- 1NHC Key Laboratory of Carcinogenesis and Hunan Key Laboratory of Translational Radiation Oncology, Hunan Cancer Hospital and The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China
- 2The Key Laboratory of Carcinogenesis and Cancer Invasion of the Chinese Ministry of Education, Cancer Research Institute, Central South University, Changsha, China
- 3Hunan Key Laboratory of Nonresolving Inflammation and Cancer, Disease Genome Research Center, The Third Xiangya Hospital, Central South University, Changsha, China
Non-coding RNA (ncRNA) plays a regulatory role in a variety of cellular activities. And long non-coding RNA (lncRNA) is one of the important kinds of ncRNA. Previous studies have shown that various lncRNAs are involved in the progression of cancer. LncRNA plasmacytoma variant translocation 1 (PVT1) is a newly discovered oncogenic factor that has been confirmed to be overexpressed in many cancer cells. Moreover, the role of PVT1 in cancer development is closely linked to microRNAs (miRNAs). PVT1 can act as a “sponge” for miRNAs to inhibit their activities, thereby affecting proliferation, invasion, and angiogenesis of cancer. In addition, PVT1 itself can be spliced and processed into several miRNAs such as miR-1204 and miR-1207, which can also regulate the development of cancer. This review summarizes various pathways through which PVT1 regulates the progression of cancer via miRNAs. We also propose additional regulatory mechanisms of PVT1 and their potential clinical applications.
Introduction
Cancer is a non-communicable disease which threatens human health, with a global death toll ranking second only to cardiovascular disease (1). An increasing number of studies have shown that non-coding RNA (ncRNA) plays an important regulatory role in the development of cancer and participates in various cellular processes, such as DNA replication, RNA transcription, and protein synthesis, transport, and degradation. There are many types of ncRNA identified in cells, of which long non-coding RNA (lncRNA) and miRNA catch most of the attention.
PVT1 is an important oncogenic lncRNA highly expressed in cancer cells. The human PVT1 gene is located in 8q24, which is widely recognized as a cancer-associated region (2). The carcinogenic effect of PVT1 has been confirmed in various tumors, such as gallbladder cancer (3), non-small-cell lung cancer (4–6), colon cancer (7, 8), leukemia (9, 10), hepatocellular cancer (11–13), breast cancer (14), and ovarian cancer (15). Multiple miRNA response elements are found on PVT1, to which specific miRNAs can bind and such that these miRNAs are silenced and the expression of certain proteins are upregulated, which ultimately affects the proliferation, invasion, and drug resistance of tumor cells. This mechanism is called the miRNA-mediated sponge interactions (MMI) effect. Currently, researches show that there are more than 20 miRNAs that can be sponged by PVT1, including miR-30a, miR-128, miR-186 etc. (16–18). In addition, PVT1 itself can also be spliced into 6 different miRNAs, namely miR-1204, miR-1205, miR-1206, miR-1207-5p, miR-1207-3p, and miR-1208, with either cancer-inducing or cancer-inhibiting function (19, 20). These discoveries point to a direction for the study of PVT1 and tumor development.
This review systematically outlines the manners through which PVT1 affects the development of the tumor via miRNAs. We propose a regulatory network that centered on PVT1, analyze the feasibility of using PVT1 as a tumor molecular marker and discuss its potential clinical applications.
PVT1 Regulates Tumor Progression Through Sponging miRNAs
PVT1 Affects Tumor Proliferation
Abnormal proliferation of tumor cells is an important feature that distinguishes tumor tissues from normal tissues. It is characterized by changes in the cell cycle, inhibition of apoptosis, and abnormality in energy metabolism. Several studies have confirmed that PVT1, as a potential oncogene, can promote tumor proliferation.
Bone morphogenetic protein (BMP) is an important member of the TGF-β superfamily and influences many important biological processes such as tumor proliferation by regulating a series of downstream genes. PVT1 can counteract the inhibitive expression of gremlin 1 (GREM1) through sponging miR-128, thereby affecting the downstream proteins BMP2 and BMP4-mediated signaling pathway, thus maintaining the proliferative activity of tumor cells (21). Notch signaling pathway affects multiple processes of normal morphogenesis, including differentiation of pluripotent progenitor cells, cell proliferation, cell boundary formation, and apoptosis. PVT1/miR-190a-5p/miR-488-3p/MEF2C/JAGGED1 pathways were shown to be involved in the promotion of tumor cell proliferation. By binding to miR-190a-5p/miR-488-3p, PVT1 promotes the overexpression of myocyte enhancer factor 2C (MEF2C), which is a direct downstream target of miR-190a-5p and miR-488-3p. MEF2C, in turn, upregulates the expression of JAGGED1 via enhancing its promoter activity (22). As a ligand of the Notch signaling pathway, JAGGED1 promotes the expression of its downstream genes such as hes1, to regulate tumor cell proliferation. In addition, PVT1 regulates the expression of Notch2 through acting on miR-488-3p (23), thereby halts the cell cycle at the G0/G1 phase. PVT1 can also regulate the expression of Golgi phosphoprotein 3 (GOLPH3) by sponging miR-186 and increase the expression of p21 and p27, and thus decreasing the phosphorylation of cyclin D1 and Rb, which ultimately shortens the cell cycle (24). Furthermore, PVT1 downregulates miR-31 to enhance the expression of cyclin-dependent kinases 1 (CDK1) and facilitates tumor cell proliferation, migration, and invasion (25) (Figure 1).
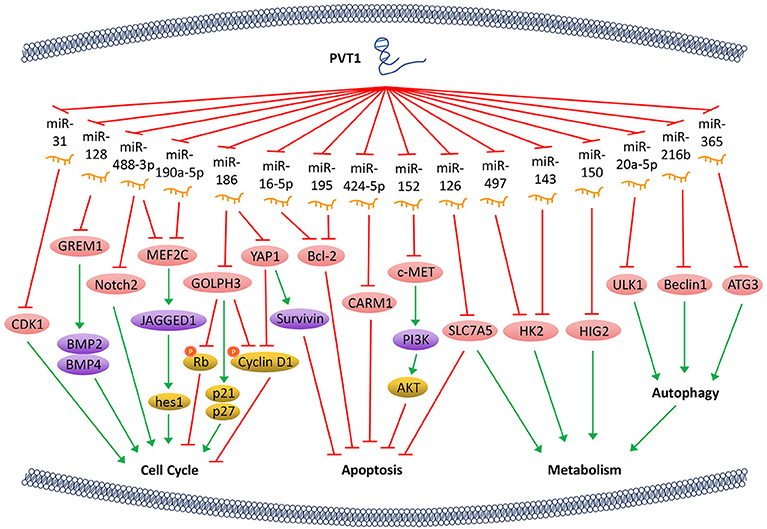
Figure 1. PVT1 regulates tumor progression. LncRNA PVT1, acting as a miRNA sponge, regulates the cell cycle, apoptosis, and energy metabolism of cancer cells through a variety of pathways, thus promotes tumor proliferation.
The Hippo-YAP pathway is involved in regulating tumorigenesis. PVT1 activates the transcriptional activator yes-associated protein 1 (YAP1) (18) via inactivating miR-186, thereby allows YAP1 to enter the nucleus and increase the expression of its downstream gene Survivin and the cell cycle protein D1. Ultimately, PVT1 shortens the cell cycle and inhibits cell apoptosis. Bcl-2 is a key factor that alters the permeability of the mitochondrial outer membrane and allows tumor cells to escape apoptosis. With PVT1 overexpression, miR-16-5p (26) or miR-195 (27) are inhibited, and thus their downstream gene Bcl-2 is overexpressed. PVT1 also plays a role in regulating the apoptosis of tumor cells through activating c-MET/PI3K/AKT and co-activator-associated arginine methyltransferase 1 (CARM1) signaling pathways by sponging miR-152 and miR-424-5p, respectively (5, 28) (Figure 1).
The PVT1/miR-497/HK2 axis which regulates tumor cell energy metabolism is another important pathway that promotes tumor cell proliferation. The overexpression of PVT1 inhibits miR-497 and restores the activity of hexokinase 2 (HK2), thereby increasing the consumption of glucose and the production of lactic acid, which promotes cell proliferation (29). PVT1 also modulates HK2 expression by competitively binding to endogenous miR-143 in tumor cells, which promotes cell proliferation and metastasis by regulating aerobic glucose metabolism (3). In addition, PVT1 acts as a sponge for miR-126 and increases the expression of energy metabolism-related enzyme SLC7A5 in mitochondria, which is another important mechanism through which PVT1 enhances energy metabolism and promotes tumor cell proliferation (30). Hypoxia-inducible protein 2 (HIG2) is found to be a target molecule of miR-150. PVT1 knockdown inhibits the expression of HIG2 via up-regulating the expression of miR-150, and thus ultimately suppresses the tumorigenesis (13). It has also been shown that PVT1 plays an important role in autophagy in tumor cells. As a sponge of miR-20a-5p, PVT1 can increase the expression of unc-51-like kinase 1 (ULK1), which promotes autophagy in tumor cells, thereby providing sufficient energy for tumor growth (31). Beclin1, an important component of PI3K complex, helps locate autophagy proteins to the autophagic vacuoles after being phosphorylated by ULK1. Hence, PVT1 can regulate autophagy via PVT1/miR-216b/Beclin-1 axis (32). Another investigation reveals that PVT1 can upregulate autophagy-related gene 3 (ATG3) expression via acting as an endogenous sponge for miR-365 (12) (Figure 1).
PVT1 Induces Tumor Metastasis
The evaluation of the invasive and metastatic ability of cancer cells is a key indicator of cancer staging and prognosis. PVT1 has been shown to be involved in the following steps during the epithelial-mesenchymal transition (EMT) and distant metastasis of cancer cells: (1) alteration of the adhesion between tumor cells and the surrounding tissues, and influence on cell detachment from the primary focus; (2) degradation of the extracellular matrix; (3) enhancement of cancer cell motility via modification of cytoskeleton; and (4) promotion of angiogenesis in tumor tissues (33).
Cadherin is an important intercellular adhesive molecule, and its decreased expression can promote the EMT of cancer cells. PVT1 regulates plasminogen activator inhibitor 1 RNA-binding protein (SERBP1), plasminogen activator inhibitor-1 (PAI-1), and other molecules by competitively binding to miR-448, thereby reducing the expression of E-cadherin and promoting the invasion of cancer cells (34–36). In addition, E-cadherin can also be down-regulated directly by PVT1 through regulation of miR-16-5p (26, 37) (Figure 2).
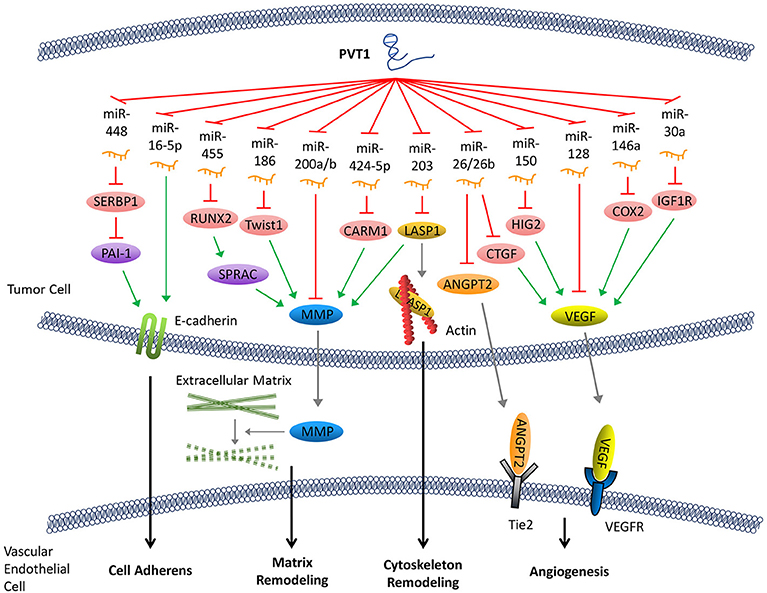
Figure 2. PVT1 promotes tumor metastasis. By sponging miRNAs, PVT1 downregulates these miRNAs and results in reduced expression of E-cadherin, enhanced production of MMP and angiogenesis factors, and remodeling of the cytoskeleton.
Following abscission from the surrounding tissues, tumor cells secrete matrix metalloproteinase (MMP), which promotes the decomposition of the surrounding matrix. The expression of MMP can be upregulated by several signaling pathways that are regulated by PVT1. Both miR-455 and miR-30d-5p can be sponged by PVT1 (8, 38). These two pathways form regulatory loops with runt-related transcription factor 2 (RUNX2), leading to the upregulation of secreted protein acid rich in cysteine (SPARC), which ultimately enhances the expression of MMP (39). Similarly, PVT1 can also upregulate MMP9 through PVT1/miR-150/HIG2 pathway (40), PVT1/miR-424-5p/CARM1 pathway (5), or by binding to miR-200a and miR-200b (41). Moreover, the discovery of the regulatory axis of PVT1/miR-186/Twist1 (42) confirmed that PVT1, through its sponging function, promotes the expression of Twist1 which is a transcription factor related to EMT, thereby promoting the EMT (Figure 2).
Remodeling of the cytoskeleton system promotes invasion and metastasis of cancer cells. PVT1 can sponge miR-203, thereby increasing the expression of Lim and SH3 domain protein 1 (LASP1) which is an actin-binding protein that binds to actin and alters its structure (43). It has been shown that an increase in LASP1 expression can promote the transformation of the cytoskeleton, rendering it more suitable for tumor cell invasion and metastasis (44). LASP1 also participates in other signaling pathways and ultimately upregulates the expression of MMP (45) (Figure 2).
Tumor angiogenesis not only facilitates the transport of nutrients required for cell growth but also creates conditions that allow for distant metastasis through blood vessels. Angiogenesis in tumor tissues is regulated by angiogenesis factors and angiogenesis inhibitors. PVT1 elevates the expression of various angiogenesis factors by binding to their corresponding regulatory miRNAs. For example, PVT1 can elevate the expression of cyclooxygenase-2 (COX2) mRNA through binding with miR-146a. COX2 can initiate the synthesis of the vascular endothelial growth factor (VEGF) family proteins, which are among the most potent factors regulating angiogenesis (46). The PVT1/miR-150/HIG2 (40) pathway also enhances the expression of VEGF. Meanwhile, PVT1 can boost the expression of insulin-like growth factor 1R (IGF1R), which in turn promotes the expression of VEGF by sponging miR-30a (16). In addition, it has been shown that miR-128 directly targets the 3'-UTR of vascular endothelial growth factor C (VEGFC). PVT1, a sponge for miR-128, eliminates the inhibitory effect of miR-128 on the expression of VEGFC, which facilitates its expression (47). Furthermore, other reports have demonstrated that PVT1 binds to and enhances the degradation of miR-26 or miR-26b, thus upregulates the expression of connective tissue growth factor (CTGF) and angiopoietin 2 (ANGPT2), both of which are important angiogenic factors (7, 48) (Figure 2).
PVT1 Regulates Tumor Progression Through Encoding miRNAs
In addition to acting as a molecular sponge for miRNAs, PVT1 itself can also be trimmed and processed into several miRNAs (miR-1204, 1205, 1206, 1207-3p, 1207-5p, 1208). These miRNAs can also regulate the development of tumors. For example, overexpression of miR-1207-5p reduces the expression of signal transducers and activators of transcription (STAT6), thereby activates cyclin-dependent kinase inhibitor 1A (CDKN1A) and CDKN1B to regulate the cell cycle and promote tumor cell proliferation (49). In addition, the elevated expression of miR-1204 not only significantly increases glucose transporters 1 (GLUT-1) expression and glucose uptake but also suppresses the expression of pitx1 and Vitamin D (1,25- dihydroxy vitamin D3) receptor (VDR), which ultimately promotes cell proliferation, invasion, and metastasis (50, 51). In addition, miR-1205 downregulates the expression of the Egl-9 family hypoxia-inducible factor 3 (EGLN3) and promotes cell proliferation and cell cycle progression and inhibits hydrogen peroxide-induced apoptosis (52). Surprisingly, miR-1204, miR-1207-3p, and miR-1207-5p have exhibited an inhibitory function of tumor progression in some studies. For instance, miR-1204 and miR-1207 are shown to enhance the sensitivity of tumor cells to chemotherapeutic drugs (53, 54). In another report, the miR-1207-3p/FNDC1/FN1/AR pathway is shown to be involved in the inhibition of tumor proliferation and migration, and induction of apoptosis (55). Furthermore, the miR-1207-5p/CSF1 axis can also inhibit tumor proliferation and migration by regulating tumor microenvironment (56). These contradictory tumor-suppressing effects caused by the inhibition of PVT1 through miR-1204, miR-1207-3p, and miR-1207-5p warrant further investigation (57) (Figure 3).
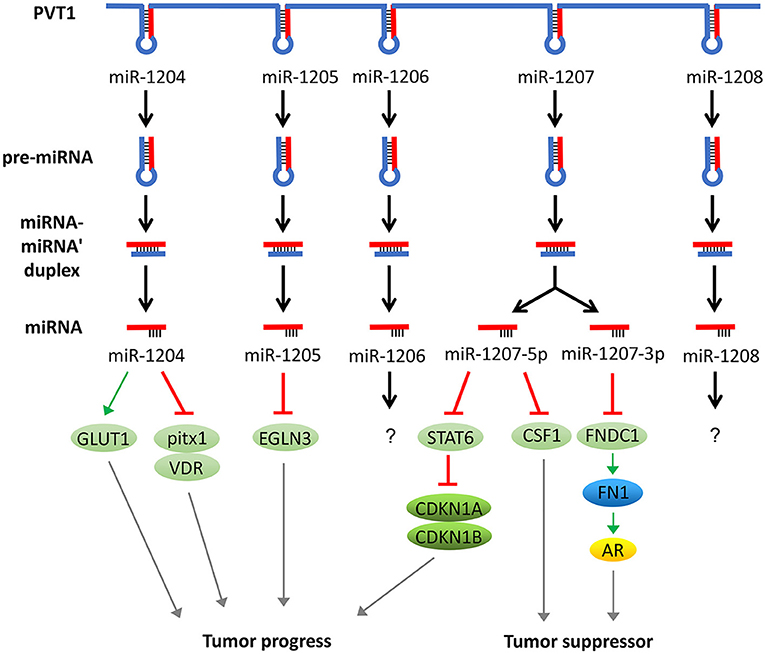
Figure 3. PVT1 regulates tumor progression through encoded miRNAs. PVT1 itself can be trimmed and processed into several miRNAs, known as miR-1204, 1205, 1206, 1207-3p, 1207-5p, and 1208. Among these miRNAs, miR-1204, 1205, 1207-3p, and 1207-5p have been shown to promote or inhibit cancer, while the effects of the remaining two miRNAs in cancer remain unknown.
Prospects
Existing studies have shown that PVT1 is overexpressed in a variety of tumors, and its expression is closely associated with tumor proliferation and apoptosis, invasion and metastasis, angiogenesis, and drug resistance. A recent study shows that knock-down of PVT1 can increase the radiosensitivity of the tumor (5), suggesting its role as an oncogene to promote tumor progression. The role of PVT1 in tumor is closely associated with a variety of miRNAs and their downstream pathways. In addition to the sponge-like effect mentioned above, PVT1 can also affect miRNAs through other mechanisms, thereby promoting tumor progression. A previous study showed that PVT1 can increase the expression of miR-214 by enhancing the binding of enhancer of zeste homolog 2 (EZH2) to the miR-214 promoter, which ultimately promotes tumor cell proliferation and invasion (58). In addition, PVT1 also downregulates the expression of miR-146a by increasing the activity of DNA methylase, which induces the methylation of the CpG island in miR-146a precursor, thereby affecting the growth of tumor cells (59). At the same time, with the discovery of PVT1-encoded miRNAs, an increasing number of studies have revealed that these miRNAs also participate in the regulation of tumor progression. Currently, there are several studies involving PVT1-encoded miR-1204, miR-1205 and miR-1207. However, not all studies show that these miRNAs promote tumor development. Additionally, whether miR-1206 and miR-1208 plays specific roles in cancer remains unknown. Overall, the effects of PVT1 on tumors are closely associated with miRNA regulation.
In addition to its long-chain form, PVT1 also exists in a circular form. The circular PVT1 (circPVT1) locus is contained within the lncPVT1, which originates from the exon 2 of the PVT1 gene. Several studies have demonstrated that circPVT1 is also abnormally expressed in tumor cells. Lorena Verduci et al. showed that the mutant p53/YAP/TEAD transcription complex enhanced the expression of circPVT1, which in turn acted as an oncogene to regulate tumor proliferation by affecting the expression of miR-497-5p (60). In addition, circPVT1 also promotes drug resistance of tumor cells (61) and interacts with miRNAs as a competing endogenous RNA (ceRNA) just like lncPVT1. Hence, not only lncPVT1 but also circPVT1 is closely linked to tumor development. There have been several studies exploring lncPVT1's clinical application. The results reveal that lncPVT1 is a potential biomarker for some tumors as its expression is abnormal and the detecting technology has been optimized (62–66). And as a more stable form, circPVT1 may be more valuable in clinic practice. In summary, PVT1 will be used for tumor screening, malignant and prognosis evaluating, or even as a molecule target for cancer treatment in the near future.
Certain mechanisms through which PVT1 affects tumor development remain unclear. It has been noticed that PVT1 and the oncogene c-MYC coexist in the same chromosomal region, namely the 8q24 region. They are coamplified (67), and c-MYC can regulate the expression of PVT1. They both can promote tumor proliferation. Moreover, Salehi et al. demonstrated that the expression of the c-MYC gene was downregulated when PVT1 was knocked out, and thus the apoptosis and necrosis of cancer cells increased (68). This suggests that PVT1 and c-MYC are related to tumorigenesis and mutually regulate each other. A recent report also proposed the mutual regulatory relationship between PVT1 and MYC. In addition to the oncogenic lncPVT1, the PVT1 promoter affects tumor development by affecting the transcription of PVT1 and c-MYC, and functions independently of lncPVT1 (69). Whether there are more oncogenes or tumor suppressors within the 8q24 region and what are the interrelationships among them warrant future explorations, which will greatly facilitate our fundamental understanding of cancer development.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (81672683, 81672993, 81702907, 81772928, 81803025, and 81872278), the Overseas Expertise Introduction Project for Discipline Innovation (111 Project, No. 111–2-12), and the Natural Science Foundation of Hunan Province (2016JC2035, 2017SK2100, 2018SK21210, 2018SK21211, 2018JJ3815, and 2018JJ3704).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This review was written under the guidance of our supervisor WX. It was his constant instructions which made our review clear and precise. We would also like to thank Mr. Yongzhen Mo, who provided many useful suggestions and assistance to this review.
Abbreviations
ATG3, autophagy-related gene 3; ANGPT2, angiopoietin 2; AR, androgen receptor; BMP, bone morphogenetic protein; CDK1, cyclin-dependent kinases 1; CARM1, co-activator-associated arginine methyltransferase 1; c-Met, cellular-mesenchymal to epithelial transition factor; COX2, cyclooxygenase-2; CTGF, connective tissue growth factor; CDKN1, cyclin-dependent kinase inhibitor 1; CpG island, 5 ’-C-phosphate-G-3’ island; circPVT1, circular PVT1; ceRNA, competing endogenous RNA; EMT, epithelial-mesenchymal transition; EGLN3, Egl-9 family hypoxia-inducible factor 3; EZH2, enhancer of zeste homolog 2; FNDC1, fibronectin type III domain containing 1; FN1, fibronectin 1; GREM1, gremlin 1; GOLPH3, Golgi phosphoprotein 3; GLUT-1, glucose transporters 1; HK2, hexokinase 2; HIG2, hypoxia-inducible protein 2; IGF1R, insulin-like growth factor 1R; lncRNA, long-chain non-coding RNA; LASP1, Lim and SH3 domain protein; miRNA, microRNA; MMI, miRNA-mediated sponge interactions; MEF2C, myocyte enhancer factor 2C; MMP, matrix metalloproteinase; ncRNA, non-coding RNA; PVT1, plasmacytoma variant translocation 1; PI3K, phosphatidylinositol 3-kinase; PAI-1, plasminogen activator inhibitor-1; RUNX2, runt-related transcription factor 2; SERBP1, plasminogen activator inhibitor 1 RNA-binding protein; SPARC, secreted protein acid rich in cysteine; STAT, signal transducers and activators of transcription; TEAD, TEA domain family member 1; ULK1, unc-51-like kinase 1; VEGF, vascular endothelial growth factor; VDR, Vitamin D (1,25- dihydroxy vitamin D3) receptor; YAP1, yes-associated protein 1.
References
1. Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. (2019) 62:640–7. doi: 10.1007/s11427-018-9461-5
2. Barsotti AM, Beckerman R, Laptenko O, Huppi K, Caplen NJ, Prives C. p53-Dependent induction of PVT1 and miR-1204. J Biol Chem. (2012) 287:2509–19. doi: 10.1074/jbc.M111.322875
3. Chen J, Yu Y, Li H, Hu Q, Chen X, He Y, et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer. (2019) 18:33. doi: 10.1186/s12943-019-0947-9
4. Li X, Zhang Z, Jiang H, Li Q, Wang R, Pan H, et al. Circular RNA circPVT1 promotes proliferation and invasion through sponging miR-125b and activating E2F2 signaling in non-small cell lung cancer. Cell Physiol Biochem. (2018) 51:2324–40. doi: 10.1159/000495876
5. Wang D, Hu Y. Long non-coding RNA PVT1 competitively binds MicroRNA-424-5p to regulate CARM1 in radiosensitivity of non-small-cell lung cancer. Mol Ther Nucleic Acids. (2018) 16:130–40. doi: 10.1016/j.omtn.2018.12.006
6. Qin S, Zhao Y, Lim G, Lin H, Zhang X, Zhang X. Circular RNA PVT1 acts as a competing endogenous RNA for miR-497 in promoting non-small cell lung cancer progression. Biomed Pharmacother. (2018) 111:244–50. doi: 10.1016/j.biopha.2018.12.007
7. Zhang R, Li J, Yan X, Jin K, Li W, Liu X, et al. Long noncoding RNA plasmacytoma variant translocation 1 (PVT1) promotes colon cancer progression via endogenous sponging miR-26b. Med Sci Monitor. (2018) 24:8685–92. doi: 10.12659/MSM.910955
8. Yu X, Zhao J, He Y. Long non-coding RNA PVT1 functions as an oncogene in human colon cancer through miR-30d-5p/RUNX2 axis. J BUON. (2018) 23:48–54.
9. Hu J, Han Q, Gu Y, Ma J, McGrath M, Qiao F, et al. Circular RNA PVT1 expression and its roles in acute lymphoblastic leukemia. Epigenomics. (2018) 10:723–32. doi: 10.2217/epi-2017-0142
10. El-Khazragy N, Elayat W, Matbouly S, Seliman S, Sami A, Safwat G, et al. The prognostic significance of the long non-coding RNAs “CCAT1, PVT1” in t(8;21) associated acute myeloid leukemia. Gene. (2019) 707:172–7. doi: 10.1016/j.gene.2019.03.055
11. Guo J, Hao C, Wang C, Li L. Long noncoding RNA PVT1 modulates hepatocellular carcinoma cell proliferation and apoptosis by recruiting EZH2. Cancer Cell Int. (2018) 18:98. doi: 10.1186/s12935-018-0582-3
12. Yang L, Peng X, Jin H, Liu J. Long non-coding RNA PVT1 promotes autophagy as ceRNA to target ATG3 by sponging microRNA-365 in hepatocellular carcinoma. Gene. (2019) 697:94–102. doi: 10.1016/j.gene.2019.02.036
13. Xu Y, Luo X, He W, Chen G, Li Y, Li W, et al. Long non-coding RNA PVT1/miR-150/ HIG2 axis regulates the proliferation, invasion and the balance of iron metabolism of hepatocellular carcinoma. Cell Physiol Biochem. (2018) 49:1403–19. doi: 10.1159/000493445
14. Guan Y, Kuo WL, Stilwell JL, Takano H, Lapuk AV, Fridlyand J, et al. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. (2007) 13:5745–55. doi: 10.1158/1078-0432.CCR-06-2882
15. Yang Q, Yu Y, Sun Z, Pan Y. Long non-coding RNA PVT1 promotes cell proliferation and invasion through regulating miR-133a in ovarian cancer. Biomed Pharmacother. (2018) 106:61–7. doi: 10.1016/j.biopha.2018.06.112
16. Feng K, Liu Y, Xu LJ, Zhao LF, Jia CW, Xu MY. Long noncoding RNA PVT1 enhances the viability and invasion of papillary thyroid carcinoma cells by functioning as ceRNA of microRNA-30a through mediating expression of insulin like growth factor 1 receptor. Biomed Pharmacother. (2018) 104:686–98. doi: 10.1016/j.biopha.2018.05.078
17. He F, Song Z, Chen H, Chen Z, Yang P, Li W, et al. Long noncoding RNA PVT1-214 promotes proliferation and invasion of colorectal cancer by stabilizing Lin28 and interacting with miR-128. Oncogene. (2018) 38:164–79. doi: 10.1038/s41388-018-0432-8
18. Lan T, Yan X, Li Z, Xu X, Mao Q, Ma W, et al. Long non-coding RNA PVT1 serves as a competing endogenous RNA for miR-186-5p to promote the tumorigenesis and metastasis of hepatocellular carcinoma. Tumour Biol. (2017) 39:1010428317705338. doi: 10.1177/1010428317705338
19. Beck-Engeser GB, Lum AM, Huppi K, Caplen NJ, Wang BB, Wabl M. Pvt1-encoded microRNAs in oncogenesis. Retrovirology. (2008) 5:4. doi: 10.1186/1742-4690-5-4
20. Huppi K, Volfovsky N, Runfola T, Jones TL, Mackiewicz M, Martin SE, et al. The identification of microRNAs in a genomically unstable region of human chromosome 8q24. Mol Cancer Res. (2008) 6:212–21. doi: 10.1158/1541-7786.MCR-07-0105
21. Fu C, Li D, Zhang X, Liu N, Chi G, Jin X. LncRNA PVT1 facilitates tumorigenesis and progression of glioma via regulation of MiR-128-3p/GREM1 axis and BMP signaling pathway. Neurotherapeutics. (2018) 15:1139–57. doi: 10.1007/s13311-018-0649-9
22. Xue W, Chen J, Liu X, Gong W, Zheng J, Guo X, et al. PVT1 regulates the malignant behaviors of human glioma cells by targeting miR-190a-5p and miR-488-3p. Biochim Biophys Acta Mol Basis Dis. (2018) 1864(5 Pt A):1783–94. doi: 10.1016/j.bbadis.2018.02.022
23. Wu XZ, Cui HP, Lv HJ, Feng L. Knockdown of lncRNA PVT1 inhibits retinoblastoma progression by sponging miR-488-3p. Biomed Pharmacother. (2019) 112:108627. doi: 10.1016/j.biopha.2019.108627
24. Hua X, Xiao Y, Pan W, Li M, Huang X, Liao Z, et al. miR-186 inhibits cell proliferation of prostate cancer by targeting GOLPH3. Am J Cancer Res. (2016) 6:1650–60.
25. Tian Z, Cao S, Li C, Xu M, Wei H, Yang H, et al. LncRNA PVT1 regulates growth, migration, and invasion of bladder cancer by miR-31/ CDK1. J Cell Physiol. (2019) 234:4799–811. doi: 10.1002/jcp.27279
26. Ren Y, Huang W, Weng G, Cui P, Liang H, Li Y. LncRNA PVT1 promotes proliferation, invasion and epithelial-mesenchymal transition of renal cell carcinoma cells through downregulation of miR-16-5p. Onco Targets Ther. (2019) 12:2563–75. doi: 10.2147/OTT.S190239
27. Zhou Q, Chen F, Zhao J, Li B, Liang Y, Pan W, et al. Long non-coding RNA PVT1 promotes osteosarcoma development by acting as a molecular sponge to regulate miR-195. Oncotarget. (2016) 7:82620–33. doi: 10.18632/oncotarget.13012
28. Sun ZY, Jian YK, Zhu HY, Li B. lncRNAPVT1 targets miR-152 to enhance chemoresistance of osteosarcoma to gemcitabine through activating c-MET/PI3K/AKT pathway. Pathol Res Pract. (2019) 215:555–63. doi: 10.1016/j.prp.2018.12.013
29. Song J, Wu X, Liu F, Li M, Sun Y, Wang Y, et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem Biophys Res Commun. (2017) 490:217–24. doi: 10.1016/j.bbrc.2017.06.024
30. Li H, Chen S, Liu J, Guo X, Xiang X, Dong T, et al. Long non-coding RNA PVT1-5 promotes cell proliferation by regulating miR-126/SLC7A5 axis in lung cancer. Biochem Biophys Res Commun. (2018) 495:2350–5. doi: 10.1016/j.bbrc.2017.12.114
31. Huang F, Chen W, Peng J, Li Y, Zhuang Y, Zhu Z, et al. LncRNA PVT1 triggers Cyto-protective autophagy and promotes pancreatic ductal adenocarcinoma development via the miR-20a-5p/ULK1 Axis. Mol Cancer. (2018) 17:98. doi: 10.1186/s12943-018-0845-6
32. Chen L, Han X, Hu Z, Chen L. The PVT1/miR-216b/Beclin-1 regulates cisplatin sensitivity of NSCLC cells via modulating autophagy and apoptosis. Cancer Chemother Pharmacol. (2019) 83:921–31. doi: 10.1007/s00280-019-03808-3
33. Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. (2017) 11:28–39. doi: 10.1002/1878-0261.12017
34. Serce NB, Boesl A, Klaman I, von Serenyi S, Noetzel E, Press MF, et al. Overexpression of SERBP1 (Plasminogen activator inhibitor 1 RNA binding protein) in human breast cancer is correlated with favourable prognosis. BMC Cancer. (2012) 12:597. doi: 10.1186/1471-2407-12-597
35. Fabre-Guillevin E, Malo M, Cartier-Michaud A, Peinado H, Moreno-Bueno G, Vallee B, et al. PAI-1 and functional blockade of SNAI1 in breast cancer cell migration. Breast Cancer Res. (2008) 10:R100. doi: 10.1186/bcr2203
36. Zhao L, Kong H, Sun H, Chen Z, Chen B, Zhou M. LncRNA-PVT1 promotes pancreatic cancer cells proliferation and migration through acting as a molecular sponge to regulate miR-448. J Cell Physiol. (2018) 233:4044–55. doi: 10.1002/jcp.26072
37. Zhang H, Yang K, Ren T, Huang Y, Tang X, Guo W. miR-16-5p inhibits chordoma cell proliferation, invasion and metastasis by targeting Smad3. Cell Death Dis. (2018) 9:680. doi: 10.1038/s41419-018-0738-z
38. Chai J, Guo D, Ma W, Han D, Dong W, Guo H, et al. A feedback loop consisting of RUNX2/LncRNA-PVT1/miR-455 is involved in the progression of colorectal cancer. Am J Cancer Res. (2018) 8:538–50.
39. Levin G, Coelho TM, Nobrega NG, Trombetta-Lima M, Sogayar MC, Carreira ACO. Spatio-temporal expression profile of matrix metalloproteinase (Mmp) modulators Reck and Sparc during the rat ovarian dynamics. Reprod Biol Endocrinol. (2018) 16:116. doi: 10.1186/s12958-018-0422-2
40. Kim SH, Wang D, Park YY, Katoh H, Margalit O, Sheffer M, et al. HIG2 promotes colorectal cancer progression via hypoxia-dependent and independent pathways. Cancer Lett. (2013) 341:159–65. doi: 10.1016/j.canlet.2013.07.028
41. Chen W, Zhu H, Yin L, Wang T, Wu J, Xu J, et al. lncRNA-PVT1 facilitates invasion through upregulation of MMP9 in nonsmall cell lung cancer cell. DNA Cell Biol. (2017) 36:787–93. doi: 10.1089/dna.2017.3725
42. Chang Z, Cui J, Song Y. Long noncoding RNA PVT1 promotes EMT via mediating microRNA-186 targeting of Twist1 in prostate cancer. Gene. (2018) 654:36–42. doi: 10.1016/j.gene.2018.02.036
43. Li PD, Hu JL, Ma C, Ma H, Yao J, Chen LL, et al. Upregulation of the long non-coding RNA PVT1 promotes esophageal squamous cell carcinoma progression by acting as a molecular sponge of miR-203 and LASP1. Oncotarget. (2017) 8:34164–76. doi: 10.18632/oncotarget.15878
44. Chew CS, Chen X, Parente JA Jr, Tarrer S, Okamoto C, Qin HY. Lasp-1 binds to non-muscle F-actin in vitro and is localized within multiple sites of dynamic actin assembly in vivo. J Cell Sci. (2002) 115(Pt 24):4787–99. doi: 10.1242/jcs.00174
45. Endres M, Kneitz S, Orth MF, Perera RK, Zernecke A, Butt E. Regulation of matrix metalloproteinases (MMPs) expression and secretion in MDA-MB-231 breast cancer cells by LIM and SH3 protein 1 (LASP1). Oncotarget. (2016) 7:64244–59. doi: 10.18632/oncotarget.11720
46. Zhang W, Xiao J, Lu X, Liu T, Jin X, Xiao Y, et al. PVT1 (rs13281615) and miR-146a (rs2910164) polymorphisms affect the prognosis of colon cancer by regulating COX2 expression and cell apoptosis. J Cell Physiol. (2019) 234:17538–48. doi: 10.1002/jcp.28377.
47. Yu C, Longfei L, Long W, Feng Z, Chen J, Chao L, et al. LncRNA PVT1 regulates VEGFC through inhibiting miR-128 in bladder cancer cells. J Cell Physiol. (2019) 234:1346–53. doi: 10.1002/jcp.26929
48. Zheng J, Hu L, Cheng J, Xu J, Zhong Z, Yang Y, et al. lncRNA PVT1 promotes the angiogenesis of vascular endothelial cell by targeting miR26b to activate CTGF/ANGPT2. Int J Mol Med. (2018) 42:489–96. doi: 10.3892/ijmm.2018.3595
49. Yan C, Chen Y, Kong W, Fu L, Liu Y, Yao Q, et al. PVT1-derived miR-1207-5p promotes breast cancer cell growth by targeting STAT6. Cancer Sci. (2017) 108:868–76. doi: 10.1111/cas.13212
50. Xu J, Gu X, Yang X, Meng Y. MiR-1204 promotes ovarian squamous cell carcinoma growth by increasing glucose uptake. Biosci Biotechnol Biochem. (2018) 83:123–8. doi: 10.1080/09168451.2018.1527208
51. Jiang W, He Y, Shi Y, Guo Z, Yang S, Wei K, et al. MicroRNA-1204 promotes cell proliferation by regulating PITX1 in non-small-cell lung cancer. Cell Biol Int. (2019) 43:253–64. doi: 10.1002/cbin.11083
52. Wang Y, Li X, Liu W, Li B, Chen D, Hu F, et al. MicroRNA-1205, encoded on chromosome 8q24, targets EGLN3 to induce cell growth and contributes to risk of castration-resistant prostate cancer. Oncogene. (2019) 38:4820–34. doi: 10.1038/s41388-019-0760-3
53. Peng X, Cao P, Li J, He D, Han S, Zhou J, et al. MiR-1204 sensitizes nasopharyngeal carcinoma cells to paclitaxel both in vitro and in vivo. Cancer Biol Ther. (2015) 16:261–7. doi: 10.1080/15384047.2014.1001287
54. You L, Wang H, Yang G, Zhao F, Zhang J, Liu Z, et al. Gemcitabine exhibits a suppressive effect on pancreatic cancer cell growth by regulating processing of PVT1 to miR1207. Mol Oncol. (2018) 12:2147–64. doi: 10.1002/1878-0261.12393
55. Das DK, Ogunwobi OO. A novel microRNA-1207-3p/FNDC1/FN1/AR regulatory pathway in prostate cancer. RNA Dis. (2017) 4:e1503. doi: 10.1017/cts.2017.37
56. Dang W, Qin Z, Fan S, Wen Q, Lu Y, Wang J, et al. miR-1207-5p suppresses lung cancer growth and metastasis by targeting CSF1. Oncotarget. (2016) 7:32421–32. doi: 10.18632/oncotarget.8718
57. Cui M, Chang Y, Fang QG, Du W, Wu JF, Wang JH, et al. Non-coding RNA Pvt1 promotes cancer stem cell-like traits in nasopharyngeal cancer via inhibiting miR-1207. Pathol Oncol Res. (2018). doi: 10.1007/s12253-018-0453-1. [Epub ahead of print].
58. Chen Y, Du H, Bao L, Liu W. LncRNA PVT1 promotes ovarian cancer progression by silencing miR-214. Cancer Biol Med. (2018) 15:238–50. doi: 10.20892/j.issn.2095-3941.2017.0174
59. Liu HT, Fang L, Cheng YX, Sun Q. LncRNA PVT1 regulates prostate cancer cell growth by inducing the methylation of miR-146a. Cancer Med. (2016) 5:3512–9. doi: 10.1002/cam4.900
60. Verduci L, Ferraiuolo M, Sacconi A, Ganci F, Vitale J, Colombo T, et al. The oncogenic role of circPVT1 in head and neck squamous cell carcinoma is mediated through the mutant p53/YAP/TEAD transcription-competent complex. Genome Biol. (2017) 18:237. doi: 10.1186/s13059-017-1368-y
61. Kun-Peng Z, Xiao-Long M, Chun-Lin Z. Overexpressed circPVT1, a potential new circular RNA biomarker, contributes to doxorubicin and cisplatin resistance of osteosarcoma cells by regulating ABCB1. Int J Biol Sci. (2018) 14:321–30. doi: 10.7150/ijbs.24360
62. Chen X, Yang Y, Cao Y, Wu C, Wu S, Su Z, et al. lncRNA PVT1 identified as an independent biomarker for prognosis surveillance of solid tumors based on transcriptome data and meta-analysis. Cancer Manag Res. (2018) 10:2711–27. doi: 10.2147/CMAR.S166260
63. Chen J, Li Y, Zheng Q, Bao C, He J, Chen B, et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. (2017) 388:208–19. doi: 10.1016/j.canlet.2016.12.006
64. Fang J, Huang J. Clinical significance of the expression of long non-coding RNA PVT1 in glioma. Cancer Biomark. (2019) 24:509–13. doi: 10.3233/CBM-182253
65. Gu JX, Zhang X, Miao RC, Xiang XH, Fu YN, Zhang JY, et al. Six-long non-coding RNA signature predicts recurrence-free survival in hepatocellular carcinoma. World J Gastroenterol. (2019) 25:220–32. doi: 10.3748/wjg.v25.i2.220
66. Qi G, Kong W, Mou X, Wang S. A new method for excavating feature lncRNA in lung adenocarcinoma based on pathway crosstalk analysis. J Cell Biochem. (2019) 120:9034–46. doi: 10.1002/jcb.28177
67. Graham M, Adams JM. Chromosome 8 breakpoint far 3′ of the c-myc oncogene in a Burkitt's lymphoma 2;8 variant translocation is equivalent to the murine pvt-1 locus. EMBO J. (1986) 5:2845–51. doi: 10.1002/j.1460-2075.1986.tb04578.x
68. Salehi M, Sharifi M, Bagheri M. Knockdown of long noncoding RNA plasmacytoma variant translocation 1 with antisense locked nucleic acid GapmeRs exerts tumor-suppressive functions in human acute erythroleukemia cells through downregulation of C-MYC expression. Cancer Biother Radiopharm. (2018). doi: 10.1089/cbr.2018.2510. [Epub ahead of print].
Keywords: PVT1, miRNA, cancer, sponge, splicing
Citation: Wang W, Zhou R, Wu Y, Liu Y, Su W, Xiong W and Zeng Z (2019) PVT1 Promotes Cancer Progression via MicroRNAs. Front. Oncol. 9:609. doi: 10.3389/fonc.2019.00609
Received: 06 January 2019; Accepted: 20 June 2019;
Published: 15 July 2019.
Edited by:
Olorunseun O. Ogunwobi, Hunter College (CUNY), United StatesReviewed by:
Zeyi Liu, Soochow University, ChinaXingming Jiang, Second Affiliated Hospital of Harbin Medical University, China
Copyright © 2019 Wang, Zhou, Wu, Liu, Su, Xiong and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Xiong, eGlvbmd3ZWlAY3N1LmVkdS5jbg==; Zhaoyang Zeng, emVuZ3poYW95YW5nQGNzdS5lZHUuY24=
 Wenxi Wang
Wenxi Wang Ruoyu Zhou
Ruoyu Zhou Yuwei Wu1,2
Yuwei Wu1,2 Wenjia Su
Wenjia Su Zhaoyang Zeng
Zhaoyang Zeng