- 1Department of Urology, University Hospitals Leuven, Leuven, Belgium
- 2Laboratory of Molecular Endocrinology, KU Leuven, Leuven, Belgium
- 3Department of Radiation Oncology, University Hospitals Leuven, Leuven, Belgium
Introduction: Salvage lymph node dissection (sLND) has been proposed as a treatment option for prostate cancer patients with lymph node (LN) recurrence following radical prostatectomy to delay or avoid palliative androgen deprivation therapy (ADT). Historically sLND has been performed using an open approach, with its associated morbidity. A limited number of studies have reported peri-operative outcomes following robot-assisted sLND. However, a direct comparison with the open approach has hitherto not yet been reported. This study investigates whether robot-assisted sLND is associated with better peri-operative outcomes compared to the open approach. Early oncological outcomes are also compared.
Patients and methods: In this retrospective study, clinical data were collected from 60 patients undergoing open sLND between 2010–2016 and 30 patients undergoing robot-assisted sLND between 2016 and 2018 at our tertiary referral center. The primary objective of the study was to compare peri-operative outcomes (length of stay, estimated blood loss, operative time, intra-operative, and postoperative complications) and LN yield between both procedures. As secondary objective early oncological outcome [biochemical recurrence-free survival (BRFS) and clinical recurrence-free survival (CRFS)] was compared. Variables of interest were compared using the chi-squared test (categorical variables), two sample t-test, and Mann-Whitney U-test (continuous variables). To compare BRFS and CRFS, Kaplan-Meier analysis, and log-rank tests were performed.
Results: Robotic sLND was associated with reduced blood loss (median 100 vs. 275cc; p < 0.0001) and shorter length of stay (median 2 vs. 7 days; p < 0.0001) compared to open sLND. Moreover, postoperative complications within 30 days after surgery were more prevalent in the open sLND group compared to the robotic group (41.6% vs. 20%, p = 0.04). No significant differences in LN yield (for each sLND template), BRFS, and CRFS were detected between both groups.
Conclusion: Robot-assisted sLND is associated with significantly reduced peri-operative morbidity compared to open sLND. No difference in LN yield, BRFS and CRFS was seen between both groups. Modern imaging techniques underestimate the tumor burden and therefore, the surgical sLND template should not be limited to the positive spots on pre-operative imaging.
Introduction
Biochemical recurrence (BCR) after radical prostatectomy (RP) for clinically localized prostate cancer occurs in 15–40% of patients (1, 2). With the emergence of new imaging modalities, such as choline and PSMA PET/CT, more patients are diagnosed with recurrence confined to a limited number of lymph nodes (LN) (3–6). These patients have a better prognosis than those with skeletal or visceral recurrence (1, 7, 8). In clinical practice, these patients are mainly treated with androgen deprivation therapy (ADT) which is a palliative option aimed at delaying symptoms (9). Recently, salvage lymph node dissection (sLND) has been proposed as a therapeutic option in “node-only” recurrence in order to postpone life-long palliative ADT or to possibly improve cancer-specific survival in selected patients (10–12). Historically, this procedure is performed using an open approach, with its associated morbidity (1, 13). Currently, a limited number of studies have reported peri-operative outcomes following robot-assisted sLND (14–17). However, a direct comparison with the open approach has hitherto not yet been published.
In this retrospective study we compared the peri-operative outcomes between open and robot-assisted sLND in patients with node-only recurrence following RP for clinically localized prostate cancer. We also compared early oncological outcomes between both procedures.
Materials and Methods
Patient Population
After obtaining approval from the institutional ethical review board (internal number: S61342), we retrospectively collected clinical data from patients undergoing open or robot-assisted sLND between 2010 and 2018 at a single tertiary referral center. Inclusion criteria were biopsy-proven diagnosis of adenocarcinoma of the prostate, BCR following RP (defined as confirmed PSA >0.2 ng/ml), at least one positive LN on imaging at the time of BCR, and open or robot-assisted sLND. Exclusion criteria were external beam radiotherapy (EBRT), brachytherapy, or high intensity focused ultrasound (HIFU) as initial treatment; visible recurrence in the prostatectomy bed; or concomitant skeletal (M1b) or visceral (M1c) recurrence on conventional or molecular-based imaging (as detected by one of the following imaging techniques at time of BCR: bone scan, abdomino-pelvic computerized tomography, MRI, and/or PET/CT).
Patient and Tumor Characteristics
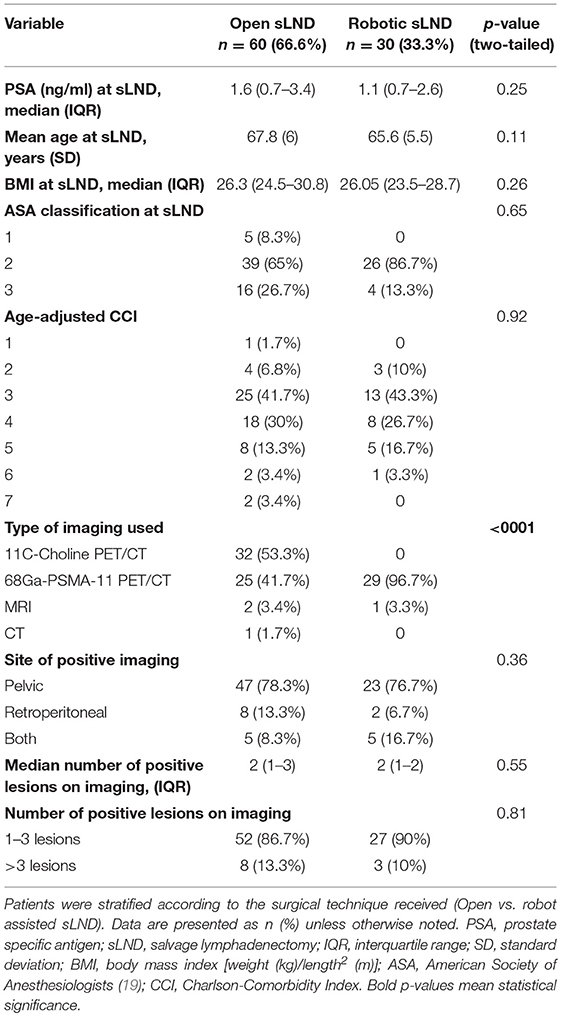
The following data were collected: clinico-pathological disease characteristics at RP, adjuvant/salvage ADT, or radiotherapy (RT) prior to sLND, imaging technique used at time of BCR, site of positive imaging (pelvic, retroperitoneal, or both), number of positive lesions on imaging, PSA at sLND, extent of sLND (pelvic, retroperitoneal, or both), number of LN removed at final pathology, perioperative blood loss (in cc), operative time (in min), and length of hospital stay (in days). Operative time was measured from skin incision to skin closure. Blood loss was estimated by the amount of blood aspirated during the procedure and weighing the surgical gauzes. Pre-operative morbidity of the patients was estimated by the age-adjusted Charlson-comorbidity index (CCI) (18). The BMI and American Society of Anesthesiologists (ASA)-classification at time of sLND was retrieved from the pre-operative anesthesia consultation (19).
Surgical Technique
The pelvic sLND template was defined as the removal of LN distal to the aortic bifurcation (Figure 1) (20):
- External iliac region: tissue overlying the external iliac vessels. Borders: bifurcation of the common iliac vessels, circumflex iliac vein, psoas muscle, and genitofemoral nerve and medial border of the external iliac vein.
- Obturator fossa region: tissue lying below the iliac vessels and above the obturator nerve. Borders: bifurcation of the common iliac vessels, pelvic floor, obturator muscle, obturator nerve, and medial border external iliac vein.
- Internal iliac region: tissue lying around the internal iliac vessels. Borders: bifurcation of the common iliac vessels, pelvic floor, bladder wall, and obturator nerve.
- Common iliac region: tissue overlying the common iliac vessels. Borders: aortic bifurcation, bifurcation of the common iliac vessels, psoas muscle and genitofemoral nerve, and medial border of the common iliac vein.
- Presacral region: tissue overlying the proximal sacral bone. Borders: Triangle between medial borders of common iliac veins and the line connecting the bifurcations of the common iliac vessels; dorsal border: promontory and proximal sacrum (S1–S2).
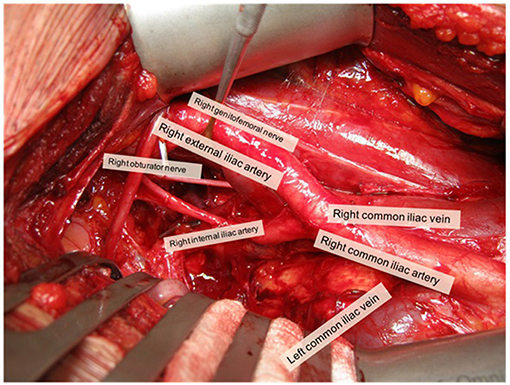
Figure 1. Overview of open pelvic sLND template (right side). Picture was taken with informed consent of the patient.
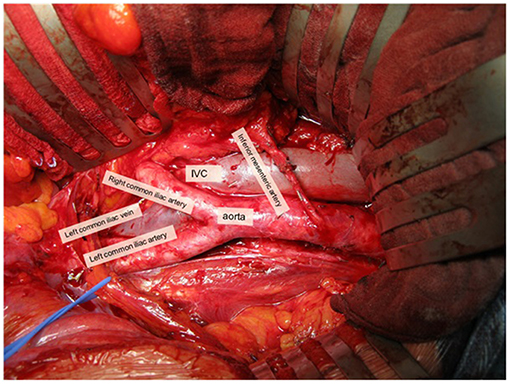
The retroperitoneal sLND template was defined as the removal of para-aortic and inter-aorto-caval LN above the aortic bifurcation up to the inferior mesenteric artery (or up to the renal hilum in case of nodal recurrence above the inferior mesenteric artery on pre-operative imaging) (Figure 2). Paracaval LN were only removed in case of a positive LN in that area on preoperative imaging. Templates were not limited to the positive spots on imaging and could be modified slightly according to the nodal recurrence site on pre-operative imaging and the extent of the prior pelvic LN dissection during RP.
All procedures were performed by three experienced surgeons (H.V.P., S.J., and W.E.). For the robot-assisted procedures, the Xi Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) was used with a six-port transperitoneal approach. Supplementary Figure 1 provides an overview of port placement in pelvic sLND and retroperitoneal sLND. In the open sLND group, pelvic LN were approached by extraperitoneal access and retroperitoneal LN by transperitoneal access.
Preoperative bowel preparation was not performed. All patients received postoperative compression stockings and subcutaneous injections with low-molecular weight heparins.
Primary Objective: Comparison of Perioperative Outcome
Intra-operative complications were retrieved from the surgical reports. Postoperative complications up to 30 days after sLND were retrieved by reviewing the electronic medical records and graded using the Clavien-Dindo classification (21). Complications later than 30 days postoperatively were not collected. Intra- and postoperative complications were reported according the recommendations of the European Association of Urology (EAU)-guidelines panel (22).
Lymph node yield for each type of sLND template (pelvic, retroperitoneal, or pelvic + retroperitoneal) was collected and compared between both approaches. Furthermore the proportion of positive LN on preoperative imaging/positive LN at final pathology was calculated and stratified by imaging technique (11C-choline vs. 68Ga PSMA-11 PET/CT) and surgical approach (open vs. robotic approach).
Secondary Objective: Comparison of Early Oncological Outcome
Biochemical recurrence free-survival (BRFS) and clinical recurrence free-survival (CRFS) were compared between both groups. BCR was defined as a PSA-value >0.2 ng/ml post sLND and clinical recurrence was defined as the onset of new lesions on imaging (or if patients became symptomatic). Decisions on performing imaging following sLND was at the discretion of the treating physician and adjuvant/salvage treatments following sLND were decided at the multidisciplinary team meeting. Patients who did not have oncological follow-up data available were excluded from analysis (BRFS and CRFS).
Statistical Analysis
Non-normally distributed continuous variables were reported by medians and interquartile ranges (IQRs) and normally distributed continuous variables by means and standard deviations (SDs). Summary statistics for categorical variables were reported using proportions and frequencies. Categorical variables were compared using the chi-squared test or Fisher's exact test and continuous variables using the two sample t-test or Mann-Whitney U-test. Kaplan-Meier analysis was performed to assess BRFS and CRFS, and log-rank test to determine a significant difference between both approaches. Statistical analyses were performed using the statistical software Medcalc, Statistical Software version 18.9 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018) with a significance level of p < 0.05.
Results
Baseline Patient Characteristics
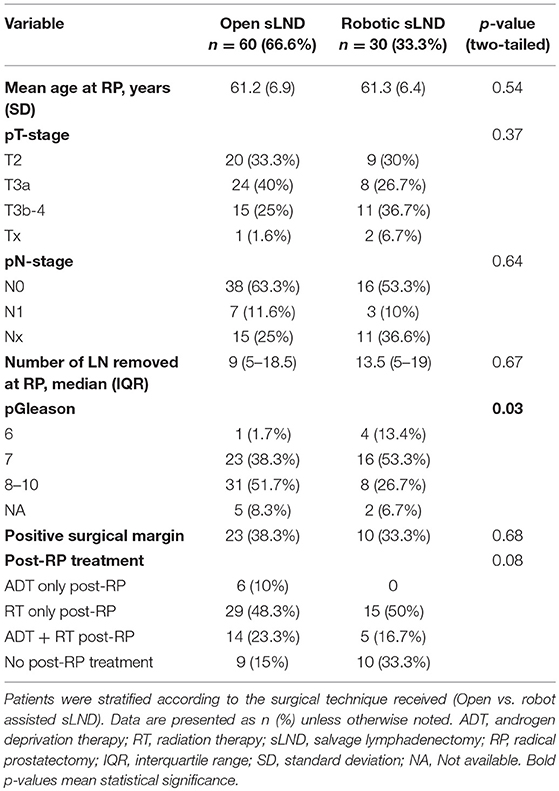
Table 1 provides an overview of the baseline demographic and tumor characteristics according to surgical technique (open vs. robot-assisted) at time of RP. We identified 60 patients undergoing open sLND between 2010–2016 and 30 patients undergoing robotic sLND between 2016 and 2018. Patients in the open SLND group more often had Gleason score 8–10 prostate cancer compared to the robotic group (p = 0.03). No difference in proportion adjuvant/salvage radiotherapy was observed between the open and robotic groups [71.6 vs. 67.7%, respectively (p = 0.7)]. The (adjuvant/salvage) radiation field (mostly 66 Gy) was confined to the prostate bed. None of the patients were castration resistant at time of sLND. In total, 45 (75%) and 18 (60%) patients received a concomitant lymphadenectomy at time of RP in the open and robot group, respectively. Of these, information on the number of LN removed during RP was available in 34 (75.6%) and 12 (66.7%) patients in the open and robotic approach, respectively. No difference was observed in median number of LN removed during RP. Table 2 provides an overview of the baseline characteristics at time of sLND. No difference in preoperative morbidity was observed in terms of BMI, ASA-classification, and age adjusted CCI. In both groups the majority of the patients had oligometastatic recurrence defined as 1–3 lesions. At time of BCR, almost all patients (96.7%) in the robot-assisted group were assessed by 68Ga-PSMA-11 PET/CT compared to only 44% in the open group (p < 0.0001). More than half of these patients were evaluated by 11C-choline PET/CT (53.3%).
Perioperative Outcomes
Table 3 provides an overview of the intra-operative and postoperative outcomes and complications. Patients treated with robot-assisted sLND had significantly less estimated blood loss during the procedure compared to open sLND (median 100 vs. 275cc; p < 0.0001). However, no intra-operative transfusions were needed in either group. Median operative time between the two procedures was equal (median 150 vs. 150 min; p = 0.89). Length of stay was significantly lower in the robot-assisted sLND group compared to the open sLND group (median 2 vs. 7 days; p < 0.0001). No difference in intra-operative complications was observed (p = 0.34), but postoperative complications within 30 days after surgery were significantly more prevalent in the open group compared to the robotic group (41.7 vs. 20%, p = 0.04). Moreover, patients in the open group had more high-grade complications [5 vs. 0 Clavien-Dindo grade III-IV complications; hydronephrosis (double-J stent), arterial bleeding (reoperation), lymphocoele drainage (2x), renal failure (biopsy was taken to exclude nephrological disease)]. Injury to the iliac veins was the most prevalent was the most prevalent intra-operative complication in both groups. Postoperatively, lymphatic complications were more prevalent in the open group.
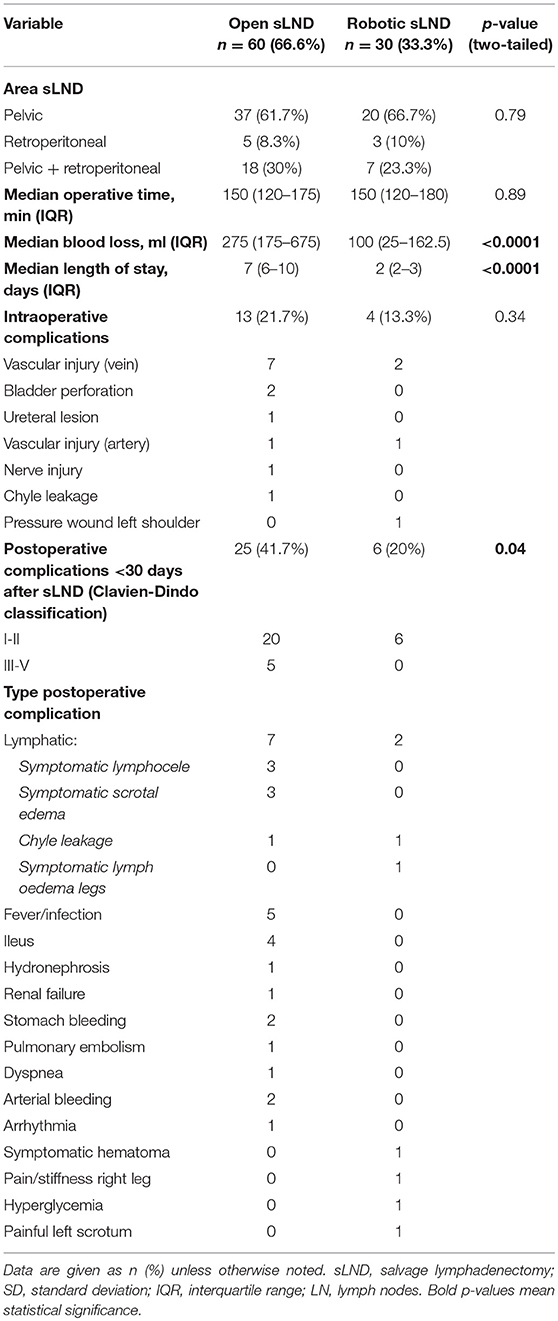
Table 3. Peri-operative outcomes of patients treated with sLND according to type of procedure (open vs. robotic).
Table 4 provides an overview of the pathological outcomes. The number of LN removed for each sLND template (pelvic, retroperitoneal, and pelvic + retroperitoneal) was equal for both groups (p = 0.88, p = 0.24, and p = 0.85, respectively). A total of 477 LN were positive at final pathology, whereas only 200 (41.9%) metastatic LN were detected on imaging. Mean numbers of metastatic LN at final pathology were 4.1 (95%-CI: 2.5–5.7) and 6 (95%-CI: 2.7–9.2) in patients assessed by 11C-choline and 68Ga-PSMA PET/CT, respectively (p = 0.30). 11C-Choline PET/CT was able to detect 57 (42.8%) out of the 133 and 68Ga-PSMA PET/CT to detect 134 (41.8%) out of 320 metastatic LN at final pathology. No significantly difference in number of metastatic LN at final pathology was observed between the open and robotic group (p = 0.11).
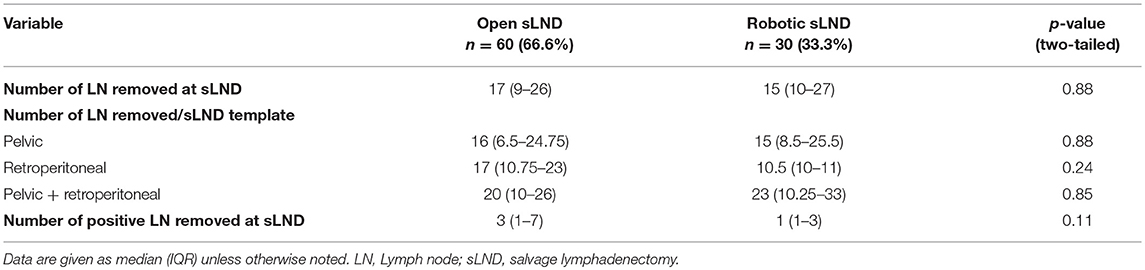
Table 4. Pathological outcomes of patients treated with sLND according to type of procedure (open vs. robotic).
Early Oncological Outcome
Mean follow-up after open and robotic sLND was 53 (median: 53mo., IQR 31.5–75) and 15 (median 15mo., IQR 10.25–21.5) months, respectively (p < 0.001). Follow-up data were available for 52 (86.7%) patients in the open group and 28 (93.3%) patients in the robotic group. Supplementary Table 1 provides information on adjuvant/salvage therapies following sLND. In the open and robotic group, 38.4 and 58% of the patients received adjuvant or salvage treatment, respectively. Median BRFS was similar in both groups (2 months, p = 0.23) (Figure 3). The majority of patients in both groups experienced BCR (90 and 89%, respectively). No difference was observed in CRFS between both groups [median 25 mo. vs. 32 mo. in the robotic and open group, respectively (p = 0.87); Figure 4].
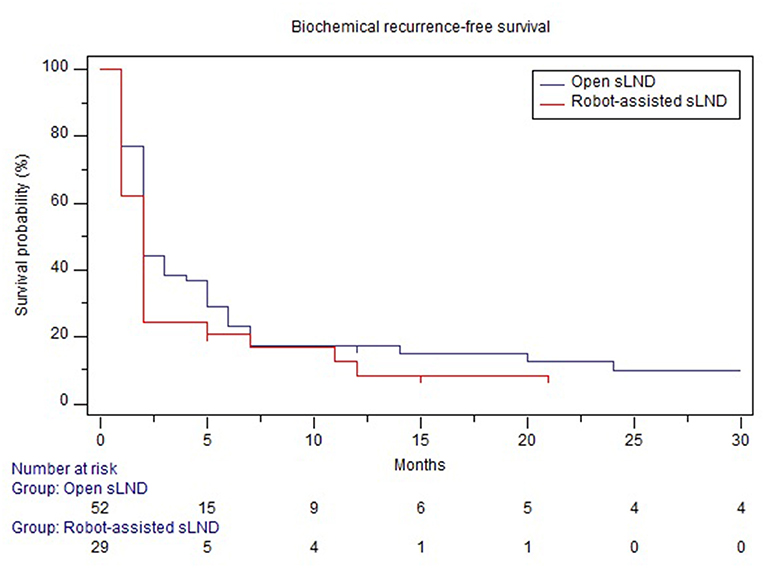
Figure 3. Comparison of biochemical recurrence-free survival between open and robotic sLND. Censored patients are marked with small vertical lines.
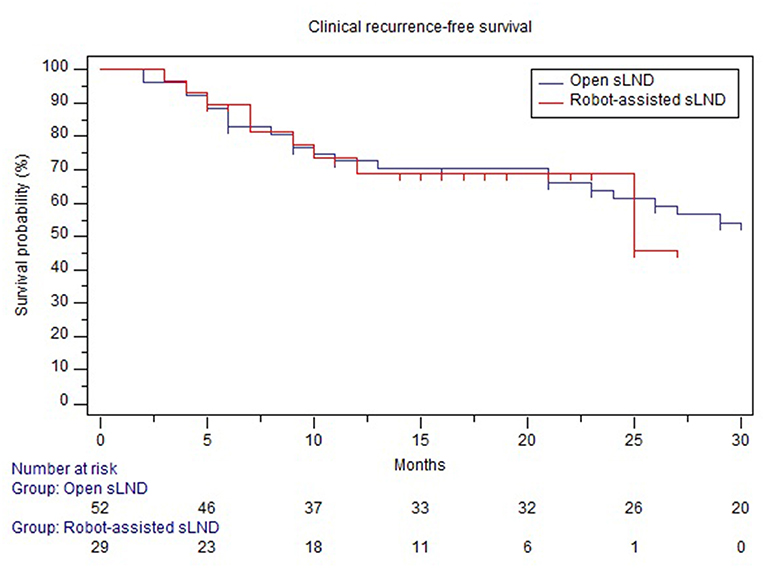
Figure 4. Comparison of clinical recurrence-free survival between open and robotic sLND. Censored patients are marked with small vertical lines.
To correct for the difference in type of preoperative imaging between both groups, a sub-analysis of patients assessed by only 68Ga-PSMA-11 PET/CT was performed. Supplementary Table 2 provides an overview of the baseline demographic and tumor characteristics according to surgical technique (open vs. robot-assisted). Baseline tumor characteristics were balanced between both groups. No difference in BRFS (median 2 mo. in both groups, p = 0.59) and CRFS (median not attained in the open group and median of 25 months in the robotic group, p = 0.79) were observed between the open and robotic approach (Supplementary Figures 2, 3).
Discussion
Patients with prostate cancer recurrence confined to a limited number of LN following primary treatment, also called oligometastatic recurrence, are potential candidates for metastasis-directed therapies. The EAU-guidelines introduced sLND as a possible therapeutic option in these patients. Salvage LND is typically performed by an open approach and associated is with substantial morbidity (1, 13). Four studies have so far investigated the feasibility and peri-operative outcomes of robot-assisted sLND, though no direct comparison has been made with the open procedure (14–17). The current study aimed to investigate the peri-operative and early oncological outcomes between open and robot-assisted sLND in patients with LN recurrence after RP.
Several observations of our study are interesting. First, robot-assisted sLND appears to be a safe alternative with favorable perioperative outcomes compared to the open approach. No high-grade postoperative complications were seen in the robotic group. This is in line with previously published robotic sLND series, where very few high-grade complications were reported (14–17). Only in the series of Linxweiler et al., five patients (13.9%) experienced high-grade (grade III according to Clavien-Dindo) complications (17). Notably, in our study lymphatic complications were more frequent in the open group (28% of all complications). This might be explained by the fact that the pelvic nodes were approached by an extraperitoneal access in the open sLND group (in case of pelvic sLND), while all nodes were removed via a transperitoneal approach in the robot-assisted group (23–25). Moreover, patients in the open group had higher metastatic burden at final pathology compared to the robotic group (median 3 vs. 1 metastatic LN). This might partially explain the higher proportion of intra- and post-operative complications as bulky nodal disease can be associated with increased risk of complications. Further, our results demonstrated significantly less blood loss and a 5-day shorter hospital stay in the robotic group compared to the open group. The higher proportion of postoperative complications with the open approach might explain this difference in hospital stay. Median operation time (150 min) and median blood loss (100 ml) in the robotic cohort were comparable with the previously published robotic sLND series (range 129–228 min and 50–250 ml, respectively) (14–17). Remarkably, the median operation time—generally one of the major drawbacks for robotic procedures—was not different between both groups. Also the number of LN removed for each sLND template (pelvic, retroperitoneal, and pelvic + retroperitoneal) was not different between both groups.
Second, only 200 (41.9%) out of 477 positive LN at final pathology were visible on preoperative imaging. Remarkably, 68Ga-PSMA-11 PET/CT was not superior to 11C-Choline PET/CT to identify metastatic LN. This might partly be explained by the fact that the mean metastatic burden in patients assessed by 68Ga-PSMA-11 PET/CT was higher compared to patients assessed by 11C-Choline PET/CT (although statistically not significant). Some patients who were assessed by 68Ga-PSMA-11 PET/CT had a very high proportion of positive LN at final pathology. For example, one patient had four suspect lesions on 68Ga-PSMA-11 PET/CT and received an open pelvic + retroperitoneal sLND resulting in 70 metastatic LN out of 78 at final pathology. Thus, despite the improved accuracy of novel imaging modalities at low PSA values compared to conventional imaging techniques, sLND should certainly not be limited to the positive spots on pre-operative imaging (26). Today, no consensus exists about the optimal extent of the sLND template. Therefore, radioguided surgery in which metastatic LN are detected intra-operatively with the use of a gamma probe, could provide an interesting alternative to reduce the morbidity of these (extensive) templates (27). Recently, Maurer et al. demonstrated that 99mTc-PSMA-based radioguided surgery had a good accuracy (93%) with promising early oncological outcomes in 31 patients with LN recurrence following RP (28). However, their technique still required an open approach with its associated morbidity (38.7% grade I; 3.2% grade IIIa complications). New promising technologies are currently developed that enable the use of radioguided surgery in combination with robotic surgery, leading to a further decrease in morbidity (29).
Finally, the majority of patients treated with sLND developed BCR independent of the surgical approach and in most cases BCR developed quickly (median time to BCR 2 months). As a consequence, it is important to counsel patients of the non-curative character of the procedure. Probably, CRFS rather than BRFS should be considered as a meaningful endpoint as CRFS in both groups extended 2 years. Patient selection appears to be of utmost importance for sLND. The identification of the “ideal” sLND candidate has already been investigated in a retrospective multi-center study in which our patients were included (30). Gleason grade group 5, a short time from RP to PSA rising, hormonal therapy at the time of sLND, positive retroperitoneal spots on imaging, ≥3 positive spots on PET scan and high PSA at time of sLND were significant predictors for early clinical recurrence (<1 year following sLND). These patients had a worse cancer-specific survival compared to patients who developed clinical recurrence >1 year following sLND. Similar prognostic factors were identified in patients treated by PSMA-based radioguided surgery (22). These findings underline the need for prospective studies to evaluate the oncological usefulness of sLND and to assess the added value of adjuvant treatments. Currently, a prospective phase-2 study (NCT03569241) is investigating the additional value of pelvic RT following sLND.
Our study is not devoid of limitations. First, this is a single center, retrospective case series comparing two techniques and is as such prone to several types of bias (31). Second, patient cohorts were not contemporary: half of the patients in the open group were assessed by 11C-choline PET/CT, whereas almost all patients in the robotic group were assessed by 68Ga-PSMA-11 PET/CT. This is important as 11C-choline PET/CT is less accurate at low PSA values than 68Ga-PSMA PET/CT (32–34). As a consequence, half of the patients in the open group might have been understaged (occult metastases) compared to their counterparts in the robotic group and more patients with local recurrence might have been missed by choline PET/CT and therefore (falsely) not excluded from the study, both resulting in a worse oncological outcome. Third, patients in the robot-assisted group had less aggressive tumor characteristics (less Gleason score 8–10 at final pathology following RP) and a shorter follow-up compared to their counterparts in the open group. Therefore, we cannot definitively conclude from this data that both sLND approaches provide similar early oncological outcomes. However, a sub-analysis of only those patients who received a PSMA PET/CT at time of BCR showed no difference in BRFS and CRFS.
Notwithstanding these limitations, the extent of surgical templates was identical with both techniques, as was the number of nodes removed within each of the templates (pelvic, retroperitoneal, and pelvic + retroperitoneal). Both groups had comparable baseline patient characteristics (e.g., no difference in post-RP adjuvant/salvage RT proportion between both groups). Moreover, no differences in terms of preoperative co-morbidities (age adjusted CCI, ASA, and BMI) were observed. We therefore believe that the conclusions on surgical feasibility, perioperative, and postoperative complications of this study are reliable. Moreover, this is the first series comparing intra-operative, postoperative and early oncological outcomes between open and robotic sLND.
Conclusions
Robotic salvage lymph node dissection appears to be a safe alternative for the open procedure with the associated benefits of minimally invasive surgery, including shorter length of stay, lower estimated blood loss, and lower early postoperative complication rates. No difference in early BRFS and CRFS was seen between both groups. Modern imaging techniques underestimate the tumor burden and therefore, the surgical sLND template should not be limited to the positive spots on pre-operative imaging.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethische commissie UZ Leuven. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
GD and SJ: study concept and design. GD, TM, and YR: acquisition of data. GD: analysis and interpretation of data, drafting of the manuscript, and statistical analysis. VC, MA, CB, GM, LM, TV, HV, WE, and SJ: critical revision of the manuscript for important intellectual content. SJ: supervision.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
SJ is a Senior Clinical Researcher of the FWO (Research Foundation Flanders). This work is supported by the Jozef De Wever Fond voor prostaatkanker preventie.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00781/full#supplementary-material
Supplementary Table 1. Post-sLND treatments according to type of procedure (open vs. robotic).
Supplementary Table 2. Baseline characteristics of patients with lymph node recurrence after radical prostatectomy treated with open or robot-assisted sLND and who were assessed by PSMA PET/CT.
Supplementary Figure 1. (A) Overview of port placement in case of pelvic robot-assisted sLND. A six-port transperitoneal approach is used. The camera-port (8 mm) is placed supra-umbilical. The robotic ports are placed at the same height as the camera-port: two on the left and one on the right side (red dots, 8 mm trocars). A 12 mm assistant port is placed on the right side (green dot) and a 5 mm assistant port (blue dot) is placed 5 cm higher between the right robotic port and the camera-port. (B) Overview of port placement in case of pelvic+retroperitoneal robot-assisted sLND. Ports are placed 5 cm higher compared to port placement in pelvic sLND. Pictures were taken with informed consent of the patient.
Supplementary Figure 2. Comparison of biochemical recurrence-free survival between open and robotic sLND for patients who received a PSMA PET/CT. Censored patients are marked with small vertical lines.
Supplementary Figure 3. Comparison of clinical recurrence-free survival between open and robotic sLND for patients who received a PSMA PET/CT. Censored patients are marked with small vertical lines.
References
1. Abdollah F, Briganti A, Montorsi F, Stenzl A, Stief C, Tombal B, et al. Contemporary role of salvage lymphadenectomy in patients with recurrence following radical prostatectomy. Eur Urol. (2015) 67:839–49. doi: 10.1016/j.eururo.2014.03.019
2. Mullins JK, Feng Z, Trock BJ, Epstein JI, Walsh PC, Loeb S. The impact of anatomical radical retropubic prostatectomy on cancer control: the 30-year anniversary. J Urol. (2012) 188:2219–24. doi: 10.1016/j.juro.2012.08.028
3. van Leeuwen PJ, Stricker P, Hruby G, Kneebone A, Ting F, Thompson B, et al. 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. (2016) 117:732–9. doi: 10.1111/bju.13397
4. Picchio M, Mapelli P, Panebianco V, Castellucci P, Incerti E, Briganti A, et al. Imaging biomarkers in prostate cancer: role of PET/CT and MRI. Eur J Nucl Med Mol Imaging. (2015) 42:644–55. doi: 10.1007/s00259-014-2982-5
5. Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68 Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. (2015) 56:668–74. doi: 10.2967/jnumed.115.154153
6. Parker WP, Evans JD, Stish BJ, Park SS, Olivier K, Choo R, et al. Patterns of recurrence after postprostatectomy fossa radiation therapy identified by C-11 choline positron emission tomography/computed tomography. Int J Radiat Oncol Biol Phys. (2017) 97:526–35. doi: 10.1016/j.ijrobp.2016.11.014
7. Gandaglia G, Karakiewicz PI, Briganti A, Passoni NM, Schiffmann J, Trudeau V, et al. Impact of the site of metastases on survival in patients with metastatic prostate cancer. Eur Urol. (2015) 68:325–34. doi: 10.1016/j.eururo.2014.07.020
8. Giovacchini G, Picchio M, Garcia-Parra R, Briganti A, Abdollah F, Gianolli L, et al. 11C-choline PET/CT predicts prostate cancer-specific survival in patients with biochemical failure during androgen-deprivation therapy. J Nucl Med. (2014) 55:233–41. doi: 10.2967/jnumed.113.123380
9. Mottet N, Bellmunt, Briers E, Bolla M, Bourke L. EAU – ESTRO – ESUR – SIOG guidelines on prostate cancer. In: The EAU Annual Congress Copenhagen. (2018). p. 1–145. Available online at: https://uroweb.org/guideline/prostate-cancer/ (accessed June 15, 2019).
10. Tilki D, Mandel P, Seeliger F, Kretschmer A, Karl A, Ergün S, et al. Salvage lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy. J Urol. (2015) 193:484–90. doi: 10.1016/j.juro.2014.08.096
11. Jeffrey Karnes R, Murphy CR, Bergstralh EJ, DiMonte G, Cheville JC, Lowe VJ, et al. Salvage lymph node dissection for prostate cancer nodal recurrence detected by 11 C-choline positron emission tomography/computerized tomography. J Urol. (2015) 193:111–6. doi: 10.1016/j.juro.2014.08.082
12. Jilg CA, Rischke HC, Reske SN, Henne K, Grosu AL, Weber W, et al. Salvage lymph node dissection with adjuvant radiotherapy for nodal recurrence of prostate cancer. J Urol. (2012) 188:2190–7. doi: 10.1016/j.juro.2012.08.041
13. Ploussard G, Gandaglia G, Borgmann H, de Visschere P, Heidegger I, Kretschmer A, et al. Salvage lymph node dissection for nodal recurrent prostate cancer: a systematic review. Eur Urol. (2018). doi: 10.1016/j.eururo.2018.10.041. [Epub ahead of print].
14. Montorsi F, Gandaglia G, Fossati N, Suardi N, Pultrone C, De Groote R, et al. Surgery in motion robot-assisted salvage lymph node dissection for clinically recurrent prostate cancer. Eur Urol. (2017) 72:432–8. doi: 10.1016/j.eururo.2016.08.051
15. Abreu A, Fay C, Park D, Quinn D, Dorff T, Carpten J, et al. Robotic salvage retroperitoneal and pelvic lymph node dissection for ‘node-only’ recurrent prostate cancer: technique and initial series. BJU Int. (2017) 120:401–8. doi: 10.1111/bju.13741
16. Siriwardana A, Thompson J, van Leeuwen PJ, Doig S, Kalsbeek A, Emmett L, et al. Initial multicentre experience of 68 gallium-PSMA PET/CT guided robot-assisted salvage lymphadenectomy: acceptable safety profile but oncological benefit appears limited. BJU Int. (2017) 120:673–81. doi: 10.1111/bju.13919
17. Linxweiler J, Saar M, Al-Kailani Z, Janssen M, Ezziddin S, Stöckle M, et al. Robotic salvage lymph node dissection for nodal-only recurrences after radical prostatectomy: perioperative and early oncological outcomes. Surg Oncol. (2018) 27:138–45. doi: 10.1016/j.suronc.2018.02.010
18. Koppie TM, Serio AM, Vickers AJ, Vora K, Dalbagni G, Donat SM, et al. Age-adjusted Charlson comorbidity score is associated with treatment decisions and clinical outcomes for patients undergoing radical cystectomy for bladder cancer. Cancer. (2008) 112:2384–92. doi: 10.1002/cncr.23462
19. Doyle DJ, Garmon EH. American Society of Anesthesiologists Classification (ASA Class). StatPearls Publishing (2018). Available online at: http://www.ncbi.nlm.nih.gov/pubmed/28722969 (accessed December 12, 2018).
20. Joniau S, Van den Bergh L, Lerut E, Deroose CM, Haustermans K, Oyen R, et al. Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol. (2013) 63:450–8. doi: 10.1016/j.eururo.2012.06.057
21. Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
22. Pinder SE, Provenzano E, Earl H, Ellis IO. Laboratory handling and histology reporting of breast specimens from patients who have received neoadjuvant chemotherapy. Histopathology. (2007) 50:409–17. doi: 10.1111/j.1365-2559.2006.02419.x
23. Binder J, Bräutigam R, Jonas D, Bentas W. Robotic surgery in urology: fact or fantasy? BJU Int. (2004) 94:1183–7. doi: 10.1046/j.1464-410x.2004.05130.x
24. Ng CK, Kauffman EC, Lee M-M, Otto BJ, Portnoff A, Ehrlich JR, et al. A comparison of postoperative complications in open versus robotic cystectomy. (2009) 7:7–4. doi: 10.1016/S0022-5347(09)60363-3
25. Yu HY, Hevelone ND, Lipsitz SR, Kowalczyk KJ, Hu JC. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol. (2012) 187:1392–9. doi: 10.1016/j.juro.2011.11.089
26. Kane CJ, Amling CL, Johnstone PA, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. (2003) 61:607–11. doi: 10.1016/S0090-4295(02)02411-1
27. van Leeuwen FWB, Winter A, van Der Poel HG, Eiber M, Suardi N, Graefen M, et al. Technologies for image-guided surgery for managing lymphatic metastases in prostate cancer. Nat Rev Urol. (2019) 16:159–71. doi: 10.1038/s41585-018-0140-8
28. Maurer T, Robu S, Schottelius M, Schwamborn K, Rauscher I, van den Berg NS, et al. 99mTechnetium-based prostate-specific membrane antigen–radioguided surgery in recurrent prostate cancer. Eur Urol. (2019) 75:659–66. doi: 10.1016/j.eururo.2018.03.013
29. van Oosterom MN, Simon H, Mengus L, Welling MM, van der Poel HG, van den Berg NS, et al. Revolutionizing (robot-assisted) laparoscopic gamma tracing using a drop-in gamma probe technology. Am J Nucl Med Mol Imaging. (2016) 6:1–17.
30. Fossati N, Suardi N, Gandaglia G, Bravi CA, Soligo M, Karnes RJ, et al. Identifying the optimal candidate for salvage lymph node dissection for nodal recurrence of prostate cancer: results from a large, multi-institutional analysis. Eur Urol. (2019) 75:176–83. doi: 10.1016/j.eururo.2018.09.009
31. Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single-center trials. Crit Care Med. (2009) 37:3114–9. doi: 10.1097/CCM.0b013e3181bc7bd5
32. Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. (2017) 44:92–101. doi: 10.1007/s00259-016-3490-6
33. Morigi JJ, Stricker PD, Van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18 F-fluoromethylcholine versus 68 Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. (2015) 56:1185–90. doi: 10.2967/jnumed.115.160382
34. Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. (2014) 41:11–20. doi: 10.1007/s00259-013-2525-5
Keywords: prostate cancer, salvage lymph node dissection, lymph node recurrence, robot-assisted approach, open approach
Citation: Devos G, Muilwijk T, Raskin Y, Calderon V, Moris L, Van den Broeck T, Berghen C, De Meerleer G, Albersen M, Van Poppel H, Everaerts W and Joniau S (2019) Comparison of Peri-operative and Early Oncological Outcomes of Robot-Assisted vs. Open Salvage Lymph Node Dissection in Recurrent Prostate Cancer. Front. Oncol. 9:781. doi: 10.3389/fonc.2019.00781
Received: 14 June 2019; Accepted: 01 August 2019;
Published: 04 September 2019.
Edited by:
Nicholas Zaorsky, Penn State Hershey Cancer Institute, United StatesReviewed by:
Sanja Štifter, University of Rijeka, CroatiaPhilipp Mandel, University Medical Center Hamburg-Eppendorf, Germany
Copyright © 2019 Devos, Muilwijk, Raskin, Calderon, Moris, Van den Broeck, Berghen, De Meerleer, Albersen, Van Poppel, Everaerts and Joniau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaëtan Devos, Z2FldGFuLmRldm9zQHV6bGV1dmVuLmJl
 Gaëtan Devos
Gaëtan Devos Tim Muilwijk
Tim Muilwijk Yannic Raskin1
Yannic Raskin1 Lisa Moris
Lisa Moris Thomas Van den Broeck
Thomas Van den Broeck Charlien Berghen
Charlien Berghen Steven Joniau
Steven Joniau