
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 28 May 2019
Sec. Radiation Oncology
Volume 9 - 2019 | https://doi.org/10.3389/fonc.2019.00377
This article is part of the Research TopicRole of SRS/SBRT in Oligometastatic DiseaseView all 15 articles
 Kevin Armstrong1
Kevin Armstrong1 Jennifer Ward1
Jennifer Ward1 Mary Dunne2
Mary Dunne2 Luke Rock3
Luke Rock3 Jennifer Westrup4
Jennifer Westrup4 Christopher R. Mascott5
Christopher R. Mascott5 Pierre Thirion3
Pierre Thirion3 Alina Mihaela Mihai3*
Alina Mihaela Mihai3*Background: The role of stereotactic radiosurgery (SRS) in the treatment of limited numbers of brain metastases in selected breast cancer patients is well-established.
Aims: To analyse outcome from a single institutional experience with SRS, to identify any significant prognostic factors and to assess the influence of Her-2, estrogen receptor status, and prescribed dose on outcome.
Methods: The medical records of 56 patients treated at in a single institution between 2009 and 2014 were reviewed. Demographic, treatment related and outcome data were analyzed to identify prognostic factors in this patient population. The primary endpoints were overall survival and local control. Secondary endpoint was distant intra-cranial progression-free survival.
Results: The median follow- up time for the entire cohort was 10.33 months (1.25–97.28). The overall median survival was 12.5months (95%CI = 5.8–19.2), with 53.3%, and 35.8% surviving at 1- and 2- years post-SRS. After adjustment for the effect of Her 2 status, uncontrolled extra-cranial disease at the time of SRS predicted for shorter survival (HR for death = 3.1, 95% CI = 1.4–6.9, p = 0.006). At the time of death, 75% of the patients had active, uncontrolled intra-cranial disease, with 56% these patients presenting intra-cranial disease only. Sustained local control was observed in 56 (59.6%) of 94 treated metastases. In univariate analysis, Her2 status, ERHer2 group status?, and prescribed SRS dose were highly significant for local progression free-survival (LPFS). After adjustment for the effect of Her 2 status, patients receiving 12–16 Gy can expect shorter LPFS than those receiving 18–20 Gy (HR = 1.7, 95% CI = 1.0–2.8, p = 0.043). After adjustment for the effect of dose group, patients with Her 2 negative cancer can expect shorter LPFS than those with Her 2 positive cancer (HR = 2.6, 95% CI = 1.5–4.4, p < 0.0005). Use of prior WBRT did not impact survival, local or distant intra-cranial progression-free survival.
Conclusions: Survival outcome is similar to the published literature. Improved outcomes are observed in patients with Her 2-positive, controlled extracranial disease at the time of SRS and higher SRS dose delivered. Achieving intra-cranial control appears to be an important factor for the survival of the breast cancer patients in the era of targeted therapies.
Brain Metastases occur in 20–40% of patients with metastatic cancer (1). Whole brain radiotherapy (WBRT) and steroid therapy have historically been used as the standard management. However, outcome is poor with this approach (2). Corticosteroid treatment has a modest impact, extending median survival by as little as 1–2 months and has significant toxicities. WBRT has a greater impact, but median survival is still measured in months (3). The biggest disadvantage with WBRT is that it doesn't result in a high prolonged local control rate, which contributes to overall low survival. Due to this limitation of WBRT investigators explored the use of surgical removal of oligometastatic (limited number) brain metastases in selected patients. In a randomized trial, Patchell et al. reported a median survival of 19 months in patients treated for solitary brain metastases, with surgical resection and WBRT compared to 9 months in those treated with WBRT alone (4). This trial which included patients with breast and other primary sites demonstrated the potential value of aggressive local intervention for oligometastatic brain tumors in patients with good performance status and controlled extracranial disease.
Historically stereotactic radiosurgery (SRS) was pioneered by Leksell for managing intracranial conditions such as arteriovenous malformations (5, 6) Following its successful use for benign brain conditions the technology was applied to brain metastases with results similar to those reported for surgery (7). SRS offers a non-invasive treatment alternative, which is performed as an outpatient procedure and generally well-tolerated.
As the systemic treatment of metastatic breast cancer has evolved and improved the prospect of achieving durable control of extracranial disease has increased dramatically. This has created a greater demand for the successful treatment of brain metastases of breast cancer patients for two reasons. Firstly, more patients fulfill the selection criteria by virtue of the control of extracranial disease and the fact that their performance status is higher systemic therapies are increasingly better tolerated. The second main reason is the identification of Her-2-Neu positive breast cancer. Up to 30% of breast cancer patients overexpress the Her-2-Neu receptor (8). This overexpression is associated with an aggressive phenotype. However, with the discovery of Trastuzumab, a monoclonal antibody against Her-2-Neu the prognosis has dramatically improved. In the metastatic setting, up to 30 to 40% of such patients will ultimately develop brain metastasis. The reason for the high incidence of brain metastasis in Trastuzumab treated patients is assumed to be because Trastuzumab (which is a large monoclonal antibody) may not cross the blood-brain barrier. As more patients achieve control of their Her-2-Neu positive extracranial disease they may develop brain metastases in a setting where SRS is clinically appropriate. It is therefore important to assess which prognostic factors will affect the outcome of this therapy and to define an optimal dose range.
The study was approved by the local Institutional Review Board.
A retrospective analysis was performed on 56 patients with metastatic breast cancer with metastases to the brain. All of the patients were treated with Stereotactic Radiosurgery (SRS) or intensity modulated radiosurgery (IMRS) between 2009 and 2014. All patients had a fine resolution 3DMRI of the brain (confirming diagnosis of brain metastases) within the 14 days preceding the treatment.
An Excel database was generated which included the patients demographics (age, date of diagnosis, treatment, pathology, brain progression details), treatment related and outcome related data. The Disease-specific graded prognostic assessment score (DS-GPA) was retrospectively calculated in all patients. DS-GPA score is a prognostic scoring system specifically designed for brain metastases. It takes account of performance status, age, number of brain metastases, and status of extracranial disease to assign a class ranging from I-IV. Class I has the best prognosis. Information was gathered on these patients using the hospital's electronic (ARIA) and paper charts.
For all patients a dedicated contrast-enhanced planning brain CT was acquired, with slice thickness of 1.25 mm, using the frame or frameless systems for localization of the lesions. Of the 56 patients receiving SRS, 37 had a frameless mouth-bite coordinate system applied to minimize patient discomfort; 19 patients had a frame-based coordinate system attached under local anesthesia due to inadequate dentition required for the frameless system.
The planning CT was co-registered with fine resolution brain MRIs (T1, T2, SPGR, FLAIR sequences with and without contrast). The use of contrast for the planning CT can help to identify small structures such as blood vessels which can be cross referenced on the planning CT and the fused MRI to assess the accuracy of image fusion. This is particularly relevant when the metastasis is not visible on the CT and target volume definition is reliant on the fused MRI. The gross tumor volume (GTV) was defined as the enhancing lesion on the CT and/or MRI T1 SPGR contrast enhanced sequence. The planning target volume (PTV) was defined as the GTV with a 1 mm circumferential margin. Varian Eclipse treatment planning system (Varian, Palo Alto, CA) was used to generate cone-based SRS or intensity modulated radiosurgery (IMRS) plans. For tumors < 3 cm maximum dimension in any plan, a single fraction of 14–24 Gy was delivered, generally using the RTOG guidelines (9). The variation in the doses prescribed, not conforming with these ranges, was due to individual physician choice, particularly when lower doses were used. For larger tumors, IMRS was used to deliver either 30 Gy/5 fx or 24 Gy/3 fx. The dose was prescribed at the 80% isodose line for cone-based SRS, while a minimum isodose of 95% prescription dose covered the target for the IMRS plans. Treatment characteristics for the 56 patient included in this study are depicted in Table 1.
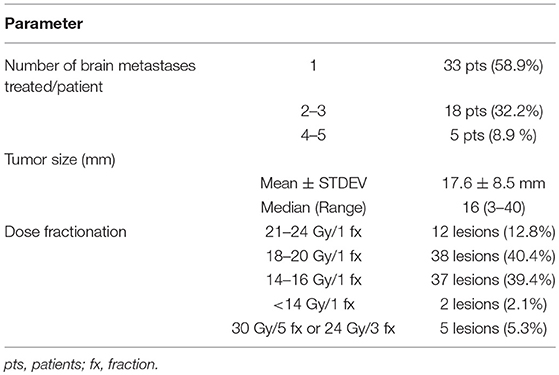
Table 1. Treatment characteristics for 94 treated brain metastases in 56 patients with primary breast cancer.
The SRS/IMRS was delivered using a Varian Trilogy Tx linear accelerator, using a cone-based or MLC based technique. Stereotactic localization was provided using the Varian SonArray infra-red localization or Vision RT surface guidance (from 2014 onwards) systems.
Steroids were not routinely recommended, however patient on steroids at the time of SRS (22 patients) were kept on the same dose (no modifications) during treatment.
Follow-up data were collected from institutional records, records from referring facilities and family physicians. After SRS, patients generally underwent routine follow-up clinical examination and imaging. MRI brain (as described above) was performed at 2 months' post SRS, then every 3 months for the first 2 years. In case of suspicion of pseudoprogression, a short-interval (6–8 weeks) MRI brain was done. For patients unable to attend our institution for follow-up, the data was retrieved from other institutional or family physician records. The data was reviewed and the response and reported toxicity were scored retrospectively. MRI images were routinely reviewed by a neurosurgeon with expertise in imaging neuroanatomy.
The primary endpoints were local control and overall survival. The secondary endpoint was distant intra-cranial progression-free survival.
Local control was defined as stability or reduction in size of the treated lesion(s) on serial MRIs. MRI response was analyzed by a neurosurgeon with expertise in brain MRI response assessment. Distant intracranial progression was defined as development of new lesion(s) outside the treated metastasis.
Patient, tumor and treatment characteristics were summarized. The following factors were analyzed for impact on local, and distant intra-cranial progression-free survival: age, clinical presentation (symptomatic vs. incidental), GPA, status of the extracranial disease at the time of SRS (controlled yes vs. no), ER status (positive vs. negative), Her 2 status (positive vs. negative), location of brain metastases (supra vs. infratentorial), number of brain metastases (targets: 1 vs. 2–3 vs. 4–5), lesion size (as a continuous variable), dose prescribed (12–16 vs. 18–20 vs. 20–24 vs. IMRS), time to development of brain metastases from the initial diagnosis (< 1 year or >1 year), and WBRT (yes vs. no).
Categorical variables were analyzed using chi-square tests. The Kaplan-Meier method was used to estimate survival times, and the log -rank tests to compare differences in survival. Survival was calculated from the date of SRS/IMRS to the date last follow-up/ death (overall survival, OS), to the date of first local progression/ death (local progression-free survival, LPFS) or to the date of first distant progression/ death (distant progression-free survival, DPFS). Overall and distant intra-cranial progression-free survival were analyzed by individual patient, while the local progression-free survival was analyzed by individual metastasis. The Cox proportional hazards model was used to assess the effects of co-variates (statistically significant in univariate analysis) on survival. All statistical tests were two-sided and assessed for a significance at 0.05 level. Statistical analyses were carried out using IMB SPSS statistical program version 24.
The cohort included 56 females with brain metastases from a breast cancer primary, with a median age of 52.8 years (30.8–82.5). Patient, tumor and treatment characteristics are detailed in Table 2. The majority of the patients (n = 54, 96.4%) had a Karnofsky performance status score (KPS) >70 and GPA of at least 2 was recorded for 68% of the patient Most patients (n = 35, 62.5%) had either no or controlled extra-cranial disease at the time of SRS, 70% of them received prior chemotherapy and 50% received systemic concurrent treatments (Herceptin or Taxanes).
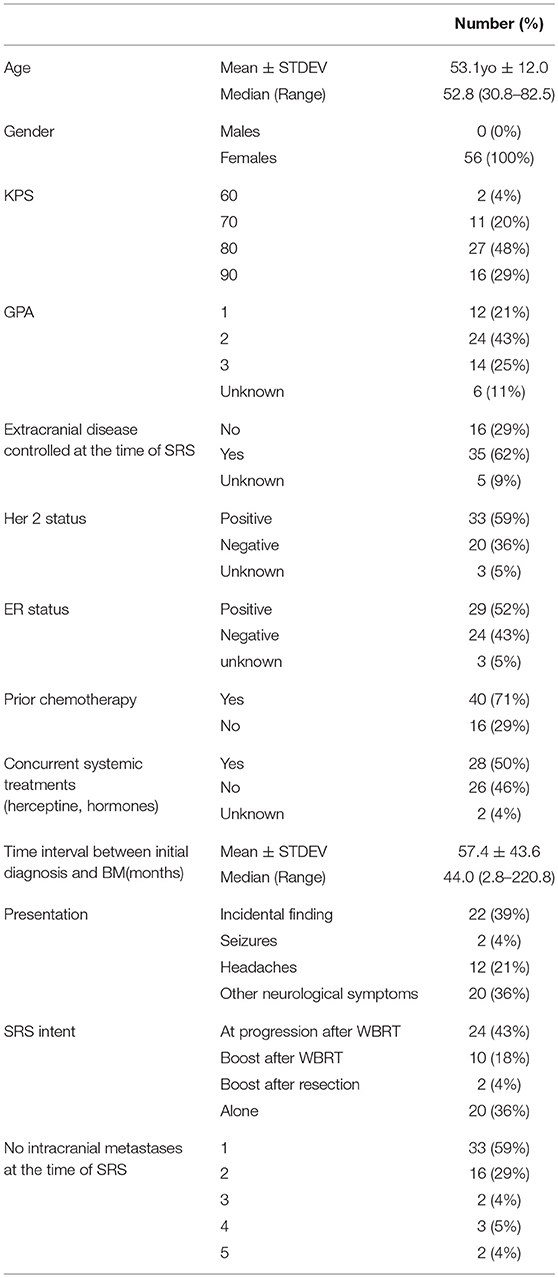
Table 2. Demographics, treatment and target characteristics in 56 patients with brain metastases from a breast cancer primary, who received stereotactic radiosurgery between 2009 and 2015.
The average age at the time of development of brain metastases was 52 years old (30–82). The median time from initial diagnosis to development of brain metastases (BM) was 44.04 months (2.82–220.8) and the median time from initial diagnosis to the SRS was 51.6 months (3.15–221.7). The average time to development of BM was significantly longer in patients with ER+ disease (ER+ vs. ER– = 76.7 vs. 32.2 months, p = 0.0001). Her 2 negative status was associated with longer time to development of BM, but it did not reach significance (Her 2– vs. Her 2+ = 69.6 vs. 47.7, p = 0.07).
Most of the patients (n = 33, 58.9%) were treated for a single brain metastasis. The median tumor size was 16 mm (3–40). However, there were five patients with more than five brain metastases at the time of SRS (one patient with six lesions, one with seven, and three patients with eight); for these patients only the progressing lesions (after prior WBRT) received SRS.
The median follow- up time for the entire cohort was 10.33 months (1.25–97.28). At the time of the last known follow-up, 17 patients (30.4%) were alive, and 39 (69.6%) have died. Among the 39 patients who died, 29 (74.35%) had uncontrolled intra-cranial disease at the time of death (13 both intra and extracranial disease uncontrolled and 16 intracranial disease only).
The overall median survival was 12.5 months (95% CI = 5.8–19.2), with 53.3%, and 35.8% surviving at 1- and 2- years post-SRS (Figure 1), with a small proportion (5–20%) surviving more than 5 years after the initial SRS.
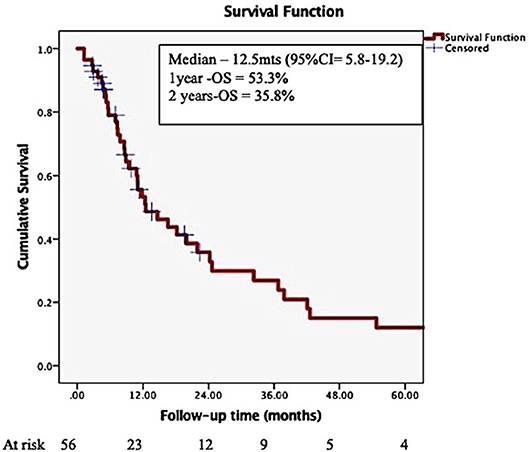
Figure 1. Overall survival in patients with brain metastases from a breast primary, treated by stereotactic radiosurgery.
In Cox multivariate analysis (MVA), after adjustment for the effect of Her 2 status, controlled extra-cranial disease at the time of SRS (HR for death if uncontrolled ECD = 2.9, 95% CI = 1.3–6.3, p = 0.009) was significantly associated with OS. The Her 2 status presented a trend toward significance (HR for death for Her 2 negative cancer = 2.1, 95% CI = 0.97–4.9, p = 0.057) after adjustment for the effect of extra-cranial disease. Addition of whole brain RT (WBRT) was not associated with increased OS. Figures 2, 3 depict the OS function of the Her 2 status and the status of extracranial disease.
During follow-up, 35 lesions (37%) have progressed, after a median of 7.3 months (1.25–97.28). Six lesions were salvaged by further local treatments (1-surgery, 2-IMRS, 3-SRS). Therefore, at the last known follow-up, of the 94 treated lesions, 61 (64.9%) were controlled locally, 29 progressed (30.9%) and we were unable to assess the response for 4 lesions (4.5%).
The median LPFS was 8.6 months (7.0–10.2), with 1- and 2 years-LPFS of 33 and 15%, respectively. LPFS is depicted in Figure 4. In univariate analysis, Her 2 status, ERHer2 group, and dose group were highly significant for LPFS (Table 3). After adjustment for the effect of Her 2 status, patients receiving 12–16 Gy can expect shorter LPFS than those receiving 18–20 Gy (HR = 1.7, 95% CI = 1.0–2.8, p = 0.043). After adjustment for the effect of dose group, patients with Her 2 negative cancer can expect shorter LPFS than those with Her 2 positive cancer (HR = 2.6, 95% CI = 1.5–4.4, p < 0.0005). Use of WBRT did not impact LC. Table 4 presents the local recurrence rates for small lesions (< 2cm) function of tumor size and dose received. Table 5 presents the local recurrence rates for all treated lesions, function of the dose received.

Figure 4. Local Progression-free survival for 94 brain metastases from a breast primary, treated by SRS.
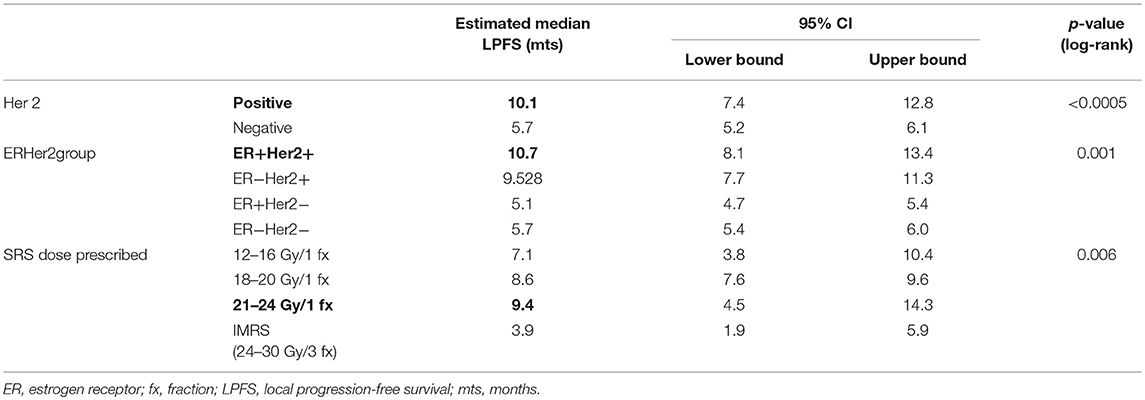
Table 3. Univariate analysis variables significant for local progression free survival in a cohort of 56 breast cancer patients with 94 brain metastases, treated by SRS.
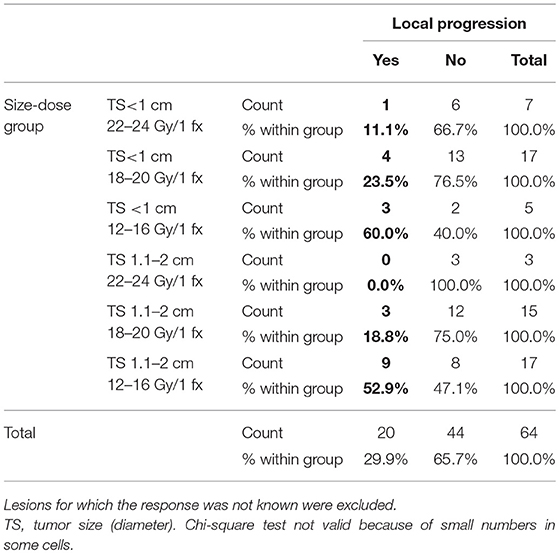
Table 4. Local progression rates for 64 small lesions (< 2cm) according to the tumor size and SRS dose prescribed.
During follow-up, 23 patients (41%) developed distant intra-cranial progression. The median DPFS was 9.85 months (7.6–12.1), with actuarial 1-, 2-years DPFS of 40.1 and 11.8%, respectively. None of the variables analyzed was significantly associated with DPFS. Particularly, WBRT either prior to, at the time to SRS or at progression, did not affect DPFS.
There was no G3 or more acute or late toxicity identified for this cohort. The most commonly identified side effect was fatigue grade 1-2, in 10 patients (17.8%).
This experience from a single institution confirms some of the findings reported from other series. The median survival of 12 months is in keeping with other publications. In this series, survival was 53.3% at 1 year after SRS and 35.8% at 2 years. Kondziolka et al. from UPMC reported the outcome for 350 breast cancer patients with 1535 brain metastases (10). Overall survival was 49% at 1 year, 26% at 2 years with a median survival of 11.2 months.
In multiple series reporting SRS for brain metastases, the primary site of origin is an important predictor of outcome (11–13). Breast cancer origin appears to be associated with improved overall survival compared to other histologies (12). It may also predict a higher prospects of achieving local control of the treated metastases. Results of several retrospective studies in patients with brain metastases from a breast primary, treated by SRS, are presented in Table 6. These series (10, 14–28) identified several factors which impact on the outcomes of these patients, with longer survival reported for higher KPS, lower RPA class, single small metastasis (< 1 cm), deep cerebral location, controlled extracranial disease and ER+ or Her2 + the biological subtypes. In this series we focused on outcome for breast cancer patients only. We observed that patients with Her-2+ brain metastases developed the metastases sooner after diagnosis than other phenotypes, in line with prior publications. This may reflect the more aggressive natural history of this subtype and its particular predilection for brain spread. Similarly, estrogen negative patients developed their brain metastases after diagnosis sooner than estrogen positive patients. However, on MVA, after controlling for other variables, the only factor associated with improved OS was controlled extra-cranial disease at the time of SRS. After adjustment for the effect of Her 2 status, LPFS was significantly correlated with the SRS dose group. After adjustment for the effect of dose group, Her 2 status was highly predictive for LPFS patients (with Her 2–status is associated with shorter LPFS with Her 2 +, HR = 2.6, 95% CI = 1.5–4.4, p < 0.0005). None of the other factors analyzed had predictive value for the studied outcomes.
Patients with Her 2 disease treated by Trastuzumab are at particular risk of developing brain metastasis. In the metastatic setting, up to 30–40% of such patients ultimately develop brain metastasis: three of the five adjuvant trails of Trastuzumab reported brain metastasis following the treatment. 1.6% of these patients ultimately develop brain metastasis (29). The reason for the high incidence of brain metastasis in Trastuzumab treated patients has been assumed to be due to the fact that Trastuzumab with 185 kDa molecular weight may not be able to cross the blood-brain barrier (BBB). Therefore, the brain might be a sanctuary site for malignant cells. Dijkers et al. (30) have performed Her2neu staining whole body scintigraphy and have demonstrated that Trastuzumab can partially penetrate the BBB. Additionally, Stemmler et al. (31) measured Trastuzumab levels in CSF of Her2neu positive brain metastasis patients and found that these levels were increased if meningeal carcinomatosis was present or if the patient had received WBRT. Analyzing these two sets of data one may postulate that BBB may be disrupted by tumor spread or by WBRT. However, it may not be disrupted by the presence of cells in the brain and this may allow brain metastasis to develop before the disruption of the BBB occurs. In other words, the cells may establish themselves as significant micro-metastatic deposits or small macroscopic deposits before the BBB is sufficiently disrupted to allow Trastuzumab to potentially treat the metastases.
The good outcome following the treatment of good prognosis limited brain metastases in Her2 positive disease may be primarily a manifestation of the overall favorable biology and efficacy of systemic therapy. However, Her 2 positive disease may be more radiosensitive than other cancer subtypes. Liang et al. (32) demonstrated in vitro Trastuzumab enhanced radiation-induced apoptosis in breast cancer cells in a Her 2 level-dependent manner. They postulated that PI3/Akt pathway may be involved in this effect. In a metanalysis by Dahabreh et al. (29), the addition of adjuvant Trastuzumab to chemotherapy resulted in a lower risk for developing locoregional recurrence (data from 3 of the 5 trials, 6,752 patients: RR 0.58, 95% CI = 0.43–0.77, p = 0.0002). However, it is not clear if this is due to concurrent radiosensitization or independent Trastuzumab activity.
Neurological death is defined as death in the presence of active intracranial or leptomeningeal disease. In 2017, McTyre (33) reported that disease specific GPA, number of brain metastases, melanoma histology and SRS dose are predictive for neurological death. Targeted therapies appear to delay neurological death. Their results are based on the analysis of outcomes of 738 patients with brain metastases (different histologies) treated by upfront SRS; in 30.6% of them neurological death occurred, while 42% died of non-neurological causes. In 2018, Shenker (19) reported the outcomes of 128 breast cancer patients treated by SRS (median doze 20 Gy/1 fx) for 1-2 brain metastases. In their series, ER+PR+ status was associated with a trend toward decreased neurological death, while status of extra-cranial disease, SRS dose and Her 2 status were associated with the non-neurological death. In our series, 75% of the patients who died had active, uncontrolled intra-cranial disease, with 56% of these patients presenting intra-cranial disease only at the time of death. Therefore, achieving intra-cranial control appears to be an important factor for the survival of the breast cancer patients in the era of targeted therapies. Moreover, 10–20% of the patients included in this cohort survived more than 5 years (Figure 1), further emphasizing the importance of intra-cranial control for survival. We could not identify any statistically significant differences between the neurological and non-neurological death groups (results not shown); however, the number of patients having uncontrolled intracranial disease at the time of death was higher for lower SRS dose delivered: 51% if 12–16 Gy/1 fx vs. 27% if 18–20 Gy vs. 20% if 21–24 Gy/1 fx (p-0.21).
In addition to clinical factors, the UPMC series (10) suggest that higher tumor dose predicted progression free survival. In patients with brain metastases from a breast primary treated by SRS, the reported 1y-LC varies between 69 and 90% (Table 5), utilizing SRS doses between 15 and 24 Gy/1 fx. However, most of the reported studies included patients who received WBRT. Very few studies report the outcomes of the patients who received SRS alone. The impact of dose on the local control was reported by two other large studies published in 2018 and 2017 (24) on patients with brain metastases from different primaries (including breast). Our study is in agreement with these previously published data: lesions treated with 21–24 Gy had a local progression rate of 8.3 %, those treated with 18–20 Gy had 21%, while 43 and 60% of those treated with 12–16 Gy and IMRS, (respectively) developed local recurrence.
Several randomized trials have assessed the value of adding whole brain radiotherapy (WBRT) to SRS for patients with limited number of brain metastases from a variety of primary sources (34). None of these trials have demonstrated an overall survival advantage to adding WBRT. It is not routinely added to SRS because it has also been demonstrated that adding WBRT to SRS increases the risk of cognitive deterioration. The use of WBRT added to SRS does however result in reduced occurrence of local progression at the treated lesions compared to SRS alone and reduces the occurrence of new brain metastases in other parts of the brain. In the trial conducted by the Alliance for Clinical Trials in Oncology local control of the treated metastases was 73% with SRS alone vs. 90% when WBRT was added to SRS (35). Patients treated with SRS alone received 24 Gy in a single fraction if lesions were < 2.0 cm or 20 Gy if lesions were 2 to 2.9 cm in maximum diameter. The focus of debate surrounding the results of these trials has understandably been the implications for the use of WBRT. However, these trials demonstrate increased local control when WBRT is added to SRS. This raises the possibility that enhanced local control of metastases treated with SRS could be achieved if the dose of SRS was increased to an optimal level, rather than adding WBRT.
A systematic review of SRS for brain metastases (arising from various multiple primary sites) demonstrates the wide variety of fraction sizes in use (36). Across the series included in the review, the range was 10 to 30 Gy. The optimal dose for single fraction SRS has therefore clearly not been identified and adopted in clinical practice. RTOG 90-05 was a dose escalation trial of single fraction SRS dose (37). That trial identified the maximum tolerated doses to be 24, 18, and 15 Gy for tumors of ≤ 20 mm, 21–30 mm, and 31–40 mm maximum diameter. These doses have since been adopted in clinical practice for previously untreated lesions often without the addition of WBRT. However, in the RTOG trial, all patients had recurrent previously irradiated primary or metastatic brain tumors. Therefore, a dose escalation trial restricted to previously unirradiated lesions would likely identify higher maximum tolerated doses. Therefore, a need is identified to conduct trials to ascertain the optimal dose for SRS in previously unirradiated cases.
In January 2019, a search on clinicaltrials.gov identified two ongoing trial escalating the dose of single fraction SRS: NCT02390518 (38) clinical trial (run by University of Utah) includes patients with 1–5 brain metastases, for whom dose escalation is preview, based on the tumor diameter and volume: for tumors < 1 cm, and < 0.52 cc: dose will be escalated to 26 Gy/1 fx, then 28 Gy/1 fx and finally to 30 Gy/1 fx. For tumors with diameters of 11–20 mm and volume 0.52–4.1 cc, dose will be escalated to 26 Gy/1 fx, then 28 Gy/1 fx and 30 Gy/fx. For large metastases with a diameters 21–30 mm and a volume 4.18–14.3 cc the dose will be escalated to 20 Gy/1 fx, then 22 Gy/1 fx and 24 Gy/1 fx. A second trial run by University of Texas (NCT02645487) (39) will escalate dose by 3 Gy/step, based on the tumor diameter: for metastasis ≤ 1 cm dose will be escalated from 24 Gy to 30 Gy/fx, for 1–2 cm size dose will escalate from 21 Gy to 27 Gy/fx; for metastases between 2 and 3 cm dose escalation from 18 to 24 Gy, and for large metastases (size 3–4 cm) dose will be escalated from 15 Gy to 21 Gy/fx.
The rapid ongoing evolution of systemic therapies targeting the individual phenotypic subtypes of breast cancer has implications for analyzing outcome of breast cancer brain SRS. The high risk of brain metastases in patients treated with the monoclonal antibody Trastuzumab, provides an illustration of how new systemic therapy can alter the risk of developing brain metastases (29, 40). It has long been recognized that systemic therapies can positively influence local control within the breast itself when radiation is used in breast conservation (33). New systemic therapies may also influence the radiosensitivity of brain metastases.
The limitations of this study are its small sample size and its retrospective nature. This analysis demonstrates encouraging results. The prolonged median survival and the moderate number of patients surviving for 2 to 3 years may be a significant advance compared to the outcome of treatment of brain metastases before SRS was developed. Further advances for such patients may result from a better understanding of biology, improved tailored systemic therapy, appropriate surgery and further developments in stereotactic radiosurgery itself.
Survival outcome is similar to the published literature. Improved outcomes are observed in patients with Her 2-positive, controlled extracranial disease at the time of SRS and higher SRS dose delivered. Achieving intra-cranial control appears to be an important factor for the survival of the breast cancer patients in the era of targeted therapies.
This is a retrospective study carried out in accordance with the recommendations of Beacon Hospital Ethics Research Committee. At the time of their treatment, all patients included in this study consented that their information can be used for the retrospective analysis of their outcomes. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Beacon Hospital Ethics Research Committee.
KA and JWa contributed to database creation, drafting of the article and editing. MD contributed to statistical analysis and editing. LR contributed to drafting of the article and editing. JWe, PT, and CM contributed to review draft and editing. AM contributed to project development, data analysis, review and editing of all versions of the draft. All authors gave final acceptance of the manuscript.
This work was supported by a public donation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
2. Mehta MP, Tsao MN, Whelan TJ, Morris DE, Hayman JA, Flickinger JC, et al. The American Society for Therapeutic Radiology and Oncology (ASTRO) evidence-based review of the role of radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. (2005) 63:37–46. doi: 10.1016/j.ijrobp.2005.05.023
3. Borgelt B, Gelber R, Kramer S, Brady LW, Chang CH, Davis LW, et al. The palliation of brain metastases: final results of the first two studies by the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. (1980) 6:1–9.
4. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. (1990) 322:494–500. doi: 10.1056/NEJM199002223220802
5. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. (1951) 102:316–9.
6. Steiner L, Leksell L, Greitz T. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Acta Chir Scand. (1972) 138:459–64.
7. Churilla TM, Chowdhury IH, Handorf E, Collette L, Collette S, Dong Y, et al. Comparison of local control of brain metastases with stereotactic radiosurgery vs. surgical resection: a secondary analysis of a randomized clinical trial. JAMA Oncol. (2019) 5:243–247. doi: 10.1001/jamaoncol.2018.4610
8. Mitri Z, Constantine T, O'Regan R. The HER2 receptor in breast cancer: pathophysiology, clinical use, and new advances in therapy. Chemother Res Pract. (2012) 2012:743193. doi: 10.1155/2012/743193
9. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. (2000) 47:291–8.
10. Kondziolka D, Kano H, Harrison GL, Yang H-C, Liew DN, Niranjan A, et al. Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. J Neurosurg. (2011) 114:792–800. doi: 10.3171/2010.8.JNS10461
11. Harris KB, Corbett MR, Mascarenhas H, Lee KS, Arastu H, Leinweber C, et al. A single-institution analysis of 126 patients treated with stereotactic radiosurgery for brain metastases. Front Oncol. (2017) 7:90. doi: 10.3389/fonc.2017.00090
12. Black PJ, Page BR, Lucas JT, Hughes RT, Laxton AW, Tatter SB, et al. Factors that determine local control with gamma knife radiosurgery: the role of primary histology. J Radiosurg SBRT. (2015) 3:281–6.
13. Flickinger JC, Kondziolka D, Lunsford LD, Coffey RJ, Goodman ML, Shaw EG, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys. (1994) 28:797–802.
14. Shenker RF, Hughes RT, McTyre ER, Lanier C, Lo HW, Metheny-Barlow L, et al. Potential prognostic markers for survival and neurologic death in patients with breast cancer brain metastases who receive upfront SRS alone. J Radiosurgery SBRT. (2018) 5:277–83.
15. Wolf A, Kvint S, Chachoua A, Pavlick A, Wilson M, Donahue B, et al. Toward the complete control of brain metastases using surveillance screening and stereotactic radiosurgery. J Neurosurg. (2018) 128:23–31. doi: 10.3171/2016.10.JNS161036
16. Pessina F, Navarria P, Cozzi L, Franceschini D, Tomatis S, Clerici E, et al. Outcome evaluation of HER2 breast cancer patients with limited brain metastasis. Anticancer Res. (2017) 37:7057–62. doi: 10.21873/anticanres.12177
17. Mix M, Elmarzouky R, O'Connor T, Plunkett R, Prasad D. Clinical outcomes in patients with brain metastases from breast cancer treated with single-session radiosurgery or whole brain radiotherapy. J Neurosurg. (2016) 125:26–30. doi: 10.3171/2016.7.GKS161541
18. Roehrig AT, Ferrel EA, Benincosa DA, MacKay AR, Ling BC, Carlson JD, et al. Pretreatment clinical prognostic factors for brain metastases from breast cancer treated with Gamma Knife radiosurgery. Surg Neurol Int. (2016) 7:S830–6. doi: 10.4103/2152-7806.194065
19. Mohammadi AM, Schroeder JL, Angelov L, Chao ST, Murphy ES, Yu JS, et al. Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J Neurosurg. (2017) 126:735–43. doi: 10.3171/2016.3.JNS153014
20. Nieder C, Oehlke O, Hintz M, Grosu AL. The challenge of durable brain control in patients with brain-only metastases from breast cancer. SpringerPlus. (2015) 4:585. doi: 10.1186/s40064-015-1384-x
21. Cho E, Rubinstein L, Stevenson P, Gooley T, Philips M, Halasz LM, et al. The use of stereotactic radiosurgery for brain metastases from breast cancer: who benefits most? Breast Cancer Res Treat. (2015) 149:743–9. doi: 10.1007/s10549-014-3242-x
22. Yang TJ, Oh JH, Folkert MR, Gupta G, Shi W, Zhang Z, et al. Outcomes and prognostic factors in women with 1 to 3 breast cancer brain metastases treated with definitive stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. (2014) 90:518–25. doi: 10.1016/j.ijrobp.2014.06.063
23. Tam M, Narayana A, Raza S, Kunnakkat S, Golfinos JG, Parker EC, et al. Role of HER2 status in the treatment for brain metastases arising from breast cancer with stereotactic radiosurgery. Med Oncol Northwood Lond Engl. (2014) 31:832. doi: 10.1007/s12032-013-0832-0
24. Yomo S, Hayashi M. The efficacy and limitations of stereotactic radiosurgery as a salvage treatment after failed whole brain radiotherapy for brain metastases. J Neurooncol. (2013) 113:459–65. doi: 10.1007/s11060-013-1138-y
25. Xu Z, Schlesinger D, Toulmin S, Rich T, Sheehan J. Impact of triple-negative phenotype on prognosis of patients with breast cancer brain metastases. Int J Radiat Oncol Biol Phys. (2012) 84:612–8. doi: 10.1016/j.ijrobp.2011.12.054
26. Kelly PJ, Lin NU, Claus EB, Quant EC, Weiss SE, Alexander BM. Salvage stereotactic radiosurgery for breast cancer brain metastases: outcomes and prognostic factors. Cancer. (2012) 118:2014–20. doi: 10.1002/cncr.26343
27. Caballero JA, Sneed PK, Lamborn KR, Ma L, Denduluri S, Nakamura JL, et al. Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys. (2012) 83:303–9. doi: 10.1016/j.ijrobp.2011.06.1987
28. Karam I, Nichol A, Woods R, Tyldesley S. Population-based outcomes after whole brain radiotherapy and re-irradiation in patients with metastatic breast cancer in the trastuzumab era. Radiat Oncol Lond Engl. (2011) 6:181. doi: 10.1186/1748-717X-6-181
29. Dahabreh IJ, Linardou H, Siannis F, Fountzilas G, Murray S. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. (2008) 13:620–30. doi: 10.1634/theoncologist.2008-0001
30. Dijkers ECF, Kosterink JGW, Rademaker AP, Perk LR, van Dongen GAMS, Bart J, et al. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl Med. (2009) 50:974–81. doi: 10.2967/jnumed.108.060392
31. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. (2007) 18:23–8. doi: 10.1097/01.cad.0000236313.50833.ee
32. Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther. (2003) 2:1113–20.
33. McTyre ER, Johnson AG, Ruiz J, Isom S, Lucas JT, Hinson WH, et al. Predictors of neurologic and nonneurologic death in patients with brain metastasis initially treated with upfront stereotactic radiosurgery without whole-brain radiation therapy. Neuro Oncol. (2017) 19:558–66. doi: 10.1093/neuonc/now184
34. Aoyama H, Tago M, Shirato H Japanese radiation oncology study group 99-1 (JROSG 99-1) investigators. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol. (2015) 1:457–64. doi: 10.1001/jamaoncol.2015.1145
35. Brown PD, Jaeckle K, Ballman KV, Farace E, Cerhan JH, Anderson SK, et al. Effect of radiosurgery alone vs. radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. (2016) 316:401–9. doi: 10.1001/jama.2016.9839
36. Nieder C, Grosu AL, Gaspar LE. Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol Lond Engl. (2014) 9:155. doi: 10.1186/1748-717X-9-155
37. Shaw E, Scott C, Souhami L, Dinapoli R, Bahary JP, Kline R, et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05). Int J Radiat Oncol Biol Phys. (1996) 34:647–54.
38. NCT02390518:An Ongoing Trial by University of Utah. Available online at: https://fdaaa.trialstracker.net/trial/NCT02390518/ (accessed January 18, 2019).
39. NCT02645487: An Ongoing Trial by University of Texas Southwestern Medical Center. Available online at: http://fdaaa.trialstracker.net/trial/NCT02645487/ (accessed January 18, 2019).
Keywords: brain metastases, SRS, Her 2 status, breast cancer, dose
Citation: Armstrong K, Ward J, Dunne M, Rock L, Westrup J, Mascott CR, Thirion P and Mihai AM (2019) Linac-Based Radiosurgery for Patients With Brain Oligometastases From a Breast Primary, in the Trastuzumab Era-Impact of Tumor Phenotype and Prescribed SRS Dose. Front. Oncol. 9:377. doi: 10.3389/fonc.2019.00377
Received: 22 January 2019; Accepted: 23 April 2019;
Published: 28 May 2019.
Edited by:
William Small Jr., Stritch School of Medicine, United StatesReviewed by:
Joshua Silverman, New York University, United StatesCopyright © 2019 Armstrong, Ward, Dunne, Rock, Westrup, Mascott, Thirion and Mihai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alina Mihaela Mihai, YWxpbmEubWloYWlAYWx1bW5pLnV0b3JvbnRvLmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.