
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Oncol. , 19 June 2019
Sec. Cancer Molecular Targets and Therapeutics
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00522
 Maria E. Mycielska1*
Maria E. Mycielska1* Markus T. J. Mohr2
Markus T. J. Mohr2 Katharina Schmidt1
Katharina Schmidt1 Konstantin Drexler3
Konstantin Drexler3 Petra Rümmele4
Petra Rümmele4 Sebastian Haferkamp3
Sebastian Haferkamp3 Hans J. Schlitt1
Hans J. Schlitt1 Andreas Gaumann5
Andreas Gaumann5 Jerzy Adamski6,7,8
Jerzy Adamski6,7,8 Edward K. Geissler1*
Edward K. Geissler1*We have recently discovered that cancer cells take up extracellular citrate through plasma membrane citrate transporter (pmCiC) and advantageously use citrate for their metabolism. Citrate uptake can be blocked with gluconate and this results in decreased tumor growth and altered metabolic characteristics of tumor tissue. Interestingly, gluconate, considered to be physiologically neutral, is incidentally used in medicine as a cation carrier, but not as a therapeutically active substance. In this review we discuss the results of our recent research with available literature and suggest that gluconate may be useful in the treatment of cancer.
Despite intense basic and clinical cancer research, progress in controlling and curing malignancies remains slow. Surgery, chemotherapy and radiotherapy are often palliative especially for advanced cancer, and can have severe side effects. Emerging immunotherapies are showing promise, but much work still needs to be done to prove their efficacy against a wide variety of tumors, and tumors with little immunogenicity will likely not respond well to these therapies.
Intense research efforts are directed toward identifying novel anti-cancer drugs and to discover known substances with potential antineoplastic effects that have been used for indications other than cancer (1). This latter approach has the advantage that new discoveries with approved substances can be moved more quickly to clinical application. In the present review we chose to concentrate on gluconate as an interesting candidate molecule that is often used as a “carrier” for putative antineoplastic substances, therefore disguising its own potential anti-cancer activities. We raise the possibility that activities thought to be attributed entirely to the putative active antineoplastic substance, may at least in part be due to the activity of gluconate. Based on our own studies and the available literature we propose that gluconate should be studied as an anti-cancer drug alone or in combination with chemotherapy. This idea is particularly attractive since gluconate use as an excipient is approved by the FDA and is considered to have little in the way of any side effects. Herein, we also present an interpretation of the existing data explaining why gluconate could also be useful in the diagnosis of cancer.
Gluconate is a glucose derivative, existing as a salt of gluconic acid known to chelate divalent metals; gluconic acid is found naturally in fruits, honey and wine. As a membrane impermeant anion, gluconate is often used in biomedical research as Cl− substitute (2, 3). In medicine, gluconate is used most commonly as a biologically neutral carrier of Zn2+, Ca2+, Cu2+, Fe2+, and K+ to treat relevant ion deficiencies. Furthermore, gluconate is also combined with other drugs such as chlorohexindine, which is used as an antiseptic in surgery (4) or dental care (5); sodium antimony (stibogluconate) is used to treat leishmaniasis (6). Importantly, however, while gluconate is exploited for these purposes in medicine, it is not considered to be physiologically active and has not been studied for its own therapeutic effects.
How can the hypothesis of gluconate's anticancer effects be rationalized scientifically? Although citrate is considered a central substrate in cancer cell metabolism, its source remains debatable. Interestingly, several recent metabolomic studies indicate a correlation between metastatic disease and decreased level of citrate in blood, tissues and urine of cancer patients (7–11), to which an increased need of the cancer for citrate could contribute. It is also possible that under these pathophysiological conditions decreased citrate could be accounted for by increased liver metabolism, especially regarding fatty acid synthesis (12). Since hepatocytes express Na+-dependent citrate transporter (NaCT), increased citrate uptake from blood could additionally be associated with increased metabolic liver function (13). We have recently put forward a hypothesis in which cancer cells take up extracellular citrate and use it to support their metabolism, especially fatty acid synthesis (14). Our work shows that cancer cells take up extracellular citrate through the plasma membrane citrate transporter (pmCiC) belonging to the SLC25 family and that expression of this transporter is mainly restricted to cancer cells (14). We have also shown that cancer cell metabolism benefits from extracellular citrate uptake by decreasing its mitochondrial activity and in consequence ROS synthesis, thereby reducing cancer cell requirements for extracellular glucose supply. Importantly, we have discovered that gluconate is a competitive and irreversible blocker of the pmCiC, thus decreasing the uptake of extracellular citrate by cancer cells, and inhibiting human tumor growth in immunodeficient mice (summarized in Figure 1). This observation together with largely cancer cell-specific expression of pmCiC provides a mechanistic explanation for why gluconate could be partially responsible for the increased anti-cancer effects observed when used with other therapeutics, like Zn2+ and Ca+2. For instance, therapeutic remissions observed when using Zn2+ gluconate for ocular melanoma and acute lymphocytic leukemia could actually be related to the presence of gluconate (15, 16), as will be detailed later in this review. Of course some of the observed effects are likely due to ions carried by gluconate, such as increased immune activity associated with Zn2+, or protection against neuropathy known to be due to Ca2+. Furthermore, from a diagnostic standpoint, our research finding that gluconate binds to the pmCiC provides an explanation for why 99mTc gluconate (technetium-99m, used in medicine as a radioactive tracer due to its short half-life), is guided specifically to malignant cancer entities. The following part of this article presents available data related to the use of gluconate in cancer treatment and diagnosis. We include an interpretation of the potential gluconate effects based on our own studies.
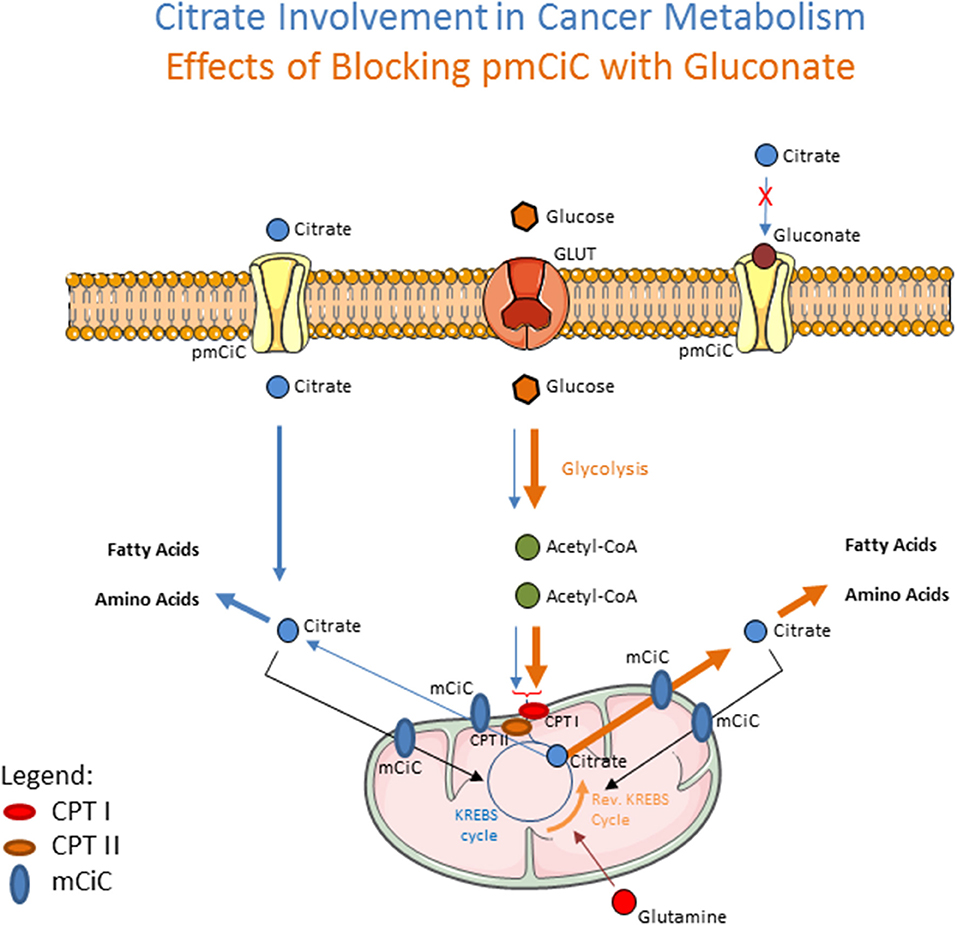
Figure 1. Schematic drawing showing the effects of extracellular citrate on cancer cell metabolism (blue arrows). Extracellular citrate is taken up by the cells through the plasma membrane citrate transporter (pmCiC). Its uptake reduces the need of mitochondrial-derived citrate (transported from mitochondria through mCiC) and is mainly used in cytoplasm to support fatty acid and amino acid synthesis. When the pmCiC is blocked with gluconate (brown arrows), extracellular citrate cannot get through the plasma membrane of cancer cells resulting in increased use of glycolysis to support mitochondrial citrate synthesis. CPTI and II (Carnitine palmitoyltransferase I and II) depict AcetylCoA transport mechanism. The thickness of lines reflects the intensity of pathway use.
Stibogluconate (sodium antimony gluconate, sodium stibugluconate, pentostam, stibium Sb gluconate) is an anti-parasitic drug that incorporates gluconate into the drug substance. It belongs to the anti-leishmaniasis group of drugs called pentavalent-(V)-antimony compounds in which antimony (Sb) is used (17–19). It has been suggested that stibogluconate acts through immune system stimulation, mainly by increasing the amount of peripheral IFN-γ+ T cells (20). Importantly, this increased immune response has been observed in mice (21), as well as in humans (22). Because of its stimulating effect on the immune system, the drug was considered a good candidate for cancer research. Indeed, application of stibogluconate in vivo in mice with implanted human tumor cells consistently resulted in either significant tumor growth inhibition or total tumor regression. Human renal cell carcinoma growth was significantly inhibited in mice using stibogluconate (21). Similar results were obtained when mice bearing human melanoma cells were treated with this substance (23). Interestingly, other pentavalent antimony compounds, not incorporating gluconate, including triphenylantimony-(V)-polyamines and monomeric species, showed limited effects on cancer cells [reviewed by (24)]. Furthermore, trivalent-(III)-antimony, not associated with gluconate, has not shown promising anti-tumor effects (applied in the form of organometallic compounds such as diphenylantimony(III) thiolates). Application of these compounds against leukemia in mice did not give a substantial survival advantage and showed high dosage-dependent toxicity (25).
Why therefore are some antimony forms effective against cancer (stibogluconate), and others are not? Possible explanations include differences in transport mechanism of alternative forms of antimony, which are still not well-defined. While transport of antimony-(III) from the extra- to intracellular space is thought to occur through aquaglyceroporins and hexose transporters, proteins involved in the uptake of antimony-(V) have not been identified [reviewed by Maciaszczyk-Dziubinska et al. (26)]. What is known is that antimony-(V) is taken up by cells at a lower rate than antimony-(III) and is metabolically reduced within the cell to antimony-(III) through the action of glutathione; this process occurs before cytotoxic effects are apparent (27, 28). Interestingly, experiments in vitro (29) show that cancer cells that are resistant to antimony-(III) action, are sensitive to stibogluconate [antimony-(V) treatment]. Together, these reports indicating that cells resistant to antimony-(III) are not resistant to stibogluconate [antimony-(V)] lead us to the hypothesis that gluconate could be an, if not the, active anti-cancer substance in this scenario.
PD-1 (programmed death 1) receptors on T lymphocytes provide an immune checkpoint that functions to prevent over-activation of the immune system to antigenic challenges. Importantly, malignant tumors often evolve to express programmed death-ligand 1 (PD-L1), which is the ligand for PD-1 that suppresses T cell activation (30) and thereby prevents the immune system from destroying cancer cells. Targeting PD-1 has indeed proved to be an effective target in cancer therapy to boost antitumor immunity (30–33). Clinical trials using anti-PD-1 antibody have produced positive results in a subgroup of patients with certain types of cancer (34–36). Unfortunately, this treatment is often accompanied by severe side-effects, including autoimmune reactions (37, 38). In an attempt to decrease these adverse effects, a self-degradable microneedle patch was developed to deliver the anti-PD1 antibody directly to melanoma lesions. To facilitate gradual release of the anti-PD-1-antibody and to control release into the cancer site, a system was invented to encapsulate antibody in pH-sensitive dextran nanoparticles. To decrease pH locally, glucose oxidase converting blood glucose to gluconic acid was encapsulated together with the anti-PD-1 antibody in dextran sensitive nanoparticles. Locally increased levels of gluconic acid in blood (decreasing pH) were expected to induce destruction of dextran nanoparticles allowing for the release of the antibody. Interestingly, addition of this enzyme significantly increased survival of mice with B16F10 melanoma cells and decreased tumor growth as compared to patches with antibody, but no glucose oxidase (39). We raise the possibility that the additional anticancer effect was at least in part due to the locally increased gluconate level, thus blocking extracellular citrate uptake by cancer cells.
Encouraging results with Zn2+ gluconate used as an adjuvant therapy to standard chemotherapy have been obtained when treating children with acute lymphocytic leukemia, with Zn2+ being applied to stimulate the immune system. Administration of Zn2+ gluconate significantly improved the effects of chemotherapy. While 30 days of chemotherapy application usually leads to bone marrow blast reduction to 3–5% when applied together with Zn2+ gluconate, it resulted in undetectable numbers of blasts. In this case Zn2+ gluconate was administered 2x per day from the start of chemotherapy until the end of 3 years of maintenance therapy (16). This treatment resulted in a complete bone marrow remission and normal hematopoietic function in all 13 children. Interestingly, application of 6-mercaptopurin accompanied by Zn2+ sulfate used to treat acute lymphoblastic leukemia did not produce this effect, but notably the application was over a significantly shorter time period (40). Nonetheless, this result is in keeping with our hypothesis regarding the potential anti-cancer effect of gluconate.
Very promising results were also obtained from the phase 1 trial of an IRX-2 (mmunostimulatory drug comprising a variety of cytokines derived from lymphocytes) regimen in patients with squamous cell carcinoma of the head and neck (41). Out of 8 patients only 2 showed progressive tumors after 21 days of treatment. Interestingly, IRX-2 treatment in this trial was accompanied by a daily intake of Zn2+ gluconate. Zn2+ was added because of its known effects on the immune system, reflected by Zn2+ deficiencies associated with decreased numbers and activity of different types of immune cells (42). Intriguingly, zinc deficiency has been correlated with several types of cancer (43). The fact that gluconate was used as part of the Zn2+ formulation in the IRX-2 study presents the possibility that pmCiC blockade could also have contributed to the anti-cancer effects that were observed.
It is also important to discuss one particular case report describing successful long-term treatment of a liver metastasis from ocular melanoma (15). In this case, intake of disulfiram was accompanied by oral administration of Zn2+ gluconate 3x per day and the treatment regimen of reduced-dose disulfiram with full dosing of Zn2+ gluconate was continued for several years (similar to the case of acute childhood leukemia described above). In our view, there were 3 substances in this case used to treat the ocular melanoma that could account for the success of the treatment: disulfiram, Zn2+ and gluconate, or a combination of these. The main substance considered to have anti-cancer activity in this study was disulfiram, while Zn2+ was intended to enhance its anti-cancer activity. However, none of the published results from clinical studies using disulfiram without heavy metal combined with gluconate as the adjuvant therapy confirmed its anti-cancer activity. In a recent study involving patients with non-small cell lung carcinoma receiving cisplatin together with disulfiram as adjuvant therapy (not accompanied by heavy metal administration), no difference in the tumor response rate was observed with chemotherapy plus or minus disulfiram (44). Similarly, a study on cisplatin-sensitive malignancies reported no significant difference in the time to progression or median survival time between patients treated with cisplatin and disulfiram, or cisplatin only (45). No improvement of non-metastatic recurrent prostate cancer was observed when different doses of disulfiram alone were applied (46). Therefore, we raise the possibility that in the case of tumor response with ocular melanoma liver metastasis, gluconate should be considered as a potential mediator of anti-cancer activity.
Gluconate has also been applied through injections to carry Ca2+ to prevent neuropathy in patients receiving oxaloplatin treatment. Oxaliplatin increases extracellular levels of oxalate which chelates Ca2+. Decreased Ca2+ levels have been postulated to induce prolonged activation of K+-independent voltage-gated Na+ channels resulting in stress and mitochondrial damage of nerve cells leading to neuropathy (47). To increase extracellular Ca2+ levels and to prevent unwanted side effects during oxaliplatin treatments for colorectal cancer, 1 g of Ca2+ gluconate was injected before and after oxaliplatin infusion. Although there is a general consensus that Ca2+ treatment does not affect the efficiency of oxaliplatin treatment, there are conflicting results regarding its use in decreasing neuropathy from no improvement (48), decrease in acute sensory neuropathy (47), to a significant reduction in neuropathy (49). While the first two studies were performed on a small number of patients (~20), the third study included a larger cohort of 161 patients. Importantly, not only a significantly better tolerance to oxaliplatin treatment was observed in the patient group injected with Ca2+ gluconate, but the anti-tumor response rate was increased from 35% (control) to 45% in the Ca2+ gluconate pretreated group (49). Since the studies were performed to test the effect of Ca2+ infusions on neuropathy, the conclusions regarding any potential anti-tumor effects of Ca2+ or gluconate can only be considered speculative at this point.
Increased uptake of radioactive glucose has been used in positron emission tomography (PET) analysis as an indicator of cancerous lesions. A drawback of this technique is that glucose uptake is not only associated with cancer, it is also typical of inflamed tissues. To overcome this problem several different labeled substances have been studied as potential diagnostic markers. Additionally, a search has been underway to find markers that distinguish between metastatic and non-metastatic lesions (50–53). These studies became particularly interesting with the development of radiolabeling techniques that led to the synthesis of new labels. One of them, presently used in many medical diagnostic tests due to its relatively high radioactivity and a short half-life, is technetium 99 (99mTc). While initial studies indicated 99mTc-glucoheptonate as a promising substrate that could help distinguish between benign and metastatic lesions in the lung, more detailed studies showed that the specificity was low. Therefore, other glucose derivatives such as gluconate have been considered (54). The use of 99mTc-labeled gluconate was efficient in the diagnosis of tumors of different origin, including the detection of intracranial metastases (55). Another trial revealed that labeled gluconate can be successfully used to distinguish between benign and malignant lesions in the lung, showing significantly better sensitivity and accuracy than radiography (54). Similarly, promising results were obtained when 99mTc-diethylenetriaminepentaacetic acid radionuclide-based angiography was combined with 99mTc-gluconate renal venography. This technique was used for the specific detection of malignant lesions in kidney and bladder cancer (56), and showed that the applied radiolabeled substances led to proper localization of the lesions and accurate assessment of malignancy. Additionally, it was observed that the use of 99mTc gluconate significantly reduced background noise as compared to other substances, and this was considered to be the major advantage (55). Our recent publication (14) finally sheds light on why gluconate binding to tumor is specific. We show that gluconate binds irreversibly to the pmCiC expressed on cancer cells (14), and the expression of pmCiC is high in metastatic lesions and more advanced disease. Therefore, labeled gluconate has the potential to distinguish between different tumor grades, although this will require additional detailed studies.
Importantly, it has been observed that the concentration of 99mTc-gluconate reaches its highest level in the kidney 1–2 h after injection (57). Moreover, there was no signal observed from adjacent organs/tissues, indicating a lack of 99mTc-gluconate uptake by healthy cells. The lack of 99mTc-gluconate uptake by healthy organs and fast clearance through the kidney was later confirmed (58). These features suggest that the potential diagnostic use of gluconate would likely have few if any side effects.
To summarize, early studies indicate that labeled gluconate is useful in cancer diagnostics because of its ability to distinguish between benign and metastatic lesions. Interestingly, until our recent research findings (14), there was no explanation as to why malignant cancers have an affinity for gluconate.
Based on our own research and the available literature data we suggest that gluconate should be studied in the context of anti-cancer treatment. Electrophysiological experiments revealed that gluconate blocking of citrate transport in cancer cells is irreversible and the effect increases with every subsequent application (14). Moreover, half-life of plasma membrane proteins is relatively long and in the case of pmCiC it has been established to be around 48h (14). Based on these data one could speculate that smaller doses of gluconate taken frequently should be more effective than large bolus doses of gluconate given e.g., IV. However, since nothing is known about oral gluconate pharmacokinetics, dosing studies should be performed before considering cancer treatment options. The possible effectiveness of oral gluconate application would be consistent with the successful treatment observed with the previously discussed ocular melanoma (15) and acute lymphocytic leukemia (16) cases where gluconate was given orally in small doses for several years.
Blocking of the pmCiC could also have indirect positive effects in cancer treatment. Indirect activity is possible since blocking citrate uptake in cancer cells in vivo has been shown to increase ROS synthesis (14). Indeed, increasing ROS synthesis to promote cancer cell apoptosis is already part of the action of several chemotherapeutics e.g., doxorubicin, daunorubicin or epirubicin (59, 60). Therefore, pretreatment of cancer patients receiving chemotherapy with gluconate should be explored for beneficial effects by decreasing cancer cell resistance and increasing efficiency of chemotherapy. For this purpose, single large doses of gluconate given 24 and 48 h before administering chemotherapy (49) should be considered.
With regard to potential side effects, there are currently no reports of gluconate specifically exerting any adverse events in patients. Side effects implicated when taking e.g., Zn2+ gluconate have been associated with increased Zn2+ absorption, rather than gluconate action. Nonetheless, a safety assessment will need to be made if gluconate does enter testing as an anti-cancer agent.
In this review we have presented several independent clinical and basic research studies in which gluconate was used incidentally together with other substances to treat cancer. Indeed, gluconate is considered to be physiologically neutral and therefore was not administered in these cases as the drug substance. Since putative anticancer agents like those described in this review tend to show positive effects mainly when combined with gluconate, an argument can be made for gluconate actually playing a role in reported anticancer activities. These examples, together with our latest research results showing that gluconate blocks citrate transport into cancer cells and inhibits tumor growth in mice, raises the intriguing hypothesis that gluconate use may offer an interesting new diagnostic and treatment option in cancer research.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was supported by the German Federal Ministry of Education and Research (BMBF) to the German Center Diabetes Research (DZD e.V.) grant to JA, DFG grant GE1188/5-1 to EG and JA and a fellowship from Bavarian Government to Women in Research and Education to MEM.
MEM, PR, and EG are co-inventors on a patent application (EP15767532.3 and US15/514,255) related to plasma membrane citrate transporter in the diagnosis and treatment of cancer, filed by the Universitätsklinikum Regensburg.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Kast RE, Boockvar JA, Brüning A, Cappello F, Chang WW, Cvek B, et al. A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International initiative for accelerated improvement of glioblastoma care. Oncotarget. (2013) 4:502–30. doi: 10.18632/oncotarget.969
2. D'Alessandro A, Reisz JA, Culp-Hill R, Korsten H, van Bruggen R, de Korte D. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion. (2018) 58:1992–2002. doi: 10.1111/trf.14620
3. Deng Z, Peng S, Zheng Y, Yang X, Zhang H, Tan Q, et al. Estradiol activates chloride channels via estrogen receptor-α in the cell membranes of osteoblasts. Am J Physiol Cell Physiol. (2017) 313:C162–72. doi: 10.1152/ajpcell.00014.2017
4. Echols K, Graves M, LeBlanc KG, Marzolf S, Yount A. Role of antiseptics in the prevention of surgical site infections. Dermatol Surg. (2015) 41:667–76. doi: 10.1097/DSS.0000000000000375
5. Zandi H, Rodrigues RC, Kristoffersen AK, Enersen M, Mdala I, Ørstavik D, et al. Antibacterial effectiveness of 2 root canal irrigants in root-filled teeth with infection: a randomized clinical trial. J Endod. (2016) 42:1307–13. doi: 10.1016/j.joen.2016.06.006
6. Alves F, Bilbe G, Blesson S, Goyal V, Goyal V, Monnerat S, et al. Recent development of visceral leishmaniasis treatments: successes, pitfalls, and perspectives. Clin Microbiol Rev. (2018) 31:e00048–18. doi: 10.1128/CMR.00048-18
7. Farrokhi Yekta R, Rezaei Tavirani M, Arefi Oskouie A, Mohajeri-Tehrani MR, Soroush AR, Akbarzadeh Baghban A. Serum-based metabolic alterations in patients with papillary thyroid carcinoma unveiled by non-targeted 1H-NMR metabolomics approach. Iran J. Basic Med. Sci. (2018) 21:1140–7. doi: 10.22038/IJBMS.2018.30375.7323
8. Tokunaga M, Kami K, Ozawa S, Oguma J, Kazuno A, Miyachi H, et al. Metabolome analysis of esophageal cancer tissues using capillary electrophoresis-time-of-flight mass spectrometry. Int J Oncol. (2018) 52:1947–58. doi: 10.3892/ijo.2018.4340
9. Karlíková R, Široká J, Friedecký D, Faber E, Hrdá M, Mičová K, et al. Metabolite profiling of the plasma and leukocytes of chronic myeloid leukemia patients. J Proteome Res. (2016) 15:3158–66. doi: 10.1021/acs.jproteome.6b00356
10. Kumar D, Gupta A, Mandhani A, Sankhwar SN. NMR spectroscopy of filtered serum of prostate cancer: a new frontier in metabolomics. Prostate. (2016) 76:1106–19. doi: 10.1002/pros.23198
11. Mycielska ME, Milenkovic VM, Wetzel CH, Rümmele P, Geissler EK. Extracellular citrate in health and disease. Curr Mol Med. (2015) 15:884–91. doi: 10.2174/1566524016666151123104855
12. Sanders FW, Griffin JL. De novo lipogenesis in the liver in health and disease: more than just a shunting yard for glucose. Biol Rev Camb Philos Soc. (2016) 91:452–68. doi: 10.1111/brv.12178
13. Gopal E, Miyauchi S, Martin PM, Ananth S, Srinivas SR, Smith SB, et al. Expression and functional features of NaCT, a sodium-coupled citrate transporter, in human and rat livers and cell lines. Am J Physiol Gastrointest Liver Physiol. (2007) 292:G402–8. doi: 10.1152/ajpgi.00371.2006
14. Mycielska ME, Dettmer K, Rümmele P, Schmidt K, Prehn C, Milenkovic VM, et al. Extracellular citrate affects critical elements of cancer cell metabolism and supports cancer development in vivo. Cancer Res. (2018) 78:2513–23. doi: 10.1158/0008-5472.CAN-17-2959
15. Brar SS, Grigg C, Wilson KS, Holder WD Jr, Dreau D, Austin C, et al. Disulfiram inhibits activating transcription factor/cyclic AMP-responsive element binding protein and human melanoma growth in a metal-dependent manner in vitro, in mice and in a patient with metastatic disease. Mol Cancer Ther. (2004) 3:1049–60.
16. Eby GA. Treatment of acute lymphocytic leukemia using zinc adjuvant with chemotherapy and radiation–a case history and hypothesis. Med Hypotheses. (2005) 64:1124–26. doi: 10.1016/j.mehy.2004.12.019
17. Dar MJ, Din FU, Khan GM. Sodium stibogluconate loaded nano-deformable liposomes for topical treatment of leishmaniasis: macrophage as a target cell. Drug Deliv. (2018) 25:1595–606. doi: 10.1080/10717544.2018.1494222
18. Kimutai R, Musa AM, Njoroge S, Omollo R, Alves F, Hailu A, et al. Safety and effectiveness of sodium stibogluconate and paromomycin combination for the treatment of visceral leishmaniasis in Eastern Africa: results from a pharmacovigilance programme. Clin Drug Investig. (2017) 37:259–72. doi: 10.1007/s40261-016-0481-0
19. Hilerowicz Y, Koren A, Mashiah J, Katz O, Sprecher E, Artzi O. Fractional ablative carbon dioxide laser followed by topical sodium stibogluconate application: a treatment option for pediatric cutaneous leishmaniasis. Pediatr Dermatol. (2018) 35:366–69. doi: 10.1111/pde.13457
20. Pathak MK, Hu X, Yi T. Effects of sodium stibogluconate on differentiation and proliferation of human myeloid leukemia cell lines in vitro. Leukemia. (2002) 16:2285–91. doi: 10.1038/sj.leu.2402692
21. Fan K, Zhou M, Pathak MK, Lindner DJ, Altuntas CZ, Tuohy VK, et al. Sodium stibogluconate interacts with IL-2 in anti-Renca tumor action via a T cell-dependent mechanism in connection with induction of tumor-infiltrating macrophages. J Immunol. (2005) 175:7003–8. doi: 10.4049/jimmunol.175.10.7003
22. Fan K, Borden E, Yi T. Interferon-gamma is induced in human peripheral blood immune cells in vitro by sodium stibogluconate/interleukin-2 and mediates its antitumor activity in vivo. J Interferon Cytokine Res. (2009) 29:451–60. doi: 10.1089/jir.2008.0061
23. Yi T, Pathak MK, Lindner DJ, Ketterer ME, Farver C, Borden EC. Anticancer activity of sodium stibogluconate in synergy with IFNs. J Immunol. (2002) 169:5978–85. doi: 10.4049/jimmunol.169.10.5978
24. Tiekink ER. Antimony and bismuth compounds in oncology. Crit Rev Oncol Hematol. (2002) 42:217–24. doi: 10.1016/S1040-8428(01)00217-7
25. Keppler BK, Silvestru C, Haiduc I. Antitumour organometallics. III. In vivo activity of diphenylantimony(III) and diorganotin(IV) dithiophosphorus derivatives against P388 leukemia. Met Based Drugs. (1994) 1:73–7. doi: 10.1155/MBD.1994.73
26. Maciaszczyk-Dziubinska E, Wawrzycka D, Wysocki R. Arsenic and antimony transporters in eukaryotes. Int J Mol Sci. (2012) 13:3527–48. doi: 10.3390/ijms13033527
27. López S, Aguilar L, Mercado L, Bravo M, Quiroz W. Sb(V) reactivity with human blood components: redox effects. PLoS ONE. (2015) 10:e0114796. doi: 10.1371/journal.pone.0114796
28. Verdugo M, Ogra Y, Quiroz W. Mechanisms underlying the toxic effects of antimony species in human embryonic kidney cells (HEK-293) and their comparison with arsenic species. J Toxicol Sci. (2016) 41:783–92. doi: 10.2131/jts.41.783
29. Naredi P, Heath DD, Enns RE, Howell SB. Cross-resistance between cisplatin, antimony potassium tartrate, and arsenite in human tumor cells. J Clin Invest. (1995) 95:1193–98. doi: 10.1172/JCI117768
30. Killock D. Lung cancer: anti-PD-1 therapy in the frontline. Nat Rev Clin Oncol. (2016) 13:715. doi: 10.1038/nrclinonc.2016.170
31. DiDomenico J, Lamano JB, Oyon D, Li Y, Veliceasa D, Kaur G, et al. The immune checkpoint protein PD-L1 induces and maintains regulatory T cells in glioblastoma. Oncoimmunology. (2018) 7:e1448329. doi: 10.1080/2162402X.2018.1448329
32. Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Anti-PD-L1/PD-1 immune therapies in ovarian cancer: basic mechanism and future clinical application. Int J Clin Oncol. (2016) 21:456–61. doi: 10.1007/s10147-016-0968-y
33. Tang J, Yu JX, Hubbard-Lucey VM, Neftelinov ST, Hodge JP, Lin Y. Trial watch: the clinical trial landscape for PD1/PDL1 immune checkpoint inhibitors. Nat Rev Drug Discov. (2018) 17:854–55. doi: 10.1038/nrd.2018.210
34. Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, et al. TIGIT and PD-1 impair tumor antigen-specific CD8? T cells in melanoma patients. J Clin Invest. (2015) 125:2046–58. doi: 10.1172/JCI80445
35. De Wolf K, Kruse V, Sundahl N, van Gele M, Chevolet I, Speeckaert R, et al. A phase II trial of stereotactic body radiotherapy with concurrent anti-PD1 treatment in metastatic melanoma: evaluation of clinical and immunologic response. J Transl Med. (2017) 15:21. doi: 10.1186/s12967-017-1123-x
36. Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. (2014) 15:69–77. doi: 10.1016/S1470-2045(13)70551-5
37. Chan MM, Kefford RF, Carlino M, Clements A, Manolios N. Arthritis and tenosynovitis associated with the anti-PD1 antibody pembrolizumab in metastatic melanoma. J Immunother. (2015) 38:37–9. doi: 10.1097/CJI.0000000000000060
38. Araújo M, Ligeiro D, Costa L, Marques F, Trindade H, Correia JM, et al. A case of fulminant Type 1 diabetes following anti-PD1 immunotherapy in a genetically susceptible patient. Immunotherapy. (2017) 9:531–5. doi: 10.2217/imt-2017-0020
39. Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. (2016) 16:2334–40. doi: 10.1021/acs.nanolett.5b05030
40. Wazewska-Czyzewska M, Wesierska-Gadek J, Legutko L. Immunostimulatory effect of zinc in patients with acute lymphoblastic leukemia. Folia Haematol Int Mag Klin Morphol Blutforsch. (1978) 105:727–32.
41. Freeman SM, Franco JL, Kenady DE, Baltzer L, Roth Z, Brandwein HJ, et al. A phase 1 safety study of an IRX-2 regimen in patients with squamous cell carcinoma of the head and neck. Am J Clin Oncol. (2011) 34:173–8. doi: 10.1097/COC.0b013e3181dbb9d8
42. Gao H, Dai W, Zhao L, Min J, Wang F. The role of zinc and zinc homeostasis in macrophage function. J Immunol Res. (2018) 2018:6872621. doi: 10.1155/2018/6872621
43. Brutcher EA, Chen Z, Pan A, Barrett T. The relationship between zinc and quality of life in patients with upper GI cancer on chemotherapy. J Adv Pract Oncol. (2017) 8:338–45. doi: 10.6004/jadpro.2017.8.4.3
44. Nechushtan H, Hamamreh Y, Nidal S, Gotfried M, Baron A, Shalev YI, et al. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist. (2015) 20:366–7. doi: 10.1634/theoncologist.2014-0424
45. Verma S, Stewart DJ, Maroun JA, Nair RC. A randomized phase II study of cisplatin alone versus cisplatin plus disulfiram. Am J Clin Oncol. (1990) 13:119–24. doi: 10.1097/00000421-199004000-00007
46. Schweizer MT, Lin J, Blackford A, Bardia A, King S, Armstrong AJ, et al. Pharmacodynamic study of disulfiram in men with non-metastatic recurrent prostate cancer. Prostate Cancer Prostatic Dis. (2013) 16:357–61. doi: 10.1038/pcan.2013.28
47. Chay WY, Tan SH, Lo YL, Ong SY, Ng HC, Gao F, et al. Use of calcium and magnesium infusions in prevention of oxaliplatin induced sensory neuropathy. Asia Pac J Clin Oncol. (2010) 6:270–7. doi: 10.1111/j.1743-7563.2010.01344.x
48. Han CH, Khwaounjoo P, Kilfoyle DH, Hill A, McKeage MJ. Phase I drug-interaction study of effects of calcium and magnesium infusions on oxaliplatin pharmacokinetics and acute neurotoxicity in colorectal cancer patients. BMC Cancer. (2013) 13:495. doi: 10.1186/1471-2407-13-495
49. Gamelin L, Boisdron-Celle M, Delva R, Guérin-Meyer V, Ifrah N, Morel A, et al. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res. (2004) 10:4055–61. doi: 10.1158/1078-0432.CCR-03-0666
50. Schechter NR, Yang DJ, Azhdarinia A, Chanda M. Technologies for translational imaging using generators in oncology. Recent Pat Anticancer Drug Discov. (2007) 2:251–8. doi: 10.2174/157489207782497253
51. Talbot JN, Paycha F, Balogova S. Diagnosis of bone metastasis: recent comparative studies of imaging modalities. Q J Nucl Med Mol Imaging. (2011) 55:374–410.
52. Huang SM, Yin L, Yue JL, Li YF, Yang Y, Lin ZC. Direct comparison of choline PET/CT and MRI in the diagnosis of lymph node metastases in patients with prostate cancer. Medicine. (2018) 97:e13344. doi: 10.1097/MD.0000000000013344
53. Zhang X, Liu R, Yuan Q, Gao F, Li J, Zhang Y, et al. The precise diagnosis of cancer invasion/metastasis via 2D laser ablation mass mapping of metalloproteinase in primary cancer tissue. ACS Nano. (2018) 12:11139–51. doi: 10.1021/acsnano.8b05584
54. Xie C, Li Y, Zhang C, Zeng S. Clinical value of lung scintigraphy with Tc-99m gluconate in distinguishing benign from malignant lung diseases. Clin Nucl Med. (1992) 17:887–3. doi: 10.1097/00003072-199211000-00012
55. Mamo L, Pannecière C, Perez R, Villa M. Value of calcium gluconate labelled with 99mTc in the detection of intracranial tumors. Nouv Presse Med. (1975) 4:795–7.
56. Ma J, Shou J, Xiao Z. Clinical value of renal radionuclide imaging for diagnosis of urinary tract tumor. Zhonghua Zhong Liu Za Zhi. (1995) 17:199–201.
57. Mori H, Futatsuya R, Ando I, Shimazu H, Koizumi K, Ando A, et al. Studies of intrarenal distribution by macroautoradiogram and tissue distribution of 99mTc-gluconate. Radioisotopes. (1979) 28:84–8. doi: 10.3769/radioisotopes.28.2_84
58. Ercan MT, Unlenen E. Accumulation of some small molecular weight complexes of 99mTc in experimental abscesses. Nucl Med Biol. (1994) 21:143–9. doi: 10.1016/0969-8051(94)90002-7
59. Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, et al. The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res. (2018) 37:266. doi: 10.1186/s13046-018-0909-x
Keywords: gluconate, cancer metabolism, citrate, metabolic targeting, transporter
Citation: Mycielska ME, Mohr MTJ, Schmidt K, Drexler K, Rümmele P, Haferkamp S, Schlitt HJ, Gaumann A, Adamski J and Geissler EK (2019) Potential Use of Gluconate in Cancer Therapy. Front. Oncol. 9:522. doi: 10.3389/fonc.2019.00522
Received: 05 February 2019; Accepted: 30 May 2019;
Published: 19 June 2019.
Edited by:
Gabi U. Dachs, University of Otago, Christchurch, New ZealandReviewed by:
Mikhail Durymanov, Moscow Institute of Physics and Technology, RussiaCopyright © 2019 Mycielska, Mohr, Schmidt, Drexler, Rümmele, Haferkamp, Schlitt, Gaumann, Adamski and Geissler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria E. Mycielska, bWFyaWEubXljaWVsc2thQHVrci5kZQ==; Edward K. Geissler, ZWR3YXJkLmdlaXNzbGVyQHVrci5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.