
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 July 2019
Sec. Thoracic Oncology
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00642
 Weidong Wang1,2,3†
Weidong Wang1,2,3† Dongni Chen1,3†
Dongni Chen1,3† Kexing Xi4†
Kexing Xi4† Yongqiang Chen1,3
Yongqiang Chen1,3 Xuewen Zhang1,5
Xuewen Zhang1,5 Yinsheng Wen1,3
Yinsheng Wen1,3 Zirui Huang1,3
Zirui Huang1,3 Xiangyang Yu1,3
Xiangyang Yu1,3 Gongming Wang1,3
Gongming Wang1,3 Rusi Zhang1,3
Rusi Zhang1,3 Lanjun Zhang1,3*
Lanjun Zhang1,3*Background: To investigate the prognostic impact of different types of lymphadenectomy with different extents of tumor resection on the outcomes of stage I non-small-cell lung cancer (NSCLC).
Methods: Patients were classified into lobectomy and sublobectomy groups, and then each group was subdivided according to the types of lymphadenectomy. The end points of the study were overall survival (OS) and disease-free survival (DFS). Propensity score matched (PSM) comparative analysis and univariate and multivariate Cox regression analyses were performed.
Result: A total of 1,336 patients were included in the current study. Lobectomy was associated with better OS and DFS. In the lobectomy group, lobectomy with bilateral mediastinal lymphadenectomy (BML) was associated with better OS than lobectomy with systematic nodal dissection (SND) or lobe-specific systematic node dissection (L-SND). Lobectomy with SND or L-SND was associated with better OS than lobectomy with systematic nodal sampling (SNS) or selected lymph node biopsy (SLNB). Additionally, lobectomy with BML or SND was associated with better DFS than lobectomy with L-SND or SNS or SLNB. After PSM, compared with lobectomy with SNS or SLNB, lobectomy with SND resulted in more favorable OS and DFS. There was no survival difference between different types of lymphadenectomy for patients who underwent sublobectomy. A multivariable analysis revealed independent associations of lobectomy with BML or SND with better OS and DFS compared with those of lobectomy with SNS or SLNB.
Conclusion: This study reveals an association of lobectomy with more systematic and complete lymph node dissection, such as BML or SND, with better prognosis in stage I NSCLC patients.
Non-small-cell lung cancer (NSCLC) is the malignancy with the highest morbidity and mortality rates worldwide (1). However, improved radiologic imaging and widespread low-dose computed tomography screening have led to increased detection of early-stage (stage I) NSCLC (2). For surgically resectable lung cancer, surgery is the best therapeutic option. Additionally, lymph node (LN) dissection during surgery is essential. For these reasons, determining the appropriate model of resection of original tumors and LN dissection has attracted more attention.
The European Society of Thoracic Surgeons (ESTS) guidelines (3) for LN dissection in NSCLC classify intraoperative LN dissection into the following five categories: (1) selected lymph node biopsy (SLNB), (2) systematic nodal sampling (SNS), (3) systematic nodal dissection (SND), (4) lobe-specific systematic node dissection (L-SND), and (5) extended lymph node dissection (ELND). Additionally, lobectomy with SND is considered to be a standard therapy for patients with NSCLC, but for some early-stage patients, sublobectomy or SNS is also acceptable (4).
Sublobar resection has been indicated to result in a similar survival rate for early-stage NSCLC patients compared with that of lobectomy, and it can retain more pulmonary function (5). However, some researchers hold the opposite view (6), and they believe that sublobectomy results in an inferior survival rate. Several previous studies have indicated that among patients who underwent sublobectomy, whether LN dissection was performed did not influence the survival outcomes (7). However, there are also some studies reporting that LN dissection can result in a better prognosis even in patients who have undergone sublobectomy (8). The need for SND has also been questioned. Whether SND compared with SNS can provide more accurate staging and survival benefit or lead to more complications in stage I NSCLC patients has not yet been confirmed (9, 10). Some studies have also shown that L-SND results in the same survival rate as that of SND, although it decreases the duration of the surgery and incidence of postoperative complications. However, some studies have found that L-SND may be ineffective in some N2-positive patients, influencing the pathological grading and adjunctive therapy (11, 12).
Therefore, in our study, we used a large cohort of patients to compare the outcomes of different LN dissection models combined with different extents of resection in stage I NSCLC patients to formulate guidelines regarding the extent of tumor resection and lymphadenectomy.
This study initially included 2,102 consecutive cases consisting of clinical and pathological stage I NSCLC patients who underwent surgical treatment between 1999 and 2014 in the Department of Thoracic Surgery in Sun Yat-sen University Cancer Center. This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center.
All patients were confirmed to be pathological stage I according to the 8th edition of the American Joint Committee on Cancer (AJCC) lung cancer staging classification (13) and met the following criteria: (1) primary NSCLC; (2) preoperatively considered node negative; and (3) pathologic stage was T1a-2aN0M0. The exclusion criteria were as follows: (1) a history of other primary cancer; (2) double primary lung cancer; (3) the patient received neoadjuvant therapy; (4) positive surgical margins; (5) the clinicopathologic and follow-up data were not complete; and (6) patients chose sublobectomy because they could not tolerate lobectomy (Figure 1). Finally, 1,336 patients were included in further analysis.
The outcomes of this study included overall survival (OS) and disease-free survival (DFS). The latest follow-up of the current study was performed on October 15, 2018.
The location and station of mediastinal LNs were based on the International Association for the Study of Lung Cancer LN map (14). For analytical purposes, we divided the patients into two major groups (Group Lobe: for those patients who underwent lobectomy, biolobectomy, or pneumonectomy; and Group Sublobe: for those patients who underwent wedge resection or segmentectomy) and then into five or three smaller groups, respectively, according to the LN dissection as follows: Group Lobe: A, patients with SLNB or SNS; B, patients with SND; C, patients with L-SND; D, patients without any LN dissection; and E, patients with bilateral mediastinal lymphadenectomy (BML); and Group Sublobe: F, patients with SND; G, patients with SLNB or SNS; and H, patients without any LN dissection (Table 1). The definitions of all options in the intraoperative LN dissection model are as follows: (1) Selected LN biopsy: one or multiple suspicious LN(s) were biopsied; (2) SNS: a predetermined selection of one or more LN stations specified by the surgeon; (3) SND: all the mediastinal tissue containing the LNs is dissected and removed systematically within anatomical landmarks, and at least three mediastinal nodal stations (but always subcarinal) should be excised, and the hilar and the intrapulmonary LNs are dissected as well; (4) L-SND: the mediastinal tissue containing specific LN stations are excised, depending on the lobar location of the primary tumor; and (5) BML: bilateral mediastinal LN dissection is performed through cervical mediastinoscopy (3).
A propensity score matched (PSM) comparative analysis was performed to control the non-random variables among groups. We adjusted for potential differences between Group A (patients with SLNB or SNS) and Group B (patients with SND) (1:1 match), and finally 468 patients (234 in each group) were included in the PSM analysis. We generated a propensity score for the matched groups using logistic regression based on the patients' potential confounding baseline characteristics, including age, tumor size, tumor location, and surgical approach. We next created a balanced cohort using an optimized performance-matching algorithm with a caliper setting of 0.02. The procedure was conducted using SPSS 21.0.
Categorical variables were calculated using the χ2 test, and continuous variables were analyzed using the t-test. All end points were estimated using the Kaplan–Meier method and compared using the log-rank test. Multivariable survival analyses were performed using the Cox proportional hazards model to identify prognostic factors for OS and DFS. For all analyses, a two-sided P < 0.05 was considered statistically significant. Hazard ratios (HRs), 95% confidence intervals (CIs), and P-values for each variable were determined using SPSS 21.0 software (IBM, Armonk, NY), and survival curves were drawn using Prism 7.0 (GraphPad software, La Jolla, CA).
In total, 44 patients underwent sublobectomy, and 1,292 patients underwent lobectomy, including 346 (25.9%) patients in Group A, 328 (24.6%) in Group B, 577 (43.2%) in Group C, 13 (1.0%) in Group D, 28 (2.1%) in Group E, 3 (0.2%) in Group F, 13 (1.0%) in Group G, and 28 (2.1%) in Group H. There were 822 males and 470 females in Group Lobe and 33 males and 11 females in Group Sublobe. The mean age of Group Lobe was 59.35 ± 10.1 years, and the median age was 60.0 years. In addition, in Group Sublobe, the mean age was 66.73 ± 11.9 years. Non-squamous cell carcinoma was the most common (1,002 and 40 patients, 77.6 and 90.9%, respectively) pathologic type in both groups. When the clinicopathologic characteristics were compared among groups, it was interesting to note that sex, histology, cell differentiation, smoking history, adjuvant therapy, and treatment after disease progression were well-balanced between the subgroups in both Group Lobe and Group Sublobe. Patients in Group Lobe were younger, had larger tumors, and had more LNs resected than those in Group Sublobe. Among the patients in Group Lobe, lobectomy was much more common than bilobectomy or pneumonectomy (1,234 vs. 33 and 25, respectively). Interestingly, in later procedures, there were more patients who underwent LN dissection rather than LN sampling in Group Lobe. Among Group Sublobe, most patients underwent wedge resection without any LN dissection. Notably, those who received SND in Group Sublobe underwent segmentectomy. The baseline characteristics of the patients in Group Lobe are summarized in Table 2, and those in Group Sublobe are summarized in Supplemental Table 1.
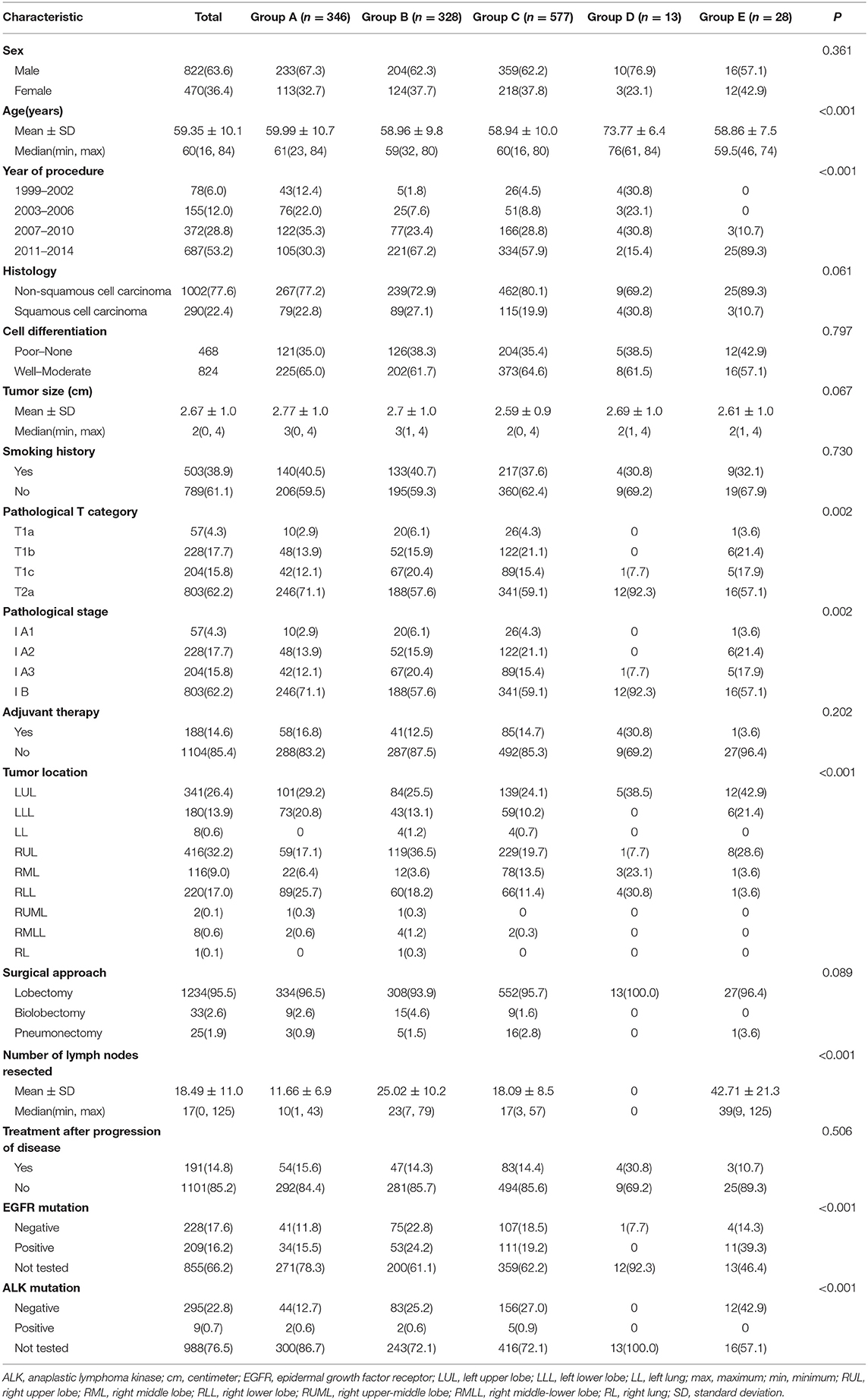
Table 2. Distribution of the clinicopathologic characteristics stratified by group in Group Lobe (n = 1,292).
1. Comparison of survival between the lobectomy group and sublobectomy group
A Kaplan–Meier survival analysis and log-rank comparison revealed that compared with the Group Sublobe, the lobectomy group (Group Lobe) was significantly associated with better OS (log rank = 9.45, P = 0.002) and DFS (log rank = 3.97; P = 0.045) in patients with stage I NSCLC (Figures 2A,B).
2. Comparison of survival between different types of lymphadenectomy in Group Lobe
When the patients who underwent lobectomy were divided into Groups A to E, it was clear that the patients who underwent lobectomy without LN dissection (Group D) had the worst OS. Among those who underwent LN dissection, patients who underwent systematic LN dissection (Group B) or L-SND (Group C) had better outcomes than patients who underwent selective LN biopsy or sampling (Group A), although the difference between Group B and Group C was not significant. Patients who underwent BML (E) had the best postoperative survival among the subgroups in Group Lobe. The 5-year survival rates were 79.5, 87.2, 85.2, 53.8, and 96.4% in Group A through Group E, respectively, and the Kaplan–Meier curves showed a significant difference in OS (log rank = 21.48, P < 0.001). Similar to OS, Group D had the worst DFS, while Group B and Group E had better DFS than Group C and Group A. The 5-year DFS rates were 72.8, 79.3, 72.4, 38.5, and 78.6% in Group A through Group E, respectively. The Kaplan–Meier curves also showed a significant difference in DFS among the subgroups (log rank = 17.12, P = 0.002) (Figures 3A,B).
3. Comparison of survival between different types of lymphadenectomy in Group Sublobe
A similar subgroup analysis was performed in Group Sublobe. However, there were no significant differences in either OS or DFS among Group F, Group G, and Group H (P = 0.621 and P = 0.954, respectively) (Figures 4A,B).
4. Comparison of survival via Cox regression
In Group Lobe, a multivariable Cox proportional hazard model revealed independent associations of lobectomy plus systematic LN dissection, lobar-selective LN dissection, or BML with better OS (HR, 0.668; 95% CI, 0.483–0.923; P = 0.015; HR, 0.770; 95% CI, 0.596–0.996; P = 0.046; and HR, 0.163; 95% CI, 0.022–0.998; P = 0.049, respectively) and lobectomy plus systematic LN dissection or BML with better DFS (HR, 0.737; 95% CI, 0.553–0.983; P = 0.038 and HR, 0.468; 95% CI, 0.073–0.978; P = 0.047, respectively) compared with that of lobectomy with selected LN biopsy or sampling only. In addition, advanced age, bronchus invasion, and pathological IB stage were identified as being negatively correlated with OS. Good-to-moderate differentiation, adjuvant therapy, and the presence of epithelial growth factor receptor (EGFR) mutations were positively correlated with OS. Patients with squamous cell carcinoma (SCC) and an earlier pathological stage had better DFS than those with other pathological subtypes and a later pathological stage, respectively (Table 3). A similar Cox regression analysis was also performed in the Group Sublobe, and the result is shown in Supplemental Table 2.
5. Comparison of survival among specific groups after PSM
After PSM, pairs were formed between Group A and Group B. A total of 468 patients (234 pairs) were included in the PSM analysis, and the baseline characteristics of these patients are summarized in Supplemental Table 3. A Kaplan–Meier survival analysis and log-rank comparison revealed that Group B had better OS (log rank = 3.97, P = 0.046) and DFS (log rank = 7.00, P = 0.008) than Group A (Figures 5A,B).
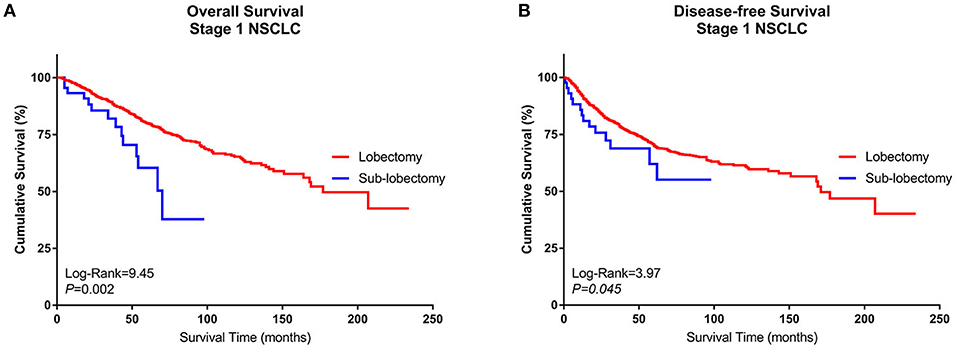
Figure 2. Kaplan–Meier curves of the survival estimates for patients who underwent lobectomy or sublobectomy. (A) Overall survival data from patients who underwent lobectomy or sublobectomy for stage I NSCLC. (B) Disease-free survival of patients who underwent lobectomy or sublobectomy for stage I NSCLC. NSCLC, non-small-cell lung cancer.
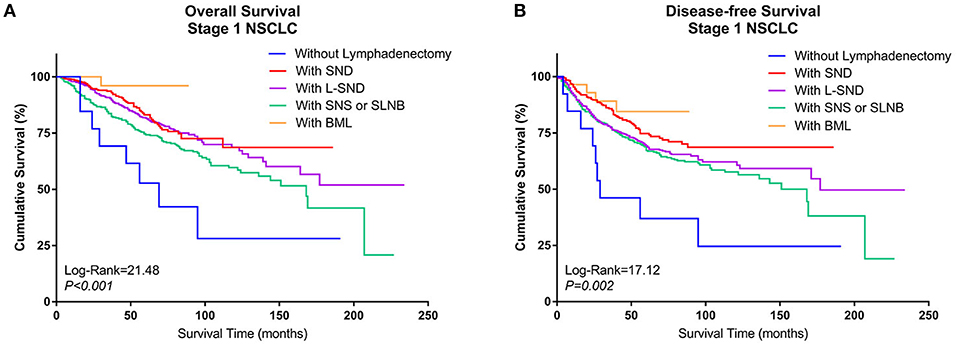
Figure 3. Kaplan–Meier curves of survival estimates for patients who underwent lobectomy with different types of lymphadenectomy. (A) Overall survival of patients who underwent lobectomy with different types of lymphadenectomy for stage I NSCLC. (B) Disease-free survival of patients who underwent lobectomy with different types of lymphadenectomy for stage I NSCLC. NSCLC, non-small-cell lung cancer.
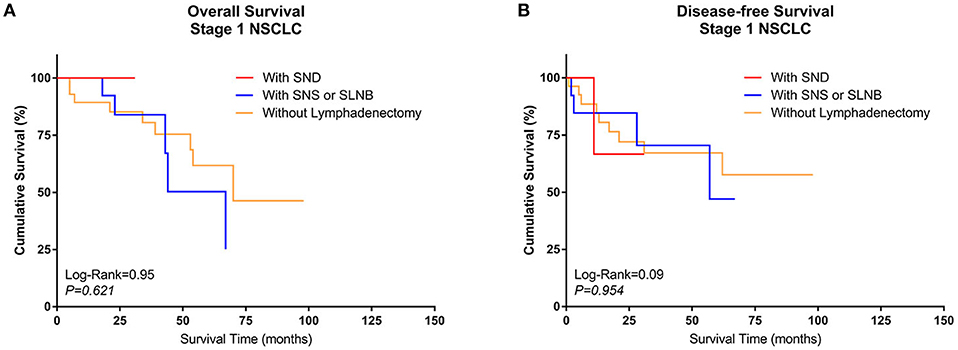
Figure 4. Kaplan–Meier curves of survival estimates for patients who underwent sublobectomy with different types of lymphadenectomy. (A) Overall survival of patients who underwent sublobectomy with different types of lymphadenectomy for stage I NSCLC. (B) Disease-free survival of patients who underwent sublobectomy with different types of lymphadenectomy for stage I NSCLC. NSCLC, non-small-cell lung cancer.
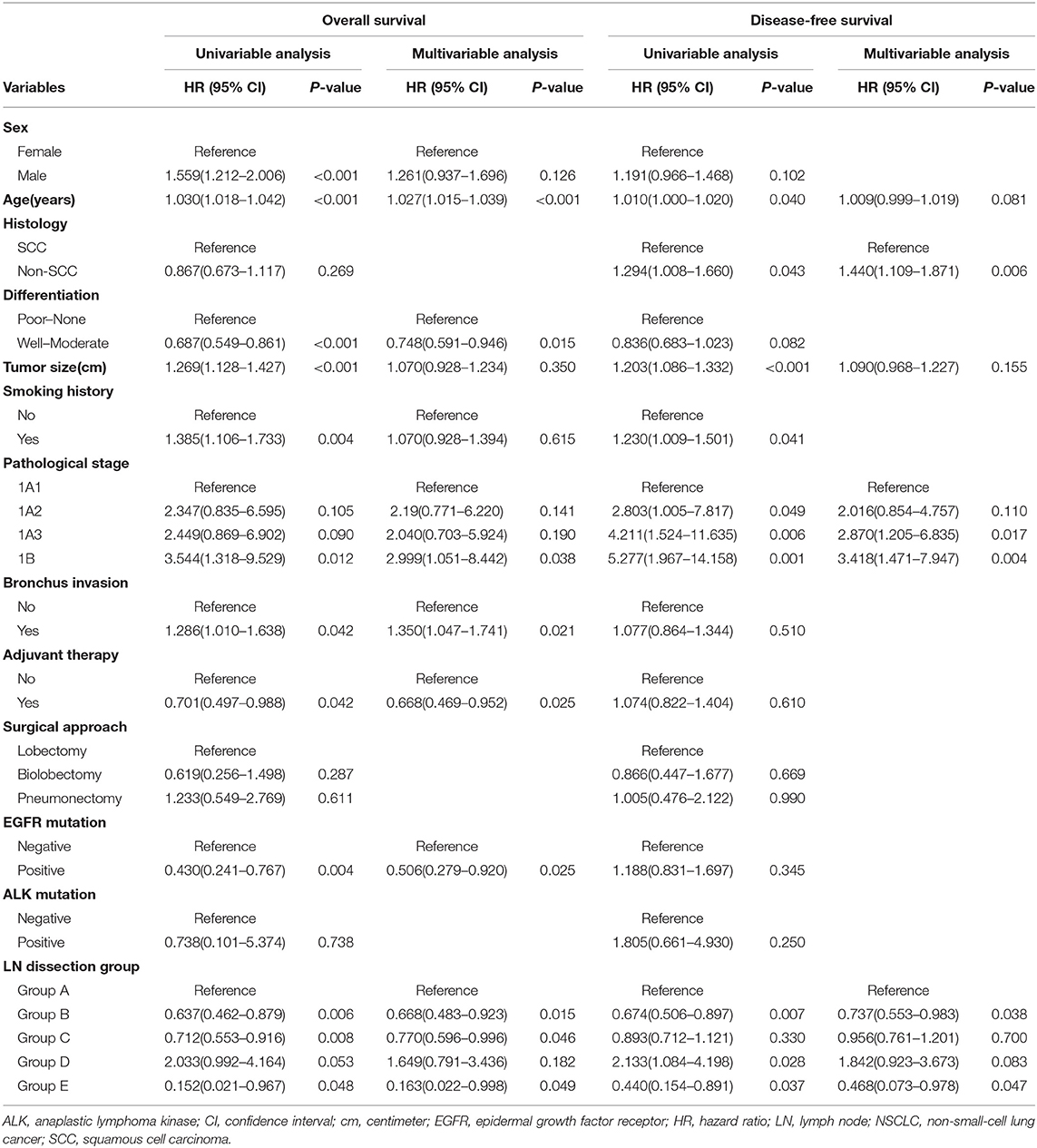
Table 3. Univariable and multivariable Cox regression analysis for Group Lobe of stage 1 NSCLC patients (n = 1,292).
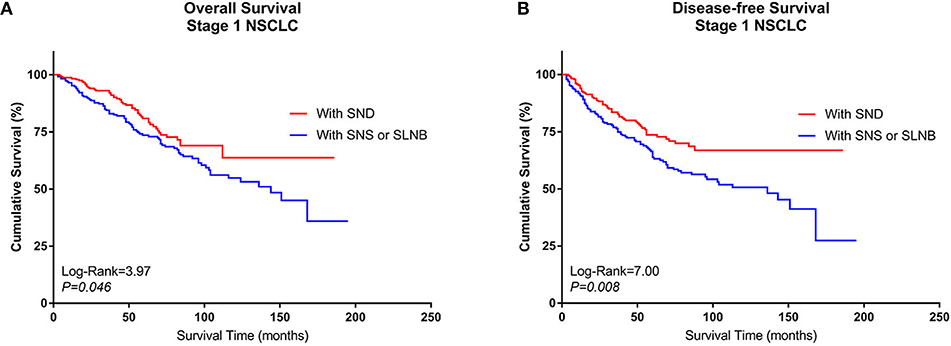
Figure 5. Kaplan–Meier curves of survival estimates for the PSM cohort who underwent lobectomy with SND or with SNS or SLNB. (A) Overall survival data for patients who underwent lobectomy with SND, SNS, or SLNB for stage I NSCLC. (B) Disease-free survival of patients who underwent lobectomy with SND, SNS, or SLNB for stage I NSCLC. NSCLC, non-small-cell lung cancer; SND, systematic nodal dissection; SNS, systematic nodal sampling; SLNB, selected lymph node biopsy.
Multivariable Cox regression analysis also revealed that lobectomy plus selective LN biopsy or sampling was independently associated with worse OS (HR, 1.305; 95% CI, 1.008–1.877; P = 0.050) and DFS (HR, 1.417; 95% CI, 1.020–1.968; P = 0.038) than other combinations. Additionally, advanced age and poor differentiation without adjuvant therapy were identified as being negatively correlated with OS. Only lobectomy plus systematic LN dissection was positively correlated with DFS (Table 4).
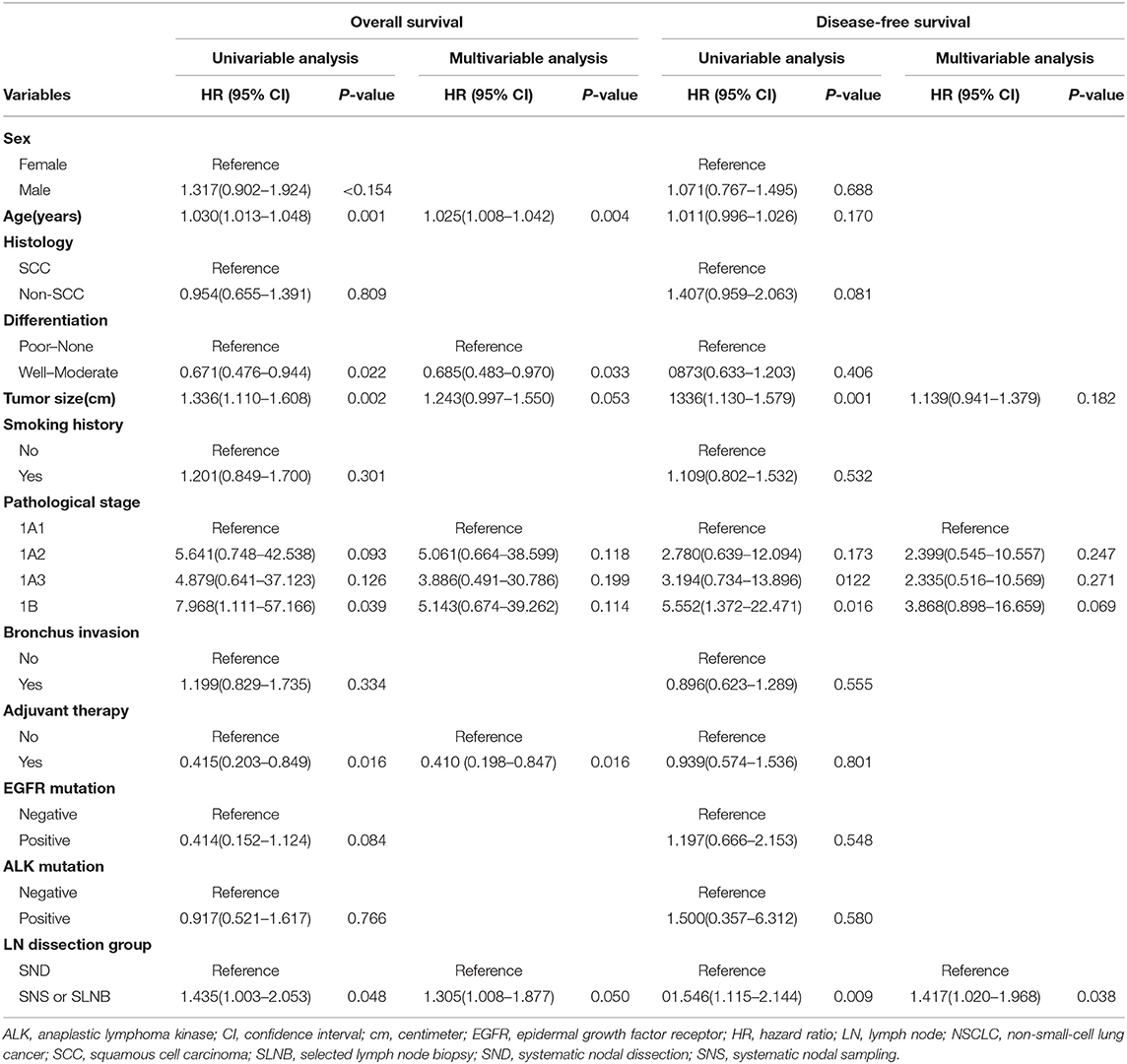
Table 4. Univariable and multivariable Cox regression analysis for stage I NSCLC patients in the propensity score matched cohort who underwent lobectomy with SND or with SNS or SLNB.
The extent of tumor resection and LN dissection are important elements in the treatment of resectable early-stage NSCLC (15, 16). Some previous studies demonstrated that sublobectomy results in similar survival outcomes to lobectomy in early-stage NSCLC patients (5, 17). However, other studies have reached different conclusions. In one study, despite adjustment for patient- and tumor-related characteristics, sublobectomy was associated with worse survival, even for stage IA patients (6). Furthermore, in another study, patients undergoing sublobectomy were more likely to have inadequate lymphadenectomies and positive margins than those undergoing lobectomies (18). The results of our study suggest that for surgically resectable stage I NSCLC, most patients undergo lobectomy and that patients who undergo sublobectomy are older and have more comorbidities, smaller tumors, and worse survival.
For LN dissection, the standard model for early-stage NSCLC remains controversial even among different guidelines. The National Comprehensive Cancer Network (NCCN) guidelines recommend that for clinical node-negative stage I NSCLC and patients who underwent sublobectomies, SNS or selected LN biopsy is also acceptable (4). The American College of Chest Physicians (ACCP) guidelines note that for preoperative stage I patients, if they are intraoperatively determined to be node negative, selective LN sampling and dissection are both recommended (19). The European Society of Thoracic Surgeons (ESTS) guidelines recommend SND or sampling for all lung cancer patients. For peripheral T1 SCC, L-SND is also acceptable (3).
Some studies suggested that SND was associated with more accurate staging and better survival, although it results in longer operative times, greater intraoperative blood loss, and recurrent nerve injury; however, the results are inconsistent among studies (20). The results of the American College of Surgery Oncology Group Z0030 trial demonstrated that if systematic sampling of the mediastinal and hilar LNs returns a negative result, mediastinal LN dissection does not improve survival, although it can provide a more accurate staging (9, 10). However, in contrast to the Z0030 trial, which included some pN1 or pN2 and other pIIIA or pIIIB patients, we focused only on pN0 and stage I patients. Moreover, we not only compared SND with SNS by PSM but also more accurately included L-SND and BML in our analysis. All of these may cause a different result between our result and the Z0030 trial result. We found that SND and BML were associated with better OS and DFS than other modalities. Regarding the controversy of LN dissection in stage I patients, the guidelines concentrate mainly on the survival outcome difference between SND and SNS. In our study, a PSM comparison was performed between these two groups, which had not been performed in the Z0030 trial. The results revealed that even after PSM, those who underwent SND had significantly better OS and DFS.
Previous studies also demonstrated that the lymphatic drainage of lung cancer might be lobe specific (21), which provides the basis for L-SND. Some studies suggested that L-SND proved to be as effective as SND (22), but the rate of detection of pN2 was significantly higher in the SND group (12). Our study showed that a significant difference was not found in OS between L-SND and SND. However, compared with SND, L-SND resulted in a worse DFS.
Our study also investigated the relationships between different types of lymphadenectomy in sublobectomy and survival outcomes. However, there were no significant differences between subgroups; this result was attributed to the small sample size. However, other studies found that LN removal appears to decrease locoregional recurrence and may be associated with a survival benefit, while it does not increase morbidity or the length of the hospital stay (23).
Several studies have also demonstrated an association between an increased number of dissected LNs following resection for NSCLC and better long-term survival rates. They indicated that examining a greater number of LNs in patients with stage I NSCLC treated with resection increases the likelihood of proper staging and affects patient outcomes (15). A more recent analysis identified a significant reduction in the mortality rate among patients with at least 16 dissected LNs and clinically certified node-negative NSCLC (24). In the current study, the numbers of resected LNs varied among groups. A greater number of resected LNs was associated with a better survival outcome. Patients who underwent lobectomy with BML may be associated with the greatest number of LNs resected in this subgroup (42.71 ± 21.3).
In our study, we specifically targeted stage I lung cancer and explored the associations between long-term survival and different extents of tumor resection and lymphadenectomy models. Meanwhile, we aimed to clarify which type of lymphadenectomy should be considered a recommended procedure for the surgical treatment of stage I NSCLC. We found that for patients who underwent lobectomy, long-term survival was associated with the type of lymphadenectomy. It is clear that patients who underwent only lobectomy had the worst OS and DFS, and BML resulted in the best OS. For DFS, BML, or SND was associated with better outcomes than that of other subgroups.
These results may be attributed to the following reasons; First, LN involvement can occur in patients with any tumor size (25), and metastasis and micrometastasis can be missed in some cases (26). Therefore, potential metastases may remain if the patient undergoes a sublobectomy or inadequate lymphadenectomy. Second, SND can resect all the possible metastatic tissue, and it can harvest more LNs, which could enhance staging accuracy (24). BML performed better in this respect; thus, it resulted in the best rates of OS and DFS. This may indicate that extended LN dissection may have survival benefits in not only advanced stage NSCLC but also stage I NSCLC. Third, it has been reported that more stringently defined mediastinal LNs are associated with better separation in the prediction of survival. BML can provide the most thorough nodal examination among the different types of lymphadenectomy, which may explain the better outcomes observed among patients who underwent BML and were declared node negative (16).
This study has several limitations of note. Given the retrospective nature of this single-institution study, selection bias is inherent in our study population. In addition, postoperative complications were not considered in our series.
In conclusion, our study shows that lobectomy with more systematic and complete LN dissection, such as BML or SND, is associated with better survival outcomes in stage I NSCLC patients. However, due to the small sample size, the efficacy of BML still needs to be further verified by a large-scale randomized clinical trial. We recommend lobectomy with SND as the recommended procedure even for stage I NSCLC patients to improve their prognosis. BML is also recommended if it is technically feasible. Despite the use of appropriate statistical methods, our study has some inevitable limitations; more evidence from large-sample-size, multicenter prospective or real-world studies is warranted.
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
This retrospective study was approved by the Institute Research Medical Ethics Committee of Sun Yat-sen University Cancer Center. The reference number is B2018-011. All patients included in this research gave written informed consent according to the Research Ethics Committee of Sun Yat-Sen University Cancer Center.
LZ was responsible for conception and design of the study. WW, DC, and KX analyzed and interpreted the patient data and were major contributors to the writing of the manuscript. YC, XZ, and YW collected and assembled the data. ZH, XY, GW, and RZ performed the follow-up survey. All authors read and approved the final manuscript.
This study was supported by the grant 2016YFC0905400 from the National Key R&D Program of China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Ningbo Fan for his helpful assistance with this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00642/full#supplementary-material
ACCP, American College of Chest Physicians; AJCC, American Joint Committee on Cancer; ALK, anaplastic lymphoma kinase; BML, bilateral mediastinal lymphadenectomy; CIs, confidence intervals; DFS, disease-free survival; EGFR, epidermal growth factor receptor; ELND, extended lymph node dissection; ESTS, The European Society of Thoracic Surgeons; HRs, hazard ratios; KM curves, Kaplan–Meier curves; LN, lymph node; L-SND, lobe-specific systematic node dissection; NCCN, National Comprehensive Cancer Network; NSCLC, non-small-cell lung cancer; OS, overall survival; PSM, propensity score matched; SLNB, selected lymph node biopsy; SND, systematic nodal dissection; SNS, systematic nodal sampling.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Aberle DR, DeMello S, Berg CD, Black WC, Brewer B, Church TR, et al. Results of the two incidence screenings in the national lung screening trial. N Engl J Med. (2013) 369:920–31. doi: 10.1056/NEJMoa1208962
3. Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardiothorac Surg. (2006) 30:787–92. doi: 10.1016/j.ejcts.2006.08.008
4. Kurzrock R, Colevas AD, Olszanski A, Akerley W, Arteaga CL, Carson WE III, et al. NCCN Oncology research program's investigator steering committee and NCCN best practices committee molecular profiling surveys. J Natl Compr Canc Netw. (2015) 13:1337–46. doi: 10.6004/jnccn.2015.0163
5. Altorki NK, Yip R, Hanaoka T, Bauer T, Aye R, Kohman L, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg. (2014) 147:754–62; discussion 762–4. doi: 10.1016/j.jtcvs.2013.09.065
6. Speicher PJ, Gu L, Gulack BC, Wang X, D'Amico TA, Hartwig MG, et al. Sublobar resection for clinical stage IA non-small-cell lung cancer in the United States. Clin Lung Cancer. (2016) 17:47–55. doi: 10.1016/j.cllc.2015.07.005
7. Moon Y, Sung SW, Namkoong M, Park JK. The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor. J Thorac Dis. (2016) 8:2617–25. doi: 10.21037/jtd.2016.08.75
8. Cao J, Xu J, He Z, Yuan P, Huang S, Lv W, et al. Prognostic impact of lymphadenectomy on outcomes of sublobar resection for stage IA non-small cell lung cancer </ = 2 cm. J Thorac Cardiovasc Surg. (2018) 156:796–805 e794. doi: 10.1016/j.jtcvs.2018.03.122
9. Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the american college of surgery oncology group Z0030 trial. J Thorac Cardiovasc Surg. (2011) 141:662–70. doi: 10.1016/j.jtcvs.2010.11.008
10. Meng D, Zhou Z, Wang Y, Wang L, Lv W, Hu J. Lymphadenectomy for clinical early-stage non-small-cell lung cancer: A systematic review and meta-analysis. Eur J Cardiothorac Surg. (2016) 50:597–604. doi: 10.1093/ejcts/ezw083
11. Asamura H, Nakayama H, Kondo H, Tsuchiya R, Naruke T. Lobe-specific extent of systematic lymph node dissection for non–small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J Thorac Cardiovasc Surg. (1999) 117:1102–11. doi: 10.1016/s0022-5223(99)70246-1
12. Adachi H, Sakamaki K, Nishii T, Yamamoto T, Nagashima T, Ishikawa Y, et al. Lobe-specific lymph node dissection as a standard procedure in surgery for non-small cell lung cancer: a propensity score matching study. J Thorac Oncol. (2017) 12:85–93. doi: 10.1016/j.jtho.2016.08.127
13. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. (2017) 151:193–203. doi: 10.1016/j.chest.2016.10.010
14. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P, et al. The IASLC lung cancer staging project: A proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. (2009) 4:568–77. doi: 10.1097/JTO.0b013e3181a0d82e
15. Gajra A, Newman N, Gamble GP, Kohman LJ, Graziano SL. Effect of number of lymph nodes sampled on outcome in patients with stage I non-small-cell lung cancer. J Clin Oncol. (2003) 21:1029–34. doi: 10.1200/JCO.2003.07.010
16. Smeltzer MP, Faris NR, Ray MA, Osarogiagbon RU. Association of pathologic nodal staging quality with survival among patients with non-small cell lung cancer after resection with curative intent. JAMA Oncol. (2018) 4:80–7. doi: 10.1001/jamaoncol.2017.2993
17. Fan J, Wang L, Jiang GN, Gao W. Sublobectomy versus lobectomy for stage I non-small-cell lung cancer, a meta-analysis of published studies. Ann Surg Oncol. (2012) 19:661–8. doi: 10.1245/s10434-011-1931-9
18. Khullar OV, Liu Y, Gillespie T, Higgins KA, Ramalingam S, Lipscomb J, et al. Survival after sublobar resection versus lobectomy for clinical stage IA lung cancer: an analysis from the national cancer data base. J Thorac Oncol. (2015) 10:1625–33. doi: 10.1097/JTO.0000000000000664
19. Jett JR, Schild SE, Kesler KA, Kalemkerian GP. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2013) 143(5 Suppl):e400S−19S. doi: 10.1378/chest.12-2363
20. Shen-Tu Y, Mao F, Pan Y, Wang W, Zhang L, Zhang H, et al. Lymph node dissection and survival in patients with early stage nonsmall cell lung cancer: a 10-year cohort study. Medicine. (2017) 96:e8356. doi: 10.1097/MD.0000000000008356
21. Cerfolio RJ, Bryant AS. Distribution and likelihood of lymph node metastasis based on the lobar location of nonsmall-cell lung cancer. Ann Thorac Surg. (2006) 81:1969–73; discussion 1973. doi: 10.1016/j.athoracsur.2005.12.067
22. Okada M, Sakamoto T, Yuki T, Mimura T, Miyoshi K, Tsubota N. Selective mediastinal lymphadenectomy for clinico-surgical stage I non-small cell lung cancer. Ann Thorac Surg. (2006) 81:1028–32. doi: 10.1016/j.athoracsur.2005.09.078
23. Stiles BM, Kamel MK, Nasar A, Harrison S, Nguyen AB, Lee P, et al. The importance of lymph node dissection accompanying wedge resection for clinical stage IA lung cancer. Eur J Cardiothorac Surg. (2017) 51:511–7. doi: 10.1093/ejcts/ezw343
24. Liang W, He J, Shen Y, Shen J, He Q, Zhang J, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the us seer database and a chinese multi-institutional registry. J Clin Oncol. (2017) 35:1162–70. doi: 10.1200/JCO.2016.67.5140
25. Moon Y, Kim KS, Lee KY, Sung SW, Kim YK, Park JK. Clinicopathologic factors associated with occult lymph node metastasis in patients with clinically diagnosed N0 lung adenocarcinoma. Ann Thorac Surg. (2016) 101:1928–35. doi: 10.1016/j.athoracsur.2015.11.056
Keywords: non-small-cell lung cancer, lymphadenectomy, prognosis, real-world study, propensity score matched
Citation: Wang W, Chen D, Xi K, Chen Y, Zhang X, Wen Y, Huang Z, Yu X, Wang G, Zhang R and Zhang L (2019) Impact of Different Types of Lymphadenectomy Combined With Different Extents of Tumor Resection on Survival Outcomes of Stage I Non-small-cell Lung Cancer: A Large-Cohort Real-World Study. Front. Oncol. 9:642. doi: 10.3389/fonc.2019.00642
Received: 09 April 2019; Accepted: 01 July 2019;
Published: 24 July 2019.
Edited by:
Marco Lucchi, University of Pisa, ItalyReviewed by:
Lorenzo Spaggiari, Istituto Europeo di Oncologia s.r.l., ItalyCopyright © 2019 Wang, Chen, Xi, Chen, Zhang, Wen, Huang, Yu, Wang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lanjun Zhang, emhhbmdsakBzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.