- 1School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, China
- 2Department of Gastrointestinal Surgery, Taizhou Hospital of Zhejiang Province, Wenzhou Medical University, Linhai, China
The clinical significance of peripheral blood parameters has been considered to be a potential prognostic indicator for malignancies. In this study, 224 colorectal cancer (CRC; ncolon = 103; nrectal = 121) patients who underwent resection were enrolled, and the pre- and post-operative clinical laboratory data within 1 week, before and after surgery, were collected. The prognostic value of the counts of white blood cell (WBC), neutrophil, lymphocyte and platelet, the neutrophil to lymphocyte ratio (NLR), and systemic immune-inflammation index (SII) were analyzed. Data revealed that pre-operative lymphocyte count (pre-LC) was much higher than that of post-LC (p < 0.001), and only rectal cancer patients with pre-LChigh (>median: 1.61 × 109/L) had a significantly better overall survival (OS) and 5-year survival rate (SR) than those with pre-LClow (OS: 62.3 vs. 49.5 months; SR: 74.0 vs. 43.0%; p = 0.006). Cox's proportional hazard models revealed that pre-LChigh was an independent, favorable prognostic factor for rectal cancer patients (hazard ratio = 0.348; p = 0.003). Moreover, when the disease stages were stratified, the pre-LChigh was significantly associated with better prognosis of rectal cancer patients with stage I + II rectal cancer (n = 65; OS: 67.5 vs. 54.3 months; p = 0.011). Taken together, our study revealed that pre-operative lymphocyte count is an independent prognostic factor for patients with stage I and II rectal cancer.
Introduction
Peripheral lymphocytes, neutrophils, and monocytes in the complete blood cell count have been considered to play important roles in cellular-mediated inflammatory response in cancers (1). In this context, peripheral immune cell alterations have been frequently observed among various cancer patients, and their clinical significance, including the prognostic value of peripheral blood test parameters, have been intensively investigated during the past decades (2, 3). A large body of studies have revealed that both pre-operative and/or post-operative peripheral blood test parameters such as the counts of white blood cells (WBC), neutrophils, lymphocytes, monocytes and platelets, and derived neutrophil to lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR), systemic immune-inflammation index (SII = count of platelet*neutrophil/lymphocyte), could be potential diagnostic and prognostic biomarkers for different types of cancers (4–6).
Colorectal cancer (CRC) ranks one of the leading cause of cancer-related death around the world (7). Although the tumor (T)-lymph nodes (N)-metastasis (M) TNM staging system has been widely used for CRC outcome prediction, the prognostic significance of peripheral blood test parameters have also been acknowledged in previous studies (8–10). However, due to the heterogeneity of the cohorts and different protocols of the study design such as the cut-off values, considerable discrepancy among the findings has been raised. Therefore, more studies are necessary to explore the predictive value of these peripheral systemic inflammation markers in CRC (11).
Previous reports revealed that PLR is an independent prognostic factor for survival in stage II CRC patients and in refractory metastatic CRC patients, where low PLR patients have a better prognosis (12, 13). In other cohorts of stage II and metastatic CRC patients, lower NLR, but not PLR is an independent favorable prognosis predictor (14, 15). An increasing number of both PLR factors and PLR associated with poor survival have been observed in CRC patients (16). Moreover, the prognostic factors, such as SII and MRR (monocyte to red blood cell count ratio), have been found to be superior to other factors including NLR, PLR, and LMR. Authors further strengthened that SII is of high prognostic stratification power in terms of particular TNM subgroups, and elevated MRR is associated with poor survival in CRC patients with early stages of rectal cancer (17, 18). Moreover, the clinical significance of the absolute count of WBC and its subsets have been explored in many studies. In this context, higher levels of pre-operative and/or post-operative lymphocyte count was found to be associated with better survival in CRC patients (19, 20).
In the current study, we retrospectively reviewed 224 CRC patients and evaluated the prognostic value of pre-operative and post-operative absolute count of WBC, neutrophils, lymphocytes, platelets and the derived NLR and SII in these CRC patients.
Patients and Methods
Patients
This study was reviewed and approved by the Institutional Ethics Review Board of Taizhou Hospital of Zhejiang Province, and written informed consent was obtained from each participant prior to the surgery.
We retrospectively reviewed 224 CRC (ncolon = 103; nrectal = 121) patients who underwent surgical R0 curative resection at Taizhou Hospital of Zhejiang Province, Wenzhou Medical University between December 15th, 2010 and March 4th, 2013. Patients received pre-operative chemoradiation treatment or other medical interventions before surgery were excluded. Detail patient characteristics including age (median: 67 years; range: 39–89), gender, clinicopathological diagnosis, date of surgical operation, pre- and post- operative laboratory data within 1 week, were retrieved from the patient medical records provided by the Tissue Bank of Taizhou Hospital of Zhejiang Province (National Human Genetic Resources Platform of China YCZYPT [2017] 02). Tumor clinicopathologic stages were determined according to the 7th TNM staging system by the American Joint Committee for Cancer and International Union for Cancer Control (21). There were 74 patients with stage I, 48 patients with stage II, 98 patients with stage III, and 4 patients with stage IV, respectively.
Follow-up has documented for all patients until the December 31st 2016. The median follow-up was 48 months. The Overall survival was defined as the interval between the date of surgery and time of patient death (event) or the time of the last follow-up (censored).
Laboratory Data
Routine laboratory data, including pre-operative and post-operative absolute count of WBC, neutrophils, lymphocytes, platelets from routine blood tests, were included in this study. Pre-operative and post-operative laboratory data were collected 1 week before and after surgery, respectively. The derived NLR and SII were calculated from the above data. NLR = count of neutrophils/lymphocytes. SII = count of platelet*neutrophil/lymphocyte (22).
Statistical Analysis
All statistical analyses were performed with SPSS 13.0 (SPSS, Inc., Chicago, IL). Normality of continuous variables were analyzed by one-sample Kolmogorov-Smirnov test. Skewed distribution variables, including WBC and subsets count, platelet, NLR and SII between groups, were analyzed with Mann–Whitney's U-test. A correlation between pre- and post-operative lymphocyte count was analyzed using the Spearman method. Overall survival probabilities were analyzed using the Kaplan-Meier method. Differences between survival curves were analyzed by the log-rank test. The prognostic power of multiple clinicopathological variables were analyzed with univariate and multivariate Cox regression model. All statistical analysis were two-sided and p < 0.05 was considered statistically significant.
Results
Relationships Between Clinicopathologic Characteristics and Laboratory Variables
In this study, 224 CRC patients (ncolon = 103; nrectal = 121) were enrolled, with 127 male and 97 female patients with a median age of 67 years (range: 30–89 years). Detail patient characteristics and their association between pre- and post-operative laboratory variables were listed in Table 1 and Supplementary Table 1, respectively.
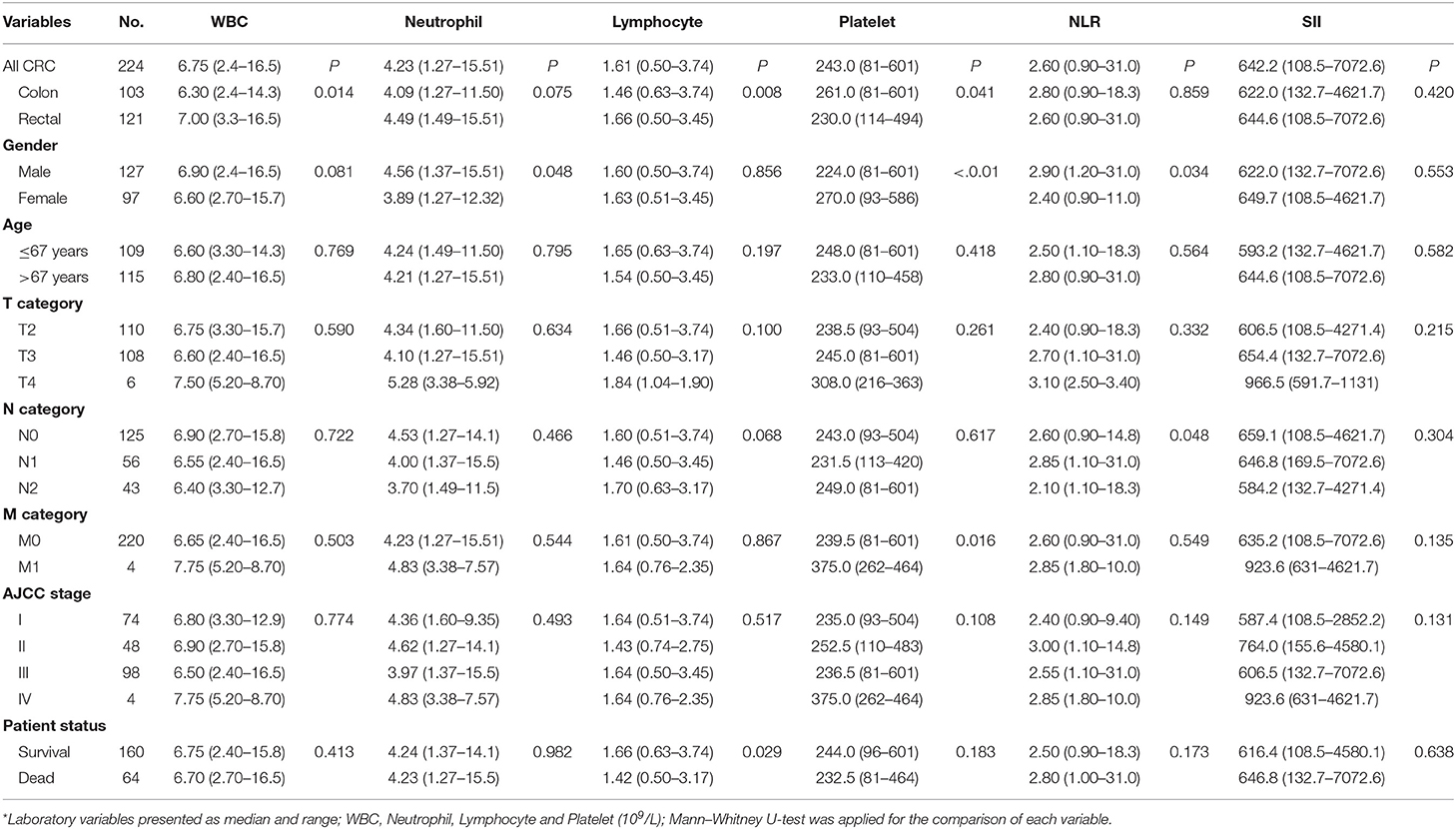
Table 1. Pre-operation laboratory data and their association with clinicopathological parameters in CRC patients*.
Data showed that only the pre-operative lymphocyte count (pre-LC) were significantly associated with the status of patient survival, that the pre-LC was much higher in survived patients than in the CRC patients who died (1.66 × 109/L vs. 1.42 × 109/L; p = 0.029; Table 1; Supplementary Table 1). Moreover, we found that pre-LC was significantly higher than post-operative lymphocyte count (post-LC) for CRC patients (1.61 × 109/L vs. 1.10 × 109/L; p < 0.001; Supplementary Figure 1A), and the levels between pre- and post-LC were significantly correlated (R = 0.502; p < 0.001; Supplementary Figure 1B). Moreover, data revealed that both pre- and post-LC were significantly higher in rectal cancer patients than in colon cancer patients (Table 1; Supplementary Table 1).
Pre-operative Lymphocyte Count Related to CRC Patient Survival
CRC patients were classified as pre-LChigh (n = 112) and pre-LClow (n = 112) groups, respectively, according to the median level of lymphocyte count (median: 1.61 × 109/L). Kaplan-Meier analysis showed that patients with pre-LChigh had significantly longer survival [mean: 63 (95% CI: 59.0–67.0) vs. 54.1 months (95% CI: 49.3–59.0)] and 5-year survival rate (SR: 76 vs. 57%) than that of patients with pre-LClow (p = 0.007; Figure 1A), respectively. However, no significant difference was observed between the patients with post-LChigh (n = 115) and post-LClow [n = 110; mean: 61.0 (95% CI: 56.9–65.2) vs. 55.7 months (95% CI: 50.7–60.8); p = 0.114; Figure 1B].
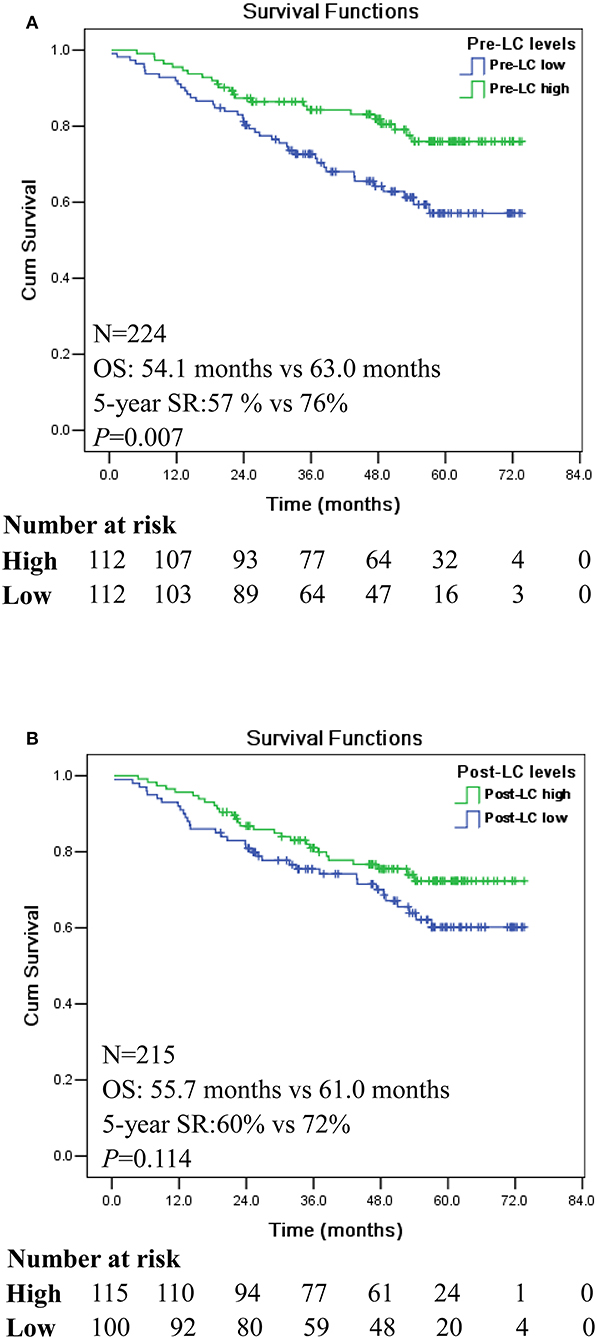
Figure 1. Kaplan-Meier survival analysis between the high and low levels of pre- and post-operation peripheral lymphocyte count (LC) in CRC patients. (A) Comparison of overall survival between pre-LChigh and pre-LClow among CRC patients. (B) Comparison of overall survival between post-LChigh and post-LClow among CRC patients.
Prognostic Stratification of Pre-operative Lymphocyte Count in CRC Patients
The prognostic stratification value of peripheral blood variables, such as high platelet count, have been found to be independent prognostic predictors only in patients with stage IV CRC patients (23). Other studies also revealed that SII was of prognostic stratification power for each disease stage (17), and elevated MRR was associated with poor survival in CRC patients with early disease stages I and II (18).
In this study, our data showed that pre-LChigh is associated with a significantly better survival in CRC patients with early disease stage of AJCC I and II (n = 122; p = 0.004; Figure 2A), whereas no significant difference was reached for patients with advanced disease stage III and IV (n = 102; p = 0.225; Figure 2B). Among disease stage I and II, patients with pre-LChigh had significantly longer survival (mean: 67.4 vs. 57.0 months) and 5-year SR (88 vs. 58%) than that of patients with pre-LClow, respectively (Figure 2A), while no significance was reached for the survival of CRC patients with stage III and IV between the pre-LClow and pre-LChigh (mean: 49.8 vs. 56.8 months) and 5-year SR (54 vs. 60%; Figure 2B).
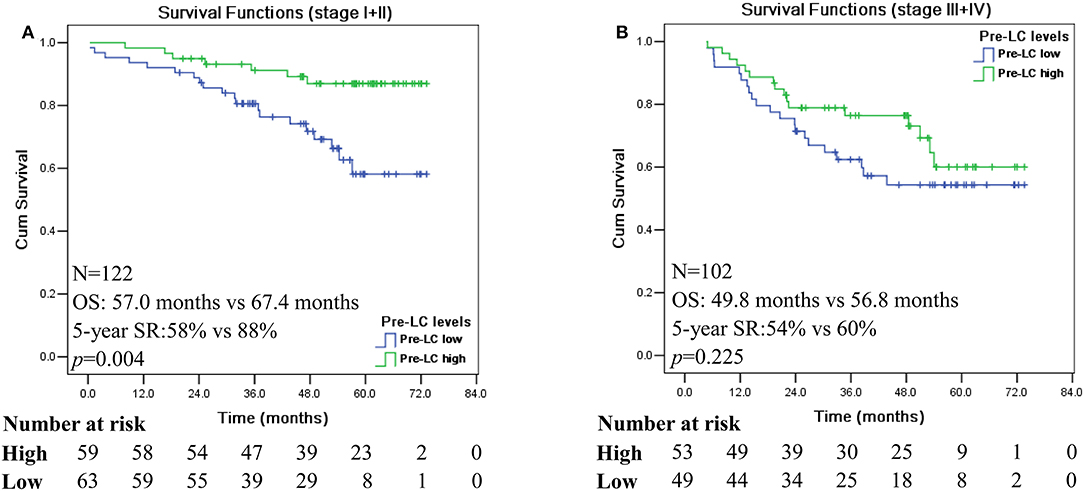
Figure 2. Kaplan-Meier survival analysis of the high and low levels of pre-operation peripheral lymphocyte count in different disease stage of CRC patients. Comparison of overall survival between pre-LChigh and pre-LClow among CRC patients with disease stage I + II (A) and disease stage III + IV (B).
Next, we analyzed the prognostic significance of pre-LC with stratification of colon and rectal cancer, respectively. A survival curve revealed that pre-operative LChigh and LClow is of prognostic stratification value only in patients with rectal cancer (n = 121; p = 0.006; Figure 3A), but not in patients with colon cancer (n = 103; p = 0.200; Figure 3B). Moreover, when the disease stages were stratified, the pre-LChigh was only significantly associated with better prognosis of rectal cancer patients with stages I + II (n = 65; p = 0.011; Figure 3C), while no prognostic significance of the status of pre-LChigh and pre-LClow was observed for the rectal cancer patients with stages III + IV (n = 56; p = 0.110; Figure 3E), as well as colon cancer patients with stages I + II (n = 57; p = 0.125; Figure 3D) and III + IV (n = 46; p = 0.680; Figure 3F).
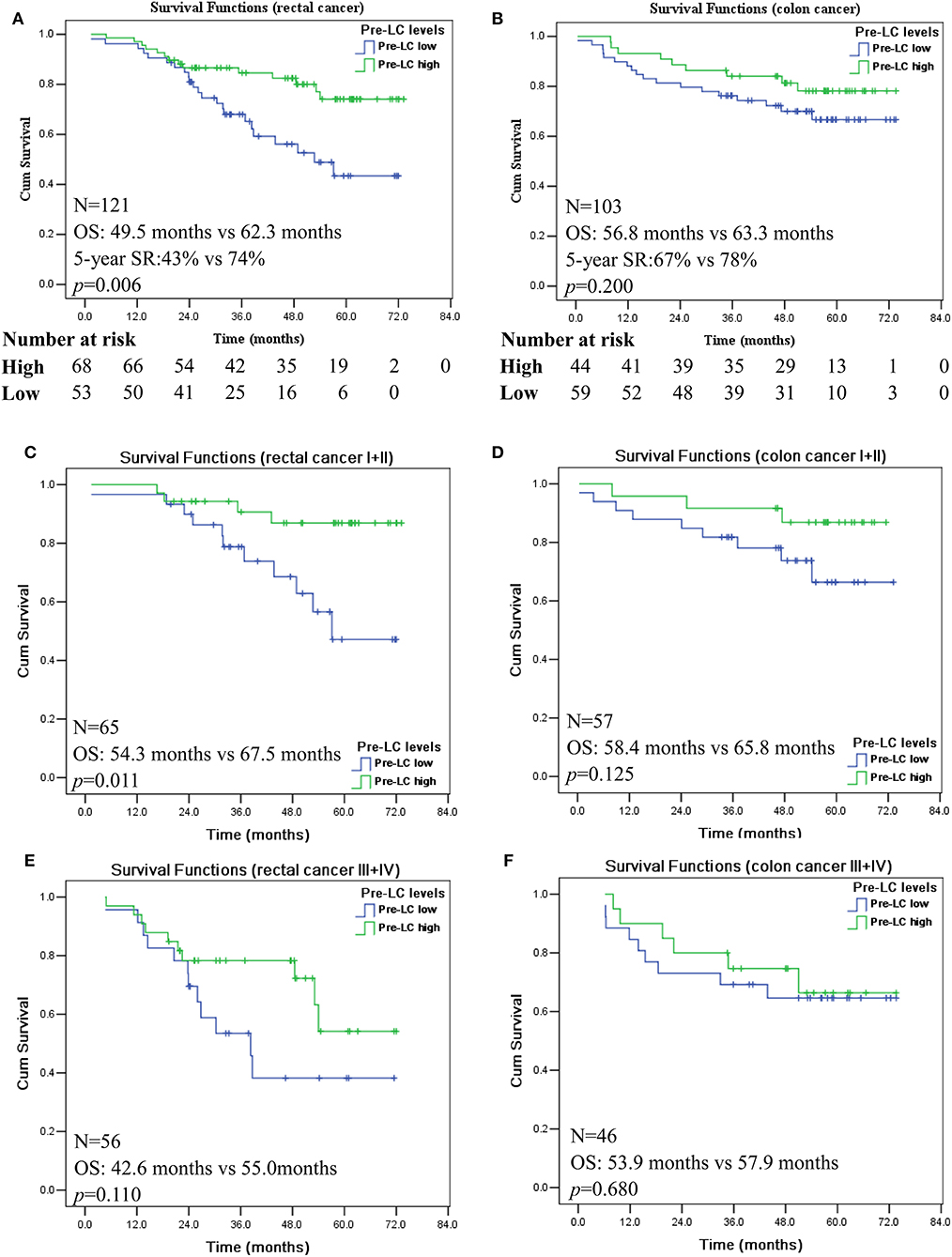
Figure 3. Kaplan-Meier survival analysis of the high and low levels of pre-operation peripheral lymphocyte count in rectal and colon cancer patients with AJCC I + II and III + IV disease stages. Comparison of overall survival between pre-LChigh and pre-LClow among patients with rectal cancer (A); colon cancer (B); rectal cancer patients with disease stage I + II (C) and III + IV (E); colon cancer patients with disease stage I + II (D) and III + IV (F).
Prognosis Value of Pre-operative Lymphocyte Count for Patients With Rectal Cancer
Finally, the prognostic value of pre-LC for patients with rectal cancer was assessed with the Cox's proportional hazards model. Univariate analysis data showed that pathological parameters including patient age (HR = 2.067, p = 0.036), pM (HR = 4.907, p = 0.030), AJCC stage (HR = 2.278, p = 0.015), and the pre-LC (LChigh vs. LClow, HR = 0.403, p = 0.008) were dramatically associated to the prognosis of patients with rectal cancer. In addition to the AJCC disease stages (HR = 2.399, p = 0.012), multivariate analysis showed that pre- LChigh vs. LClow remains statistically significant and can be an independent prognostic factor (HR = 0.348, p = 0.003), indicating that pre-LChigh is an favorable predictor for patients with rectal cancer (Table 2).
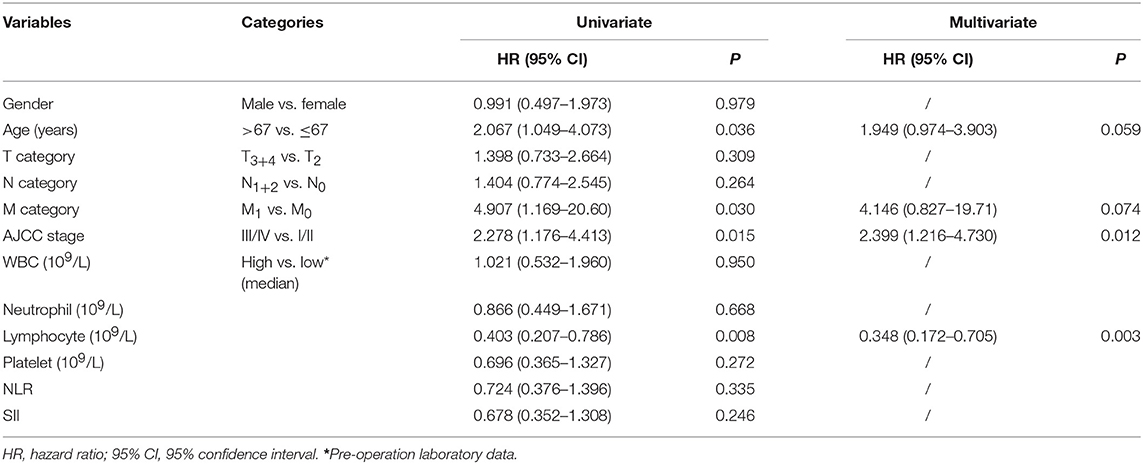
Table 2. Cox proportional hazards model for variables affecting overall survival in rectal cancer patients.
Discussion
In addition to widely used risk factors, including tumor itself features (TNM and AJCC stages, histopathological types, etc.) and patient individual characteristics (gender, age, immunological status, genetic background, etc.), the prognostic value of the peripheral hematological markers, such as WBC and subset count and their derived parameters, such as SII, NLR, MLR, and MRR, has suggested this to be a promising field that has been carried out in many malignancies (11, 24, 25).
In this study, we found that only the higher pre-operative lymphocyte count (above the median level), is an independent favorable prognostic predictor for patients with stage I and II rectal cancer. However, no prognostic significance of pre-operative lymphocyte count was observed for patients with rectal cancer stage III and IV, and for patients with colon cancer. In previous studies, the prognostic significance of peripheral hematological markers and their derived parameters PLR, NLR, or SII have been investigated. However, conclusions remain discrepant. Among these studies, different prognostic significance of PLR and NLR have been found in CRC patients with disease stage II and in refractory metastatic CRC patients (13, 14). Chen et al. (17) reported that SII was an independent risk factor for both overall survival and disease-free survival; however, in another study, SII was a risk factor only for the disease-free survival (26). The discrepancy of findings might be due to the heterogeneity of the cohorts and different protocols of the study design, such as the cut-off values, or even the genetic background of a particular patient. In this context, NLR has been found of prognostic value only in mismatch repair-proficient (pMMR), but not in CRC patients with mismatch repair-deficient (dMMR) (27). Other factors like pre-operative circulating eosinophil and basophil count have been reported to affect the predictive value of the PLR and NLR in CRC patients with stage I–III (28).
In the current study, our data revealed a strong correlation between the pre-operative LC and post-operative LC, while the levels of post-operative LC were dramatically decreased. Our findings also showed that only the higher levels of pre-operative LC were significantly associated with a better survival and an independent predictive factor for patients with stage I and II rectal cancer. A disease stage dependent predictive value of other parameters, such as NLR, LMR, and MRR, have been observed in previous studies. However, the immunological mechanism for this phenomenon remains to be explored. In this context, LMR has been found to be a favorable prognostic factor for early stage of CRC; however, LMR and MRR have a better prognostic effect on advanced stage rather than early stage of CRC (18, 29, 30). Though the prognostic significance of either pre- or post-operative LC has been investigated, studies comparison between pre- or postoperative LC are less available (31–35). A strong correlation between pre-operative LC and post-operative LC in CRC patients had been reported in a previous study by Yamamoto et al. (19), and the study also showed that the post-operative lymphocyte count was dramatically lower than the pre-operative lymphocyte count. However, in that study, the authors found that both pre-operative and post-operative LC were related to prognosis of CRC patients, and the combination of pre- and post-operative LC had a more powerful prognostic value than either pre- or post-operative LC alone. In other studies, Iseki et al. (31) investigated the prognostic value of count and percentage of pre-operative peripheral lymphocyte in 362 patients with colorectal cancer. With the cut-off of 1.7 × 109/L for pre-operative lymphocyte count, authors found that the 5-year relapse-free survival (RFS) and overall survival (OS) rate in the high lymphocyte count group was significantly higher than that in the low lymphocyte count group. In line with this, another study with the cut-off of 1.0 × 109/L for pre-operative lymphocyte count, Shibutani et al. (32) showed that RFS and OS rates were significantly higher in the high-lymphocyte group compared with the low-lymphocyte group. On the contrary, no prognostic significance of pre-operative lymphocyte count was observed for both RFS and OS in another cohort of patients with colorectal cancer (33). The discrepancy findings between previous studies might be owing to the different cut-off values, and actually no reference of cut-off values has been recommended or established (36). Other factors can also affect the predictive value of laboratory markers, such as heterogeneous cohorts of CRC patients, by simultaneous inclusion of colon and rectal cancer patients, and by different treatment options especially in the administration of different adjuvant chemotherapy regimens or not (37).
In summary, our findings revealed that pre-operative lymphocyte count is an independent prognostic factor for patients with stage I and II rectal cancer. However, the limited size of the cohort and the fact that only colorectal cancer patients who underwent radical resection were included in this retrospective study, are the main limitations. Therefore, larger prospective cohorts with a multicenter are necessary to validate the prognostic values of these non-invasive, cost-effective, and convenient systemic inflammatory indices.
Data Availability Statement
All datasets generated for this study are included in the manuscript/Supplementary Files.
Ethics Statement
This study was reviewed and approved by the Institutional Ethic Committee and Review Board of Taizhou Hospital of Zhejiang Province, written informed consent was obtained from each participant prior to the surgery.
Author Contributions
AL and W-HY: study design, statistical analysis, and manuscript draft. Y-YZ, W-QL, Z-FL, X-HG, and S-KZ: clinical data review and acquisition. All authors read and approved the final manuscript.
Funding
This work was supported by grants from Taizhou Technology Project (1801ky07, 1901ky01, 1901ky04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00960/full#supplementary-material
Supplementary Figure 1. (A) Comparison of levels between pre-operation (n = 224) and post-operation (n = 215) peripheral lymphocyte count in CRC patients with Mann–Whitney U-test. Blue bar represents the median. (B) Correlation between pre-operative and post-operative lymphocyte count with the Spearman method (n = 215).
Supplementary Table 1. Post-operation laboratory data and their association with clinicopathological parameters in CRC patients*.
References
1. Upadhyay S, Sharma N, Gupta KB, Dhiman M. Role of immune system in tumor progression and carcinogenesis. J Cell Biochem. (2018) 119:5028–42. doi: 10.1002/jcb.26663
2. Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
3. Menetrier-Caux C, Ray-Coquard I, Blay JY, Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with Cytokines? J Immunother Cancer. (2019) 7:85. doi: 10.1186/s40425-019-0549-5
4. Mei Z, Shi L, Wang B, Yang J, Xiao Z, Du P, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev. (2017) 58:1–13. doi: 10.1016/j.ctrv.2017.05.005
5. Zhang Y, Lin S, Yang X, Wang R, Luo L. Prognostic value of pretreatment systemic immune-inflammation index in patients with gastrointestinal cancers. J Cell Physiol. (2019) 234:5555–63. doi: 10.1002/jcp.27373
6. Dupre A, Malik HZ. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol. (2018) 44:566–70. doi: 10.1016/j.ejso.2018.02.209
7. Buccafusca G, Proserpio I, Tralongo AC, Rametta Giuliano S, Tralongo P. Early colorectal cancer: diagnosis, treatment and survivorship care. Crit Rev Oncol Hematol. (2019) 136:20–30. doi: 10.1016/j.critrevonc.2019.01.023
8. Mercier J, Voutsadakis IA. The platelets-neutrophils to lymphocytes ratio: a new prognostic marker in metastatic colorectal cancer. J Gastrointest Oncol. (2018) 9:478–86. doi: 10.21037/jgo.2018.03.13
9. Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer. (2014) 134:2403–13. doi: 10.1002/ijc.28536
10. Lam M, Roszik J, Kanikarla-Marie P, Davis JS, Morris J, Kopetz S, et al. The potential role of platelets in the consensus molecular subtypes of colorectal cancer. Cancer Metastasis Rev. (2017) 36:273–88. doi: 10.1007/s10555-017-9678-9
11. Rossi S, Basso M, Strippoli A, Schinzari G, D'Argento E, Larocca M, et al. Are markers of systemic inflammation good prognostic indicators in colorectal cancer? Clin Colorectal Cancer. (2017) 16:264–74. doi: 10.1016/j.clcc.2017.03.015
12. Song A, Eo W, Lee S. Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol. (2015) 21:12410–20. doi: 10.3748/wjg.v21.i43.12410
13. Ozawa T, Ishihara S, Nishikawa T, Tanaka T, Tanaka J, Kiyomatsu T, et al. The preoperative platelet to lymphocyte ratio is a prognostic marker in patients with stage II colorectal cancer. Int J Colorectal Dis. (2015) 30:1165–71. doi: 10.1007/s00384-015-2276-9
14. He W, Yin C, Guo G, Jiang C, Wang F, Qiu H, et al. Initial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancer. Med Oncol. (2013) 30:439. doi: 10.1007/s12032-012-0439-x
15. Dimitriou N, Felekouras E, Karavokyros I, Alexandrou A, Pikoulis E, Griniatsos J. Neutrophils to lymphocytes ratio as a useful prognosticator for stage II colorectal cancer patients. BMC Cancer. (2018) 18:1202. doi: 10.1186/s12885-018-5042-x
16. Li Y, Jia H, Yu W, Xu Y, Li X, Li Q, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. (2016) 139:220–31. doi: 10.1002/ijc.30071
17. Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, et al. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. (2017) 23:6261–72. doi: 10.3748/wjg.v23.i34.6261
18. Peng F, Hu D, Lin X, Chen G, Liang B, Li C, et al. The monocyte to red blood cell count ratio is a strong predictor of postoperative survival in colorectal cancer patients: the Fujian prospective investigation of cancer (FIESTA) study. J Cancer. (2017) 8:967–75. doi: 10.7150/jca.18000
19. Yamamoto M, Saito H, Uejima C, Tanio A, Takaya S, Ashida K, et al. Combined pre- and postoperative lymphocyte count accurately predicts outcomes of patients with colorectal cancer. Dig Surg. (2018) 14:1–8. doi: 10.1159/000492340
20. Liang L, Zhu J, Jia H, Huang L, Li D, Li Q, et al. Predictive value of pretreatment lymphocyte count in stage II colorectal cancer and in high-risk patients treated with adjuvant chemotherapy. Oncotarget. (2016) 7:1014–28. doi: 10.18632/oncotarget.5835
21. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
22. Jomrich G, Gruber ES, Winkler D, Hollenstein M, Gnant M, Sahora K, et al. Systemic Immune-Inflammation Index (SII) predicts poor survival in pancreatic cancer patients undergoing resection. J Gastrointest Surg. (2019). doi: 10.1007/s11605-019-04187-z. [Epub ahead of print].
23. Pedrazzani C, Mantovani G, Fernandes E, Bagante F, Luca Salvagno G, Surci N, et al. Assessment of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and platelet count as predictors of long-term outcome after R0 resection for colorectal cancer. Sci Rep. (2017) 7:1494. doi: 10.1038/s41598-017-01652-0
24. Kim HS, Ku JH. Systemic inflammatory response based on neutrophil-to-lymphocyte ratio as a prognostic marker in bladder cancer. Dis Markers. (2016) 2016:8345286. doi: 10.1155/2016/8345286
25. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. (2010) 6:149–63. doi: 10.2217/fon.09.136
26. Yang J, Guo X, Wu T, Niu K, Ma X. Prognostic significance of inflammation-based indexes in patients with stage III/IV colorectal cancer after adjuvant chemoradiotherapy. Medicine (Baltimore). (2019) 98:e14420. doi: 10.1097/MD.0000000000014420
27. He WZ, Hu WM, Kong PF, Yang L, Yang YZ, Xie QK, et al. Systemic neutrophil lymphocyte ratio and mismatch repair status in colorectal cancer patients: correlation and prognostic value. J Cancer. (2018) 9:3093–100. doi: 10.7150/jca.26669
28. Wei Y, Zhang X, Wang G, Zhou Y, Luo M, Wang S, et al. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage - colorectal cancer. Asia Pac J Clin Oncol. (2018) 14:e243–51. doi: 10.1111/ajco.12871
29. Zou ZY, Liu HL, Ning N, Li SY, Du XH, Li R. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. (2016) 11:2241–8. doi: 10.3892/ol.2016.4216
30. Kubo H, Murayama Y, Arita T, Kuriu Y, Nakanishi M, Otsuji E. The prognostic value of preoperative neutrophil-to-lymphocyte ratio in colorectal cancer. World J Surg. (2016) 40:2796–802. doi: 10.1007/s00268-016-3595-x
31. Iseki Y, Shibutani M, Maeda K, Nagahara H, Tamura T, Ohira G, et al. The impact of the preoperative peripheral lymphocyte count and lymphocyte percentage in patients with colorectal cancer. Surg Today. (2017) 47:743–54. doi: 10.1007/s00595-016-1433-2
32. Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative lymphocyte-to-monocyte ratio in patients with colorectal cancer. Oncol Lett. (2017) 13:1000–6. doi: 10.3892/ol.2016.5487
33. Solak Mekić M, Pedišić I, Šobat H, Vučićević Boras V, Kirac I, Štefančić L, et al. The role of complete blood count parameters in patients with colorectal cancer. Acta Clin Croat. (2018) 57:624–9. doi: 10.20471/acc.2018.57.04.03
34. Saroha S, Uzzo RG, Plimack ER, Ruth K, Al-Saleem T. Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma. J Urol. (2013) 189:454–61. doi: 10.1016/j.juro.2012.09.166
35. Kozak MM, von Eyben R, Pai JS, Anderson EM, Welton ML, Shelton AA, et al. The prognostic significance of pretreatment hematologic parameters in patients undergoing resection for colorectal cancer. Am J Clin Oncol. (2017) 40:405–12. doi: 10.1097/COC.0000000000000183
36. Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol. (2017) 115:470–9. doi: 10.1002/jso.24523
Keywords: colorectal cancer, blood lymphocyte count, survival, prognosis, biomarker
Citation: Zhang Y-Y, Li W-Q, Li Z-F, Guo X-H, Zhou S-K, Lin A and Yan W-H (2019) Higher Levels of Pre-operative Peripheral Lymphocyte Count Is a Favorable Prognostic Factor for Patients With Stage I and II Rectal Cancer. Front. Oncol. 9:960. doi: 10.3389/fonc.2019.00960
Received: 09 May 2019; Accepted: 10 September 2019;
Published: 24 September 2019.
Edited by:
Cornelis F. M. Sier, Leiden University, NetherlandsReviewed by:
Johan Nicolay Wiig, Oslo University Hospital, NorwayLukas Hawinkels, Leiden University Medical Center, Netherlands
Copyright © 2019 Zhang, Li, Li, Guo, Zhou, Lin and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aifen Lin, YWlmZW5saW5AeWFob28uY29t; Wei-Hua Yan, eWFud2hjb21AeWFob28uY29t
†These authors have contributed equally to this work as co-first authors
 Ying-Ying Zhang1†
Ying-Ying Zhang1† Wei-Hua Yan
Wei-Hua Yan