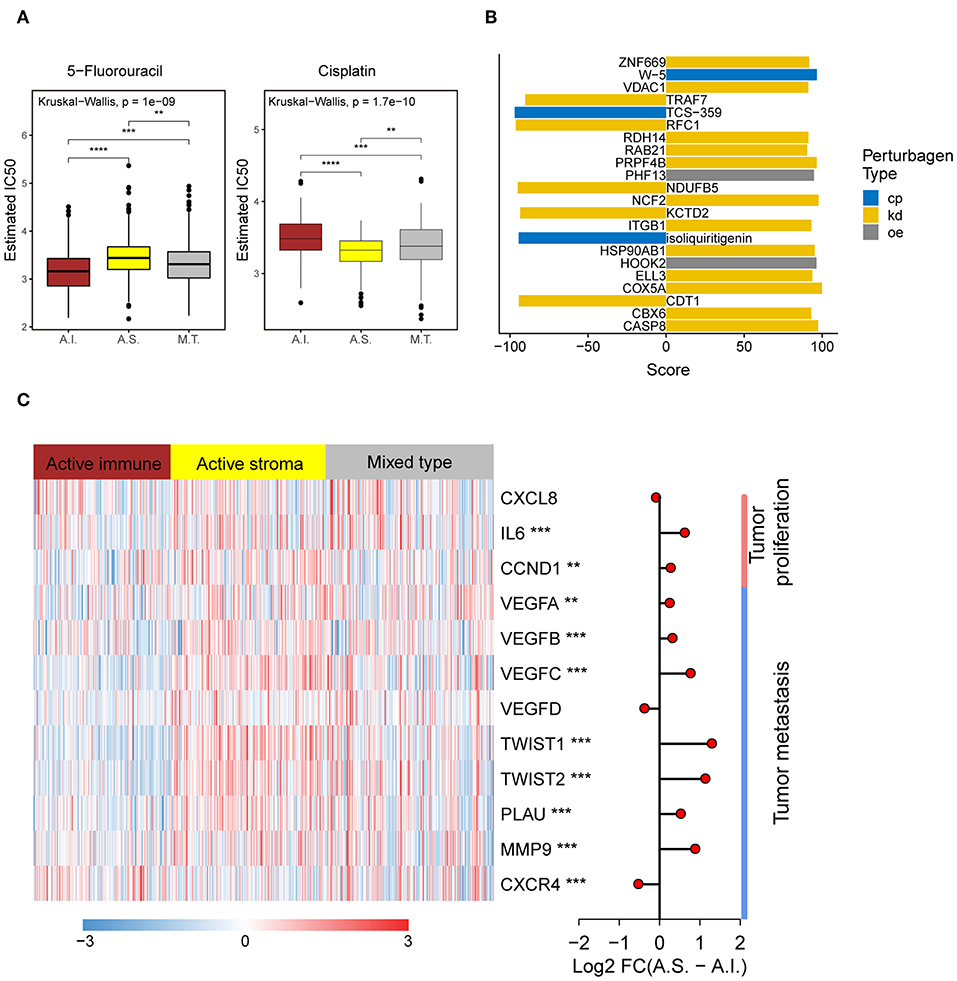
Figure 5. Different chemotherapeutic sensitivities across immune subtypes and activation of the NF-kB pathway in the active stroma. (A) Distribution of the estimated IC50 of 5-Fluorouracil and Cisplatin among the TME subtypes in TCGA-COADREAD cohort. The statistical significance of pairwise comparisons is annotated with symbols in which ns, *, **, and *** represent not significant (P > 0.05), P ≤ 0.05, P ≤ 0.001, and P ≤ 0.0001, respectively. A.I., A.S., and M.T. represent the active immune, active stroma and mixed type, respectively. (B) Bar plot of the candidate perturbations inferred from connectivity map analysis. Cp, kd, and oe represent compound, knockdown and overexpression, respectively. The score value placed on x-axis represents a holistic measurement of the relationship between the query gene set and the perturbation. The higher positive score, the more similar between the query and the perturbation. On the contrary, the lower negative score, the more opposing. (C) Complex heat map of the downstream genes of the NF-kB pathway. Genes with a significant difference between the active immune and active stroma subtypes are annotated with symbols in which **, and *** represent P ≤ 0.01, and P ≤ 0.001, respectively. Log2-fold change between the active stroma and active immune subtypes is shown on the right panel.
To identify candidate drugs and small molecules targeting the active stroma, we employed Connectivity Map (CMap) tools (34). CMap is a data-driven algorithm connecting genes, drugs and diseases and is widely used to discover potential therapeutic drugs and small molecules and to explore the mechanism of action underlying these drugs (51). We identified 22 candidate small molecules with absolute connectivity scores >90 (Figure 5B). We observed that knocking down the TRAF7-derived gene signature was strongly anticonnected with active stroma patients. TRAF7 is a signal transducer for members of the TNF receptor superfamily, indicating the activation of the NF-kB pathway in the active stroma. It has been reported that the activation of NF-kB in pancreatic stellate cells prevents the infiltration of cytotoxic T cells by upregulating CXCL12 in pancreatic cancer (52). Overexpression of its downstream genes, such as MMP9 and IL6, was observed in the active stroma subtype compared with the active immune subtype (Figure 5C). Thus, strategies to deactivate the NF-kB pathway, such as blocking TRAF7, might be useful to enforce the infiltration of cytotoxic T cells and to kill colorectal cancer cells.
The colorectal gland epithelia and surrounding tumor environment interact and are mutually restricted during tumorigenesis. Normal colorectal glands mainly participate in digestion, iron metabolic processes and immunologic processes, including T cell activation, B cell activation and lymphocyte differentiation; these processes are enriched in its matching microenvironment to provide a defense mechanism against hostile factors to reach self-stabilization. In contrast, the tumor epithelium proliferates rapidly with the corresponding immunosuppressive environment, and thus, cancer cells are not expunged. Complementary changes in the tumor gland and microenvironment lead to cancer progression, metastasis and drug resistance. To date, no studies have systematically elaborated on the molecular interaction patterns of the epithelium and stroma during colorectal cancer initiation. Recently, rapidly developed technologies, such as single-cell RNA-seq, have allowed us to clarify the complex and dynamic relationship between cells (53, 54). However, the spatial location information of cells, which is of vital importance during tumorigenesis and progression, has been overlooked. Combining microdissection transcriptome profiling with single-cell RNA-seq or other developing technologies, such as Slide-seq (55), might help us determine spatial cell communication patterns.
We first revealed three molecular subsets, namely, the “active immune,” “active stroma” and “mixed type” subtypes based on the NTP method using gene templates established from stromal and immune compartments. The active immune and active stroma groups share similar characteristics with typically defined “hot tumors” and “cold tumors,” respectively. Hot tumors are characterized by a high degree of T cell and cytotoxic T cell infiltration and the overexpression of immune checkpoints such as PD-1, PD-L1, and LAG3 compared with cold tumors (56, 57). These identified subsets exhibited a distinct immune context, prognosis, and immunotherapy benefit, which supports the idea that the immune environment is of vital importance in predicting patient prognosis and evaluating the response rate of checkpoint inhibitor immunotherapies.
Genomic analysis revealed a distinct mutation and copy number change landscape. Patients in the active immune group exhibited a higher TMB, a lower copy number burden and enriched mutations that correlated with local immune cytolytic activity compared with those in the active stroma group. A significant negative correlation between TMB and the copy number alteration level has been observed in colorectal cancer (58). Ciriello et al. categorized colorectal cancer as an M class that was characterized by recurrent mutations other than recurrent copy number alterations, and McGrail et al. showed that neoantigen levels were predictive of cytotoxic T lymphocyte (CTL) infiltration (59, 60). However, Davoli et al. showed that arm/chromosome SCNAs provided a larger contribution to the immune signature than the total number of mutations, and the focal SCNA level failed to be selected for the prediction model. Immune cell infiltration mainly driven by mutations or copy number alterations is still a controversial issue. Our identified immune subtypes attached importance to focal number alterations. Nevertheless, these results revealed that distinct immunologic phenotypes have distinct genomic features (61).
Distinct immune cell infiltration patterns revealed a distinct tumor escape mechanism (62) and a remarkable difference in the prognosis of colorectal cancer (63).
The systematic analysis underscored the role of NF-kB pathway activation in leading low immune infiltration. Taken together, our results provide a systematic analysis of the biological changes in the epithelial and stromal compartments of colorectal cancer. Immunotherapy for colorectal cancer is approved only for microsatellite instability-high (MSI-H) patients, which comprise only a small subset (64), by the Food and Drug Administration (FDA). Since the TMB level of colorectal cancer ranks higher than that in other cancers (e.g., TCGA pan-cancer) (65), we have good reason to believe that the prospects for immunotherapy in colorectal cancer are optimistic. Strategies that block the NF-kB pathway might turn an active stroma compartment into an active immune compartment, thus increasing the opportunity for a response to ICB treatment and improving survival. The close interplay between intrinsic traits (mutation landscape and copy number alterations) and extrinsic characteristics (infiltrated immune cells) in our identified immune subtypes and their different tendencies toward the ICB treatment response might help guide immunotherapy decisions in colorectal cancer.
The datasets generated in this study can be found in the Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) under the accession numbers GSE136735, and the access to other datasets used in this study can be found in the article when they are mentioned.
The studies involving human participants were reviewed and approved by Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. The patients/participants provided their written informed consent to participate in this study.
All authors have reviewed the data analyses. SC and LF conceived and designed the whole study. RS performed the data analyses and wrote the manuscript. BL collected clinical samples, and PL performed the microdissection and microarray experiments. BZ provided help with the computational analysis. All authors read, critically revised, and approved the final manuscript.
This study was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-3-005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank all the patients who selflessly donated their tumor specimens to this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.01497/full#supplementary-material
Figure S1. Representative histopathological images of enrolled samples and the schematic diagram of manual microdissection. Representative histopathological image in hematoxylin-eosin staining of colorectal cancer tissue (A) in x100 and adjacent normal tissue (B) in 100x enrolled in this study (left) and the selected stromal compartment subjected to microdissection is noted in black circle (right).
Figure S2. Immune cell infiltration pattern in the epithelium and stroma of colorectal cancer and adjacent normal tissues and weighted gene coexpression network construction and evaluation. (A) Unsupervised hierarchical clustering analysis of the MCP-counter quantified immune scores in microdissection-processed colorectal cancer and adjacent normal tissues. The cluster distance was calculated with the Ward.D2 method. (B) Volcano plot showing immune cell enrichment differences between the normal stroma and tumor stroma. The Wilcoxon rank-sum test was used to compare differences, and the BH method was adopted to adjust P-values. (C) Scale-free fit index (left) and network mean connectivity (right) for various soft-thresholding powers. Red line in left plot indicates the predefined high value which is 0.9. Here, we choose the power 10 as the soft-thresholding power, which is the lowest power before the scale-free topology fit index curve reaching the predefined high value. (D) The scale-free plot indicates that our constructed network has a power-law degree distribution. (E,F) Network visualization of the selected gene template in the brown (E) and yellow (F) modules identified by WGCNA.
Figure S3. Identification of immune subtypes with distinct immune contexts and immunotherapeutic responses. (A) Heat map of the functional signature genes and immune cell infiltration extent across immune subtypes in the GSE39582 cohort. The immune cell infiltration score was generated by MCP-counter. The TME subtype, CMS subtype, MSI status, tumor site, stage, sex, and RFS are annotated in the lower panel. (B) SubMap analysis of the GSE39582 cohort and four independent preimmunotherapeutic treatment datasets. The active stroma subtype shares high similarity with the immunotherapeutic resistance class in the GSE78220 and IMvigor210 cohorts, while the active immune subtype shares high similarity with the immunotherapeutic response class in the GSE35640 and GSE63557 cohorts, and the mixed type is not associated with either responders or non-responders. The colors labeled in each cell reflect the P-values for each subclass association. A.I., A.S., and M.T. represent the active immune, active stroma and mixed type, respectively. (C) Distribution of tumor purity across immune subtypes in the TCGA-COADREAD (left) and GSE39582 (right) cohorts in which ns represents no significant difference been detected. (D) Box plot of prognostic genes in the POPLAR study, with expression profiling among the immune classes. The gene expression level was normalized by log2 (TPM+1) transformation. The statistical significance of pairwise comparisons is annotated with symbols in which ns, *, **, ***, and **** represent not significant (P > 0.05), P ≤ 0.05, P ≤ 0.01, P ≤ 0.001, and P ≤ 0.0001, respectively. (E) Alluvial diagram showing associations among the TME subtype, CMS subtype and MSI status. (F) Distribution of the estimated IC50 of 5-Fluorouracil and Cisplatin among the TME subtypes in GSE39582 cohort. The statistical significance of pairwise comparisons is annotated with symbols in which *, **, and **** represent P > 0.05, P ≤ 0.01, and P ≤ 0.0001, respectively. A.I., A.S., and M.T. represent the active immune, active stroma and mixed type, respectively. The Wilcoxon rank-sum test was used for comparisons between two groups, and the Kruskal–Wallis test was used for comparisons between more than two groups (C,D,F).
Figure S4. Focal alterations in the active stroma and mixed type groups. (A) Detailed focal amplification (left) and focal deletion (right) in the active immune group generated with GISTIC_2.0 software. (B) Detailed focal amplification (left) and focal deletion (right) in the mixed type group generated with GISTIC_2.0 software.
Table S1. Clinical characteristics of enrolled samples in WGCNA analysis.
Table S2. Top 8000 genes with highest standard deviation in microdissection microarray.
Table S3. Gene Ontology-Biology process enrichment analysis of selected four module.
Table S4. Subtype template genes.
Table S5. Gene Set Enrichment Analysis of hallmark geneset derived from Molecular Signatures Database (MSigDB) in active immune and active stroma class.
Table S6. Nearest template prediction analysis on TCGA COAD-READ cohort and GSE39582 cohort.
Table S7. Wilcox test analysis on identified significant mutated genes between active stroma and active immune class.
Table S8. Tumor mutation burden and copy number burden among TME subtype.
Table S9. Tumor purity in TCGA COAD-READ cohort and GSE39582 cohort.
Table S10. Paired comparison detail among immune subtypes.
Table S11. Dataset and gene sets enrolled in this study.
ICB, immune checkpoint blockade; LCM, laser capture microdissection; TME, tumor microenvironment; AJCC, American Joint Committee on Cancer; TPM, transcripts per kilobase million; FPKM, fragments per kilobase million; CIBERSORT, cell type identification by estimating relative subset of known RNA transcripts; MCP-counter, Microenvironment Cell Populations-counter; PCA, principal component analysis; WGCNA, weighted gene coexpression network analysis; TMB, tumor mutation burden; CMS, consensus molecular subtype; NTP, nearest template prediction; TCGA, The Cancer Genome Atlas; SCNA, somatic copy number alteration; MSI-H, microsatellite instability-high; FDA, Food and Drug Administration. CMap, Connectivity Map.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Oh EY, Christensen SM, Ghanta S, Jeong JC, Bucur O, Glass B, et al. Extensive rewiring of epithelial-stromal co-expression networks in breast cancer. Genome Biol. (2015) 16:128. doi: 10.1186/s13059-015-0675-4
3. Tyekucheva S, Bowden M, Bango C, Giunchi F, Huang Y, Zhou C, et al. Stromal and epithelial transcriptional map of initiation progression and metastatic potential of human prostate cancer. Nat Commun. (2017) 8:420. doi: 10.1038/s41467-017-00460-4
4. Yeung TL, Sheng J, Leung CS, Li F, Kim J, Ho SY, et al. Systematic identification of druggable epithelial-stromal crosstalk signaling networks in ovarian cancer. J Natl Cancer Inst. (2019) 111:272–82. doi: 10.1093/jnci/djy097
5. Nishida N, Nagahara M, Sato T, Mimori K, Sudo T, Tanaka F, et al. Microarray analysis of colorectal cancer stromal tissue reveals upregulation of two oncogenic miRNA clusters. Clin Cancer Res. (2012) 18:3054–70. doi: 10.1158/1078-0432.CCR-11-1078
6. Sadanandam A, Lyssiotis CA, Homicsko K, Collisson EA, Gibb WJ, Wullschleger S, et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat Med. (2013) 19:619–25. doi: 10.1038/nm.3175
7. De Sousa EMF, Wang X, Jansen M, Fessler E, Trinh A, de Rooij LP, et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat Med. (2013) 19:614–8. doi: 10.1038/nm.3174
8. Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. (2015) 21:1350–6. doi: 10.1038/nm.3967
9. Goncalves-Ribeiro S, Sanz-Pamplona R, Vidal A, Sanjuan X, Guillen Diaz-Maroto N, Soriano A, et al. Prediction of pathological response to neoadjuvant treatment in rectal cancer with a two-protein immunohistochemical score derived from stromal gene-profiling. Ann Oncol. (2017) 28:2160–8. doi: 10.1093/annonc/mdx293
10. Mahajan UM, Langhoff E, Goni E, Costello E, Greenhalf W, Halloran C, et al. Immune cell and stromal signature associated with progression-free survival of patients with resected pancreatic ductal adenocarcinoma. Gastroenterology. (2018) 155:1625–39 e2. doi: 10.1053/j.gastro.2018.08.009
11. Wang Z, Tang Y, Tan Y, Wei Q, Yu W. Cancer-associated fibroblasts in radiotherapy: challenges and new opportunities. Cell Commun Signal. (2019) 17:47. doi: 10.1186/s12964-019-0362-2
12. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. (2018) 24:541–50. doi: 10.1038/s41591-018-0014-x
13. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. (2019) 19:133–50. doi: 10.1038/s41568-019-0116-x
14. Wang L, Saci A, Szabo PM, Chasalow SD, Castillo-Martin M, Domingo-Domenech J, et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat Commun. (2018) 9:3503. doi: 10.1038/s41467-018-05992-x
15. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to Anti-PD-1 therapy in metastatic melanoma. Cell. (2016) 165:35–44. doi: 10.1016/j.cell.2016.02.065
16. Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. (2017) 18:248–62. doi: 10.1016/j.celrep.2016.12.019
17. Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. (2015) 43:e47. doi: 10.1093/nar/gkv007
18. Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. (2007) 23:1846–7. doi: 10.1093/bioinformatics/btm254
19. Colaprico A, Silva TC, Olsen C, Garofano L, Cava C, Garolini D, et al. TCGAbiolinks: an R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. (2016) 44:e71. doi: 10.1093/nar/gkv1507
20. Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. (2013) 31:2388–95. doi: 10.1200/JCO.2012.44.3762
21. Lesterhuis WJ, Rinaldi C, Jones A, Rozali EN, Dick IM, Khong A, et al. Network analysis of immunotherapy-induced regressing tumours identifies novel synergistic drug combinations. Sci Rep. (2015) 5:12298. doi: 10.1038/srep12298
22. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. (2018) 554:544–8. doi: 10.1038/nature25501
23. Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. (2008) 9:559. doi: 10.1186/1471-2105-9-559
24. Hoshida Y. Nearest template prediction: a single-sample-based flexible class prediction with confidence assessment. PLoS ONE. (2010) 5:e15543. doi: 10.1371/journal.pone.0015543
25. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. (2018) 48:812–30 e14. doi: 10.1016/j.immuni.2018.03.023
26. Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. (2012) 30:413–21. doi: 10.1038/nbt.2203
27. Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. (2013) 4:2612. doi: 10.1038/ncomms3612
28. Hoshida Y, Brunet JP, Tamayo P, Golub TR, Mesirov JP. Subclass mapping: identifying common subtypes in independent disease data sets. PLoS ONE. (2007) 2:e1195. doi: 10.1371/journal.pone.0001195
29. Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. (2016) 32:2847–9. doi: 10.1093/bioinformatics/btw313
30. Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. (2013) 499:214–8. doi: 10.1038/nature12213
31. Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. (2011) 12:R41. doi: 10.1186/gb-2011-12-4-r41
32. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. (2012) 16:284–7. doi: 10.1089/omi.2011.0118
33. Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. (2016) 17:218. doi: 10.1186/s13059-016-1113-y
34. Subramanian A, Narayan R, Corsello SM, Peck DD, Natoli TE, Lu X, et al. A Next Generation Connectivity Map: L1000 platform and the First 1,000,000 Profiles. Cell. (2017) 171:1437–52 e17. doi: 10.1016/j.cell.2017.10.049
35. Geeleher P, Cox N, Huang RS. pRRophetic: an R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS ONE. (2014) 9:e107468. doi: 10.1371/journal.pone.0107468
36. Geeleher P, Cox NJ, Huang RS. Clinical drug response can be predicted using baseline gene expression levels and in vitro drug sensitivity in cell lines. Genome Biol. (2014) 15:R47. doi: 10.1186/gb-2014-15-3-r47
37. Nagasaki T, Hara M, Nakanishi H, Takahashi H, Sato M, Takeyama H. Interleukin-6 released by colon cancer-associated fibroblasts is critical for tumour angiogenesis: anti-interleukin-6 receptor antibody suppressed angiogenesis and inhibited tumour-stroma interaction. Br J Cancer. (2014) 110:469–78. doi: 10.1038/bjc.2013.748
38. Benci JL, Johnson LR, Choa R, Xu Y, Qiu J, Zhou Z, et al. Opposing functions of interferon coordinate adaptive and innate immune responses to cancer immune checkpoint blockade. Cell. (2019) 178:933–48 e14. doi: 10.1016/j.cell.2019.07.019
39. Rahma OE, Hodi FS. The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res. (2019) 25:5449–57. doi: 10.1158/1078-0432.CCR-18-1543
40. Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. (2018) 24:1550–8. doi: 10.1038/s41591-018-0136-1
41. Ock CY, Hwang JE, Keam B, Kim SB, Shim JJ, Jang HJ, et al. Genomic landscape associated with potential response to anti-CTLA-4 treatment in cancers. Nat Commun. (2017) 8:1050. doi: 10.1038/s41467-017-01018-0
42. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. (2017) 127:2930–40. doi: 10.1172/JCI91190
43. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. (2017) 168:542. doi: 10.1016/j.cell.2017.01.010
44. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. (2016) 387:1837–46. doi: 10.1016/S0140-6736(16)00587-0
45. Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. (2015) 160:48–61. doi: 10.1016/j.cell.2014.12.033
46. Mlecnik B, Bindea G, Kirilovsky A, Angell HK, Obenauf AC, Tosolini M, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. (2016) 8:327ra26. doi: 10.1126/scitranslmed.aad6352
47. Munoz-Fontela C, Mandinova A, Aaronson SA, Lee SW. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat Rev Immunol. (2016) 16:741–50. doi: 10.1038/nri.2016.99
48. Pai SG, Carneiro BA, Mota JM, Costa R, Leite CA, Barroso-Sousa R, et al. Wnt/beta-catenin pathway: modulating anticancer immune response. J Hematol Oncol. (2017) 10:101. doi: 10.1186/s13045-017-0471-6
49. Roh W, Chen PL, Reuben A, Spencer CN, Prieto PA, Miller JP, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med. (2017) 9:eaah3560. doi: 10.1126/scitranslmed.aah3560
50. Sia D, Jiao Y, Martinez-Quetglas I, Kuchuk O, Villacorta-Martin C, Castro de Moura M, et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology. (2017) 153:812–26. doi: 10.1053/j.gastro.2017.06.007
51. Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, et al. Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell. (2018) 173:338–54 e15. doi: 10.1016/j.cell.2018.03.034
52. Garg B, Giri B, Modi S, Sethi V, Castro I, Umland O, et al. NFkappaB in pancreatic stellate cells reduces infiltration of tumors by cytotoxic T cells and killing of cancer cells, via up-regulation of CXCL12. Gastroenterology. (2018) 155:880–91 e8. doi: 10.1053/j.gastro.2018.05.051
53. Vento-Tormo R, Efremova M, Botting RA, Turco MY, Vento-Tormo M, Meyer KB, et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature. (2018) 563:347–53. doi: 10.1038/s41586-018-0698-6
54. Dong J, Hu Y, Fan X, Wu X, Mao Y, Hu B, et al. Single-cell RNA-seq analysis unveils a prevalent epithelial/mesenchymal hybrid state during mouse organogenesis. Genome Biol. (2018) 19:31. doi: 10.1186/s13059-018-1416-2
55. Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, et al. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science. (2019) 363:1463–7. doi: 10.1126/science.aaw1219
56. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. (2019) 18:197–218. doi: 10.1038/s41573-018-0007-y
57. Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. (2017) 17:559–72. doi: 10.1038/nri.2017.49
58. Davoli T, Uno H, Wooten EC, Elledge SJ. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. (2017) 355:eaaf8399. doi: 10.1126/science.aaf8399
59. Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. (2013) 45:1127–33. doi: 10.1038/ng.2762
60. McGrail DJ, Federico L, Li Y, Dai H, Lu Y, Mills GB, et al. Multi-omics analysis reveals neoantigen-independent immune cell infiltration in copy-number driven cancers. Nat Commun. (2018) 9:1317. doi: 10.1038/s41467-018-03730-x
61. Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. (2015) 5:43–51. doi: 10.1158/2159-8290.CD-14-0863
62. Rosenthal R, Cadieux EL, Salgado R, Bakir MA, Moore DA, Hiley CT, et al. Neoantigen-directed immune escape in lung cancer evolution. Nature. (2019) 567:479–85. doi: 10.1038/s41586-019-1032-7
63. Angelova M, Charoentong P, Hackl H, Fischer ML, Snajder R, Krogsdam AM, et al. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol. (2015) 16:64. doi: 10.1186/s13059-015-0620-6
64. Franke AJ, Skelton WP, Starr JS, Parekh H, Lee JJ, Overman MJ, et al. Immunotherapy for colorectal cancer: a review of current and novel therapeutic approaches. J Natl Cancer Inst. (2019) 111:1131–41. doi: 10.1093/jnci/djz093
Keywords: microdissection, colorectal cancer, tumor environment, weighted gene coexpression network analysis, immune subtypes, immunotherapy
Citation: Shen R, Li P, Li B, Zhang B, Feng L and Cheng S (2020) Identification of Distinct Immune Subtypes in Colorectal Cancer Based on the Stromal Compartment. Front. Oncol. 9:1497. doi: 10.3389/fonc.2019.01497
Received: 05 October 2019; Accepted: 12 December 2019;
Published: 10 January 2020.
Edited by:
Lucia Conti, Istituto Superiore di Sanità (ISS), ItalyReviewed by:
Ying Ma, University of Texas MD Anderson Cancer Center, United StatesCopyright © 2020 Shen, Li, Li, Zhang, Feng and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Feng, ZmVuZ2xpbkBjaWNhbXMuYWMuY24=; Shujun Cheng, Y2hlbmdzaGpAY2ljYW1zLmFjLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.