
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 21 June 2019
Sec. Genitourinary Oncology
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00479
This article is part of the Research Topic Tyrosine Kinase Therapy for Metastatic Renal Cell Carcinoma View all 7 articles
Purpose: Sorafenib and sunitinib are extensively used as first-line medications for metastatic renal cell carcinoma (mRCC). This meta-analysis was conducted to assess the antitumor efficacy, toxicity, and costs of the two drugs among mRCC patients.
Materials and methods: PubMed, ScienceDirect, Scopus, Web of Science, Ovid MEDLINE, the Cochrane Library, Embase, and Google Scholar were searched for eligible articles. The endpoints consisted of progression-free survival (PFS), overall survival (OS), objective response rate (ORR), adverse effects (AEs), and per-patient-per-month (PPPM) costs.
Results: We included 14 studies with 2,925 patients. Both drugs were valid for treating mRCC with equivalent PFS [hazard ratio (HR) = 0.98, 95% confidence interval (CI): 0.88–1.10, P = 0.74] and disease control rates [DCRs; risk ratio (RR) = 1.03, 95% CI: 0.98–1.08, P = 0.28], but sunitinib had a better OS (HR = 1.10, 95% CI: 1.01–1.20, P = 0.04) and higher ORR (HR = 0.66, 95% CI: 0.45–0.97, P = 0.03) than sorafenib. Furthermore, sunitinib induced more incidences of severe hematologic AEs (anemia, neutropenia, and thrombocytopenia) and stomatitis/mucositis than sorafenib. In the subanalysis, Asian patients treated with sorafenib reported a longer PFS than those treated with sunitinib (HR = 0.87, 95% CI: 0.83–0.90, P = 0.01), and European patients treated with sunitinib had a longer OS than those treated with sorafenib (HR = 1.17, 95% CI: 1.01–1.30, P = 0.04). Moreover, the pooled results of the high-quality studies reported a higher ORR with sunitinib than with sorafenib, and medium-quality studies showed a longer OS with sunitinib than with sorafenib.
Conclusions: Sunitinib has more benefits (longer OS and better ORR) than sorafenib as a first-line therapy for mRCC. However, sunitinib has higher toxicity than sorafenib. Sorafenib might be more suitable than sunitinib among Asian patients, and sunitinib might be superior to sorafenib in European patients. Nevertheless, more large-scale, high-quality studies are required.
Renal cell carcinoma (RCC) is a common tumor in the urological system, with an expected 73,820 cases and 14,770 deaths in 2019 (1). Moreover, more than 30% of the patients had metastases when initially diagnosed, and 20–40% developed systemic spread after undergoing surgery (2). The National Comprehensive Cancer Network (NCCN) guidelines recommend sunitinib and sorafenib as first-line drugs for metastatic clear cell RCC (mRCC) (3).
Sorafenib and sunitinib are both small-molecule tyrosine kinase inhibitors (TKIs). Both of these drugs have many targets and can inhibit vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptor tyrosine kinases. Furthermore, as a kind of RAF kinase inhibitor, sorafenib can interrupt the RAS/RAF/MEK intracellular signaling pathway (4, 5). A phase II clinical trial has indicated that sorafenib has a better progression-free survival (PFS) than anticipated as therapy for mRCC (6). Sunitinib is also used as a first-line TKI as targeted treatment for mRCC and has shown excellent antitumor efficacy and safety for treating mRCC in a randomized controlled trial (RCT) (NCT00130897) (7). Although both TKIs have shown great benefits for treating mRCC, the best patient profile for the use of the two targeted drugs is still unclear. In a retrospective study, Cai et al. suggested that sorafenib had an equivalent treatment efficacy to but a lower toxicity than sunitinib as first-line therapy for mRCC (8). However, Di Fiore et al. reported that sunitinib had worse survival and more severe clinical adverse events (AEs) for advanced RCC (aRCC) than sorafenib (9).
To address this discrepancy, a meta-analysis of the relevant articles was performed to compare the treatment efficacy, toxicity, and costs of sorafenib and sunitinib and provide evidence-based suggestions for the optimal first-line treatment in patients with mRCC.
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (Table S1).
PubMed, ScienceDirect, Embase, Web of Science, Ovid MEDLINE, the Cochrane Library, Scopus, and Google Scholar were searched up to November 2018 to select relevant studies that compared sorafenib and sunitinib as first-line therapy for mRCC. The following terms were used: “sorafenib,” “sunitinib,” and “renal cell carcinoma.” We used the complete search function in PubMed for the following terms: (sorafenib [MeSH Terms] OR sorafenib [Text Word] OR BAY 545-9085 [Text Word] OR BAY 43-9006 [Text Word] OR Nexavar [Text Word]) AND (sunitinib [MeSH Terms] OR sunitinib [Text Word] OR Sutent [Text Word] OR SU011248 [Text Word]) AND (renal cell carcinoma [MeSH Terms] OR renal cell carcinoma [Text Word]). We also searched the references of all included studies for further eligible articles. All included articles were written in English.
We included studies that satisfied the following criteria according to PICOS (population, interventions, comparators, outcomes, and study designs): (1) population: enrolled patients who were diagnosed with mRCC (defined as having distant metastasis); (2) interventions and comparators: compared sorafenib and sunitinib as first-line therapy; (3) outcomes: PFS, overall survival (OS), objective response rate (ORR), disease control rate (DCR), AEs, and per-patient-per-month (PPPM) costs; (4) study designs: RCTs or retrospective observational studies; and (5) were written in English. We excluded reviews without raw data and conference abstracts, case reports, meta-analyses, and articles with repeated data.
Two investigators (HD and WZ) extracted the following information independently: first author, the time of publication, country, number of patients in the sorafenib and sunitinib groups, study design, patient characteristics (age, sex, pathological type, pretreatment, and initial dosage), antitumor effectiveness indicators (PFS, OS, ORR, and DCR), number of AEs (all-grade AEs and grade 3–4 AEs), and PPPM costs. The data about the healthcare costs of sorafenib and sunitinib were converted into PPPM costs (US dollar) through mathematical operations. A third researcher (ZH) settled disagreements under various circumstances. We used hazard ratios (HRs), which consider the number and time of events, instead of odds ratios to analyze the PFS and OS. HRs with 95% confidence intervals (CIs) were obtained directly if a Cox multivariate survival analysis was performed. Otherwise, HRs and 95% CIs were extracted from the Kaplan–Meier curves in accordance with the protocol from Tierney et al. (10).
We evaluated the quality of the retrospective observational studies through the 9-point Newcastle–Ottawa Scale, which included a questionnaire on the following three major aspects: selection, comparability, and exposure. A total score of 8–9 points suggested that a study was high quality, and a study with 6–7 points was medium quality (11).
We performed this meta-analysis using Review Manager (version 5.2) and STATA (version 12.0). HRs and 95% CIs were used to analyze the PFS and OS (HR < 1 supports the sorafenib group; HR > 1 supports the sunitinib group). Risk ratios (RRs) and 95% CIs were used to analyze the ORR, DCR (RR < 1 supports the sunitinib group; RR > 1 supports the sorafenib group), and AEs (RR < 1 supports the sorafenib group; RR > 1 supports the sunitinib group). Weighted mean differences (WMDs) with 95% CIs were used to analyze the PPPM. Subgroup analyses of the PFS, OS, and ORR were performed to determine if these outcomes vary based on nationality, initial dosage, and study quality. Heterogeneity was assessed through the χ2 test and I2 statistic. If I2 > 50% or P < 0.1, then the study showed significant heterogeneity. We would use the random-effects model rather than the fixed-effects model in all results (including subanalysis) in case we draw misleading conclusions. To enhance robustness, sensitivity analyses of the PFS, OS, ORR, and DCR were performed to determine if these effects were variables. We assessed publication bias through Begg's test and Egger's test. Significant differences were considered as P < 0.05.
Figure 1 shows the study selection process. An eventual 14 studies involving 2,925 patients (sorafenib, 1,150; sunitinib, 1,775) were selected for this meta-analysis (9, 12–24). All included articles were retrospective observational studies. In fact, two studies originated from the same patient population; one study reported the PPPM, and the other reported anti-efficacy and toxicity (22, 23). Nine articles were considered high quality (two articles scored 9 points, seven articles scored 8 points). Five articles were considered medium quality (three articles scored 7 points, two articles scored 6 points; Table S2). Table 1 lists the baseline characteristics and key evaluation indicators of all included articles.
The antitumor effectiveness regarding PFS, OS, ORR, and DCR between the two groups was evaluated.
Eight articles compared the PFS (heterogeneity: P < 0.00001, I2 = 90%). Significant differences were not found between sorafenib and sunitinib (HR = 0.98, 95% CI: 0.88–1.10, P = 0.74; Figure 2A).
Eight articles compared the OS (heterogeneity: P = 0.04, I2 = 54%). Sunitinib-treated patients had a better OS than sorafenib-treated patients (HR = 1.10, 95% CI: 1.01–1.20, P = 0.04; Figure 2B).
Seven articles compared the ORR (heterogeneity: P = 0.002, I2 = 71%). The sunitinib group had a higher ORR than the sorafenib group (RR = 0.66, 95% CI: 0.45–0.97, P = 0.03; Figure 3A).
Eight articles compared the DCR (heterogeneity: P = 0.23, I2 = 25%). Significant differences were not found between the groups (RR = 1.03, 95% CI: 0.98–1.08, P = 0.28; Figure 3B).
We compared the toxicity between sorafenib and sunitinib based on the total number of AEs and performed subgroup analyses of the 10 most common toxic events.
Two articles compared the total number of AEs (heterogeneity: P = 0.31, I2 = 2%). No significant differences were found between the two TKIs (RR = 0.98, 95% CI: 0.95–1.01, P = 0.16; Figure 4).
Some patients experienced reductions, interruptions, or discontinuations with their drug treatments. Three studies compared drug reductions; no significant difference was found between the two TKIs (RR = 0.97, 95% CI: 0.66–1.42, P = 0.87; Figure 5A). Three studies compared the drug reductions due to serious AEs; no significant difference was found between the two groups (RR = 0.79, 95% CI: 0.57–1.09, P = 0.15; Figure 5B). Two studies compared drug interruptions; no significant difference was found between the two groups (RR = 1.49, 95% CI: 0.66–3.37, P = 0.34; Figure 5C). Three studies compared drug interruptions due to serious AEs; no significant difference was found between the two groups (RR = 1.46, 95% CI: 0.90–2.37, P = 0.12; Figure 5D). Two studies compared drug discontinuations due to serious AEs; no significant difference was found between the two groups (RR = 1.06, 95% CI: 0.54–2.10, P = 0.86; Figure 5E).
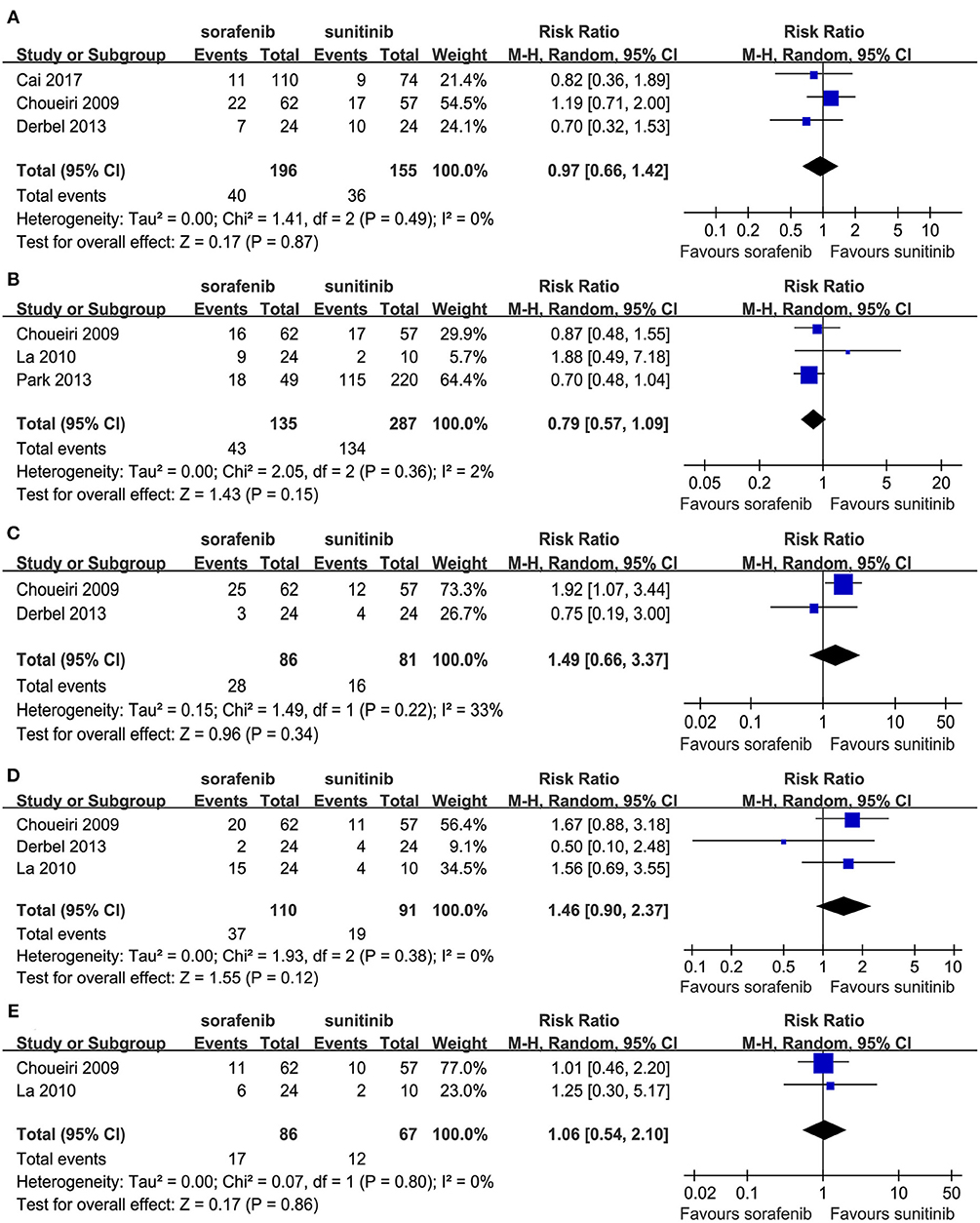
Figure 5. Forest plots of the RR of drug reductions (A), drug reductions due to serious AEs (B), drug interruption (C), drug interruption due to serious AEs (D), and drug discontinuations due to serious AEs (E) associated with sorafenib vs. sunitinib.
In the subgroup analyses of the 10 most common AEs (in order of incidence: hand–foot syndrome, diarrhea, nausea/vomiting, fatigue/asthenia, neutropenia, rash, thrombocytopenia, hypertension, anemia, and stomatitis/mucositis), the outcomes of these all-grade AEs indicated that there were no significant differences in the incidence of hand–foot syndrome, diarrhea nausea/vomiting, rash, hypertension, and anemia between sorafenib and sunitinib. For all-grade AEs, sunitinib had higher incidences of fatigue/asthenia (RR = 0.75, 95% CI: 0.63–0.89, P = 0.001), neutropenia (RR = 0.33, 95% CI: 0.23–0.48, P < 0.00001), thrombocytopenia (RR = 0.27, 95% CI: 0.20–0.37, P < 0.00001), and stomatitis/mucositis (RR = 0.14, 95% CI: 0.03–0.60, P = 0.008, Table 2) than sorafenib. The outcomes of grade 3–4 AEs demonstrated that there were no significant differences for hand–foot syndrome, diarrhea, nausea/vomiting, fatigue/asthenia, rash, and hypertension between the two groups. For grade 3–4 AEs, sunitinib had more neutropenia (RR = 0.20, 95% CI: 0.05–0.73, P = 0.01), anemia (RR = 0.37, 95% CI: 0.08–0.88, P = 0.03), thrombocytopenia (RR = 0.11, 95% CI: 0.04–0.26, P < 0.00001), and stomatitis/mucositis (RR = 0.62, 95% CI: 0.40–0.98, P = 0.04, Table 3) than sorafenib.
We assessed the costs between the two groups based on the PPPM. Only one included article provided mean and standard deviation values and reported that sorafenib had lower PPPM costs than sunitinib ($6,990.36 ± 3,073.11 vs. $7,944.91 ± 2,993.36) (23).
To determine if the treatment efficacy of sorafenib vs. sunitinib changed over time, the pooled results of PFS, OS, and ORR were calculated according to nationality, initial dosage, and study quality (Table 4). Interestingly, Asian patients treated with sorafenib had a longer PFS than European patients (HR = 0.87, 95% CI: 0.83–0.90, P = 0.01); European studies (all from France) indicated that sunitinib led to a longer OS than sorafenib (HR = 1.17, 95% CI: 1.01–1.30, P = 0.04). The pooled results of the high-quality studies indicated that sunitinib had a higher ORR than sorafenib (HR = 0.57, 95% CI: 0.35–0.93, P = 0.02), and the medium-quality studies showed that sunitinib led to a longer OS than sorafenib (HR = 1.32, 95% CI: 1.07–1.61, P = 0.008).
The PFS (Figure S1A) and OS (Figure S1B) were all robust, with consistent findings from the sensitivity analysis. In addition, the ORR (Figure S2A) and DCR (Figure S2B) were all robust, and the sensitivity analysis showed that no estimates were beyond the 95% CIs.
No proof of publication bias was found for the PFS (Begg's test, P = 0.386, Egger's test, P = 0.187; Figure S3A), OS (Begg's test, P = 0.803; Egger's test, P = 0.071; Figure S3B), ORR (Begg's test, P = 1.000; Egger's test, P = 0.651; Figure S4A), and DCR (Begg's test, P = 0.711; Egger's test, P = 1.000; Figure S4B).
This may be the first meta-analysis to compare the antitumor efficacy, toxicity, and costs between these two TKIs as first-line treatment for mRCC. Our analysis of 14 medium- to high-quality studies shows that sunitinib was associated with more benefits (improved OS and better ORR) than sorafenib as first-line therapy for mRCC. However, sunitinib was associated with more all-grade and grade 3–4 neutropenia, thrombocytopenia, and stomatitis/mucositis than sorafenib. However, sorafenib might have lower PPPM costs than sunitinib, although only one study reported PPPM costs. In the subgroup analyses, Asian patients using sorafenib had a longer PFS than those using sunitinib, and European patients using sunitinib had a superior OS than those using sorafenib as first-line therapy for mRCC; the pooled outcomes of the high-quality studies reported that sunitinib had a higher ORR than sorafenib, and the medium-quality studies showed that sunitinib had a longer OS than sorafenib.
Antitumor efficacy is the most predominant cornerstone to consider when comparing sorafenib and sunitinib. The pooled analysis indicated that there were no significant differences between the two groups in PFS and DCR. However, sunitinib had a better OS than sorafenib. In a retrospective analysis with 251 consecutive patients, Levy et al. reported that sunitinib-treated patients were associated with a longer median OS than sorafenib-treated patients (26.3 vs. 16.4 months) (18). This finding was also confirmed by the subgroup analysis of medium-quality studies for OS, which showed that sunitinib had a better OS than sorafenib (HR = 1.32, 95% CI: 1.07–1.61, P = 0.008). Moreover, sunitinib had a higher ORR than and a similar DCR to sorafenib. In other words, although sorafenib-treated patients had a lower ORR, they had more stable disease (SD), which is also considered as a kind of disease control. SD is defined as when the total length of the baseline tumor lesions is reduced in size but does not reach 30% of the original size or increases <20% in size, based on RECIST (Response Evaluation Criteria in Solid Tumors) version 1.1 (25). Furthermore, a retrospective study of the 5-year experiences of two large oncology centers demonstrated that among patients with mRCC, sorafenib led to more SD than sunitinib (69% vs. 45%) (22). Similarly, a large single-center retrospective study indicated that no significant difference was found in terms of preventing progressive disease (PD), although sunitinib was associated with higher objective responses than sorafenib (12). Furthermore, the subgroup analysis of the ORR in the high-quality studies showed that sunitinib had a better ORR than sorafenib (HR = 0.57, 95% CI: 0.35–0.93, P = 0.02). Notably, the subgroup analysis showed that the Asian studies reported a longer PFS (Table 4), which suggests that Asian patients using sorafenib as first-line therapy for mRCC might achieve better antitumor efficacy than those using sorafenib. Additionally, the European studies reported a better OS (Table 4), which demonstrates that sunitinib might provide superior antitumor efficacy for European patients compared to sorafenib. Undoubtedly, these positive findings of subanalysis have the effect of revealing the tendency. Our conclusions need to be accepted carefully, especially the findings of the subanalysis. More high-impact, large-sample studies are required to verify these conclusions.
Drug toxicity is an indispensable factor when choosing between sorafenib and sunitinib. Although the incidence of all-grade AEs were not significantly different between the two drugs (Figure 4), sunitinib was associated with more severe AEs, especially grade 3–4 hematologic AEs, than sorafenib (Table 3). Within the grade 3–4 AEs, higher rates of stomatitis/mucositis, anemia, neutropenia, and thrombocytopenia were observed among patients using sunitinib than those using sorafenib. A probable reason might be the use of an inappropriate dose and schedule alterations when using sunitinib. In fact, a phase II RCT showed that therapy with 50 mg sunitinib daily using a 2/1 dosing schedule (2 weeks on and 1 week off) was associated with less toxicity and superior tolerability among patients with mRCC than a standard 4/2 schedule (4 weeks on and 2 weeks off) (NCT00570882) after 30 months of follow-up (26). Similarly, a retrospective study of 99 patients showed that sunitinib on a 2/1 schedule had fewer grade 3–4 AEs among Chinese patients with mRCC than a 4/2 schedule (27). In a systematic review of the side effects of TKIs, Bhojani et al. found that sunitinib led to the most grade 3/4 AEs, and sorafenib led to the fewest grade 3–4 AEs out of sorafenib, sunitinib, and temsirolimus (28). Furthermore, although the two targeted drugs were equally effective among elderly patients with aRCC, sunitinib was less well-tolerated than sorafenib (16). Notably, this difference in toxicity might be of vital importance for patients, as treatment is administered continuously over months. Although toxicity might not reduce the survival time, it could significantly decrease patient compliance, influence the patients' quality of life, and undermine treatment efficacy. The timely prevention, recognition, and prompt management of AEs are essential to avoid unnecessary dose reductions, interruption, or discontinuation of treatment, which may impair the antitumor efficacy.
The cost effects are also a significant factor when choosing between the two TKIs. The only included study that provided mean and standard deviation values reported that patients treated with sorafenib had lower PPPM costs than sunitinib as first-line treatment for mRCC ($6,990.36 ± 3,073.11 vs. $7,944.91 ± 2,993.36) (23). In a retrospective claims database analysis, Duh et al. found that the mean medical costs of sorafenib were less than that of sunitinib in the treatment of aRCC ($6,998 and $8,213, respectively) (29). Similarly, in an analysis of 18 American community oncology clinics, Chen et al. reported that sunitinib was associated with higher PPPM costs than sorafenib ($9,417.35 ± 670.78 vs. $7,992.48 ± 682.29) (30). Although the costs of the two drugs were not greatly different, sorafenib therapy might help relieve the financial pressure on patients and their families to some extent, extend the time of using TKIs while providing equivalent treatment efficacy as sunitinib, and even relieve the psychological burden of patients facing high medical expenses, especially for patients from low-income families or developing countries.
Several limitations should be addressed when considering our results. First, the lack of RCTs weakens the quality of these outcomes. Second, the significant heterogeneity of some comparisons (PFS and ORR) may impair the reliability of these results. Third, the number of patients in the two groups was not large enough, which might have resulted in relatively unreliable estimates. Fourth, selection bias may exist because the included articles were limited to the literature published in English. Fifth, our subgroup analysis of Asia only included three Asian countries (China, Korea, and Japan), and the Europe analysis only included two European countries (France and Italy), which may decrease the representativeness of the positive subgroup results. Sixth, we could not completely control for confounding factors (pathological status, pretreatment, and metastatic sites) as they were unavailable for some studies, but these factors could influence the final results.
Our meta-analysis demonstrates that sunitinib has more benefits (longer OS and better ORR) than sorafenib as first-line therapy for mRCC. However, sunitinib has higher toxicity and higher PPPM costs than sorafenib. The subanalysis reveals that sorafenib might be more suitable among Asian patients, and European patients using sunitinib might achieve better survival than those using sorafenib. Nevertheless, the inherent limitations of this meta-analysis mean that additional high-impact studies with large samples are needed to better determine the roles of sorafenib and sunitinib under complicated clinical conditions.
No datasets were generated or analyzed for this study.
HD, ZH, WL, TH, and WZ conceived and designed the study. HD, ZH, and FY performed the literature search, data extraction, quality assessment for the included studies, and statistical analysis. HD and WZ wrote the paper. YW reviewed and edited the manuscript. All authors read and approved the manuscript.
This study was supported by National Natural Science Foundation of China (NSFC; grant number: 81560345), Natural Science Foundation of Jiangxi Province (grant number: 20161BAB215237).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank professor Jichun Liu, MD (Department of Cardio-Thoracic Surgery, The Second Affiliated Hospital of Nanchang University) for his advice and professor Xiaoshu Cheng, MD, PhD (Department of Cardiology, The Second Affiliated Hospital of Nanchang University) for his data collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00479/full#supplementary-material
Table S1. PRISMA 2009 checklist.
Table S2. Quality assessment of all included studies.
Figure S1. Sensitivity analysis of PFS (A) and OS (B) associated with sorafenib vs. sunitinib.
Figure S2. Sensitivity analysis of ORR (A) and DCR (B) associated with sorafenib vs. sunitinib.
Figure S3. Begg's and Egger's tests for comparisons of OS (A) and PFS (B) associated with sorafenib vs. sunitinib.
Figure S4. Begg's and Egger's tests for comparisons of ORR (A) and DCR (B) associated with sorafenib vs. sunitinib.
mRCC, metastatic renal cell carcinoma; aRCC, advanced renal cell carcinoma; AEs, adverse effects; DCR, disease control rate; HRs, hazard ratios; RRs, risk ratios; PPPM, per-patient-per-month costs; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PRISMA, preferred reporting items for systematic review and meta-analysis; RCT, randomized controlled trial; TKI, tyrosine kinase inhibitor; WMD, weighted mean difference; CIs, confidence intervals; NCCN, National Comprehensive Cancer Network; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; SD, stable disease; PD, progressive disease; PICOS, population, interventions, comparators, outcomes and study designs; RECIST, Response Evaluation Criteria in Solid Tumors.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Wersäll PJ, Blomgren H, Lax I, Kälkner KM, Linder C, Lundell G, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol. (2005) 77:88–95. doi: 10.1016/j.radonc.2005.03.022
3. Motzer RJ, Agarwal N, Beard C, Bolger GB, Boston B, Carducci MA, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw. (2009) 7:618–30. doi: 10.6004/jnccn.2009.0043
4. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. (2007) 356:125–34. doi: 10.1056/NEJMoa060655
5. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. (2009) 27:3584–90. doi: 10.1200/JCO.2008.20.1293
6. Escudier B, Szczylik C, Hutson TE, Demkow T, Staehler M, Rolland F, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon Alfa-2a in patients with metastatic renal cell carcinoma. J Clin Oncol. (2009) 27:1280–9. doi: 10.1200/JCO.2008.19.3342
7. Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. (2009) 10:757–63. doi: 10.1016/S1470-2045(09)70162-7
8. Cai W, Kong W, Dong B, Zhang J, Chen Y, Xue W, et al. Comparison of efficacy, safety, and quality of life between sorafenib and sunitinib as first-line therapy for Chinese patients with metastatic renal cell carcinoma. Chin J Cancer. (2017) 36:64. doi: 10.1186/s40880-017-0230-7
9. Di Fiore F, Rigal O, Ménager C, Michel P, Pfister C. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer. (2011) 105:1811–3. doi: 10.1038/bjc.2011.507
10. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
11. Wells GA, Shea BJ, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomised Studies in Meta-analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
12. Sheng X, Chi Z, Cui C, Si L, Li S, Tang B, et al. Efficacy and safety of sorafenib versus sunitinib as first-line treatment in patients with metastatic renal cell carcinoma: largest single-center retrospective analysis. Oncotarget. (2016) 7:27044–54. doi: 10.18632/oncotarget.7395
13. Zhang HL, Sheng XN, Li XS, Wang HK, Chi ZH, He ZS, et al. Sorafenib versus sunitinib as first-line treatment agents in Chinese patients with metastatic renal cell carcinoma: the largest multicenter retrospective analysis of survival and prognostic factors. BMC Cancer. (2017) 17:16. doi: 10.1186/s12885-016-3016-4
14. La Vine DB, Coleman TA, Davis CH, Carbonell CE, Davis WB. Frequent dose interruptions are required for patients receiving oral kinase inhibitor therapy for advanced renal cell carcinoma. Am J Clin Oncol. (2010) 33:217–20. doi: 10.1097/COC.0b013e3181a650a6
15. Harrison MR, George DJ, Walker MS, Chen C, Korytowsky B, Kirkendall DT, et al. “Real world” treatment of metastatic renal cell carcinoma in a joint community–academic cohort: progression-free survival over three lines of therapy. Clin Genitourin Cancer. (2013) 11:441–50. doi: 10.1016/j.clgc.2013.05.002
16. Derbel Miled O, Dionne C, Terret C, Segura-Ferlay C, Flechon A, Neidhart EM, et al. Sorafenib and sunitinib for elderly patients with renal cell carcinoma. J Geriatr Oncol. (2013) 4:255–61. doi: 10.1016/j.jgo.2013.04.004
17. Maroun R, Fleury L, Nachbaur G, Maunoury F, Vanhille JL, Durand-Zaleski I. Real-world costs and outcomes in metastatic renal cell carcinoma patients treated by targeted therapies: a cohort study from the French health insurance database. Curr Med Res Opin. (2017) 33:1755–62. doi: 10.1080/03007995.2017.1360850
18. Levy A, Menard J, Albiges L, Loriot Y, Di Palma M, Fizazi K, et al. Second-line treatment of metastatic renal cell carcinoma: the Institut Gustave Roussy experience with targeted therapies in 251 consecutive patients. Eur J Cancer. (2013) 49:1898–904. doi: 10.1016/j.ejca.2013.02.003
19. Busch J, Seidel C, Weikert S, Wolff I, Kempkensteffen C, Weinkauf L, et al. Intrinsic resistance to tyrosine kinase inhibitors is associated with poor clinical outcome in metastatic renal cell carcinoma. BMC Cancer. (2011) 11:295. doi: 10.1186/1471-2407-11-295
20. Park SJ, Lee JL, Park I, Park K, Ahn Y, Ahn JH, et al. Comparative efficacy of sunitinib versus sorafenib as first-line treatment for patients with metastatic renal cell carcinoma. Chemotherapy. (2012) 58:468–74. doi: 10.1159/000346484
21. Ishihara H, Kondo T, Fukuda H, Yoshida K, Omae K, Takagi T, et al. Evaluation of renal function change during first-line tyrosine kinase inhibitor therapy for metastatic renal cell carcinoma. Jpn J Clin Oncol. (2017) 47:1175–81. doi: 10.1093/jjco/hyx161
22. Choueiri TK, Duh MS, Clement J, Brick AJ, Rogers MJ, Kwabi C, et al. Angiogenesis inhibitor therapies for metastatic renal cell carcinoma: effectiveness, safety and treatment patterns in clinical practice-based on medical chart review. BJU Int. (2010) 105:1247–54. doi: 10.1111/j.1464-410X.2009.08972.x
23. Choueiri TK, McDermott D, Sheng Duh M, Sarda SP, Neary MP, Oh WK. Costs associated with angiogenesis inhibitor therapies for metastatic renal cell carcinoma in clinical practice: results from a medical chart review study. Urol Oncol. (2012) 30:848–55. doi: 10.1016/j.urolonc.2010.07.009
24. Santoni M, Conti A, Porta C, Procopio G, Sternberg CN, Basso U, et al. Sunitinib, pazopanib or sorafenib for the treatment of patients with late-relapsing metastatic renal cell carcinoma. J Urol. (2015) 193:41–7. doi: 10.1016/j.juro.2014.07.011
25. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
26. Lee JL, Kim MK, Park I, Ahn JH, Lee DH, Ryoo HM, et al. Randomized phase II trial of sunitinib four weeks on and two weeks off versus two weeks on and one week off in metastatic clear-cell type renal cell carcinoma: RESTORE trial. Ann Oncol. (2015) 26:2300–5. doi: 10.1093/annonc/mdv357
27. Zhang X, Sun G, Zhao J, Shu K, Zhao P, Liu J, et al. Improved long-term clinical outcomes and safety profile of sunitinib dosing schedule with 4/2 switched to 2/1 in patients with metastatic renal cell carcinoma. J Cancer. (2018) 79:3303–10. doi: 10.7150/jca.25693
28. Bhojani N, Jeldres C, Patard JJ, Perrotte P, Suardi N, Hutterer G, et al. Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol. (2008) 53:917–30. doi: 10.1016/j.eururo.2007.11.037
29. Duh MS, Dial E, Choueiri TK, Fournier AA, Antras L, Rodermund D, et al. Cost implications of IV versus oral anti-angiogenesis therapies in patients with advanced renal cell carcinoma: retrospective claims database analysis. Curr Med Res Opin. (2009) 25:2081–90. doi: 10.1185/03007990903084800
30. Chen K, Sarda SP, Antràs L, Whittemore S, Luka A, Ramamurthy P, et al. Health care costs associated with angiogenesis inhibitors (AIS) and mtor inhibitors (MTORS) in patients with metastatic renal cell carcinoma (MRCC) treated at us community oncology clinics. Value Health. (2010) 13:A33. doi: 10.1016/S1098-3015(10)72140-9
Keywords: sorafenib, sunitinib, targeted therapy, renal cell carcinoma, meta-analysis
Citation: Deng H, Liu W, He T, Hong Z, Yi F, Wei Y and Zhang W (2019) Comparative Efficacy, Safety, and Costs of Sorafenib vs. Sunitinib as First-Line Therapy for Metastatic Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 9:479. doi: 10.3389/fonc.2019.00479
Received: 22 March 2019; Accepted: 20 May 2019;
Published: 21 June 2019.
Edited by:
Janice P. Dutcher, Cancer Research Foundation, United StatesReviewed by:
Riccardo Autorino, Virginia Commonwealth University, United StatesCopyright © 2019 Deng, Liu, He, Hong, Yi, Wei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxiong Zhang, end4MTIzZHJAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.