- 1Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, Beijing, China
- 2Department of Pathology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou, China
- 3Department of Radiobiology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou, China
- 4Department of Cardiology, First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 5Department of General Surgery, China-Japan Friendship Hospital, Beijing, China
Objectives: We sought to determine the optimal cutting points for two inflammatory biomarkers, neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR), to assess their prognostic value in patients with postoperative digestive tract cancers overall and by cancer sites, and further to construct an inflammation-related index based on the two biomarkers and assess its predictive performance.
Methods: Total 6,865 assessable patients with digestive tract cancers who underwent tumor resection were consecutively enrolled from Fujian Cancer Hospital between January 2000 and December 2010, including 2535/3012/1318 patients with esophageal/gastric/colorectal cancer. The latest follow-up (median: 44.9 months) ended in December 2015. Optimal cutting points were determined using survival tree analysis overall and by cancer sites.
Results: Among all study patients, the optimal cutting points were 2.07 and 168.50 to define high and low NLR and PLR, respectively. High NLR (hazard ratio [HR]: 1.48, 95% confidence interval [CI]: 1.37–1.61) and high PLR (HR: 1.41, 95% CI: 1.29–1.53) were associated with a significantly increased risk for the mortality of digestive tract cancers as a whole. By cancer sites, effect-size estimates were comparable and statistically significant. Elevation over the selected optimal cutting points for both NLR and PLR was associated with 1.69-fold increased risk of cancer-specific mortality compared to patients with simultaneously low NLR and PLR among all study patients, and this association persisted by cancer sites, especially for gastric cancer.
Conclusions: Our findings demonstrate that the preoperative integrated NLR and PLR, as an inflammation-related index, is a significant independent predictor for postoperative mortality in Chinese patients with digestive tract cancers both overall and by cancer sites.
Introduction
Digestive tract cancers are common and pose a heavy health burden in both developed and developing countries. In China, esophageal cancer (EC), gastric cancer (GC), and colorectal cancer (CRC) constitute three most frequently occurring cancers in digestive tract system, with the corresponding incidence of 477.9, 679.1, and 376.3 per 100,000 and the mortality of 375.0, 498.0, and 191.0 per 100,000, respectively in 2015 (1). Despite the advances made in multidisciplinary cancer management over the past years, a poor prognosis in patients with digestive tract cancers remains, even after receiving tumor resection (2). Currently, the debates regarding how to improve the prognosis and prolong survival time in patients with resectable digestive tract cancers are ongoing and unsettled. Identification of non-invasive and easy-to-obtain biomarkers has proven to be feasible and effective, yet no consensus has been reached, probably due to the differences in population background, sample size, follow-up interval or cancer site.
Evidence is mounting supporting an important role of systemic inflammation response in carcinogenesis (3). Further, a preoperative systemic inflammation score has been suggested as a useful indicator of postoperative survival in patients with GC (4). Of clinical biomarkers in systemic inflammation, neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are extensively studied in the medical literature, mainly because they are easily measured, reproducible and inexpensive. In theory, the neutrophils act as cancer-promoting leukocytes, capable of stimulating tumorigenesis and suppressing anti-cancer immune response, while the host's anti-cancer immune response greatly depends on the lymphocytes (5). The platelets can provide a procoagulant surface facilitating amplification of cancer-related coagulation, and facilitate cancer growth and dissemination (6). Many clinical and epidemiological studies have examined the association of NLR and PLR with postoperative survival in patients with digestive tract cancers using different cutting points (7–10), limiting between-study comparisons. It is widely accepted that the accuracy of cutting points mainly depends on statistical power and follow-up interval.
To derive a more reliable estimate, we, in the ongoing Fujian prospective investigation of cancer (FIESTA) cohort (11–23), sought to determine the optimal cutting points for both NLR and PLR before surgery when assessing their prognostic value in patients with postoperative digestive tract cancers overall and by cancer sites. We further attempted to construct an inflammation-related index based on the integration of NLR and PLR, and assessed its predictive performance.
Methods
Study Patients
As we previously recorded in the FIESTA study for each type of digestive tract cancers (11–23), a total of 7,757 patients were consecutively enrolled from the Department of Thoracic Surgery, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital (the former Fujian Provincial Cancer Hospital) during the period between January 2000 and December 2010. Of all study patients, there were 2,886 patients with EC who underwent three-field lymphadenectomy, 3,413 patients with GC who underwent radical gastrectomy, and 1,458 patients with CRC who underwent radical resection. The Ethics Committee of Fujian Cancer Hospital & Fujian Medical University Cancer Hospital approved the present study, and informed consents were signed by all patients.
Tissue Collection and Diagnosis
Primary cancer and adjacent normal tissue samples were resected during the surgery and fixed in 10% neutral-buffered formalin, and further paraffin-embedded using standard procedures. All pathological assays were completed at the Department of Pathology, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital.
Follow-Up Assessment
Postoperative patients were followed up every 6–12 months by face-to-face interviews at the Out-Patient Department, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, or by phone calls or postal mails if they missed appointments. The follow-up began from initial admission after the surgery since January 2000 to the date of deaths attributable to the causes other than digestive tract cancers or the end of follow-up visits until December 31, 2015, whichever came first.
Demographic and Clinicopathologic Characteristics
Demographic characteristics at baseline, including age (at the time of surgery), gender, smoking status (categorized as never, former and current smoking, with the latter two combined as ever smoking), drinking status (categorized as never, former and current alcohol drinking, with the latter two combined as ever drinking), and family cancer history (one or more direct relatives diagnosed with cancers except non-melanoma skin cancer within three generations) were obtained by a self-designed questionnaire after agreeing to participation.
Body weight and height were measured after removal of shoes and when wearing light clothing. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Blood pressure was measured by trained and certified examiners according to the standard protocols recommended by the American Heart Association (24).
Routine blood biomarkers, including neutrophil, lymphocyte, monocyte, eosinophil, basophil, white blood cell count, red blood cell count, hemoglobin, red cell distribution width, and platelet count were measured using fasting venous blood samples taken at the morning of receiving the surgery by the SYSMEX XE-2100 Automatic Blood Cell Analyzer (Sysmex, Kobe, Japan) at the Clinical Laboratory, Fujian Cancer Hospital & Fujian Medical University Cancer Hospital. The interval from blood drawing to clinical assays was <4 h. NLR and PLR were calculated accordingly.
Clinicopathologic characteristics were obtained from medical charts and/or pathological reports, including tumor node metastasis (TNM) stage [according to the 7th Edition of the UICC/AJCC TNM Staging system (25)], depth of invasion (T1-T4), regional lymph node metastasis (LNM) (N0-N3), distant metastasis (M0 and M1), tumor size (in centimeters) and tumor embolus.
Statistical Analysis
Data are expressed as median (interquartile range) or number (proportion) where appropriate. Differences between two groups were compared by the Wilcoxon rank-sum or Chi-square test where appropriate. Survival rates were compared by the Kaplan-Meier curves and differences in survival time were judged by the Log-rank tests. Survival tree analysis by the STREE program (available at the website: http://c2s2.yale.edu/software/stree/) was used to determine the optimal cutting points for both NLR and PLR among all study patients and separately by three cancer sites. The survival tree algorithm can recursively split patients into two groups according to many cutoff points, and the cutoff point is optimal when the two groups have the most different Kaplan-Meier survival curves, meaning that the two groups have the minimum p-value for the log-rank test. Proportional hazards assumption was checked by Weighted Schoenfeld residuals. Hazard ratio (HR) and 95% confidence interval (CI) for postoperative mortality were estimated by adjusted and unadjusted Cox proportional hazard models. In addition, permutation testing using 1,000 bootstrap replications was performed to internally validate the results. Predictive accuracy of the basic model gained by adding integrated NLR and PLR (namely INP) was appraised from both calibration and discrimination aspects. Specifically, calibration statistics included Akaike information criterion (AIC) and Bayesian information criterion (BIC), as well as the −2 log likelihood ratio test and the area under the receiver operating characteristic (ROC) curve (AUC) to assess how closely the prediction probability for the addition of INP reflected the actual observed risk and the global fit of modified risk model. Discrimination statistics included net reclassification improvement (NRI) and integrated discrimination improvement (IDI) (26, 27) to justify the improvement in prediction performance, as well as the Harrell's C index to inspect whether the addition of INP to the basic model can differentiate among patients who died or survived.
All statistical tests were two-sided, and p < 0.05 was considered to be statistically significant. SAS software, version 9.4 (SAS Institute Inc.) and STATA software, version 14.1 (Stata Corp, College Station, TX) were adopted to complete statistical analyses, unless otherwise indicated.
Results
Baseline Characteristics
After removing patients with incomplete data and dying from causes other than digestive tract cancers as we previously reported (11–23), 6,865 patients were assessable in the current analysis, including 2,535 patients with EC, 3,012 patients with GC, and 1,318 patients with CRC. The follow-up time ranged from 1.0 month to 188.9 months (median: 44.9 months). Total 2,808 deaths occurred during the follow-up, including 1,065 patients with EC, 1,331 patients with GC, and 412 patients with CRC. Baseline characteristics differed significantly except BMI and family cancer history between non-survivors (n = 2,808) and survivors (n = 4,057) (Supplementary Table 1).
Cutting Point Determination
Weighted Schoenfeld residuals did not indicate major departures from the proportional hazards assumption, and so Cox proportional hazard model was employed. As continuous variables, after adjusting for age, gender, smoking, drinking, BMI and family cancer history, per standard deviation increments (NLR: 2.48 and PLR: 94.68) in preoperative NLR (HR: 1.07, 95% CI: 1.05–1.10, p < 0.001) and PLR (HR: 1.07, 95% CI: 1.05–1.09, p < 0.001) were significantly associated with poor survival of digestive tract cancers as a whole.
To determine the optimal cutting points for NLR and PLR among all study patients and in patients separately with EC, GC, and CRC, we adopted the survival tree analysis, and found that the optimal cutting points for NLR and PLR were 2.07 and 168.50 among all study patients, 2.36 and 159.23 in patients with EC, 1.97 and 188.0 in patients with GC and 3.37 and 264.29 for patients with CRC, respectively. The optimal cutting points selected can split patients into two groups with the maximal difference in survival time. The estimates of predictive performance of selected optimal cutting points for NLR and PLR in predicting cancer-specific mortality are presented in Supplementary Table 2. Among all study patients, the cutting points selected has sensitivity and specificity of 77.68 and 75.68% for NLR, and of 84.21 and 73.89% for PLR, respectively, and the corresponding AUC was 0.743 (95% CI: 0.728–0.757) and 0.715 (95% CI: 0.701–0.730). By cancer sites, patients with CRC had the best sensitivity, specificity, and AUC, followed by patients with gastric cancer.
In patients with digestive tract cancers overall and by cancer sites, those with NLR or PLR greater than selected cutting points were classified as high NLR or high PLR group, whereas those with NLR or PLR less than or equal to cutting points were classified as low NLR or low PLR group. After adjusting for age, gender, smoking, drinking, BMI and family cancer history, high NLR, and high PLR were significantly associated with 1.48-fold (95% CI: 1.37–1.61, p < 0.001) and 1.41-fold (95% CI: 1.29–1.53, p < 0.001) increased mortality risk of digestive tract cancers as a whole relative to low NLR and low PLR among all study patients, 1.32-fold (95% CI: 1.16–1.50, p < 0.001) and 1.39-fold (95% CI: 1.21–1.60, p < 0.001) increased risk in patients with EC, 1.83-fold (95% CI: 1.62–2.07, p < 0.001) and 1.58-fold increased risk in patients with GC, and 1.89-fold (95% CI: 1.50–2.38, p < 0.001) and 1.82-fold (1.35–2.43, p < 0.001) increased risk in patients with CRC, respectively (Table 1).
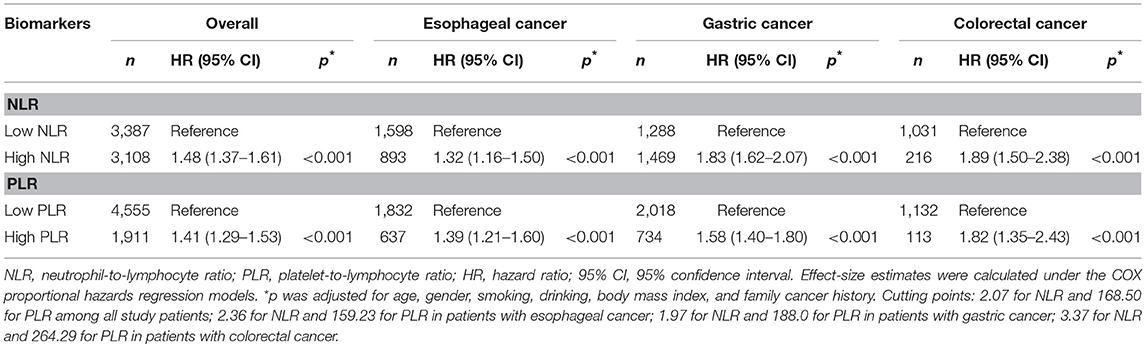
Table 1. Risk prediction of NLR and PLR as categorical variables for cancer-specific mortality in patients with postoperative digestive tract cancers overall and by cancer sites.
Further subgroup analyses were conducted according to clinicopathologic characteristics, and high NLR and high PLR were found to be associated with significantly high risk for cancer-specific mortality within each subgroup among all study patients except high PLR in invasion depth T1/T2 group (Table 2). Subgroup analyses in patients with GC revealed that only patients with positive distant metastasis showed a non-significant association between high PLR, high NLR and cancer-specific mortality (Table 2).
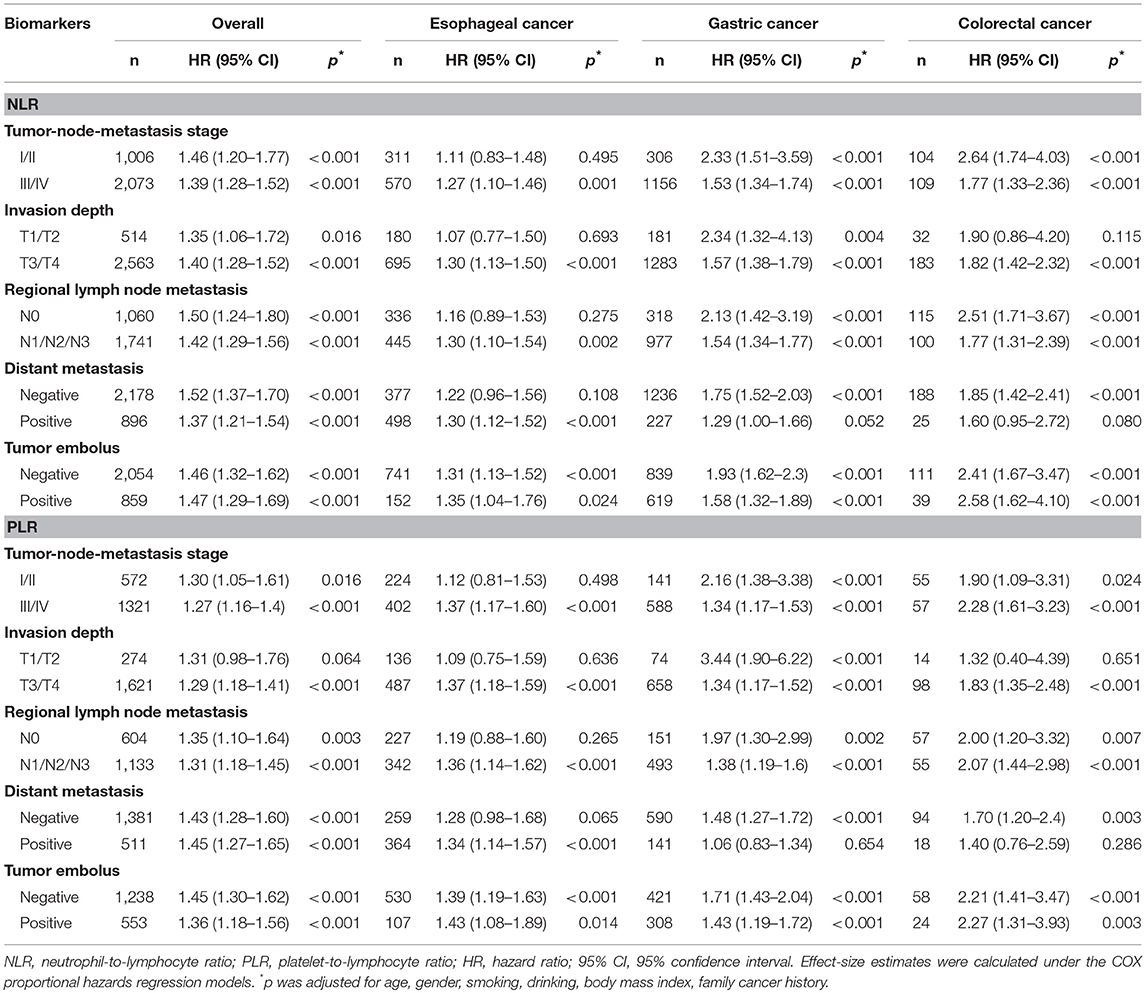
Table 2. Stratified risk prediction of NLR and PLR as categorical variables for cancer-specific mortality in patients with postoperative digestive tract cancers overall and by cancer sites.
Integrated NLR and PLR in Predicting Cancer-Specific Mortality
On a continuous scale, a three-dimension surface was plotted to show joint increments in preoperative NLR and PLR in predicting the mortality risk of digestive tract cancers as a whole (Supplementary Figure 1).
As correlation analysis indicated a strong positive relation between NLR and PLR (r = 0.60, p < 0.001) among all study patients, we generated the integrated NLR and PLR, namely INP, according to selected cutting points both overall and by cancer sites, which was defined as follows: patients with neither elevated NLR (≤ cutting point) nor PLR (≤ cutting point) were assigned a score of 0; patients with only elevated PLR (> cutting point) were assigned a score of 1; patients with only elevated NLR (> cutting point) were assigned a score of 2; patients with both elevated NLR (>cutting point) and PLR (> cutting point) were assigned a score of 3. For INP ranging from 0 to 3, there were 3,369 (49.08%), 388 (5.65%), 1,585 (23.09%), and 1,523 (22.18%) patients, respectively. The median survival time for patients with INP equal to 3 (55.7 months, Log-rank test p < 0.001) was significantly shorter than the other three INP groups (Figure 1), and HRs of cancer-specific mortality for patients with INP equal to 1, 2, and 3 were 1.06 (95% CI: 0.88–1.27), 1.32 (95% CI: 1.19–1.46), and 1.69 (95% CI: 1.53–1.86) relative to INP equal to 0 among all study patients, respectively. In patients with GC, INP equal to 1 (HR: 1.49, 95% CI: 1.07–2.08), 2 (HR: 1.73, 95% CI: 1.50–2.00), and 3 (HR: 2.10, 95% CI: 1.81–2.45) were associated with significantly increased risk of cancer-specific mortality. The risk for INP equal to three was 1.82 times as high as the sum of the risk in INP equal to 1 and 2 (synergy index: 1.82, 95% CI: 0.97–3.39). Relative excess risk due to the additive interaction between NLR and PLR was 0.31 (95% CI: 0.06–0.56), and the additive interaction accounted for 18% of mortality in patients with both risk factors (attributable proportion due to interaction: 0.18, 95% CI: 0.04–0.33) (Table 3).
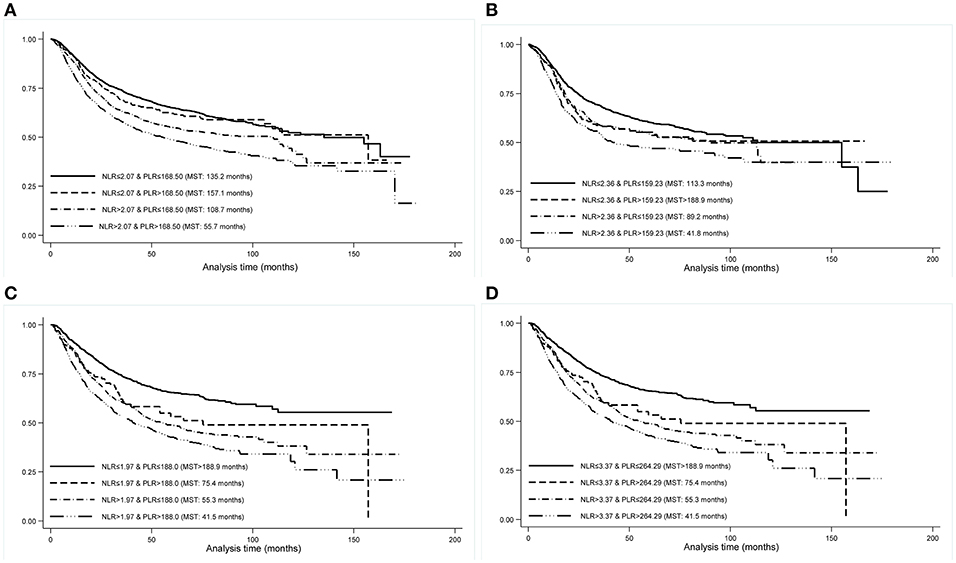
Figure 1. Kaplan-Meier curves by INP groups in all study patients (A), patients with esophageal cancer (B), patients with gastric cancer (C), and patients with colorectal cancer (D). INP, integrated neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio; MST, median survival time.

Table 3. Risk prediction of INP for cancer-specific mortality in patients with postoperative digestive tract cancers overall and by cancer sites.
Additionally, for the risk prediction of INP for cancer-specific mortality, robust permutation testing was performed using 1,000 bootstrap replications subsequently (Table 3), and no change in significance level was detected.
The predictive accuracy of the basic model (including age, gender, smoking, drinking, BMI, family cancer history, TNM stage, and tumor embolus) with and without INP for postoperative digestive tract cancers overall and by cancer sites is presented in Supplementary Table 3. Among all study patients and in patients separately with EC, GC, and CRC, adding INP to the basic model produced small AIC and BIC statistics, and likelihood ratio test indicated that INP was indeed a part of true model and carried a better fit. The AUC differed significantly between the basic model with and without adding INP. Moreover, the probabilities of NRI and IDI were statistically significant after adding INP to the basic model. Harrell's C index showed that the basic model with and without INP was well-performed.
Patients with both evaluated NLR and PLR showed higher risk for cancer-specific mortality in all subgroups stratified by clinicopathologic characteristics among all study patients and in patients by three cancer sites (Supplementary Table 4).
Discussion
Via a comprehensive analysis of the long-term FIESTA cohort, we identified the optimal cutting points for two inflammatory biomarkers, NLR and PLR, in 6,865 patients with digestive tract cancers overall and by cancer sites. Importantly, we have generated an inflammation-related index based on the integrated NLR and PLR, namely INP, and found that this index exhibited better performance of survival prediction for cancer-specific mortality in Chinese patients with digestive tract cancers overall and by cancer sites. The findings of this study will advance our understanding on the clinical relevance of NLR and PLR, as well as their integration form in the development and progression of digestive tract cancers.
It is widely recognized that inflammation plays a contributory role in the initiation, progression and prognosis of various types of cancers, especially in digestive tract system (3, 28–30). Several systemic inflammation-based prognostic biomarkers have been identified, such as NLR, PLR, lymphocyte-to-monocyte ratio and C-reactive protein, as potential cancer risk or prognostic factors (31–35). In particular, NLR and PLR are two inflammatory biomarkers that are extensively evaluated in the medical literature, and they were found to be associated with the significant risk of EC, GC, and CRC in our prior studies (13, 15, 16). However, a common problem facing scientific community is to seek optimal cutting points for both biomarkers, which are constrained by some methodological issues, such as statistical power and follow-up interval.
The majority of prior studies have employed the receiver operating characteristic (ROC) curve or quantile to determine optimal cutting point. These cutting points are heterogeneous across studies, even for the same type of cancer or at the same place (31, 36–41). A lack of sufficient power has been cited as a major reason for inconsistencies. Several splitting criteria have been developed, such as classification and regression trees (CART) and multivariate adaptive regression splines (MARS) (42, 43). Although the relative merits of these criteria are not clearly resolved, survival tree-based method has been applicable to more general situations based on scientific judgement (44). Therefore, we employed survival tree analysis to determine the optimal cutting points in predicting the cancer-specific mortality postoperatively, and further performed validation in patients with digestive tract cancer overall and by cancer sites. Using the derived optimal cutting points among all study patients, we found that both high NLR and high PLR were associated with an ~1.5-fold increased risk of cancer-specific mortality in the present study, and this association persisted for three types of digestive tract cancers, especially GC.
Cancer is a highly complex family of diseases, to which multiple factors contribute interactively, and so the contribution of any single biomarker, by itself, might be small and depends on the others. Given this fact, we thereby, on the basis of the integration of NLR and PLR at their optimal cutting points, developed the INP, as an inflammation-related index to assess its association with the risk of digestive tract cancer-specific mortality overall and by cancer sites. Although the integration of NLR and PLR as a composite biomarker has been widely investigated, comparisons between the results of different studies are difficult due to diverse cutting points selected (38, 45). For instance, Tao and colleagues found a strong predictive effect for combined NLR-PLR index in 153 patients with CRC who received adjuvant chemotherapy (46). Feng et al. also found that INP was an independent prognostic marker in patients with EC without neoadjuvant or adjuvant treatment (47). By contract in this present study, on a binary scale we found that the effect of NLR on prognosis was greater than that of PLR in terms of hazard ratio, and the parameter INP was an independent predictor, with high NLR and high PLR together predicting poor postsurgical survival. Although broad replication offers valuable information for a better understanding of NLR and PLR in cancer survival, the exact mechanisms are elusive currently. It is possible that platelet can regulate immune response, inflammation and angiogenesis, in cooperation with neutrophils and lymphocytes (48). Activated platelets promote cancer metastasis and angiogenesis via releasing various cytokines and forming cancer embolus, so that it can escape from the immunocyte (49, 50). Moreover, platelet activation can trigger platelet-neutrophils interaction, alter the immunocyte subpopulations and enhance the differentiation and cytokine production of T-effector cell (51–53).
There were several potential limitations for the present study. Firstly, this study was performed in a single hospital, which restricted the generalizability, although it can facilitate consistency of evaluation. Additionally, external validation is necessary. Secondly, due to the difficulty in identifying an external group, we are unable to validate our findings in an independent population. Thirdly, only cancer-specific mortality was analyzed in this study, because information on deaths from causes other than digestive tract cancers is incomplete, which precludes further competing risk analysis. Fourthly, patients were exclusively enrolled from a southern city in China, which restricted racial or ethnical extrapolation. Fifthly, the recruitment period was as long as 10 years, during which the advances in surgical therapies might introduce a possible bias and impact the prognosis of patients due to time effect.
Taken together, our findings indicate that preoperative INP, as an inflammation-related index, is a significant independent predictor for postoperative cancer-specific mortality in patients with digestive tract cancers overall and by cancer sites in Chinese. For practical reasons, data from this study may provide basic evidence that patients with digestive tract cancers especially GC who have elevated INP based on the optimal cutting points of NLR and PLR, presumably need close monitoring for prolonging survival and improving quality of life after the surgery, and are thus of significant clinical value.
Ethics Statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Author Contributions
DH, FP, and WN planned and designed the study, and directed its implementation. FP, DH, JL, and XZhe drafted the protocol. DH, XL, HZ, and YX obtained statutory and ethics approvals. DH, XL, HZ, and YX contributed to data acquisition. XZha, JJ, and WN conducted statistical analyses. WN, DH, FP, JJ, JL, and XZhe had access to all raw data. XZha, FP, DH, and WN did the data preparation and quality control. XZha, FP, JJ, and WN wrote and revised the manuscript. All authors read and approved the final manuscript prior to submission.
Funding
This study was supported by the Scientific and Technological Project of Qinghai Province (Grant No. 2015-ZJ-742), the Fundamental Research Funds for the Central Universities (Grant No. 3332018170), the Innovation of Science and Technology of Fujian Province (Grant No. 2017Y9090 and 2017Y9082), the Natural Science Foundation of Fujian Province (Grant No. 2018Y0024), and the Ministry of Health P.R. China (WKJ2016-2-05). The funders of this study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our colleagues over the years at Fujian Cancer Hospital—particularly Gang Chen, Chao Li, Binying Liang, Xiaohui Chen, Yuzhen Zheng, Qingfeng Zheng, Shuoyan Liu, Zhilian She, Kunshou Zhu, Weidong Zang, Weizhong Ruan, Weimin Fang, Lin Li, Mingqiang Chen, Derong Zhang, Shaofeng Lin, Shunjin Chen, Yigui Chen, and Guohong Zhao for performing the surgery, Yanni Gao, Zhenzhou Xiao, Su Lin, Xuehong Liao, Wenhui Jiang, Jieqiong Lin, Xinjing Li, Yi Shi, Xiaojiang Wang, Shanfeng Jin, Hongfei Wang, Wucheng Shen, Weifeng Zhu, Xiaowen Cai, Baozhen Chen, Tongmei Chen, Xueyan Chen, and Lifang Chen for collecting the blood/tissue samples and performing the follow-up investigations.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00427/full#supplementary-material
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
2. Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. (2009) 374:477–90. doi: 10.1016/s0140-6736(09)60617-6
3. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
4. Lin JX, Lin JP, Xie JW, Wang JB, Lu J, Chen QY, et al. Prognostic importance of the preoperative modified systemic inflammation score for patients with gastric cancer. Gastric Cancer. (2018) 22:403–12. doi: 10.1007/s10120-018-0854-6
5. Faria SS, Fernandes PC Jr, Silva MJ, Lima VC, Fontes W, Freitas-Junior R, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermed Sci. (2016) 10:702. doi: 10.3332/ecancer.2016.702
6. Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. (2011) 9:237–49. doi: 10.1111/j.1538-7836.2010.04131.x
7. Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
8. Yu J, Ding Z, Yang Y, Liu S. Increased platelet-to-lymphocytes ratio is associated with poor long-term prognosis in patients with pancreatic cancer after surgery. Medicine. (2018a) 97:e11002. doi: 10.1097/md.0000000000011002
9. Zhang H, Shang X, Ren P, Gong L, Ahmed A, Ma Z, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. (2018) 234:1794–802. doi: 10.1002/jcp.27052
10. Zhao Z, Zhao X, Lu J, Xue J, Liu P, Mao H. Prognostic roles of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in ovarian cancer: a meta-analysis of retrospective studies. Arch Gynecol Obstet. (2018) 297:849–57. doi: 10.1007/s00404-018-4678-8
11. Hu D, Peng F, Lin X, Chen G, Liang B, Li C, et al. The elevated preoperative fasting blood glucose predicts a poor prognosis in patients with esophageal squamous cell carcinoma: the Fujian prospective investigation of cancer (FIESTA) study. Oncotarget. (2016) 7:65247–56. doi: 10.18632/oncotarget.11247
12. Peng F, Hu D, Lin X, Chen G, Liang B, Zhang H, et al. Preoperative metabolic syndrome and prognosis after radical resection for colorectal cancer: the Fujian prospective investigation of cancer (FIESTA) study. Int J Cancer. (2016) 139:2705–13. doi: 10.1002/ijc.30404
13. Hu D, Lin X, Chen Y, Chang Q, Chen G, Li C, et al. Preoperative blood-routine markers and prognosis of esophageal squamous cell carcinoma: the Fujian prospective investigation of cancer (FIESTA) study. Oncotarget. (2017a) 8:23841–50. doi: 10.18632/oncotarget.13318
14. Hu D, Peng F, Lin X, Chen G, Zhang H, Liang B, et al. Preoperative metabolic syndrome is predictive of significant gastric cancer mortality after gastrectomy: the Fujian prospective investigation of cancer (FIESTA) study. EBio Med. (2017b) 15:73–80. doi: 10.1016/j.ebiom.2016.12.004
15. Hu D, Zhang H, Lin X, Chen G, Li C, Liang B, et al. Elevated preoperative neutrophil-to-lymphocyte ratio can predict poor survival in early stage gastric cancer patients receiving radical gastrectomy: the Fujian prospective investigation of cancer (FIESTA) study. J Cancer. (2017c) 8:1214–22. doi: 10.7150/jca.18707
16. Peng F, Hu D, Lin X, Chen G, Liang B, Li C, et al. The monocyte to red blood cell count ratio is a strong predictor of postoperative survival in colorectal cancer patients: the Fujian prospective investigation of cancer (FIESTA) study. J Cancer. (2017a) 8:967–75. doi: 10.7150/jca.18000
17. Peng F, Hu D, Lin X, Chen G, Liang B, Zhang H, et al. Analysis of preoperative metabolic risk factors affecting the prognosis of patients with esophageal squamous cell carcinoma: the Fujian prospective investigation of cancer (FIESTA) study. EBio Med. (2017b) 16:115–23. doi: 10.1016/j.ebiom.2017.01.035
18. Fan G, Hu D, Peng F, Xu G, Lin X, Liang B, et al. Different risk profiles for the postsurgical prognosis of gastric cancer patients with different blood types: the FIESTA study. J Cancer. (2018a) 9:2885–94. doi: 10.7150/jca.25408
19. Fan G, Hu D, Zhang X, Peng F, Lin X, Chen G, et al. Interaction between prediabetes and the abo blood types in predicting postsurgical esophageal squamous cell carcinoma-specific mortality: the FIESTA study. Front Oncol. (2018b) 8:461. doi: 10.3389/fonc.2018.00461
20. Hu D, Peng F, Lin X, Chen G, Liang B, Chen Y, et al. Prediction of three lipid derivatives for postoperative gastric cancer mortality: the Fujian prospective investigation of cancer (FIESTA) study. BMC Cancer. (2018) 18:785. doi: 10.1186/s12885-018-4596-y
21. Peng F, Hu D, Lin X, Chen G, Liang B, Chen Y, et al. An in-depth prognostic analysis of baseline blood lipids in predicting postoperative colorectal cancer mortality: the FIESTA study. Cancer Epidemiol. (2018a) 52:148–57. doi: 10.1016/j.canep.2018.01.001
22. Peng F, Hu D, Lin X, Liang B, Chen Y, Zhang H, et al. Impact of long-term antihypertensive and antidiabetic medications on the prognosis of post-surgical colorectal cancer: the Fujian prospective investigation of cancer (FIESTA) study. Aging. (2018b) 10:1166–81. doi: 10.18632/aging.101459
23. Sha H, Hu D, Wu S, Peng F, Xu G, Fan G, et al. Baseline metabolic risk score and postsurgical esophageal cancer-specific mortality: the Fujian prospective investigation of cancer (FIESTA) study. J Cancer. (2018) 9:1173–81. doi: 10.7150/jca.23631
24. Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, et al. Human blood pressure determination by sphygmomanometry. Circulation. (1993) 88 (5 Pt 1):2460–70.
25. Edge SB, Compton CC. The american joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. (2010) 17:1471–4. doi: 10.1245/s10434-010-0985-4
26. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. valuating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. (2008) 27:157–72; discussion 207–112. doi: 10.1002/sim.2929
27. Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. (2010) 48:1703–11. doi: 10.1515/CCLM.2010.340
28. Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. (2007) 117:60–9. doi: 10.1172/jci30111
29. John BJ, Abulafi AM, Poullis A, Mendall MA. Chronic subclinical bowel inflammation may explain increased risk of colorectal cancer in obese people. Gut. (2007) 56:1034–5. doi: 10.1136/gut.2007.125955
30. Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol. (2016) 70:395–411. doi: 10.1146/annurev-micro-102215-095513
31. Liu D, Huang Y, Li L, Song J, Zhang L, Li W. High neutrophil-to-lymphocyte ratios confer poor prognoses in patients with small cell lung cancer. BMC Cancer. (2017) 17:882. doi: 10.1186/s12885-017-3893-1
32. Yang J, Guo X, Wang M, Ma X, Ye X, Lin P. Pre-treatment inflammatory indexes as predictors of survival and cetuximab efficacy in metastatic colorectal cancer patients with wild-type RAS. Sci Rep. (2017) 7:17166. doi: 10.1038/s41598-017-17130-6
33. Semeniuk-Wojtas A, Lubas A, Stec R, Syrylo T, Niemczyk S, Szczylik C. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and C-reactive protein as new and simple prognostic factors in patients with metastatic renal cell cancer treated with tyrosine kinase inhibitors: a systemic review and meta-analysis. Clin Genitourin Cancer. (2018) 16:e685–93. doi: 10.1016/j.clgc.2018.01.010
34. Wen J, Bedford M, Begum R, Mitchell H, Hodson J, Whiting J, et al. The value of inflammation based prognostic scores in patients undergoing surgical resection for oesophageal and gastric carcinoma. J Surg Oncol. (2018) 117:1697–707. doi: 10.1002/jso.25057
35. Yang J, Xu H, Guo X, Zhang J, Ye X, Yang Y, et al. Pretreatment inflammatory indexes as prognostic predictors for survival in colorectal cancer patients receiving neoadjuvant chemoradiotherapy. Sci Rep. (2018a) 8:3044. doi: 10.1038/s41598-018-21093-7
36. Zhou XL, Li YQ, Zhu WG, Yu CH, Song YQ, Wang WW, et al. Neutrophil-to-lymphocyte ratio as a prognostic biomarker for patients with locally advanced esophageal squamous cell carcinoma treated with definitive chemoradiotherapy. Sci Rep. (2017) 7:42581. doi: 10.1038/srep42581
37. Jones HG, Qasem E, Dilaver N, Egan R, Bodger O, Kokelaar R, et al. Inflammatory cell ratios predict major septic complications following rectal cancer surgery. Int J Colorectal Dis. (2018) 33:857–62. doi: 10.1007/s00384-018-3061-3
38. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. (2018) 12:342–52. doi: 10.5009/gnl17216
39. Yang Y, Xu H, Zhou L, Deng T, Ning T, Liu R, et al. Platelet to lymphocyte ratio is a predictive marker of prognosis and therapeutic effect of postoperative chemotherapy in non-metastatic esophageal squamous cell carcinoma. Clin Chim Acta. (2018b) 479:160–5. doi: 10.1016/j.cca.2018.01.013
40. Yu X, Wen Y, Lin Y, Zhang X, Chen Y, Wang W, et al. The value of preoperative glasgow prognostic score and the C-reactive protein to albumin ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J Cancer. (2018b) 9:807–15. doi: 10.7150/jca.22755
41. Zhu M, Feng M, He F, Han B, Ma K, Zeng X, et al. Pretreatment neutrophil-lymphocyte and platelet-lymphocyte ratio predict clinical outcome and prognosis for cervical Cancer. Clin Chim Acta. (2018) 483:296–302. doi: 10.1016/j.cca.2018.05.025
42. Put R, Xu QS, Massart DL, Vander Heyden Y. Multivariate adaptive regression splines (MARS) in chromatographic quantitative structure-retention relationship studies. J Chromatogr A. (2004) 1055:11–9. doi: 10.1016/j.chroma.2004.07.112
43. Austin PC. A comparison of regression trees, logistic regression, generalized additive models, and multivariate adaptive regression splines for predicting AMI mortality. Stat Med. (2007) 26:2937–57. doi: 10.1002/sim.2770
44. Zhang H, Singer BH. Analysis of Censored Data: Survival Trees and Random Forests. (1999). 93–103 p.
45. Wu G, Yao Y, Bai C, Zeng J, Shi D, Gu X, et al. Combination of platelet to lymphocyte ratio and neutrophil to lymphocyte ratio is a useful prognostic factor in advanced non-small cell lung cancer patients. Thorac Cancer. (2015) 6:275–87. doi: 10.1111/1759-7714.12178
46. Tao Y, Ding L, Yang GG, Qiu JM, Wang D, Wang H, et al. Predictive impact of the inflammation-based indices in colorectal cancer patients with adjuvant chemotherapy. Cancer Med. (2018). 7:2876–86. doi: 10.1002/cam4.1542
47. Feng JF, Huang Y, Liu JS. Combination of neutrophil lymphocyte ratio and platelet lymphocyte ratio is a useful predictor of postoperative survival in patients with esophageal squamous cell carcinoma. Onco Targets Ther. (2013) 6:1605–12. doi: 10.2147/ott.s52501
48. Li N. CD4+ T cells in atherosclerosis: regulation by platelets. Thromb Haemost. (2013) 109:980–90. doi: 10.1160/th12-11-0819
49. Placke T, Orgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, et al. Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res. (2012) 72:440–8. doi: 10.1158/0008-5472.can-11-1872
50. Li N. Platelets in cancer metastasis: to help the “villain” to do evil. Int J Cancer. (2016) 138:2078–87. doi: 10.1002/ijc.29847
51. Gerdes N, Zhu L, Ersoy M, Hermansson A, Hjemdahl P, Hu H, et al. Platelets regulate CD4(+) T-cell differentiation via multiple chemokines in humans. Thromb Haemost. (2011) 106:353–62. doi: 10.1160/th11-01-0020
52. Schrottmaier WC, Kral JB, Badrnya S, Assinger A. Aspirin and P2Y12 Inhibitors in platelet-mediated activation of neutrophils and monocytes. Thromb Haemost. (2015) 114:478–89. doi: 10.1160/th14-11-0943
Keywords: digestive tract cancer, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, mortality, prognosis, FIESTA study
Citation: Zhang X, Hu D, Lin X, Zhang H, Xia Y, Lin J, Zheng X, Peng F, Jie J and Niu W (2019) Prognostic Value of an Inflammation-Related Index in 6,865 Chinese Patients With Postoperative Digestive Tract Cancers: The FIESTA Study. Front. Oncol. 9:427. doi: 10.3389/fonc.2019.00427
Received: 08 October 2018; Accepted: 07 May 2019;
Published: 22 May 2019.
Edited by:
Farhad Islami, American Cancer Society, United StatesReviewed by:
Guangwen Cao, Second Military Medical University, ChinaJerry Polesel, Centro di Riferimento Oncologico di Aviano (IRCCS), Italy
Copyright © 2019 Zhang, Hu, Lin, Zhang, Xia, Lin, Zheng, Peng, Jie and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianzheng Jie, amllamlhbnpoZW5nMDEmI3gwMDA0MDtzaW5hLmNvbQ==
Wenquan Niu, bml1d2VucXVhbiYjeDAwMDVGO3NoY24mI3gwMDA0MDsxNjMuY29t
†These authors share first authorship
 Xinran Zhang
Xinran Zhang Dan Hu2†
Dan Hu2† Wenquan Niu
Wenquan Niu