- 1Colorectal Unit, Department of Surgery, Royal Adelaide Hospital, Adelaide, SA, Australia
- 2Department of Surgery, Amsterdam University Medical Centers, Amsterdam, Netherlands
- 3Department of Surgery, Catharina Hospital, Eindhoven, Netherlands
- 4Discipline of Surgery, Faculty of Health and Medical Science, School of Medicine, University of Adelaide, Adelaide, SA, Australia
- 5Department of Surgery, Leiden University Medical Center, Leiden, Netherlands
- 6GROW, School of Oncology and Developmental Biology, University of Maastricht, Maastricht, Netherlands
- 7Department of Surgery, The Netherlands Cancer Institute-Antoni van Leeuwenhoek, Amsterdam, Netherlands
Background: In the West, pre-treatment abnormal lateral lymph nodes (LLN+) in patients with a low locally advanced rectal cancer (AJCC Stage III), are treated with neoadjuvant (chemo)radiotherapy (nCRT), without a lateral lymph node dissection (LLND). It has been suggested, however, that LLN+ patients have higher local recurrence (LR) rates than similarly staged patients with abnormal mesorectal lymph nodes only (LLN−), but no comparative data exist. Therefore, we conducted this international multi-center study in the Netherlands and Australia of Stage III rectal cancer patients with either LLN+ or LLN− to compare oncological outcomes from both groups.
Materials and Methods: Patients with Stage III low rectal cancer with (LLN+ group) or without (LLN− group) abnormal lateral lymph nodes on pre-treatment MRI were included. Patients underwent nCRT followed by rectal resection surgery with curative intent between 2009 and 2016 with a minimum follow-up of 2-years. No patient had a LLND. Propensity score matching corrected differences in baseline characteristics.
Results: Two hundred twenty-three patients could be included: 125 in the LLN+ group and 98 in the LLN− group. Between groups, there were significant differences in cT-stage and in the rate of adjuvant chemotherapy administered. Propensity score matching resulted in 54 patients in each group, with equal baseline characteristics. The 5-year LR rate in the LLN+ group was 11 vs. 2% in the LLN− group (P = 0.06) and disease-free survival (DFS) was 64 vs. 76%, respectively (P = 0.09). Five-year overall survival was similar between groups (73 vs. 80%, respectively; P = 0.90).
Conclusions: In Western patients with Stage III low rectal cancer, there is a trend toward worse LR rate and DFS rates in LLN+ patients compared to similarly staged LLN− patients. These results suggest that LLN+ patients may currently not be treated optimally with nCRT alone, and the addition of LLND requires further consideration.
Introduction
Local recurrences (LR) in patients who have previously been treated curatively for locally advanced low rectal cancer [American Joint Committee on Cancer (AJCC) Stage III] are associated with severe morbidities such as pain and reduced quality of life. Over the past three decades, the introduction of neoadjuvant (chemo)radiotherapy (nCRT) and the broad application of rectal resections according to the principle of total mesenteric excision (TME) have reduced 5-year LR rates to 5–10% (1–3). However, it has been suggested that patients with pre-treatment abnormal lateral lymph nodes (LLN+), which are present in approximately 15–20% of patients with Stage III rectal cancer, still have increased LR rates (4–6).
With the aim of reducing LR rates, treatment strategies for patients with LLN+ have evolved differently around the world. In the West, treatment consists of nCRT followed by TME surgery, typically without resecting the LLN+ (7). In contrast, in the East, the standard treatment is surgery combining TME with a lateral lymph node dissection (LLND), often without nCRT (8).
Interestingly, despite these differences in treatment approach, comparable LR rates have been reported: 6.9% in Eastern patients undergoing TME and LLND, and 5.8% in Western patient receiving nCRT and TME (9) while others have suggested favorable results following the Eastern approach (10, 11). Furthermore, some studies demonstrated that LLN+ cannot be eradicated completely by nCRT only, suggesting that the Western treatment may not be sufficient for local disease-control (12–14). On the other hand, a LLND is a complex surgical procedure with some risks of post-operative complications and long-term morbidity such as sexual and urinary dysfunction (15, 16). Therefore, if not oncologically necessary, it may be in the patient's best interest to omit this procedure. In addition, it is unclear whether combining nCRT and LLND adds anything over each alone.
Current knowledge about the behavior of LLN+ as distinct from mesorectal node positivity (N+) is inconclusive. There is only one small single-center study available looking at oncological outcomes following treatment of the two subcategories of Stage AJCC III patients: patients with LLN+ compared to those with abnormal mesorectal lymph nodes only (LLN−) (17). Therefore, this international multi-center cohort study was conducted to compare long-term oncological outcomes of patients with LLN+ and LLN−, who were both treated the same according to the Western protocol of nCRT followed by TME surgery.
Patients and Methods
The current study was conducted at three hospitals in the Netherlands (Antoni van Leeuwenhoek-Netherlands Cancer Institute in Amsterdam, Catharina Hospital in Eindhoven and Leiden University Medical Center in Leiden) and two hospitals in Australia (Royal Adelaide Hospital and St Andrew's Hospital, both in Adelaide).
Included were patients of 18 years of age and over, who were treated with curative intent between 2009 and 2016 for a low (within 8 cm of the anal verge) AJCC clinical Stage III locally advanced rectal cancer with abnormal mesorectal lymph nodes with or without LLN+ on pre-treatment magnetic resonance imaging (MRI). All patients had pre-treatment abnormal mesorectal lymph nodes on MRI imaging and/or LLN+ in the obturator, internal iliac, external iliac, and/or common iliac basins. Abnormal mesorectal lymph nodes and LLN+ were defined as nodes with a short-axis of ≥5 mm on MRI with or without malignant features (10, 14, 18). In order to identify eligible patients, during the study period pelvic MRI scans for rectal cancer were re-reviewed by senior radiologists at each site. All patients underwent nCRT followed by TME surgery. Excluded were patients with concurrent distant metastatic disease in para-aortic lymph nodes or distant organs at the time of diagnosis, those with previous radiotherapy (RT) to the pelvis precluding nCRT, and those who did not undergo TME surgery. The study was approved by the human research ethics committee at each site.
After tissue diagnosis, all patients were clinically staged according to the 7th edition of the AJCC Colon and Rectum Cancer Staging and discussed the local multidisciplinary team meetings (MDT) (4). Neoadjuvant therapy was conducted according to the hospital's local protocol and consisted of either short-course RT (5 × 5 Gray) or long-course CRT (45–50.4 Gray) applied in 28 fractions over 7 weeks applied to the pelvis with individually shaped portals and the use of a three-field or four-field box technique with concomitant one of the following chemotherapy regimens: FOLFOX (folinic acid, fluorouracil, and oxaliplatin) on the first day of each week of RT, daily oral capecitabine, or 5-fluorouracil for five 2-week cycles. For LLN+ patients, it was confirmed for each center that standard practice involved including the obturator and internal iliac compartments in the irradiated field. Following completion of nCRT, all patients underwent TME surgery with curative intent by means of a low anterior resection (LAR), an abdominoperineal resection (APR) or a pelvic exenteration in case of involvement of adjacent organs. Post-operative histopathological staging was performed on the surgical specimen. The patient was then again discussed at the MDT where consensus was reached for adjuvant treatment and follow-up. Adjuvant chemotherapy consisted of FOLFOX, 5-fluorouracil, capecitabine or capecitabine+oxaliplatin (CAPOX) for three 6–8 week cycles. LR were defined as tumor regrowth in the lower pelvis at the anastomotic site, at the site of the previously resected mesorectal tissues, or in the lateral compartment.
Included patients were divided into two groups: a LLN+ and a LLN− group (all stage III). Continuous variables are shown as medians with range, and categorical variables are presented as absolute numbers with percentages. Differences in characteristics between the LLN+ and the LLN− group were evaluated with the Mann Whitney U-test for continuous variables, and the Chi-square or the Fisher's exact test for categorical variables (19). LR-free survival (LRFS: defined as recurrent disease in the pelvis), distant metastatic-free survival (DMFS: defined as recurrent disease distally), disease-free survival (DFS: defined as recurrent disease anywhere), and overall survival (OS; defined as death due to any cause) were estimated using the Kaplan-Meier method, with the log-rank test from the day of surgery until detection of LR, distant metastases or date of death (20). To minimize the effect of confounding factors on the outcome between both groups, a propensity score matching was performed for the covariates gender, clinical tumor (cT)-stage, resection margin, adjuvant chemotherapy, and follow-up time. LLN+ patients in whom selected lateral lymph nodes were harvested were excluded from the propensity score matching. A p ≤ 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 25.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism version 8.0.2 (GraphPad Software Inc., San Diego, CA, USA).
Results
Complete Cohort
Baseline patient and tumor characteristics are shown in Table 1. A total of 223 patients with Stage III rectal cancer were included: 125 patients in the LLN+ group (lateral lymph node size range 5.1–48.0 mm), and 98 in the LLN− group. There was a significant difference in median height of the tumor from the anal verge on MRI: 3.2 cm in the LLN+ group (range 0.0–8.0 cm) and 6.0 cm in the LLN− group (range 0.1–8.0 cm, P < 0.0001). LLN+ patients had more cT4 disease compared to LLN− group (37 vs. 11%, P < 0.0001) and received more long-course CRT (83 vs. 69%, P = 0.02). All other baseline characteristics were similar between groups.
Low anterior resection was more frequently performed in the LLN− group (67 vs. 46% for LLN+ patients, P = 0.03), while LLN+ patients underwent more APRs (53 vs. 31% for the LLN− group, P = 0.003, Table 2). More lymph nodes were harvested in the LLN+ group (median 16 vs. 13, P = 0.002). In the LLN+ group, selected lateral lymph nodes were harvested in 10 patients (so-called “cherry-picking surgery”: no complete lateral lymph node dissection was performed, median nodes harvested: 1 node, range 1–3 nodes) of which in 6 tumor was harvested upon histopathological examination. Pathological TNM staging and negative resection margins (R0) were similar between groups (90 vs. 94% in the LLN+ and LLN− group, respectively, P = 0.28). More LLN− patients received adjuvant chemotherapy (70 vs. 29% for LLN+ patients, P < 0.0001).
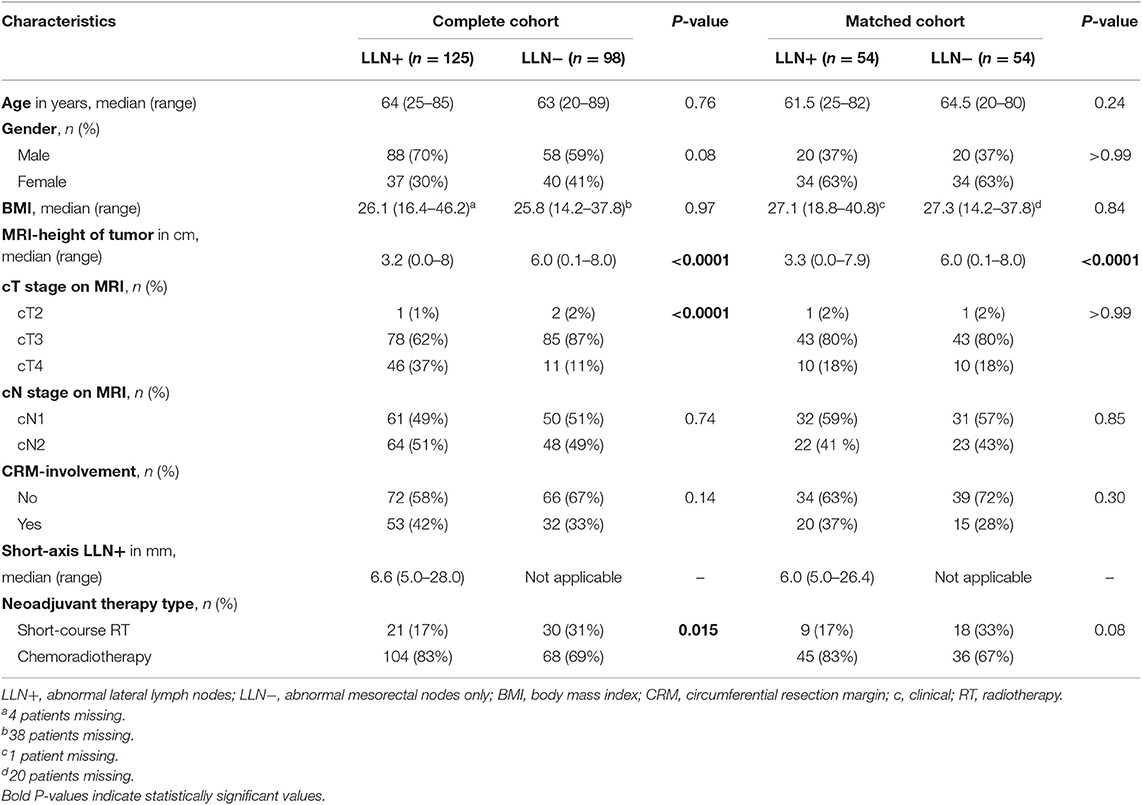
Median follow-up time for the LLN+ group was 57 months (range 1–98) and 59 months in the LLN− group (range 1–99; p = 0.55). The 5-year LRFS was significantly shorter in the LLN+ group compared to the LLN− group [88 vs. 98%, P = 0.009, hazard ratio (HR) 5.72, 95%CI 2.07–15.77, Figure 1A]. No differences between groups were seen in the 5-year DMFS (69 vs. 75%, P = 0.30, HR 1.33, 95%CI 0.78–2.28, Figure 1B), or DFS (63 vs. 73%, P = 0.15, HR 1.45, 95%CI 0.89–2.37, Figure 1C). Five-year OS in LLN+ patients was 72% and was 83% in the LLN− group (P = 0.06, HR 1.64, 95%CI 0.98–2.73, Figure 1D).
Figure 1. (A) Complete cohort local recurrence-free survival (LRFS) of LLN+ and LLN− patients [5-year LRFS 88 vs. 98%, respectively, P = 0.009, hazard ratio (HR) 5.72, 95%CI 2.07–15.77]. (B) Complete cohort distant metastatic-free survival (DMFS) of LLN+ and LLN− patients (5-year DMFS 69 vs. 75%, respectively, P = 0.30, HR 1.33, 95%CI 0.78–2.28). (C) Complete cohort disease-free survival (DFS) of LLN+ and LLN− patients (5-year DFS 63 vs. 73%, respectively, P = 0.15, HR 1.45, 95%CI 0.89–2.37). (D) Complete cohort overall survival (OS) of LLN+ and LLN− patients (5-year OS 72 vs. 83%, respectively, P = 0.06, HR 1.64, 95%CI 0.98–2.73).
Matched Cohort
After propensity score matching, 54 patients remained in each group. In the matched cohort, age, gender, body mass index (BMI), cT-stage, clinical nodal (cN) stage, pre-treatment tumor involvement of the circumferential resection margin and nCRT were similar between both groups (Table 1). The distance of the tumor from the anal verge remained significantly different with a median of 3.3 cm (range 0.0–7.9 cm) in the LLN+ group compared to 6.0 cm in LLN− patients (range 0.1–8.0 cm, p < 0.001).
The majority of patients in the LLN+ group underwent an APR while LAR remained the most performed procedure in the LLN− group (56 vs. 65%, P = 0.001). More lymph nodes were harvested in LLN+ patients (median 18 vs. 13 for LLN− patients, P = 0.004). The number of patients receiving adjuvant chemotherapy was similar between both groups after matching for pre-operative variables (p > 0.99). All other peri-operative characteristics and post-operative histopathological findings were similar between both groups (Table 2).
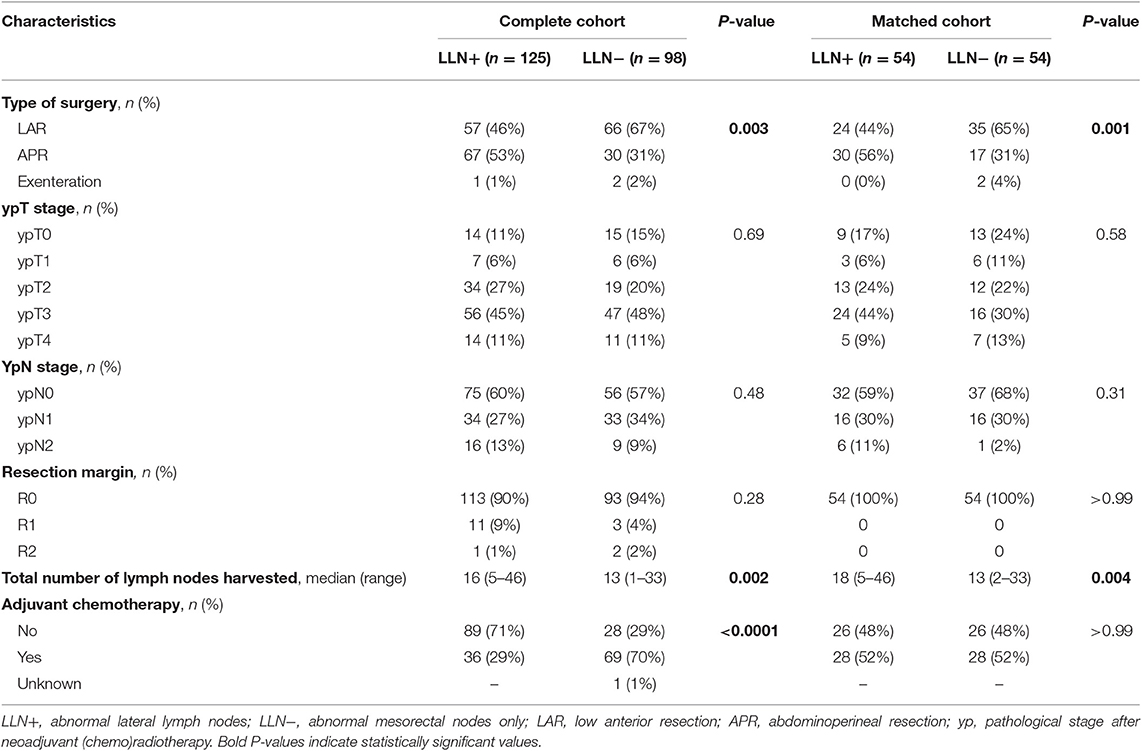
In the matched cohort, median follow-up time was 58 months in the LLN+ group (range 1–98) and 60 months in the LLN− group (range 1–99; p = 0.61). Five-year LRFS was 89% in LLN+ patients and 98% in LLN− patients (P = 0.07, HR 5.89, 95%CI 0.91–22.87, Figure 2A). During follow-up, four of the LLN+ patients had a lateral LR and one had a central LR, while of the LLN− patients, one central and no lateral LR were observed. One LLN+ patient developed a synchronous lateral LR and distant metastases to the liver at 67 months. One LLN+ patient had metachronous recurrent disease with a lateral LR at 19 months and distant metastases to the liver at 21 months. The other patients with LR in either group (n = 4) did not develop distant metastases. Five-year DMFS was 71% in LLN+ patients vs. 78% in LLN− patients (P = 0.21, HR 1.70, 95%CI 0.75–3.85, Figure 2B) and 5-year DFS was 64% in LLN+ patients and 76% in LLN− patients (P = 0.09, HR 1.96, 95%CI 0.92–4.17, Figure 2C). Finally, 5-year OS was similar between both groups (74% for LLN+ vs. 80% for LLN− patients, P = 0.90, HR 1.05, 95%CI 0.48–2.27, Figure 2D).
Figure 2. (A) Matched cohort local recurrence-free survival (LRFS) of LLN+ and LLN− patients (5-year LRFS 89 vs. 98.0%, respectively, P = 0.07, HR 5.89, 95%CI 0.91–22.87). (B) Matched cohort distant metastatic-free survival (DMFS) of LLN+ and LLN− patients (5-year DM 71.2 vs. 77.8%, respectively, P = 0.21, HR 1.70, 95%CI 0.75–3.85). (C) Matched cohort disease-free survival (DFS) of LLN+ and LLN− patients (5-year DFS 63.5 vs. 76.0%, respectively, P = 0.09, HR 1.96, 95%CI 0.92–4.17). (D) Matched cohort overall survival (OS) of LLN+ and LLN− patients (5-year OS 73.5 vs. 80.1%, respectively, P = 0.90, HR 1.05, 95%CI 0.48–2.27).
Discussion
The current study suggests that patients who suffer from an AJCC stage III low rectal cancer with LLN+ have a worse LRFS and DFS, both showing a trend toward significance compared to similarly staged LLN− patients. These results suggest that in the West rectal cancer patients with LLN+ may not be treated optimally.
Metastases to the lateral lymph nodes in patients with Stage III low rectal cancer occur via extra-mesorectal spread (5, 21). This means that these LLN+ are not resected during standard TME surgery and are therefore a potential site for LR (6, 22). When these patients are treated with surgery only, LLN+ patients have worse survival rates compared to those with mesorectal lymph node metastases only (22–25). For this reason, LLN+ patients in the East, mainly Japan, undergo a LLND at the time of TME surgery, however, without undergoing nCRT in most cases (8). In contrast, Western patients with LLN+ are treated similarly to LLN− patients, both with nCRT, increasing the RT field to include the lateral nodal basins in most LLN+ patients, followed TME surgery without performing a LLND. It has previously been suggested that this Western approach may result in worse oncological outcomes, mainly LR rates, for Stage III patients with LLN+ compared to similarly staged LLN− patients, but comparative data have been scarce thus far (1–3, 10, 17, 26).
After analyzing the complete cohort, there were some significant baseline differences between both groups, likely causing a bias in the long-term oncological results. Therefore, we performed a propensity score matching for gender, cT-stage and adjuvant chemotherapy with two similar groups in baseline characteristics (27).
In the matched cohort, there was a clinically relevant (though non-significant) trend toward worse 5-year LRFS and DFS in LLN+ patients compared to LLN− patients. The absolute differences were 9 and 12%, respectively (P = 0.07 and P = 0.09). Similar findings have been reported by Ogura et al. (26). Although they analyzed a larger cohort of patients in their study, they included patients both from the East and the West treated by a variety of different strategies, making it difficult to draw firm conclusions on the oncological behavior of LLN+ as Stage III disease in the West. In contrast, the current study was carried out in comparable patient groups who were all treated in the West, all receiving nCRT.
There are three studies suggesting that the current Western treatment of nCRT followed by TME is effective to achieve local control in LLN+ patients. To date, one study has been performed that is comparable to the current study (17). In their study, Dharmarajan et al., included a total of 53 patients and showed no difference in 5-year LRFS, DFS, and OS between LLN− and LLN+ patients undergoing nCRT followed by TME surgery. Based on these results, they concluded that in LLN+ patients a LLND is not justified as it is unlikely to provide a survival benefit. However, their study was a single-center experience reporting on a small number of patients only, meaning it was likely underpowered to detect differences in long-term oncological outcomes between LLN+ and LLN− patients. Also, due to the small number of patients, they were unable to perform a propensity score matching analysis to correct for differences baseline criteria. Interestingly though, the DFS and OS rates they reported in both groups were worse than in our study, possibly the result of the more advanced cT and cN-stages. In addition, Syk et al. performed a retrospective cohort study, suggesting that LLN+ are not a major cause of LR after TME (28). Finally, the MERCURY study included 325 patients, in which LLN+ patients were compared to LLN− patients, and showed that LLN+ on pre-treatment MRI have little impact on outcome if nCRT is administered (29). However, both studies also included patients with lower stages of disease (AJCC Stage I and II) and only a portion of the patients underwent nCRT. Furthermore, neither study performed a propensity score matching or multivariate analysis.
On the contrary, other studies, mostly originating from the East, have suggested that nCRT followed by TME alone may not be sufficient treatment for LLN+ (11, 12). For instance, three studies showed that in 33–66% of the LLN+, metastases were found during pathological examination when a LLND was performed after nCRT (12–14). Interestingly, the study by Ishihara et al. reported a 0% LR rate in LLN+ patients, which is considerably lower compared to the current and previous studies reporting on LR rates in LLN+ patients treated with nCRT + TME only (11, 13, 26). Furthermore, they also reported an improved 5-year OS rate of 81.2%, compared with 69% in the current study. However, the median follow-up in their study was considerably shorter: 39 months compared to 58 months in the present study, creating potential bias. Nonetheless, their study, combined with the low LR rates from other recent studies suggest favorable results when a LLND is combined with TME after nCRT, but it remains unclear if these results can be extrapolated to Western patients (12, 26).
Some limitations of the current study have to be addressed. Firstly, this is a retrospective cohort conducted at multiple centers, resulting in heterogeneity of patients and treatment modalities, such as the n(C)RT and adjuvant chemotherapy regimens used. Also, it was clear that LLN+ were not treated the same as mesorectal nodes, with surprisingly few patients receiving adjuvant chemotherapy in the LLN+ group. This was largely due to difference in practice between countries, where in the Netherlands, chemotherapy is mostly reserved as palliative or induction treatment prior to further surgery if a patient develops recurrent disease. In an attempt to overcome these issues, we conducted propensity score matching leading to comparable groups at baseline. However, we did not correct for the MRI-height of the tumor, since the occurrence of LLN+ is closely related to the anatomical height of rectal cancer (22, 30). As a result, the type of nCRT and type of surgery also remained significantly different as these variables are also related to tumor height. We also excluded from the matched cohort patients in whom lateral lymph nodes were selectively removed and all R1 and R2 resections, since independently of the lateral lymph node status, non-radical resections are associated with worse LRFS, DFS and OS and thus would severely influence the long-term oncological outcomes (31, 32). Based on previous publications, a cut-off short-axis size of ≥5 mm for LLN+ was chosen, however, in literature the definition of a LLN+ varies between 5–10 mm for the short-axis, making comparisons challenging (10, 14, 18, 22, 26, 30, 33). Also, we were not able to evaluate the response of the LLN to nCRT as most patients did not undergo a restaging MRI. Finally, although we included patients from five large international tertiary referral centers, the study population was relatively small and potentially underpowered for survival outcomes. In the future, larger multi-center collaborations are required to answer the questions posed in this study using a larger prospective dataset.
In the future, a study comparing Western LLN+ patients undergoing a LLND after nCRT to patients undergoing nCRT only would be important and such a study is currently being undertaken by our group. A recent study by Malakorn et al. showed that a LLND may only be advantageous in patients with persistent LLN+ (>5 mm) on restaging MRI after nCRT. The soon to open Lateral Nodal Recurrence in Rectal Cancer (LaNoReC) study will also be of great interest (34).
In conclusion, in Western patients with Stage III low rectal cancer, there is a trend toward worse LR and DFS rates in LLN+ patients compared to similarly staged LLN− patients. These results suggest that LLN+ patients may currently not be treated optimally with nCRT alone, and the addition of LLND requires further consideration.
Data Availability Statement
The datasets generated for this study will not be made publicly available. Data cannot be shared as per Ethics approval.
Ethics Statement
The study was approved by the human research ethics committee at each site. Written informed consent for participation was not required for this study in accordance with the national legislations and the institutional requirements.
Author Contributions
AH, HK, MK, and TS contributed conception and design of the study. AH, HK, DS, HL, JL, MV, HR, GB, and MK organized the database. AH, HK, and SB performed the statistical analysis. AH and HK wrote the first draft of the manuscript. DS, SB, ND-V, HL, MT, JL, MV, HR, GB, MK, and TS wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
AH was supported by the Stichting Prof. Michaël-van Vloten Fonds, Stichting Bekker-la Bastide-Fonds, Stichting Dr. Hendrik Muller's Vaderlandsch Fonds, and Stichting Sacha Swarttouw-Hijmans.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. (2006) 355:1114–23. doi: 10.1056/NEJMoa060829
2. Kodeda K, Johansson R, Zar N, Birgisson H, Dahlberg M, Skullman S, et al. Time trends, improvements and national auditing of rectal cancer management over an 18-year period. Colorectal Dis. (2015) 17(9):168–79. doi: 10.1111/codi.13060
3. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. (2004) 351:1731–40. doi: 10.1056/NEJMoa040694
4. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
5. Hojo K, Koyama Y, Moriya Y. Lymphatic spread and its prognostic value in patients with rectal cancer. Am J Surg. (1982) 144:350–4. doi: 10.1016/0002-9610(82)90018-6
6. Yano H, Moran BJ. The incidence of lateral pelvic side-wall nodal involvement in low rectal cancer may be similar in Japan and the West. Br J Surg. (2008) 95:33–49. doi: 10.1002/bjs.6061
7. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28(Suppl. 4):iv22–40. doi: 10.1093/annonc/mdx224
8. Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. (2018) 23:1–34. doi: 10.1007/s10147-017-1101-6
9. Kusters M, Beets GL, van de Velde CJ, Beets-Tan RG, Marijnen CA, Rutten HJ, et al. A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Ann Surg. (2009) 249:229–35. doi: 10.1245/s10434-009-0320-0
10. Kim MJ, Kim TH, Kim DY, Kim SY, Baek JY, Chang HJ, et al. Can chemoradiation allow for omission of lateral pelvic node dissection for locally advanced rectal cancer? J Surg Oncol. (2015) 111:459–64. doi: 10.1002/jso.23852
11. Kim TH, Jeong SY, Choi DH, Kim DY, Jung KH, Moon SH, et al. Lateral lymph node metastasis is a major cause of locoregional recurrence in rectal cancer treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol. (2008) 15:729–37. doi: 10.1245/s10434-007-9696-x
12. Akiyoshi T, Ueno M, Matsueda K, Konishi T, Fujimoto Y, Nagayama S, et al. Selective lateral pelvic lymph node dissection in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy based on pretreatment imaging. Ann Surg Oncol. (2014) 21:189–96. doi: 10.1245/s10434-013-3216-y
13. Ishihara S, Kawai K, Tanaka T, Kiyomatsu T, Hata K, Nozawa H, et al. Oncological outcomes of lateral pelvic lymph node metastasis in rectal cancer treated with preoperative chemoradiotherapy. Dis Colon Rectum. (2017) 60:469–76. doi: 10.1097/DCR.0000000000000752
14. Oh HK, Kang SB, Lee SM, Lee SY, Ihn MH, Kim DW, et al. Neoadjuvant chemoradiotherapy affects the indications for lateral pelvic node dissection in mid/low rectal cancer with clinically suspected lateral node involvement: a multicenter retrospective cohort study. Ann Surg Oncol. (2014) 21:2280–7. doi: 10.1245/s10434-014-3559-z
15. Georgiou P, Tan E, Gouvas N, Antoniou A, Brown G, Nicholls RJ, et al. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol. (2009) 10:1053–62. doi: 10.1016/S1470-2045(09)70224-4
16. Nagawa H, Muto T, Sunouchi K, Higuchi Y, Tsurita G, Watanabe T, et al. Randomized, controlled trial of lateral node dissection vs. nerve-preserving resection in patients with rectal cancer after preoperative radiotherapy. Dis Colon Rectum. (2001) 44:1274–80. doi: 10.1007/BF02234784
17. Dharmarajan S, Shuai D, Fajardo AD, Birnbaum EH, Hunt SR, Mutch MG, et al. Clinically enlarged lateral pelvic lymph nodes do not influence prognosis after neoadjuvant therapy and TME in stage III rectal cancer. J Gastrointest Surg. (2011) 15:1368–74. doi: 10.1007/s11605-011-1533-7
18. Lim SB, Yu Cs, Kim CW, Yoon YS, Park SH, Kim TW, et al. Clinical implication of additional selective lateral lymph node excision in patients with locally advanced rectal cancer who underwent preoperative chemoradiotherapy. Int J Colorectal Dis. (2013) 28:1667–74. doi: 10.1007/s00384-013-1761-2
19. Mann HB, Whitney D. R. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. (1947) 18:50–60. doi: 10.1214/aoms/1177730491
20. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. (1958) 53:457–81. doi: 10.1080/01621459.1958.10501452
21. Matalon SA, Mamon HJ, Fuchs CS, Doyle LA, Tirumani SH, Ramaiya NH, et al. Anorectal cancer: Critical anatomic and staging distinctions that affect use of radiation therapy. Radiographics. (2015) 35:2090–107. doi: 10.1148/rg.2015150037
22. Ueno M, Oya M, Azekura K, Yamaguchi T, Muto T. Incidence and prognostic significance of lateral lymph node metastasis in patients with advanced low rectal cancer. Br J Surg. (2005) 92:756–63. doi: 10.1002/bjs.4975
23. Ueno H, Mochizuki H, Hashiguchi Y, Ishiguro M, Miyoshi M, Kajiwara Y, et al. Potential prognostic benefit of lateral pelvic node dissection for rectal cancer located below the peritoneal reflection. Ann Surg. (2007) 245:80–7. doi: 10.1097/01.sla.0000225359.72553.8c
24. Sugihara K, Kobayashi H, Kato T, Mori T, Mochizuki H, Kameoka S, et al. Indication and benefit of pelvic sidewall dissection for rectal cancer. Dis Colon Rectum. (2006) 49:1663–72. doi: 10.1007/s10350-006-0714-z
25. Akiyoshi T, Watanabe T, Miyata S, Kotake K, Muto T, Sugihara K, et al. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: is it regional or distant disease? Ann Surg. (2012) 255:1129–34. doi: 10.1097/SLA.0b013e3182565d9d
26. Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, et al. Neoadjuvant (chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: Results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. J Clin Oncol. (2019) 37:33–43. doi: 10.1200/JCO.18.00032
27. Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Hoff PM, et al. Clinical and pathologic predictors of locoregional recurrence, distant metastasis, and overall survival in patients treated with chemoradiation and mesorectal excision for rectal cancer. Am J Clin Oncol. (2006) 29:219–24. doi: 10.1097/01.coc.0000214930.78200.4a
28. Syk E, Torkzad MR, Blomqvist L, Ljungqvist O, Glimelius B. Radiological findings do not support lateral residual tumour as a major cause of local recurrence of rectal cancer. Br J Surg. (2006) 93:113–9.29. doi: 10.1002/bjs.5233
29. Group MS, Shihab OC, Taylor F, Bees N, Blake H, Jeyadevan N, et al. Relevance of magnetic resonance imaging-detected pelvic sidewall lymph node involvement in rectal cancer. Br J Surg. (2011) 98:1798–804. doi: 10.1002/bjs.7662
30. Takahashi T, Ueno M, Azekura K, Ohta H. Lateral node dissection and total mesorectal excision for rectal cancer. Dis Colon Rectum. (2000) 43(10 Suppl.):S59–68. doi: 10.1007/BF02237228
31. Rahbari NN, Ulrich AB, Bruckner T, Munter M, Nickles A, Contin P, et al. Surgery for locally recurrent rectal cancer in the era of total mesorectal excision: is there still a chance for cure? Ann Surg. (2011) 253:522–33. doi: 10.1097/SLA.0b013e3182096d4f
32. Alberda WJ, Verhoef C, Schipper ME, Nuyttens JJ, Rothbarth J, de Wilt JH, et al. The importance of a minimal tumor-free resection margin in locally recurrent rectal cancer. Dis Colon Rectum. (2015) 58:677–85. doi: 10.1097/DCR.0000000000000388
33. Ogura A, Konishi T, Beets GL, Cunningham C, Garcia-Aguilar J, Iversen H, et al. Lateral nodal features on restaging magnetic resonance imaging associated with lateral local recurrence in low rectal cancer after neoadjuvant chemoradiotherapy or radiotherapy. JAMA Surg. (2019) 3:e192172. doi: 10.1001/jamasurg.2019.2172
34. Rectal Cancer Surgery. Amsterdam: Amsterdam UMC; 2019. LaNoReC trial. Available from: https://rectalcancersurgery.eu/lanorec-trial/ (accessed October 25, 2019).
Keywords: lateral lymph nodes, locally advanced low rectal cancer, neoadjuvant (chemo)radiotherapy, oncological outcomes, survival
Citation: Haanappel A, Kroon HM, Schaap DP, Bedrikovetski S, Dudi-Venkata NN, Lee HX, Thomas ML, Liu J, van der Valk MJM, Rutten HJT, Beets GL, Kusters M and Sammour T (2019) Lateral Lymph Node Metastases in Locally Advanced Low Rectal Cancers May Not Be Treated Effectively With Neoadjuvant (Chemo)Radiotherapy Only. Front. Oncol. 9:1355. doi: 10.3389/fonc.2019.01355
Received: 09 September 2019; Accepted: 18 November 2019;
Published: 03 December 2019.
Edited by:
Des Winter, St. Vincent's University Hospital, IrelandReviewed by:
Yoshiharu Sakai, Kyoto University, JapanFergal Flemingfergal, Medical Center, University of Rochester, United States
Copyright © 2019 Haanappel, Kroon, Schaap, Bedrikovetski, Dudi-Venkata, Lee, Thomas, Liu, van der Valk, Rutten, Beets, Kusters and Sammour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hidde M. Kroon, aGlkZGUua3Jvb25Ac2EuZ292LmF1
 Anouck Haanappel
Anouck Haanappel Hidde M. Kroon
Hidde M. Kroon Dennis P. Schaap
Dennis P. Schaap Sergei Bedrikovetski1,4
Sergei Bedrikovetski1,4 Nagendra N. Dudi-Venkata
Nagendra N. Dudi-Venkata