- Cancer Institute in Hebei Province, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
This study aimed at estimating the effects of epidemiological risk factors for lung cancer in never-smokers. A multicenter and matched case-control study was conducted in the cities of Shijiazhuang, Xingtai, Qinhuangdao, Baoding, and Chengde in North China. It comprised 1,086 cases and 2,172 healthy subjects as controls, all of whom had smoked fewer than 100 cigarettes in their lifetimes. Patients were newly diagnosed with lung cancer between January 2015 and December 2017. Each patient was matched to two control participants for sex and age (±5 years). Both univariate analysis and multivariate conditional logistic regression models were used to estimate the odds ratio (OR) and 95% confidence interval (95% CI). Subsequently, data were stratified by participant sex and different air quality conditions for analysis. Type of job, exposure to environmental tobacco smoke in the workplace or at home, above-average exposure to cooking oil fumes, depression, poor sleep quality, occupational exposure, cardiovascular diseases, and family history of cancer were revealed as significant risk factors for lung cancer in never-smokers. However, higher educational level, frequent use of a PM2.5 mask, cooking using clean fuels, and consumption of dietary supplements and tea reduced the risk of lung cancer. Risk factors varied between males and females. In areas with air pollution, the number of risk factors was greater than elsewhere, and the magnitudes of their effects were different. Hence, focusing on these risk factors is important for the prevention and control of lung cancer in never-smokers.
Introduction
Lung cancer accounts for 11.6% of all newly diagnosed cancer cases and 18.4% of cancer-related deaths worldwide (1). In China, lung cancer is the leading cause of cancer incidence and mortality (2). Several prospective and retrospective studies have reported that smoking to be the major and most well-established risk factor for lung cancer. However, ~15% of males and 53% of females with lung cancer have no history of smoking (3). Approximately 10–20% of lung cancer patients in Europe and the United States are never-smokers, and the proportion of never-smokers among lung cancer patients in Asia is 40–50% (4). In China, 44.9% of cases of lung cancers in males and 86.1% of cases of lung cancers in females are attributed to risk factors other than smoking (5, 6). The proportion of never-smokers in lung cancer patients increased from 31% in 1999–2002 to 48% in 2008–2011 (7). The clinicopathological and molecular mechanisms underlying lung cancer in never-smokers are different from those in smokers. Moreover, mortality from lung cancer in never-smokers was the seventh leading cause of cancer-related death. Furthermore, the incidence rate of lung cancer in never-smokers has been increasing in the last 30 years (8).
The incidence rate of lung cancer is related to the quantity of cigarettes consumed by smokers. Lung cancer incidence among Chinese women is no different from that in Western European countries, despite substantial differences in smoking prevalence (1). Thus, risk factors other than smoking, including genetic susceptibility, poor diet, and occupational exposure, have received considerable attention in recent years (9). However, some studies of lung cancer in never-smokers have only focused on females, and research on lung cancer risk in never-smokers in different air quality areas is limited. Hebei Province, which is in North China, has poor air quality and persistently experiences fog and haze (10, 11). Therefore, a multicenter matched case-control study was conducted to estimate the effects of epidemiological risk factors for lung cancer in never-smokers.
Materials and Methods
Study Design and Participants
Participants and controls were recruited between January 1, 2015, and December 10, 2017, in Shijiazhuang City, Baoding City, Xingtai City, Chengde City, and Qinhuangdao City in North China. The first three have poor air quality, and the others have standard air quality.
Two controls were matched for sex and age (±5 years) with one lung cancer patient. The following patients were included in this study: patients who were never-smokers but were diagnosed with primary lung cancer by pathology; patients who were newly diagnosed with lung cancer for the first time between January 1st, 2015, and December 10th, 2017, and were recruited prior to chemotherapy and radiotherapy; patients who were never-smokers (in this study, a never-smoker was defined as someone who had never smoked or smoked fewer than 100 cigarettes in his/her lifetime or someone who stopped smoking longer than 15 years before recruitment) (12); patients residing in local areas (Shijiazhuang City, Baoding City, Xingtai City, Chengde City, and Qinhuangdao City); and patients with no other cancers. Subjects with the following characteristics were included in the control group in this study: never-smokers; participants who were matched for sex and age (±5 years) with cases; participants in the control group with no familial relationship with the matched case; participants with no cancer history; participants with no diseases that could influence lung function. All cases and controls were Han Chinese. There were a total of 1,086 cases (Shijiazhuang: 250 cases from the Fourth Hospital of Hebei Medical University; Baoding: 250 cases collected by the centers for disease control of Baoding City; Xingtai: 245 cases from the People's Hospital of Xingtai city; Chengde: 250 cases from the Third Hospital of Chengde city; Qinhuangdao: 91 cases from the Fourth Hospital of Qinhuangdao city) and 2,172 controls (Shijiazhuang: 500 controls; Baoding: 500 controls; Xingtai: 490 controls; Chengde: 500 controls; Qinhuangdao: 182 controls). This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University.
Data Collection and Measurements
As part of the recruitment process, participants who enrolled in this study provided written informed consent for inclusion. However, if the participants were under the age of 18 years, both the participants and their parents or guardians provided written informed consent. Subsequently, a trained investigator distributed a structured questionnaire. Participants' basic information (including age, sex, educational level, and occupation), exposure to environmental tobacco smoke (ETS), condition of living quarters, cooking habits, physical exercise habits, stress level, eating habits, alcohol consumption, occupational exposure, history of chronic diseases, and family history of cancer were collected. On-site training and ongoing monitoring were conducted to ensure standardized and uniform data collection between different areas. The database was established by the Hebei Provincial Cancer Institute with double-entry and high-quality control to ensure consistency.
ETS was identified in participants living with a smoker on a regular basis in the workplace or at home (13). The variable “more than 5 kg weight lost” was based on weight loss over the past year, and people who had taken steps to lose weight were not included. For cooking fires, low fire was defined as burning the inner flame of a stove, and high fire was defined as burning the inner and outer flame. Data on exposure to cooking oil fumes, type of cooking fuel, cooking habits, physical exercise habits, and eating habits were continuously and frequently obtained for at least 6 months. Emotional status was defined by several questions that are part of the Eysenck Personality Questionnaire-RSC. High stress was defined as participants feeling tense or anxious for more than 6 months in the previous 3 years. Participants with good mental and social activity the next day and having had more than 6 h of sleep were reported as having experienced good quality sleeping conditions for the previous 1 year. A family history of cancer was defined as participants having first-degree (parents, daughters, sons, brothers, and sisters) or second-degree (uncles, aunts, and grandparents) relatives with cancer. Consumption of dietary supplements was defined as participants' regular intake of vitamins, minerals, herbs, botanicals, or other types of dietary supplements for the previous 30 days (14). Participants consuming alcohol were defined as those imbibing 50 ml of alcohol or 750 ml of beer per day for at least 1 year.
Statistical Analysis
Demographic variables and risk factors were compared between cases and matched controls using a univariate conditional logistic regression model. Categorical data were presented as numbers and percentages. Multivariate conditional logistic regression models were applied to estimate the odds ratio (OR) and 95% confidence interval (CI) for risk factors with P < 0.10 in univariate analysis. All data analyses were performed using Statistical Package for the Social Sciences (SPSS) (version 20, SPSS Inc., Chicago, IL, USA) and Statistical Analysis System (SAS) (version 9.3, SAS Institute Inc., Cary, NC, USA) software. P ≤ 0.05, established on two-sided probabilities, was considered statistically significant.
Results
Characteristics of the Participants
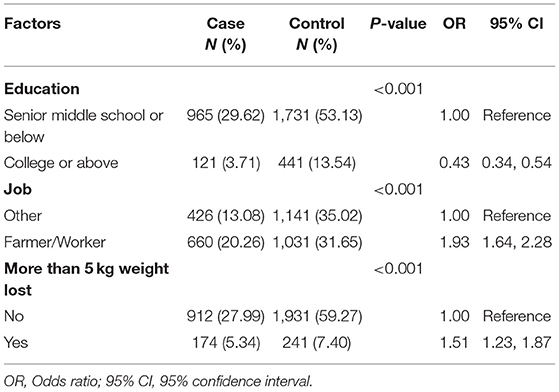
In this study, a total of 3,258 never-smokers, comprising 1,086 cases with lung cancer and 2,172 healthy controls, were included in the analysis. Females comprised 63.54% (690 cases for the case group, and 1,380 cases for the control group) and males 36.46% (396 cases for the case group, and 792 cases for the control group) of total participants. Participants who were never-smokers with a higher educational level (college or above) had a lower probability of developing lung cancer (OR, 0.43; 95% CI, 0.34–0.54) compared to participants with a lower educational level (senior middle school or below), who were considered the reference group. Compared with other kinds of jobs, farmers or workers who were never-smokers had a higher probability of developing lung cancer (OR, 1.93; 95% CI, 1.64–2.28). Participants who had lost more than 5 kg weight in the previous year had a 51% increased risk of lung cancer (OR, 1.51; 95% CI, 1.23–1.87) (Table 1).
Exposure to Environmental Tobacco Smoke and Smog
A significant association was observed between lung cancer and ETS in the workplace or at home. A 2.23-fold increased risk (OR, 2.23; 95% CI, 1.84–2.71) and a 2.33-fold increased risk (OR, 2.33; 95% CI, 1.99–2.72) of lung cancer were observed for never-smokers who were exposed to smoke in the workplace and at home. Using a fine particulate matter (PM2.5) mask (OR, 0.26; 95% CI, 0.17–0.40) and cooking using clean fuels (OR, 0.56; 95% CI, 0.47–0.66) were revealed as protective factors. Compared to participants who cooked 0–1 time per day, increased risk of lung cancer was observed in participants who cooked 2–3 times per day (OR, 1.36; 95% CI, 1.08–1.71). Compared to smokeless cooking, both normal exposure (ORnormal, 2.49; 95% CI, 1.91–3.25) and above-average exposure (ORmore, 3.08; 95% CI, 2.31–4.11) to cooking oil fumes were significantly associated with lung cancer (Table 2).
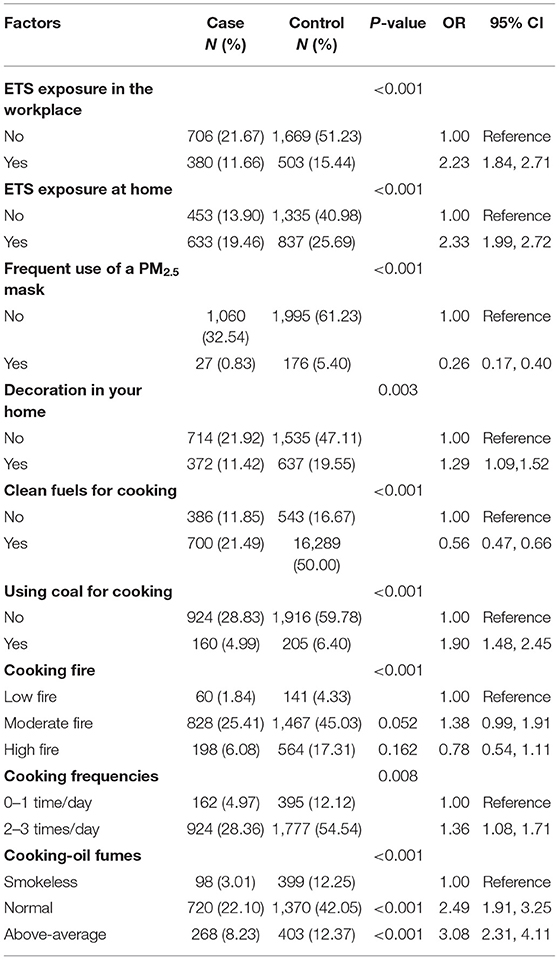
Table 2. Estimated risks of lung cancer associated with ETS, smog, and cooking habits in never-smokers.
Disease History, Lifestyle Habits, Family History of Cancer, and Occupational Exposure
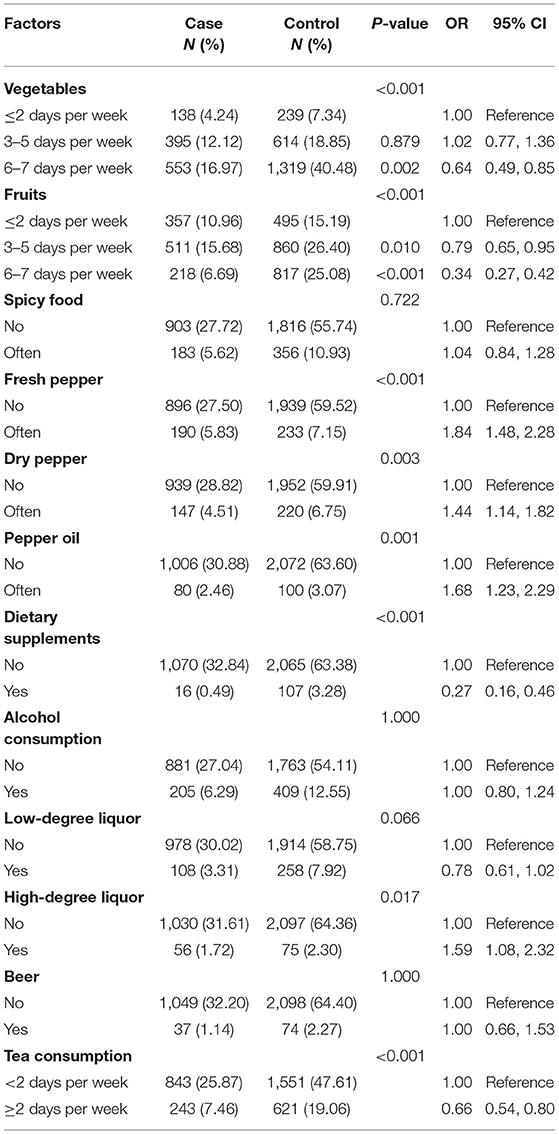
The ORs of cardiovascular diseases, diabetes, and diseases of the digestive system, considered as chronic diseases, were 1.77 (95% CI, 1.47–2.13), 1.33 (95% CI, 0.98–1.79), and 1.23 (95% CI, 0.92–1.63), respectively. Depression (OR, 1.65; 95% CI, 1.39–1.94) and high stress (OR, 1.49; 95% CI, 1.18–1.90) were revealed as risk factors for lung cancer. Quality of sleep was associated with lung cancer, with poor sleep quality being harmful to participants' health (OR, 1.29; 95% CI, 1.10–1.52). A family history of cancer was significantly associated with increased risk (OR, 1.57; 95% CI, 1.26–1.96) of lung cancer. However, physical exercise habits and lung cancer showed no significant association (Table 3).
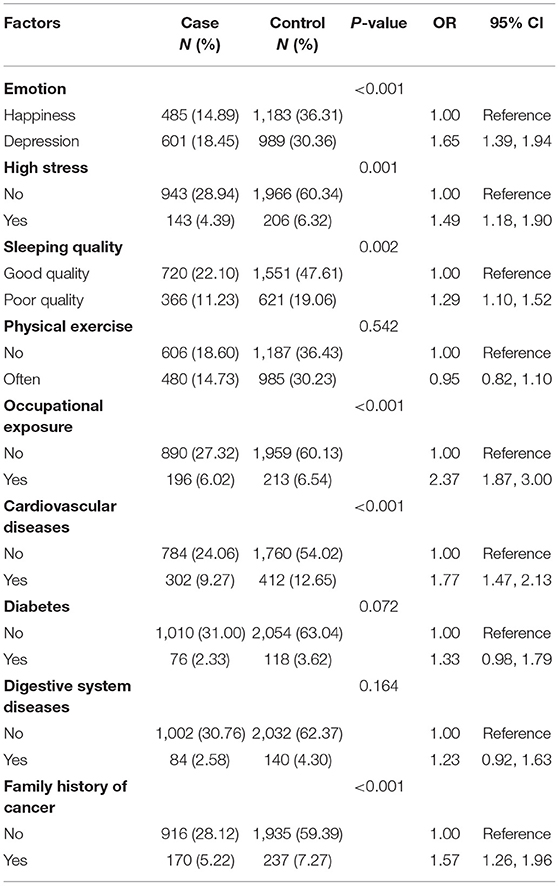
Table 3. Estimated risks of lung cancer associated with emotion, lifestyle, occupational exposures, history of disease, and family history of cancer in never-smokers.
Dietary Factors
Fruit intake 3–5 days per week (OR, 0.79; 95% CI, 0.65–0.95) and 6–7 days per week (OR, 0.34; 95% CI, 0.27–0.42) in never-smokers was considered a protective factor for lung cancer. The frequent consumption of spicy food was not significantly associated with lung cancer. However, there was an increased risk of lung cancer for specific types of pepper (fresh pepper, dry pepper, and pepper oil), while chili sauce and other types of pepper had no significant association with lung cancer. The consumption of high-degree liquor was significantly associated with lung cancer (OR, 1.59; 95% CI, 1.08–2.32), while the consumption of low-degree wine and beer had no significant association. On the other hand, the consumption of dietary supplements (OR, 0.27; 95% CI, 0.16–0.46) and tea (OR, 0.66; 95% CI, 0.54–0.80) reduced the risk of developing lung cancer (Table 4).
Multivariate Analysis of Risk Factors
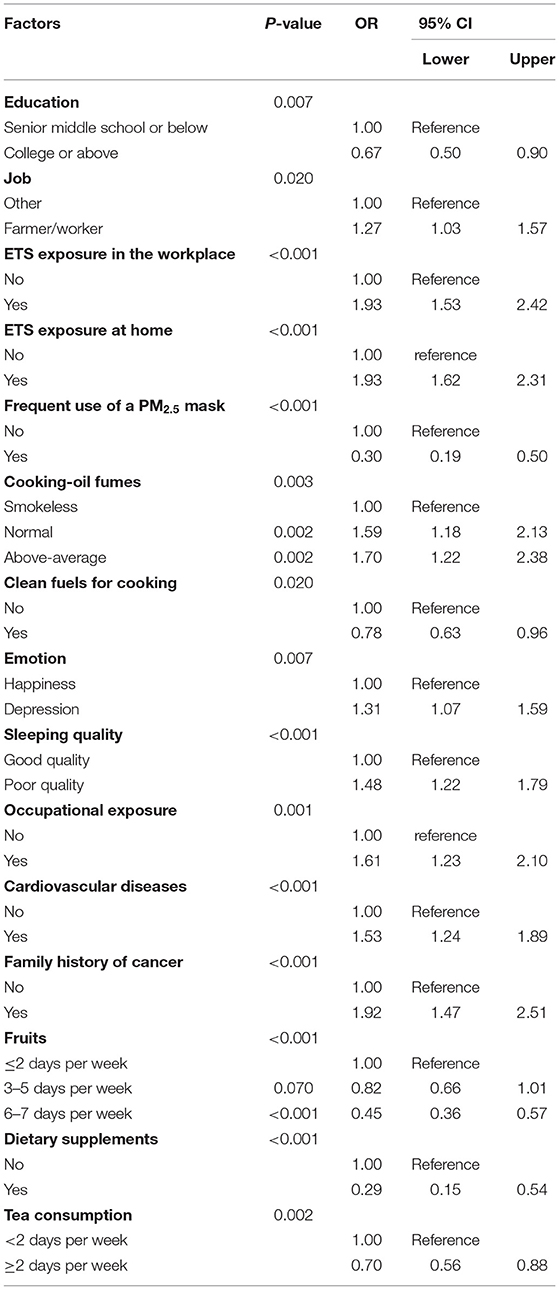
Table 5 shows the significant associations between risk factors and lung cancer in never-smokers. Higher educational level, frequent use of a PM2.5 mask, regular fruit intake, cooking using clean fuels, and consumption of dietary supplements and tea were revealed as protective factors for lung cancer in never-smokers. Compared with other types of jobs, farmers and workers had a higher probability of developing lung cancer (OR, 1.27; 95% CI, 1.03–1.57). ETS exposure in the workplace (OR, 1.93; 95% CI, 1.53–2.42) or at home (OR, 1.93; 95% CI, 1.62–2.31) was associated with lung cancer. Normal exposure (OR, 1.59; 95% CI, 1.18–2.13) and above-average exposure (OR, 1.70; 95% CI, 1.22–2.38) to cooking oil fumes were significantly associated with lung cancer. Occupational exposure (OR, 1.61; 95% CI, 1.23–2.10) increased the risk of lung cancer. Poor sleep quality, depression, cardiovascular diseases, and family history of cancer in never-smokers were shown to be risk factors for lung cancer (Table 5).
Participants were categorized by sex for analysis. According to the multivariate model, the significant predictive factors of lung cancer in females were similar to those in the overall model. In females, a higher educational level, consumption of dietary supplements, frequent use of a mask, cooking using clean fuels, and regular fruit intake were identified as protective factors, while ETS exposure in the workplace (OR, 1.74; 95% CI, 1.29–2.33) or at home (OR, 1.58; 95% CI, 1.25–2.00) and family history of cancer (OR, 1.91; 95% CI, 1.38–2.64) were identified as risk factors. Furthermore, above-average exposure to cooking oil fumes, specific types of jobs, and occupational exposure significantly increased the risk of lung cancer in never-smokers. Moreover, participants' mental states and habits, including depression, high stress, poor sleep quality, and frequent consumption of spicy food, were revealed as risk factors for lung cancer in never-smokers. In males, consumption of tea (OR, 0.68; 95% CI, 0.50–0.93) and regular fruit intake were significant protective factors. ETS exposure in the workplace or at home were risk factors in males, with ORs of 2.24 and 1.92, respectively. Occupational exposure, above-average exposure to cooking oil fumes, weight loss of more than 5 kg, and family history of cancer were significantly associated with lung cancer (Figure 1).
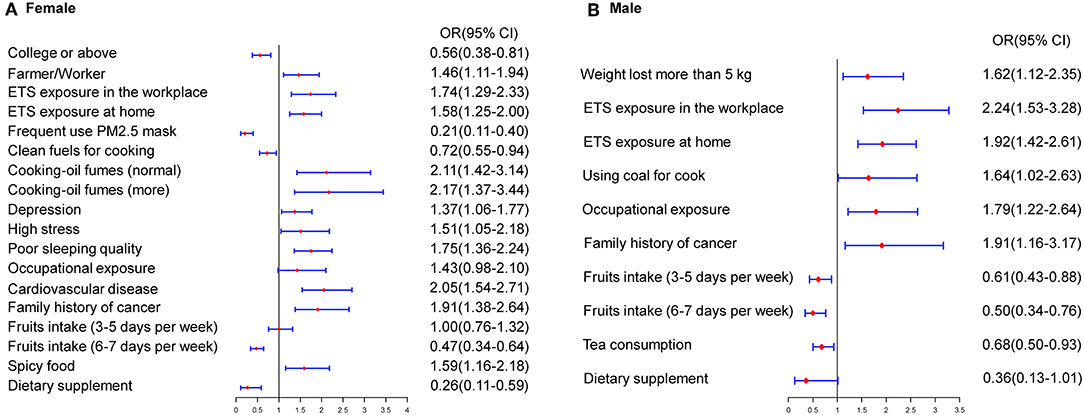
Figure 1. Estimated risks of lung cancer in never-smokers by gender. (A) Multivariate risk factors model in females. (B) Multivariate risk factors model in males.
The risk factors for lung cancer differed between areas with and without air pollution. The multivariate model for polluted areas included a series of variables as related factors for lung cancer, such as type of job, occupational exposure, ETS exposure in the workplace, high pressure, cooking using clean fuels, frequent use of a PM2.5 mask, family history of cancer, cardiovascular diseases, consumption of dietary supplements and tea, and regular fruit intake. In areas without air pollution, variables such as type of job, ETS exposure in the workplace or at home, educational level, family history of cancer, regular fruit intake, consumption of tea, frequent consumption of spicy foods, and depression were significantly associated with lung cancer in never-smokers (Figure 2).
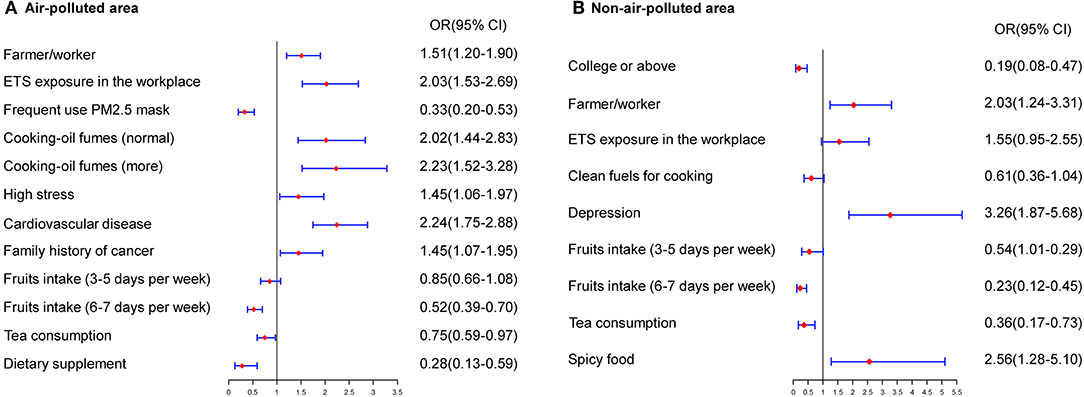
Figure 2. Estimated risks of lung cancer in never-smokers by air quality. (A) Multivariate risk factors model in air-polluted areas. (B) Multivariate risk factors model in non-air-polluted areas.
Discussion
Studies on lung cancer have revealed that risk factors, including genetic susceptibility, poor diet, and occupational exposure, might either be considered as independent risk factors for lung cancer or be associated with smoking-related lung cancer. Risk factors differ between smoking-related and non-smoking-related lung cancer, especially with respect to sex and air quality (15–17). In China, several studies have aimed at confirming the above hypothesis, but corroborating results have been limited. The present study used epidemiological data to assess the risk factors and protective factors significantly associated with lung cancer in never-smokers and carried out stratified analysis by sex and air quality in North China.
According to previous studies, a critical risk factor for lung cancer was smoking, with population-attributable risk proportions of 75.04% and 18.35% in males and females, respectively (6). Tobacco smoking causes deoxyribonucleic acid damage in bronchial epithelial cells, causing dysfunction in the immune system in the lungs. In some Asian regions, smoking rates in females are low, and the incidence rates of female lung cancer are inconsistent with expected incidence rates (18). According to a retrospective analysis of data from 192 countries, ~21,000 deaths by lung cancer could be attributed to secondhand smoking (19). In China, 740 million never-smokers are exposed to secondhand smoke, resulting in ~100,000 deaths (20). In this study, ETS exposure in the workplace (OR, 1.93; 95% CI, 1.53–2.42) or at home (OR, 1.93; 95% CI, 1.62–2.31) had a significant effect on lung cancer. Similarly, a large-scale prospective European study estimated that the proportion of lung cancers in never- and ex-smokers attributable to ETS was between 16 and 24%, mainly due to work-related exposure (21), while a few studies reported lower proportions of ETS exposure in the living place compared to the abovementioned study. Gorlova et al. reported that ETS exposure in the workplace (OR, 3.94; 95% CI, 1.54–10.06) was more significantly associated with lung cancer than ETS exposure at home (OR, 2.02; 95% CI, 1.06–3.85) (13). “Sidestream” smoke, which is defined as the smoke released from the end of a burning cigarette, could directly contribute to 80% of the smoke in secondhand smoking. Because sidestream smoke is generated at a lower temperature than “mainstream” smoke, it can lead to a thicker density of smoke containing at least 17 kinds of carcinogens compared to mainstream smoke (22).
This study found that males and females were differently affected by ETS exposure. The OR of secondhand smoking was higher for males than for females, with ORs of ETS exposure at home of 1.92 and 1.58 for males and females, respectively. In the workplace, the OR for females (1.74) was lower than that for males (OR, 2.24). An International Agency for Research on Cancer (IARC) analysis estimated that the risk of lung cancer was increased by more than 35% in males and 25% in females who were exposed to secondhand smoke compared to non-exposed males and females (23). Approximately 26.51% of cases of never-smokers with undiagnosed lung cancer can be attributed to passive smoking; thus, reducing exposure to secondhand smoke in never-smokers is beneficial with respect to the prevention and treatment of lung cancer (24).
According to some studies, a diet rich in vegetables and fruits, especially cruciferous vegetables, may help prevent lung cancer. However, the results of several studies with detailed information on dietary intake are inconsistent (25, 26). This study revealed that regular fruit intake and consumption of tea and dietary supplements were protective factors for lung cancer in never-smokers. Dose-response relationships for diets rich in fruits were identified in this study. Compared to participants who consumed fruits <2 days per week, the adjusted OR was lower (OR, 0.45; 95% CI, 0.36–0.57) for participants who consumed fruits 6 or 7 days per week. Some case-control studies also revealed that never-smokers who regularly consumed fruits had a decreased risk of developing lung cancer. According to a study by Vieira et al., regular fruit intake in never-smokers had no significant association with lung cancer, with a relative risk of 0.88 (95% CI, 0.68–1.15) (25). The inconsistency between the results of the studies may be attributable to the different study populations and confounding factors of lung cancer, such as passive smoking. Moreover, the target population in this study were Chinese, and we performed multivariate analysis to control for confounding factors. The protective effects of fruits may be due to biologically active compounds, such as sulforaphane, glucosamine, and indole derivatives, which have antioxidant functions. Tea contains several catechins, such as epigallocatechin gallate, epigallocatechin, and epicatechin gallate, which prevent the development of cancer (27). Consumption of tea and regular fruit intake prevent the development of diseases in humans.
Regarding the Chinese population, their preferred cooking methods are one of the risk factors of lung cancer. Compared to smoke-free cooking, the OR of above-average exposure to cooking oil fumes in never-smokers with lung cancer was 1.70 (95% CI, 1.22–2.38) in this study. Studies also revealed that different types of fuels could be considered to result in different risks of lung cancer, and cooking using clean fuels could be a protective factor in lung cancer, with an OR of 0.78 (95% CI, 0.63–0.96). Furthermore, Mu et al. reached similar conclusions: individuals with no separate kitchen had higher risk of lung cancer than those with a separate kitchen. Additionally, the absence of ventilation equipment could increase the risk of lung cancer (OR, 1.78; 95 CI, 1.09–2.90) (28). Cooking fuels generated from unclean fuels and high-temperature cooking have become one of the most serious indoor environmental pollutants, and they were classified as indoor carcinogenic pollutants by the IARC (25, 29). Completely eliminating these harmful cooking habits could reduce the risk of developing lung cancer in never-smokers.
This study revealed that a family history of lung cancer could contribute to the development of lung cancer in never-smokers, increasing risk 1.92-fold (95% CI, 1.47–2.51). The ORs of a family history of lung cancer in male and female never-smokers were 1.91 (95% CI, 1.38–2.64) and 1.91 (95% CI, 1.16–3.17), respectively. Gorlova et al. revealed that the risk of lung cancer in females was significantly higher compared to in males (21). The results of this study were similar to those of Lo et al. (24). Another meta-analysis revealed that a family history of cancer was closely associated with lung cancer in never-smokers, with a 1.5-fold increased risk (30). Hence, individuals who have a family history of cancer should undergo regular screening for lung cancer.
The Global Burden of Diseases, Injuries, and Risk Factors Study 2015 (GBD 2015) reported that exposure to air pollution could increase the incidence and mortality of lung cancer and is the leading cause of the global disease burden (31). Air pollution is a major, insufficiently appreciated cause of non-communicable disease. Some other risk factors for lung cancer in never-smokers might be influenced by air pollution and differed in areas with different air qualities. Particle mass with an aerodynamic diameter < 2.5 μm (PM2.5) is the most consistent and robust predictor of mortality (32, 33). Long-term exposure to PM2.5 has been associated with an increased risk of developing lung cancer (34). According to data from China's Ministry of Environmental Protection, the cities of Baoding, Shijiazhuang, and Xingtai are among the most heavily polluted cities in China, with PM2.5 concentrations of ~60–80 μg/m3 per year. The PM2.5 concentrations in Chengde and Qinhuangdao were reported to be below 30 μg/m3 per year (35). The relative risk of PM2.5 and lung cancer mortality in Hebei province was 1.20 (95% CI, 1.10-1.26) for females (35). A study by Gharibvand et al. also reached a similar conclusion, that PM2.5 might contribute to lung cancer incidence (HR, 1.43; 95% CI, 1.11–1.84) in never-smokers (36). Exposure in the workplace and air pollution might have synergistic effects. In air polluted areas, the adjusted OR of ETS exposure was higher (OR, 2.03; 95% CI, 1.53–2.69) than that of all areas (OR, 1.93; 95% CI, 1.53–2.42). After air quality conditions were stratified, above-average exposure to cooking oil fumes was significantly more harmful than smokeless exposure (OR, 2.23; 95% CI, 1.52–3.28). Furthermore, household air pollution from the burning of fuels has also been shown to be a major cause of lung cancer in low-income and middle-income countries and, with ambient air pollution, poses a substantial public health challenge (31). Because of the relationship between PM2.5 and lung cancer, it could lead to variation in the effect magnitude in the other risk factors. Hence, the data was stratified by air quality so as to provide more information to targeted prevention. In areas with air pollution, individuals should protect themselves from ETS and exposure cooking oil fumes and should wear a PM2.5 mask frequently to prevent the occurrence of lung cancer.
This study has the following strengths: a sufficiently large sample size and the use of stratified analysis by sex and air quality conditions. The epidemiological risk factors for lung cancer were exposure to passive smoking, cooking habits, disease history, and family history of cancer. This study has the limitation that smoking status and other variables were self-reported. Hence, never-smokers may have been misclassified, especially participants who were ex-smokers and had stopped smoking <15 years before. The development of lung cancer is a complex process, as it involves several factors, including epidemiological and genetic factors and gene–environment interactions.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by This study was approved by the Ethics Committee of Hebei Medical University Fourth Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
DLia and YH wrote the main text and performed data analysis. YH and BS designed the study. DLi, JS, JJ, and JW collected the data. All authors reviewed the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully thank all of the population-based cancer registries in Hebei Province for their cooperation and for providing participants' information. We are grateful to the participants for agreeing to take part in this study. The authors assume full responsibility for the analyses and interpretation of the data.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, et al. Report of cancer epidemiology in China, 2015. Chin J Oncol. (2019) 41:1–10. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005
3. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. (2005) 55:74–108. doi: 10.3322/canjclin.55.2.74
4. Cho J, Choi SM, Lee J, Lee CH, Lee SM, Kim DW, et al. Proportion and clinical features of never-smokers with non-small cell lung cancer. Chin J Cancer. (2017) 36:20. doi: 10.1186/s40880-017-0187-6
5. Chen WQ, Xia CF, Zheng RS, Zhou MG, Lin CQ, Zeng HM, et al. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Glob Health. (2019) 7:E257–69. doi: 10.1016/S2214-109X(18)30488-1
6. Wang JB, Jiang Y, Wei WQ, Yang GH, Qiao YL, Boffetta P. Estimation of cancer incidence and mortality attributable to smoking in China. Cancer Causes Control. (2010) 21:959–65. doi: 10.1007/s10552-010-9523-8
7. Toh CK, Ong WS, Lim WT, Tan WDS, Ng QS, Kanesvaran R, et al. A decade of never-smokers among lung cancer patients-increasing trend and improved survival. Clin Lung Cancer. (2018) 19:E539–50. doi: 10.1016/j.cllc.2018.03.013
8. Thun MJ, Hannan LM, Adams-Campbell LL, Boffetta P, Buring JE, Feskanich D, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry Studies. PLoS Med. (2008) 5:1357–71. doi: 10.1371/journal.pmed.0050185
9. Malhotra J, Malvezzi M, Negri E, CLa Vecchia, Boffetta P. Risk factors for lung cancer worldwide. Eur Respir J. (2016) 48:889–902. doi: 10.1183/13993003.00359-2016
10. He QS, Geng FH, Li CC, Yang SQ, Wang YY, Mu HZ, et al. Long-term characteristics of satellite-based PM2.5 over East China. Sci Total Environ. (2018) 612:1417–23. doi: 10.1016/j.scitotenv.2017.09.027
11. Feng LW, Ye B, Feng H, Ren F, Huang SC, Zhang XT, et al. Spatiotemporal changes in fine particulate matter pollution and the associated mortality burden in China between 2015 and 2016. Int J Environ Res Public Health. (2017) 14:E1321. doi: 10.3390/ijerph14111321
12. Couraud S, Zalcman G, Milleron B, Morin F, Souquet PJ. Lung cancer in never smokers - A review. Eur J Cancer. (2012) 48:1299–311. doi: 10.1016/j.ejca.2012.03.007
13. Gorlova OY, Zhang YQ, Schabath MB, Lei L, Zhang Q, Amos CI, et al. Never smokers and lung cancer risk: a case-control study of epidemiological factors. Int J Cancer. (2006) 118:1798–804. doi: 10.1002/ijc.21561
14. Gahche JJ, Bailey RL, Potischman N, Dwyer JT. Dietary supplement use was very high among older adults in the United States in 2011-2014. J Nutr. (2017) 147:1968–76. doi: 10.3945/jn.117.255984
15. Bae JM. Modifiable risk factors of lung cancer in “never-smoker” women. Epidemiol Health. (2015) 37:e2015047. doi: 10.4178/epih/e2015047
16. Clément-Duchêne C, Vignaud JM, Stoufflet A, Bertrand O, Gislard A, Thiberville L, et al. Characteristics of never smoker lung cancer including environmental and occupational risk factors. Lung Cancer. (2010) 67:144–50. doi: 10.1016/j.lungcan.2009.04.005
17. Lee YJ, Cho BC, Jee SH, Moon JW, Kim SK, Chang J, et al. Impact of environmental tobacco smoke on the incidence of mutations in epidermal growth factor receptor gene in never-smoker patients with non-small-cell lung cancer. J Clin Oncol. (2010) 28:487–92. doi: 10.1200/jco.2010.28.15_suppl.7577
18. Lee PN, Forey BA. Indirectly estimated absolute lung cancer mortality rates by smoking status and histological type based on a systematic review. BMC Cancer. (2013) 13:189. doi: 10.1186/1471-2407-13-189
19. Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. (2011) 377:139–46. doi: 10.1016/S0140-6736(10)61388-8
20. National Health and Family Planning Commission of China. China Reported Health Hazards of Smoking. Beijing: People's Health Publishing House (2012)
21. Vineis P, Hoek G, Krzyzanowski M, Vigna-Taglianti F, Veglia F, Airoldi L, et al. Lung cancers attributable to environmental tobacco smoke and air pollution in non-smokers in different European countries: a prospective study. Environ Health. (2007) 6:7. doi: 10.1186/1476-069X-6-7
22. Kim AS, Ko HJ, Kwon JH, Lee JM. Exposure to secondhand smoke and risk of cancer in never smokers: a meta-analysis of epidemiologic Studies. Int J Environ Res Public Health. (2018) 15:E1981. doi: 10.3390/ijerph15091981
23. International Agency for Research on. Cancer. $$Tobacco smoke and involuntary smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 1-1438 (2004)
24. Lo YL, Hsiao CF, Chang GC, Tsai YH, Huang MS, Su WC, et al. Risk factors for primary lung cancer among never smokers by gender in a matched case-control study. Cancer Causes Control. (2013) 24:567–76. doi: 10.1007/s10552-012-9994-x
25. Vieira AR, Abar L, Vingeliene S, Chan DSM, Aune D, Navarro-Rosenblatt D, et al. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol. (2016) 27:81–96. doi: 10.1093/annonc/mdv381
26. Wu QJ, Xie L, Zheng W, Vogtmann E, Li HL, Yang G, et al. Cruciferous vegetables consumption and the risk of female lung cancer: a prospective study and a meta-analysis. Ann Oncol. (2013) 24:1918–24. doi: 10.1093/annonc/mdt119
27. Suganuma M, Takahashi A, Watanabe T, Iida K, Matsuzaki T, Yoshikawa HY, et al. Biophysical approach to mechanisms of cancer prevention and treatment with green tea catechins. Molecules. (2016) 21:E1566. doi: 10.3390/molecules21111566
28. Mu LN, Liu L, Niu RG, Zhao BX, Shi JP, Li YL, et al. Indoor air pollution and risk of lung cancer among Chinese female non-smokers. Cancer Causes Control. (2013) 24:439–50. doi: 10.1007/s10552-012-0130-8
29. Chiu YL, Wang XR, Qiu H, Yu ITS. Risk factors for lung cancer: a case-control study in Hong Kong women. Cancer Causes Control. (2010) 21:777–85. doi: 10.1007/s10552-010-9506-9
30. Matakidou A, Eisen T, Houlston RS. Systematic review of the relationship between family history and lung cancer risk. Br J Cancer. (2005) 93:825–33. doi: 10.1038/sj.bjc.6602769
31. Hay SI, Jayaraman SP, AGManzano C, Millear A, Kemmer L, Bell B, et al. GBD 2015 risk factors collaborators. global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015 (vol 388, pg 1659, 2016). Lancet. (2017) 389:1345–422. doi: 10.1016/S0140-6736(17)32366-8
32. Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, et al. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health. (2013) 12:43. doi: 10.1186/1476-069X-12-43
33. Villeneuve PJ, Weichenthal SA, Crouse D, Miller AB, To T, Martin RV, et al. Long-term exposure to fine particulate matter air pollution and mortality among Canadian women. Epidemiology. (2015) 26:536–45. doi: 10.1097/EDE.0000000000000294
34. Yang WS, Zhao H, Wang X, Deng Q, Fan WY, Wang L. An evidence-based assessment for the association between long-term exposure to outdoor air pollution and the risk of lung cancer. Eur J Cancer Prevent. (2016) 25:163–72. doi: 10.1097/CEJ.0000000000000158
35. He Y, Gao Z, Guo T, Qu F, Liang D, Li D, et al. Fine particulate matter associated mortality burden of lung cancer in Hebei Province, China. Thorac Cancer. (2018) 9:820–6. doi: 10.1111/1759-7714.12653
Keywords: lung cancer, never-smokers, multicenter case-control study, risk factors, protective factors
Citation: Liang D, Wang J, Li D, Shi J, Jing J, Shan B and He Y (2019) Lung Cancer in Never-Smokers: A Multicenter Case-Control Study in North China. Front. Oncol. 9:1354. doi: 10.3389/fonc.2019.01354
Received: 08 July 2019; Accepted: 18 November 2019;
Published: 10 December 2019.
Edited by:
Tianhui Chen, Zhejiang Cancer Hospital, ChinaReviewed by:
Xiangqian Guo, Henan University, ChinaLiu Yun Yong, Liaoning Cancer Hospital, Cancer Hospital of China Medical University, China
Copyright © 2019 Liang, Wang, Li, Shi, Jing, Shan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yutong He, aHl0b25nNjlAeWFob28uY29t
 Di Liang
Di Liang Jingxi Wang
Jingxi Wang