
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Oncol., 23 September 2019
Sec. Hematologic Malignancies
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00863
 Vlad Moisoiu1
Vlad Moisoiu1 Patric Teodorescu1,2†
Patric Teodorescu1,2† Lorand Parajdi3
Lorand Parajdi3 Sergiu Pasca1†
Sergiu Pasca1† Mihnea Zdrenghea1
Mihnea Zdrenghea1 Delia Dima2
Delia Dima2 Radu Precup3
Radu Precup3 Ciprian Tomuleasa2,4*
Ciprian Tomuleasa2,4* Simona Soverini5
Simona Soverini5Chronic myelogenous leukemia (CML) is a malignancy of the myeloid cell lineage characterized by a recurrent chromosomal abnormality: the Philadelphia chromosome, which results from the reciprocal translocation of the chromosomes 9 and 22. The Philadelphia chromosome contains a fusion gene called BCR-ABL1. The BCR-ABL1 codes for an aberrantly functioning tyrosine kinase that drives the malignant proliferation of the founding clone. The advent of tyrosine kinase inhibitors (TKI) represents a landmark in the treatment of CML, that has led to tremendous improvement in the remission and survival rates. Since the introduction of imatinib, the first TKI, several other TKI have been approved that further broadened the arsenal against CML. Patients treated with TKIs require sensitive monitoring of BCR-ABL1 transcripts with quantitative real-time polymerase chain reaction (qRT-PCT), which has become an essential part of managing patients with CML. In this review, we discuss the importance of the BCR-ABL1 assay, and we highlight the growing importance of BCR-ABL1 dynamics. We also introduce a mathematical correction for the BCR-ABL1 assay that could help homogenizing the use of the ABL1 as a control gene. Finally, we discuss the growing body of evidence concerning treatment-free remission. Along with the continuous improvement in the therapeutic arsenal against CML, the molecular monitoring of CML represents the avant-garde in the struggle to make CML a curable disease.
CML is a malignant proliferation driven by a characteristic fusion gene called BCR-ABL1 (1). The BCR-ABL1 gene results from the reciprocal translocation between chromosome 22 and 11 (the Philadelphia chromosome – Ph+) (2, 3). BCR-ABL1 codes a constitutionally active tyrosine kinase that inflicts growth advantage to the leukemic clone harboring the mutation. Based on cytology and cytogenetic studies, CML is further subdivided into chronic phase (CP), accelerated phase (AP), and blast phase (BP) CML (4). In CML, the presence of the Ph+ cells can be detected both in the peripheral blood and in the bone marrow using cytogenetic-based protocols that have been extensively used in the past for monitoring treatment response (5, 6). Moreover, cytogenetics is also important for detecting additional chromosomal alterations (ACA) such as trisomy eight, isochromosome 17q, second Ph, and trisomy 19 that bear prognostic value (7, 8).
The introduction of imatinib, the first tyrosine kinase inhibitor (TKI), has improved the treatment outcomes to such an extent that in many patients the disease burden decreases quickly below the detection limit of classical assays such as bone marrow cytogenetics (9–11). Therefore, the presence of measurable residual disease (MRD) must be monitored using quantitative polymerase chain reaction (qRT-PCR) of the BCR-ABL1 transcript, an assay which can detect as few as one malignant cell in 100.000 non-malignant ones (12–16). As the number of patients on long term therapy has increased, the MRD status evaluation has become increasingly important. Moreover, MRD can also be used as a convenient surrogate outcome in clinical trials (17).
The main goal of TKI therapy is to achieve a complete cytogenetic response (CCyR), defined as the lack of any detectable Ph+ cells in the bone marrow, within 12 months after starting treatment. CCyR is roughly equivalent to BCR-ABL1 IS ≤ 1%. The therapy for CP-CML is mainly represented by TKIs, which have improved the treatment outcomes compared to previous therapies. For instance, in the IRIS trial, a phase III trial that included more than 1,000 CML patients, and which eventually led to the approval of imatinib, the rate of complete cytogenetic response (CCyR) at 18 months in patients treated with imatinib was 76 vs. 15% for patients treated with interferon (IFN) plus cytarabine (10). Ever since, several other TKIs have been approved, namely dasatinib, nilotinib, bosutinib, and ponatinib (18–21). The front line TKI therapy for CP-CML includes imatinib and next-generation front-line TKIs: dasatinib, nilotinib and bosutinib. Next generation TKIs result in faster and deeper responses as well as in a lower risk of progression to advanced CML (13–15, 22). Nonetheless, randomized control trials showed no overall survival benefit for next-generation TKIs (13, 22). Next-generation TKIs are more appropriate for high-risk patients as well as in young and/or female patients for which treatment discontinuation is particularly important for fertility purposes. The TKI of choice for primary treatment is also dictated by the comorbidity profile of the patients. Hematopoietic stem cell transplantation (HSCT) is no longer recommended for CP-CML (23–25). Resistance to TKIs is determined by aberrant expressions of drug transporters (26, 27), plasma protein binding of TKIs (28–31), and mutations in the BCR-ABL1 tyrosine kinase domain that prevent the proper binding of the TKIs to their epitopes (31–36). There are more that 100 point mutations described so far, which have to be specifically treated with certain TKI: Y253H, E255K/V, or F359V/C/I mutations should be treated with dasatinib, F317L/V/I/C, T315A, or V299L mutations should be treated with nilotinib, while E255K/V, F317L/V/I/C, F359V/C/I, T315A, or Y253H mutations respond to ponatinib or omacetaxine (13, 37–44), the latter being a synthetic analog of a natural product isolated from the Japanese plum yew (45–49).
AP or BP CML should be treated by allogeneic HSCT after inducing remission with TKI and/or chemotherapy, regardless of whether the event occurred at diagnosis or progressed from CP-CML (50). Depending whether the BP is dominated by myeloid or lymphoid cells, acute myeloid leukemia or acute lymphocytic leukemia type protocols should be employed (51–53). Accelerated phase CML patients that progressed on TKI therapy also benefit from omacetaxine (54, 55).
In the era of TKIs, for most patients only qRT-PCR is sensitive enough for detecting residual disease in the long term. CML burden monitoring via qRT-PCR was considered even before the advent of TKI (56, 57), but it was the unprecedented response to TKIs that pushed toward the implementation and standardization of the BCR-ABL1 assay.
The implementation of the BCR-ABL1 assay in the clinical practice was also aided by the development of an international scale (IS). The IS reference is represented by the median value of 30 samples collected from patients with newly diagnosed CP CML who were enrolled in the IRIS trial (58). Since these samples have been exhausted, the calibration to IS is currently achieved by either exchanging a set of samples with reference laboratories that maintain strict quality control or by using the World Health Organization (WHO) primary reference standard of four reference reagents (stably stored lyophilized cell line mixtures) assigned a fixed BCR-ABL1 IS value (10, 1, 0.1, 0.01%) BCR-ABL1 RNA (59, 60). It should be noted that BCR-ABL1 IS = 100% is just a reference ratio between BCR-ABL1 mRNA and control mRNA and it does not mean that the number of BCR-ABL1 mRNA is equal to that of control mRNAs. Alternatively, it is customary to express response as the log reduction compared to BCR-ABL1 IS = 100%. For example, a BCR-ABL1 IS = 0.1% represents a log three reduction compared to baseline (MR 3.0).
The reference gene for the BCR-ABL1 assay is usually represented by the ABL1. The ABL1 gene was chosen through an international initiative out of a panel of 14 candidate genes and has since become the standard control gene for most laboratories performing BCR-ABL1 quantification (61). The major criteria for selecting the control gene were the following: (i) absence of pseudogenes; (ii) not very high or very low expression; (iii) no significantly different expression levels between normal and leukemic samples; (iv) no significantly different expression levels between peripheral blood and bone marrow. Despite fulfilling these criteria, the ABL gene has an important drawback in that the amplicons also include the BCR-ABL1 gene. Thus, when ABL is used as a reference, the BCR-ABL1 assay expresses not the BCR-ABL1/ABL ratio but the proportion of BCR-ABL1 transcripts, i.e., BCR-ABL1/BCR-ABL1+ABL. The two are approximately equal however when BCR-ABL1 < < ABL. In other words, the BCR-ABL1/BCR-ABL1+ABL is an acceptable proxy for the BCR-ABL1/ABL for BCR-ABL1 IS values below 10%. We will show below that this bias can be mitigated based on a mathematical correction, allowing the ABL1 gene to be used as control even for BCR-ABL1 IS values >10%.
The evidence for the prognostic and predictive value of BCR-ABL1 IS is compelling and serial assessment of BCR-ABL1 IS is now an integral part of CML guidelines. According to the ELN guidelines, BCR-ABL1 testing should be performed every 3 months for 2 years and then every 3–6 months thereafter, provided that the patient fulfilled all required milestones (4). In the case of each milestone, the ELN guidelines define threshold values, based on which the response to the treatment can be labeled as Optimal, Warning, or Failure (Figure 1A).
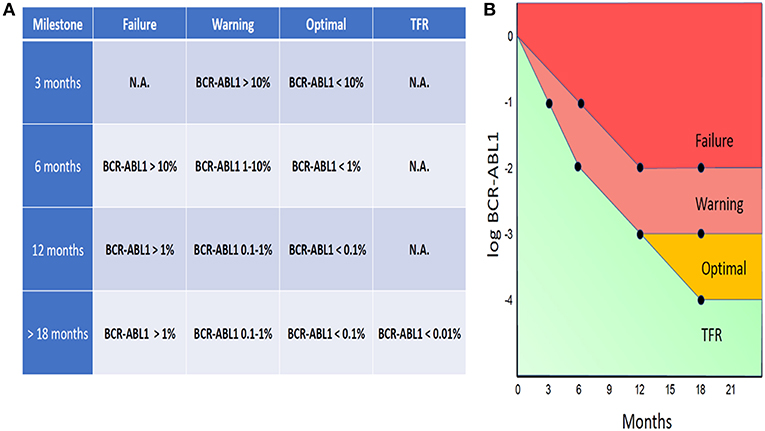
Figure 1. ELN-based guidelines threshold values, labeled as Optimal, Warning, or Failure (A). BCR-ABL1 IS “trajectories” designated as Failure, Warning, or Optimal (B).
By uniting the threshold values defined by the ELN guidelines, there is a whole spectrum of BCR-ABL1 IS “trajectories” that can be designated as Failure, Warning, or Optimal (Figure 1B). The ELN guidelines also define special conditions for attempting treatment-free remission (TFR) (see below the discussion o TFR).
According to the ELN guidelines, the optimal response threshold at 12 months is BCR-ABL1 IS <0.1% [also called major molecular response (MMR)]. Achieving MMR is associated with a negligible risk of disease progression compared to patients that failed to achieve this milestone (62–66). However, the prognostic significance of MMR has been challenged by the lack of statistical significance when adjusting for multiple comparisons (62, 64, 65). Thus, there is an ongoing research regarding the appropriate management of patients having BCR-ABL1 IS between 1 and 0.1% in the long run. This issue has been recently tackled by the German CML Study IV group, which showed evidence suggesting that the optimal waiting time for patients to achieve MMR is about 2.5 years (67), after which a change in the treatment should be considered.
Beyond MMR, achieving deeper remission is an important concept as well. Deep molecular remission (DMR) is variably defined as either BCR-ABL1 IS <0.01% (MR 4.0) or BCR-ABL IS <0.0032% (MR 4.5). In the German CML study IV, the life expectancy of patients with MR4 or MR4.5 was the same as that of age-matched population (68). The same study also showed that among 792 patients who achieved MR4, only four patients (5%) displayed disease progression. MMR and DMR also corelate with improved EFS, PFS and OS (69, 70). Thus, DMR has been taken into consideration as a treatment goal in itself and a proper surrogate endpoint in clinical trials (17).
The absence of any detectable BCR-ABL1 transcripts is termed complete molecular remission (CMR) or molecular undetectable leukemia and it corelates with better outcomes in terms of event-free survival (EFS), progression-free survival (PFS), and overall survival (OS) (69, 70). Designating CMR must always be accompanied by the sensitivity and the control gene of the assay. However, one of the most important implication of DMR and CMR regards the achievement of treatment-free remission, which was shown to be feasible in selected patients.
At the other end of the spectrum, there is an ongoing research regarding the role of early molecular response (EMR) as a means of predicting outcomes and treatment responses. EMR is defined as BCR-ABL1 IS <10% at 3 months. Achieving EMR is associated with improved PFS and OS compared to patients not achieving this milestone (71–73).
Several studies have shown that the dynamics of the BCR-ABL1 values can be used for devising independent predictors of outcome (74–76). For instance, Branford et al. have shown that for patients with >10% BCR-ABL1 IS after 3 months of imatinib, the rate of BCR-ABL1 decline at 3 months defines distinct prognostic subgroups. Patients with a BCR-ABL1 decline by at least one-half at 76 days (74 out of 95 patients, 78%) had significantly superior outcomes compared with the patients (21 out of 95 patients, 22%), where the halving time was >76 days in terms of OS, PFS EFS, and MMR (77).
Similarly, Hanfstein et al. showed that that the velocity of the BCR-ABL1 decay successfully predicts outcome and that a 0.35-fold reduction of BCR-ABL1 compared to baseline levels at 3 months (0.46-log reduction, that is, roughly half-log) defines distinct risk subgroups in terms of overall survival (78). In order to calculate the fold decrease compared to baseline, the authors had to use beta glucuronidase (GUSB) as control gene, since many BCR-ABL1 IS values were above 10% at the time of diagnosis. Thus, the authors suggested that implementing dynamical parameters will require abandoning ABL1 as the control gene and using alternative control genes such as GUSB. However, we believe that the substituting ABL1 as the control gene is not required and that the situation can be mitigated using a mathematical transformation.
The reasoning behind the mathematical transformation lies in the distinction between ratio and proportion. The aim of the BCR-ABL1 assay is to calculate the ratio between the BCR-ABL1 transcripts and the ABL1 transcripts (i.e., BCR-ABL1/ABL1). However, the actual results of the assay are represented by the proportion of BCR-ABL1 transcripts from the total number of BCR-ABL1 plus ABL1 transcripts (i.e., BCR-ABL1/BCR-ABL1+ABL1). The distinction between ratio and proportion is unimportant when the results of the assay is small, as it is most often the case in clinical practice due to the excellent response to TKIs. In a situation when out of the total number of BCR-ABL1 plus ABL1 transcripts, the BCR-ABL1 transcripts represent 1 part, and the ABL1 transcripts represent 99 parts (Figure 2 top, green box). In this case, the proportion of BCR-ABL1 transcripts is 1% (1/99+1), while the ratio between BCR-ABL1 transcripts and ABL1 transcripts is 1.01% (1/99). Thus, the proportion and ratio of BCR-ABL1 transcripts are almost equal, since the number of BCR-ABL1 transcripts is negligible compared to the ABL1 transcripts.
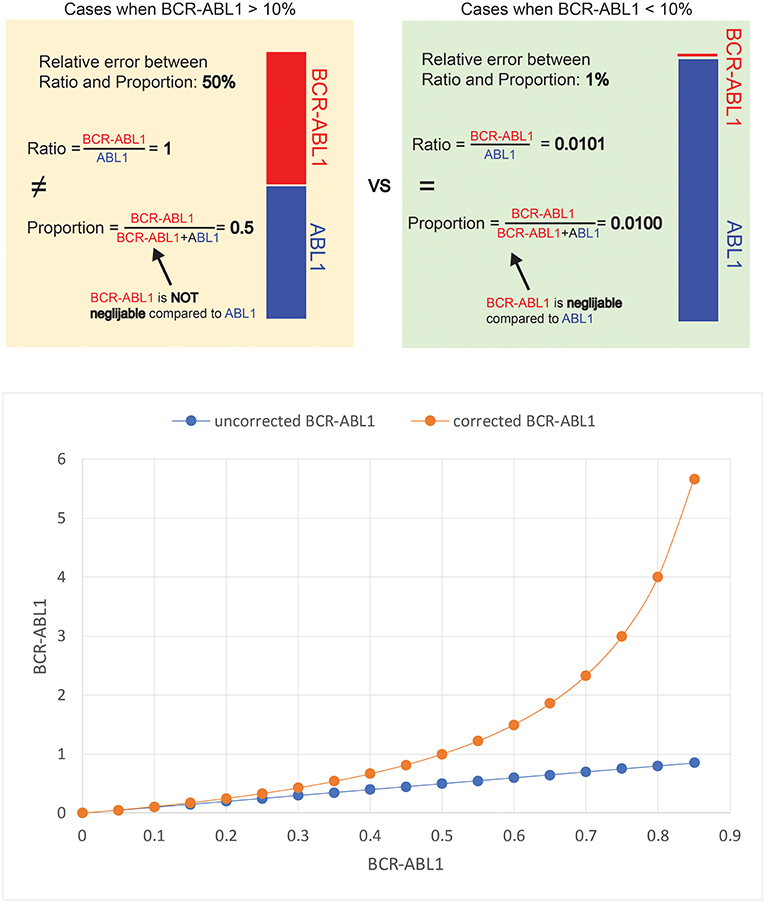
Figure 2. Scenario when out of the total number of BCRABL1 plus ABL1 transcripts, the BCR-ABL1 transcripts represent 1 part, and the ABL1 transcripts represent 99 parts.
Still, a sample from a patient might have an equal number of BCR-ABL1 transcripts and ABL1 transcripts (Figure 2 top, yellow box). The BCR-ABL1 transcripts represent 50 parts and the ABL1 transcripts also represent 50 parts. In this case, the BCR-ABL1 represent 50% of the total number of BCR-ABL1 plus ABL1 transcripts (50/50+50), while the ratio between the two is 100% (50/50). Fifty percent differs significantly from 100%, whereas 1% is very close to 1.01%. The threshold for deciding whether the proportion of BCR-ABL1 transcripts is an acceptable proxy for the ration between BCR-ABL1 and ABL1 transcripts has been arbitrarily set to BCR-ABL1 IS = 10%. The relation between the mathematically corrected and experimental BCR-ABL1 values is shown in Figure 2 (below). The red curve in Figure 2 is the depiction of Equation (1) and it can be considered a nomogram for correcting the results of the BCR-ABL1 assay. The correction can be applied for the whole range of experimental BCR-ABL1 results, but the correction will be significant only when the experimental results of the BCR-ABL1 assay are large (i.e., BCR-ABL1 IS >10%).
The mathematical transformation proposed here is a method of extracting the ratio of two numbers when the proportion of one of the numbers is known. In other words, we describe a strategy of retrieving the BCR-ABL1/ABL1 from the BCR-ABL1/(ABL1 + BCR-ABL1) ratio using a mathematical correction, such that the ABL1 control gene could be used even for BCA-ABL1 IS values higher than 10% (see below).
It can be showed that the BCR-ABL1/ABL1 ratio (X) can be extracted from the BCR-ABL1/(ABL + BCR-ABL1) proportion (Y) by the following transformation (see Supplementary Materials for the deduction of the formula):
The relation between the mathematically corrected and experimental BCR-ABL1 values is shown in Figure 2 (bottom). The red curve in Figure 2 is the depiction of Equation (1) and it can be considered a nomogram for correcting the results of the BCR-ABL1 assay. The correction can be applied for the whole range of experimental BCR-ABL1 results, but the correction will be significant only when the experimental results of the BCR-ABL1 assay are large (i.e., BCR-ABL1 IS >10%).
Thus, we propose a mathematical correction to help removing the bias caused by counting both malignant and non-malignant transcripts in the denominator of the BCR-ABL1 assay when using ABL1 as a control gene. This corrected BCR-ABL1 values represent the hypothetical results of the BCR-ABL1 assay is the ABL1 amplicon would not be contained in the BCR-ABL1 amplicon. For further explanations on the bias caused by the ABL1 gene, see the argument described in the study by Hanfstein et al. for employing an alternative gene as control (namely GUSB) (78).
With increasing evidence in support of the predictive and prognostic value of dynamical parameters, it is expected that situations when calculation of BCR-ABL1 IS values higher 10% is required will be more and more often. Using the mathematical transformation presented here, BCR-ABL1 transcripts values above 10% could be reliably calculated without changing ABL1 as the control gene, thus spearing laboratories from the effort of redesigning their assays and helping to translate dynamical parameters in the clinical setting.
The possibility to achieve treatment free remission (TFR), a term defined as sustained MMR after TKI treatment discontinuation, was put forward more than a decade ago (79–82). Since then, the concept was validated in several trials involving patients who were on sustained DMR. The results showed that treatment discontinuation leads to sustained MMR in around 50% of patients (83). Consequently, in the United States the Food and Drug Administration (FDA) has updated the label of nilotinib to reflect the possibility to achieve TFR.
The ELN guidelines consider that discontinuing therapy is feasible in low-risk CP CML patients achieving MR4.5 that remained in DMR (at least MR4.0) for >2 years after having been on first line TKI therapy at least 5 years. Attempting treatment discontinuation also requires no history of accelerated or blast phase CML and no history of TKI treatment resistance, among others. Access to reliable and quick BCR-ABL1 IS assay with a sensitivity of at least 4.5 MRD is also important.
Interestingly, the mechanism behind TFR is not yet fully understood. Several studies brought strong evidence for the fact that TKI therapy does not directly eliminate CML stem cells (84, 85). Another possibility is that TKI therapy eliminates CML stem cells indirectly, or that the immune surveillance can take care of MRD. This later hypothesis is supported by several studies linking successful TKI discontinuation with mechanistic insights of the immune system such as an increased proportion of mature NK cells or decreased PD-1 and immune suppressors (86, 87). However, a direct cause-effect relation is yet to be proven. Moreover, epidemiologic studies in immunocompromised patients failed to attribute immunity a pivotal role in CML progression (88).
Another possible explanation for successful TFR is that the time for developing a full-blown CML can take several years and that the process could be delayed by stochastic events (89, 90). As data from TFR clinical trials mature, the lengths of remission after treatment discontinuation will certainly bring more insights regarding the role of disease latency. In the meanwhile, treatment-free remission continues to be looked upon with care outside the setting of clinical trials. The manuscript has its limitations and this correction typically is more impactful for transcript levels above 10% and the question is of the clinical applicability as below this is more meaningful disease response criteria of major molecular remission, deep molecular remission, and complete molecular remissions.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
All authors contributed in the design of the hypothesis. VM and CT wrote the manuscript. SS supervised the work. MZ significantly contributed to the revision of the initial submitted manuscript, figures as well as in re-designing and adding new, important paragraphs to the manuscript, and thus contributing to the acceptance of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
PT was funded by an internal grant of the Iuliu Hatieganu University-School of Doctoral Studies (PCD 2019-2020). CT was supported from five ongoing Grants of the Romanian Government: Institutional Development Fund (CNFIS-FDI-2019-0636), Mobility Projects for Researchers MC-2019, a joint Romania–China Bilateral Collaboration Grant 2018-2019 (contract 14/2018), by a National Research Grant awarded for Frontiers Research Projects (PN-III-P4-ID-PCCF-2016-112) awarded to the Babes Bolyai University in collaboration with the Ion Chiricuta Oncology Institute Cluj Napoca, as well as by an Institutional Grant (ECHITAS-No. nr.29PFE/18.10.2018).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00863/full#supplementary-material
1. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am J Hematol. (2016) 91:252–65. doi: 10.1002/ajh.24275
2. Quintás-Cardama A, Cortes J. Molecular biology of bcr-abl1–positive chronic myeloid leukemia. Blood. (2009) 113:1619–30. doi: 10.1182/blood-2008-03-144790
3. Koretzky GA. The legacy of the Philadelphia chromosome. J Clin Invest. (2007) 117:2030–2. doi: 10.1172/JCI33032
4. Hochhaus A, Saussele S, Rosti G, Mahon FX, Janssen JJWM, Hjorth-Hansen H, et al. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2017) 28 (Suppl. 4):iv41–iv51. doi: 10.1093/annonc/mdx219
5. Chase A, Huntly BJP, Cross NCP. Cytogenetics of chronic myeloid leukaemia. Best Pract Res Clin Haematol. (2001) 14:553–71. doi: 10.1053/beha.2001.0154
6. Morris CM. Chronic myeloid leukemia: cytogenetic methods and applications for diagnosis and treatment. Methods Mol Biol. (2011) 730:33–61. doi: 10.1007/978-1-61779-074-4_4
7. Wang W, Cortes JE, Tang G, Khoury JD, Wang S, Bueso-Ramos CE, et al. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. (2016) 127:2742–50. doi: 10.1182/blood-2016-01-690230
8. Chen Z, Shao C, Wang W, Zuo Z, Mou X, Hu SJ, et al. Cytogenetic landscape and impact in blast phase of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Leukemia. (2017) 31:585–92. doi: 10.1038/leu.2016.231
9. Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. (2017) 376:917–27. doi: 10.1056/NEJMoa1609324
10. O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. (2003) 348:994–1004. doi: 10.1056/NEJMoa022457
11. Hochhaus A, Druker BJ, Larson RA, O'Brien SG, Gathmann I, Guilhot F. IRIS 6-year follow-up: sustained survival and declining annual rate of transformation in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood. (2007) 110:25.
12. Luu MH, Press RD. BCR-ABL PCR testing in chronic myelogenous leukemia: molecular diagnosis for targeted cancer therapy and monitoring. Expert Rev Mol Diagnos. (2013) 13:749–62. doi: 10.1586/14737159.2013.835573
13. Cortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J Clin Oncol. (2016) 34:2333–40. doi: 10.1200/JCO.2015.64.8899
14. Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. (2016) 30:1044–54. doi: 10.1038/leu.2016.5
15. Cortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. (2018) 36:231–7. doi: 10.1200/JCO.2017.74.7162
16. Lipton JH, Chuah C, Guerci-Bresler A, Rosti G, Simpson D, Assouline S, et al. Epic: a phase 3 trial of ponatinib compared with imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CP-CML). Blood. (2014) 124:519. doi: 10.1016/S1470-2045(16)00080-2
17. Mahon F-X, Etienne G. Deep molecular response in chronic myeloid leukemia: the new goal of therapy? Clin Cancer Res. (2014) 20:310–22. doi: 10.1158/1078-0432.CCR-13-1988
18. Keating GM. Dasatinib: a review in chronic myeloid leukaemia and Ph+ acute lymphoblastic leukaemia. Drugs. (2017) 77:85–96. doi: 10.1007/s40265-016-0677-x
19. Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. (2006) 354:2542–51. doi: 10.1056/NEJMoa055104
20. Cortes JE, Kim DW, Kantarjian HM, Brummendorf TH, Dyagil I, Griskevicius L, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. (2012) 30:3486–92. doi: 10.1200/JCO.2011.38.7522
21. O'Hare T, Shakespeare WC, Zhu X, Eide CA, Rivera VM, Wang F, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. (2009) 16:401–12. doi: 10.1016/j.ccr.2009.09.028
22. Verma D, Kantarjian HM, Jones D, Luthra R, Borthakur G, Verstovsek S, et al. Chronic myeloid leukemia (CML) with P190 BCR-ABL: analysis of characteristics, outcomes, and prognostic significance. Blood. (2009) 114:2232–5. doi: 10.1182/blood-2009-02-204693
23. Zabriskie MS, Eide CA, Tantravahi SK, Vellore NA, Estrada J, Nicolini FE, et al. BCR-ABL1 compound mutations combining key kinase domain positions confer clinical resistance to ponatinib in Ph chromosome-positive leukemia. Cancer Cell. (2014) 26:428–42. doi: 10.1016/j.ccr.2014.07.006
24. Kantarjian HM, Cortes JE, Kim DW, Khoury HJ, Brummendorf TH, Porkka K, et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood. (2014) 123:1309–18. doi: 10.1182/blood-2013-07-513937
25. Rossari F, Minutolo F, Orciuolo E. Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy. J Hematol Oncol. (2018) 11:84. doi: 10.1186/s13045-018-0624-2
26. Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. (2004) 104:3739–45. doi: 10.1182/blood-2003-12-4276
27. Hegedus C, Ozvegy-Laczka C, Apati A, Magocsi M, Nemet K, Orfi L, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. (2009) 158:1153–64. doi: 10.1111/j.1476-5381.2009.00383.x
28. Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. (2007) 109:3496–9. doi: 10.1182/blood-2006-07-036012
29. Larson RA, Druker BJ, Guilhot F, O'Brien SG, Riviere GJ, Krahnke T, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. (2008) 111:4022–8. doi: 10.1182/blood-2007-10-116475
30. Bouchet S, Titier K, Moore N, Lassalle R, Ambrosino B, Poulette S, et al. Therapeutic drug monitoring of imatinib in chronic myeloid leukemia: experience from 1216 patients at a centralized laboratory. Fundam Clin Pharmacol. (2013) 27:690–7. doi: 10.1111/fcp.12007
31. Branford S, Rudzki Z, Walsh S, Parkinson I, Grigg A, Szer J, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. (2003) 102:276–83. doi: 10.1182/blood-2002-09-2896
32. Soverini S, Martinelli G, Rosti G, Bassi S, Amabile M, Poerio A, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. (2005) 23:4100–9. doi: 10.1200/JCO.2005.05.531
33. Nicolini FE, Corm S, Le QH, Sorel N, Hayette S, Bories D, et al. Mutation status and clinical outcome of 89 imatinib mesylate-resistant chronic myelogenous leukemia patients: a retrospective analysis from the French intergroup of CML (Fi(phi)-LMC GROUP). Leukemia. (2006) 20:1061–6. doi: 10.1038/sj.leu.2404236
34. Soverini S, Colarossi S, Gnani A, Rosti G, Castagnetti F, Poerio A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. (2006) 12:7374–9. doi: 10.1158/1078-0432.CCR-06-1516
35. Khorashad JS, de Lavallade H, Apperley JF, Milojkovic D, Reid AG, Bua M, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol. (2008) 26:4806–13. doi: 10.1200/JCO.2008.16.9953
36. Soverini S, Gnani A, Colarossi S, Castagnetti F, Abruzzese E, Paolini S, et al. Philadelphia-positive patients who already harbor imatinib-resistant Bcr-Abl kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. (2009) 114:2168–71. doi: 10.1182/blood-2009-01-197186
37. Itonaga H, Tsushima H, Hata T, Matsuo E, Imanishi D, Imaizumi Y, et al. Successful treatment of a chronic-phase T-315I-mutated chronic myelogenous leukemia patient with a combination of imatinib and interferon-alfa. Int J Hematol. (2012) 95:209–13. doi: 10.1007/s12185-012-1005-1
38. Tomuleasa C, Dima D, Frinc I, Patiu M, Petrushev B, Cucuianu A, et al. BCR-ABL1 T315I mutation, a negative prognostic factor for the terminal phase of chronic myelogenous leukemia treated with first- and second-line tyrosine kinase inhibitors, might be an indicator of allogeneic stem cell transplant as the treatment of choice. Leuk Lymphoma. (2015) 56:546–7. doi: 10.3109/10428194.2014.940582
39. Yun S, Vincelette ND, Segar JM, Dong Y, Shen Y, Kim DW, et al. Comparative effectiveness of newer tyrosine kinase inhibitors versus imatinib in the first-line treatment of chronic-phase chronic myeloid leukemia across risk groups: a systematic review and meta-analysis of eight randomized trials. Clin Lymphoma Myeloma Leuk. (2016) 16:e85–94. doi: 10.1016/j.clml.2016.03.003
40. Hughes TP, Saglio G, Quintas-Cardama A, Mauro MJ, Kim DW, Lipton JH, et al. BCR-ABL1 mutation development during first-line treatment with dasatinib or imatinib for chronic myeloid leukemia in chronic phase. Leukemia. (2015) 29:1832–8. doi: 10.1038/leu.2015.168
41. Jabbour E, Kantarjian H, Jones D, Breeden M, Garcia-Manero G, O'Brien S, et al. Characteristics and outcomes of patients with chronic myeloid leukemia and T315I mutation following failure of imatinib mesylate therapy. Blood. (2008) 112:53–5. doi: 10.1182/blood-2007-11-123950
42. Jabbour E, Kantarjian HM, Jones D, Reddy N, O'Brien S, Garcia-Manero G, et al. Characteristics and outcome of chronic myeloid leukemia patients with F317L BCR-ABL kinase domain mutation after therapy with tyrosine kinase inhibitors. Blood. (2008) 112:4839–42. doi: 10.1182/blood-2008-04-149948
43. Muller MC, Cortes JE, Kim DW, Druker BJ, Erben P, Pasquini R, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. (2009) 114:4944–53. doi: 10.1182/blood-2009-04-214221
44. Hughes T, Saglio G, Branford S, Soverini S, Kim DW, Muller MC, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. (2009) 27:4204–10. doi: 10.1200/JCO.2009.21.8230
45. Okabe S, Tauchi T, Tanaka Y, Katagiri S, Kitahara T, Ohyashiki K. Activity of omacetaxine mepesuccinate against ponatinib-resistant BCR-ABL-positive cells. Blood. (2013) 122:3086–8. doi: 10.1182/blood-2013-04-494773
46. Li YF, Deng ZK, Xuan HB, Zhu JB, Ding BH, Liu XN, et al. Prolonged chronic phase in chronic myelogenous leukemia after homoharringtonine therapy. Chin Med J. (2009) 122:1413–7. doi: 10.3760/cma.j.issn.0366-6999.2009.12.011
47. Quintas-Cardama A, Kantarjian H, Cortes J. Homoharringtonine, omacetaxine mepesuccinate, and chronic myeloid leukemia circa 2009. Cancer. (2009) 115:5382–93. doi: 10.1002/cncr.24601
48. Deininger MW, Hodgson JG, Shah NP, Cortes JE, Kim DW, Nicolini FE, et al. Compound mutations in BCR-ABL1 are not major drivers of primary or secondary resistance to ponatinib in CP-CML patients. Blood. (2016) 127:703–12. doi: 10.1182/blood-2015-08-660977
49. Saglio G, Fava C. BCR-ABL1 mutation not equal ponatinib resistance. Blood. (2016) 127:666–7. doi: 10.1182/blood-2015-12-685149
50. Chhabra S, Ahn KW, Hu ZH, Jain S, Assal A, Cerny J, et al. Myeloablative vs reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chronic myeloid leukemia. Blood Adv. (2018) 2:2922–36. doi: 10.1182/bloodadvances.2018024844
51. Jain P, Kantarjian HM, Ghorab A, Sasaki K, Jabbour EJ, Nogueras Gonzalez G, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: cohort study of 477 patients. Cancer. (2017) 123:4391–402. doi: 10.1002/cncr.30864
52. Saussele S, Silver RT. Management of chronic myeloid leukemia in blast crisis. Ann Hematol. (2015) 94 (Suppl. 2):S159–65. doi: 10.1007/s00277-015-2324-0
53. Hehlmann R. How I treat CML blast crisis. Blood. (2012) 120:737–47. doi: 10.1182/blood-2012-03-380147
54. Rosshandler Y, Shen AQ, Cortes J, Khoury HJ. Omacetaxine mepesuccinate for chronic myeloid leukemia. Expert Rev Hematol. (2016) 9:419–24. doi: 10.1586/17474086.2016.1151351
55. Nicolini FE, Khoury HJ, Akard L, Rea D, Kantarjian H, Baccarani M, et al. Omacetaxine mepesuccinate for patients with accelerated phase chronic myeloid leukemia with resistance or intolerance to two or more tyrosine kinase inhibitors. Haematologica. (2013) 98:e78–9. doi: 10.3324/haematol.2012.083006
56. Soverini S, Rosti G, Baccarani M, Martinelli G. Molecular monitoring. Curr Hematol Malig Rep. (2014) 9:1–8. doi: 10.1007/s11899-013-0192-z
57. Menif S, Zarrouki S, Jeddi R, ben Alaya N, Ali ZB, Ben Abid H, et al. Quantitative detection of bcr-abl transcripts in chronic myeloid leukemia. Pathol Biol. (2009) 57:388–91. doi: 10.1016/j.patbio.2007.12.010
58. Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. (2003) 349:1423–32. doi: 10.1056/NEJMoa030513
59. Press RD, Kamel-Reid S, Ang D. BCR-ABL1 RT-qPCR for monitoring the molecular response to tyrosine kinase inhibitors in chronic myeloid leukemia. J Mol Diagnos. (2013) 15:565–76. doi: 10.1016/j.jmoldx.2013.04.007
60. Branford S, Fletcher L, Cross NC, Muller MC, Hochhaus A, Kim DW, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. (2008) 112:3330–8. doi: 10.1182/blood-2008-04-150680
61. Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) - a Europe against cancer program. Leukemia. (2003) 17:2474–86. doi: 10.1038/sj.leu.2403136
62. Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. (2006) 355:2408–17. doi: 10.1056/NEJMoa062867
63. Press RD, Galderisi C, Yang R, Rempfer C, Willis SG, Mauro MJ, et al. A half-log increase in BCR-ABL RNA predicts a higher risk of relapse in patients with chronic myeloid leukemia with an imatinib-induced complete cytogenetic response. Clin Cancer Res. (2007) 13:6136–43. doi: 10.1158/1078-0432.CCR-07-1112
64. de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. (2008) 26:3358–63. doi: 10.1200/JCO.2007.15.8154
65. Marin D, Milojkovic D, Olavarria E, Khorashad JS, de Lavallade H, Reid AG, et al. European leukemianet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. (2008) 112:4437–44. doi: 10.1182/blood-2008-06-162388
66. Hehlmann R, Lauseker M, Jung-Munkwitz S, Leitner A, Muller MC, Pletsch N, et al. Tolerability-adapted imatinib 800 mg/d versus 400 mg/d versus 400 mg/d plus interferon-alpha in newly diagnosed chronic myeloid leukemia. J Clin Oncol. (2011) 29:1634–42. doi: 10.1200/JCO.2010.32.0598
67. Saussele S, Hehlmann R, Fabarius A, Jeromin S, Proetel U, Rinaldetti S, et al. Defining therapy goals for major molecular remission in chronic myeloid leukemia: results of the randomized CML Study IV. Leukemia. (2018) 32:1222–8. doi: 10.1038/s41375-018-0055-7
68. Hehlmann R, Lauseker M, Hanfstein B, Müller MC, Schreiber A, Proetel U, et al. Complete molecular remission (CMR 4.5) of CML is induced faster by dose – optimized imatinib and predicts better survival - results from the randomized CML-study IV. Blood. (2012) 120:67.
69. Falchi L, Kantarjian HM, Quintas-Cardama A, Brien S, Jabbour EJ, Ravandi F, et al. Clinical significance of deeper molecular responses with four modalities of tyrosine kinase inhibitors as frontline therapy for chronic myeloid leukemia. Blood. (2012) 120:164.
70. Etienne G, Nicolini NE, Dulucq S, Schmitt A, Hayette S, Lippert É, et al. Achieving a complete molecular remission under imatinib therapy is associated with a better outcome in chronic phase chronic myeloid leukaemia patients on imatinib frontline therapy. Blood. (2012) 120:3754. doi: 10.3324/haematol.2013.095158
71. Hehlmann R, Lauseker M, Saussele S, Pfirrmann M, Krause S, Kolb HJ, et al. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. (2017) 31:2398–406. doi: 10.1038/leu.2017.253
72. Hanfstein B, Muller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia. (2012) 26:2096–102. doi: 10.1038/leu.2012.85
73. Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. (2012) 30:232–8. doi: 10.1200/JCO.2011.38.6565
74. Huet S, Cony-Makhoul P, Heiblig M, Tigaud I, Gazzo S, Belhabri A, et al. Major molecular response achievement in CML Patients can be predicted by BCR-ABL1/ABL1 or BCR-ABL1/GUS ratio at an earlier time point of follow-up than currently recommended. PLoS ONE. (2014) 9:e106250-e. doi: 10.1371/journal.pone.0106250
75. Iriyama N, Fujisawa S, Yoshida C, Wakita H, Chiba S, Okamoto S, et al. Shorter halving time of BCR-ABL1 transcripts is a novel predictor for achievement of molecular responses in newly diagnosed chronic-phase chronic myeloid leukemia treated with dasatinib: results of the D-first study of Kanto CML study group. Am J Hematol. (2015) 90:282–7. doi: 10.1002/ajh.23923
76. Fava C, Rege-Cambrin G, Dogliotti I, Gottardi E, Berchialla P, Di Gioacchino B, et al. Early BCR-ABL1 reduction is predictive of better event-free survival in patients with newly diagnosed chronic myeloid leukemia treated with any tyrosine kinase inhibitor. Clin Lymphoma Myeloma Leuk. (2016) 16 (Suppl.):S96–100. doi: 10.1016/j.clml.2016.03.008
77. Branford S, Yeung DT, Parker WT, Roberts ND, Purins L, Braley JA, et al. Prognosis for patients with CML and >10% BCR-ABL1 after 3 months of imatinib depends on the rate of BCR-ABL1 decline. Blood. (2014) 124:511–8. doi: 10.1182/blood-2014-03-566323
78. Hanfstein B, Shlyakhto V, Lauseker M, Hehlmann R, Saussele S, Dietz C, et al. Velocity of early BCR-ABL transcript elimination as an optimized predictor of outcome in chronic myeloid leukemia (CML) patients in chronic phase on treatment with imatinib. Leukemia. (2014) 28:1988–92. doi: 10.1038/leu.2014.153
79. Gale RP, Hochhaus A. Therapy-free remission in chronic myeloid leukemia: possible mechanism. Expert Rev Hematol. (2018) 11:269–72. doi: 10.1080/17474086.2018.1442213
80. Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. (2007) 109:58–60. doi: 10.1182/blood-2006-03-011239
81. Mahon FX, Delbrel X, Cony-Makhoul P, Faberes C, Boiron JM, Barthe C, et al. Follow-up of complete cytogenetic remission in patients with chronic myeloid leukemia after cessation of interferon alfa. J Clin Oncol. (2002) 20:214–20. doi: 10.1200/JCO.2002.20.1.214
82. Cortes J, O'Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. (2004) 104:2204–5. doi: 10.1182/blood-2004-04-1335
83. Saußele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. (2016) 30:1638–47. doi: 10.1038/leu.2016.115
84. Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. (2011) 121:396–409. doi: 10.1172/JCI35721
85. Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. (2012) 119:1501–10. doi: 10.1182/blood-2010-12-326843
86. Ilander M, Olsson-Stromberg U, Schlums H, Guilhot J, Bruck O, Lahteenmaki H, et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia. (2017) 31:1108–16. doi: 10.1038/leu.2016.360
87. Hughes A, Clarson J, Tang C, Vidovic L, White DL, Hughes TP, et al. CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood. (2017) 129:1166–76. doi: 10.1182/blood-2016-10-745992
88. Gale RP, Opelz G. Is there immune surveillance against chronic myeloid leukaemia? Possibly, but not much. Leuk Res. (2017) 57:109–11. doi: 10.1016/j.leukres.2017.03.003
89. Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, et al. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radi Res. (2013) 179:361–82. doi: 10.1667/RR2892.1
Keywords: chronic myeloid leukemia, mathematical modeling, BCR-ABL, IS, treatment free remission
Citation: Moisoiu V, Teodorescu P, Parajdi L, Pasca S, Zdrenghea M, Dima D, Precup R, Tomuleasa C and Soverini S (2019) Assessing Measurable Residual Disease in Chronic Myeloid Leukemia. BCR-ABL1 IS in the Avant-Garde of Molecular Hematology. Front. Oncol. 9:863. doi: 10.3389/fonc.2019.00863
Received: 30 April 2019; Accepted: 20 August 2019;
Published: 23 September 2019.
Edited by:
Naval Daver, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Alice Mims, The Ohio State University, United StatesCopyright © 2019 Moisoiu, Teodorescu, Parajdi, Pasca, Zdrenghea, Dima, Precup, Tomuleasa and Soverini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ciprian Tomuleasa, Y2lwcmlhbi50b211bGVhc2FAdW1mY2x1ai5ybw==
†These authors have contributed equally to this work as co-first authors
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.