- 1Department of Radiation Oncology, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ, United States
- 2Division of Surgical Oncology, Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ, United States
- 3Division of Medical Oncology, Rutgers Cancer Institute of New Jersey, Rutgers University, New Brunswick, NJ, United States
- 4Division of Gastroenterology and Hepatology, Rutgers Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ, United States
- 5Division of Surgical Oncology, Department of Surgery, Emory University School of Medicine, Atlanta, GA, United States
- 6Department of Radiology, Rutgers Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ, United States
Hepatocellular carcinoma (HCC) is the second most common cause of cancer death worldwide, with a majority of HCC patients not suitable for curative therapies. Approximately 70% of initially diagnosed patients cannot undergo surgical resection or transplantation due to locally advanced disease, poor liver function/underlying cirrhosis, or additional comorbidities. Local therapeutic options for patients with unresectable HCC, who are not suitable for thermal ablation, include transarterial embolization (bland, chemoembolization, radioembolization) and/or external beam radiation therapy (EBRT). Regarding EBRT specifically, technological advancements provide a means for safe and effective radiotherapy delivery in a wide spectrum of HCC patients. In multiple prospective studies, EBRT delivery in a variety of different fractionation schemes or in combination with transcatheter arterial chemoembolization (TACE) demonstrate improved outcomes, particularly with combination therapy. The Barcelona Clinic Liver Cancer classification provides a framework for treatment selection; however, given the growing complexity of treatment strategies, this classification system tends to simplify decision-making. In this review, we discuss the current literature regarding unresectable HCC and propose a modified treatment algorithm that emphasizes the role of radiation therapy for Child-Pugh score A or B patients with ≤3 nodules measuring >3 cm, multinodular disease or portal venous thrombosis.
Introduction
Hepatocellular carcinoma (HCC) is the second most common cause of cancer death worldwide (1). While the incidence of HCC is highest in Asia, the rate has been increasing significantly in North America (2). HCC can result in patients with liver cirrhosis, and known major risk factors for cirrhosis include viruses [chronic hepatitis B virus (HBV) and hepatitis C virus (HCV)], toxins (e.g., alcohol, tobacco, and aflatoxins), and metabolic disorders (e.g., nonalcoholic steatohepatitis, and diabetes) and other conditions, such as hereditary hemochromatosis (3). The American Association for the Study of Liver Diseases (AASLD) recommends surveillance of patients with cirrhosis using ultrasound, with or without alpha-fetoprotein (AFP), every 6 months (4). Patients with HCC are often asymptomatic at the time of diagnosis leading to a delay in diagnosis for patients not being screened for HCC in the setting of viral hepatitis infection. Classic imaging characteristics of arterial enhancement and venous or delayed-phase washout of lesions >1cm in patients with cirrhosis or chronic HBV are considered by some as diagnostic for HCC even in the absence of histologic confirmation (5).
Local control (LC) is the most important prognostic factor for HCC, because up to 92% of deaths can be directly correlated to local progression leading to liver failure rather than distant metastases (6, 7). While liver transplantation or surgical resection remains the principal curative option for patients with HCC, only 30% are suitable for this therapy. Patients are often deemed non-surgical candidates due to locally advanced disease, poor liver function, additional comorbidities, and/or poor performance status (8). For HCC patients who are non-surgical candidates, an alternative curative therapeutic option is radiofrequency ablation (RFA), which has optimal outcomes in tumors <3 cm that are primarily located away from major blood vessels, bile ducts, and abdominal organs (9). Some unresectable HCC patients are also not candidates for RFA due to location of the tumor, intrahepatic bile duct dilation, and volume of disease; for these patients other local therapies include transcatheter arterial bland embolization, chemoembolization (TACE), radioembolization (TARE) with Yttrium-90 (90Y) microspheres, and external beam radiation therapy (EBRT). Inoperable lesions for which local ablation is not possible are treated with TACE since non-randomized studies suggest it may improve survival compared to best supportive care (10, 11). Historically, liver EBRT was not employed due to risk of radiation-induced liver disease (12, 13). However, modern imaging techniques, advances in EBRT planning and delivery, and improvements in biological understanding of radiation dose tolerances to liver parenchyma have led to reconsideration of EBRT in the context of other local treatment options. Moreover, with a better understanding of the dose-volume effects of partial liver radiation and utilization of advanced radiation technology, severe toxicity rates following EBRT are now less than 10% (14).
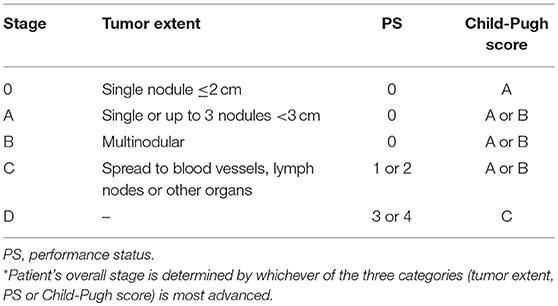
The Barcelona Clinic Liver Cancer (BCLC) staging classification combines tumor number, size, extent of spread, performance status, and Child Pugh (CP) score to provide treatment recommendations for patients with HCC (Table 1) (15, 16). While the BCLC is the most commonly used treatment algorithm for newly diagnosed HCC, it may inadequately reflect therapeutic advances and simplify decision making, particularly in patients with BCLC Stage B or C disease. For example, the algorithm does not provide treatment recommendations for patients with ≤3 nodules measuring >3 cm, a common presentation in the era of advanced imaging techniques. Furthermore, in light of growing prospective data evaluating the role of modern radiation therapy (RT) for HCC, EBRT has emerged as a potential treatment option in select patients with BCLC Stage B and C disease and EBRT is not incorporated into this algorithm.
Given this constantly evolving treatment paradigm, herein, we evaluate the published data on local therapeutic options for unresectable HCC and propose a functional treatment algorithm for CP-A or B patients with ≤3 nodules measuring >3 cm, multinodular disease, or portal venous thrombosis (PVT) (Figure 1).
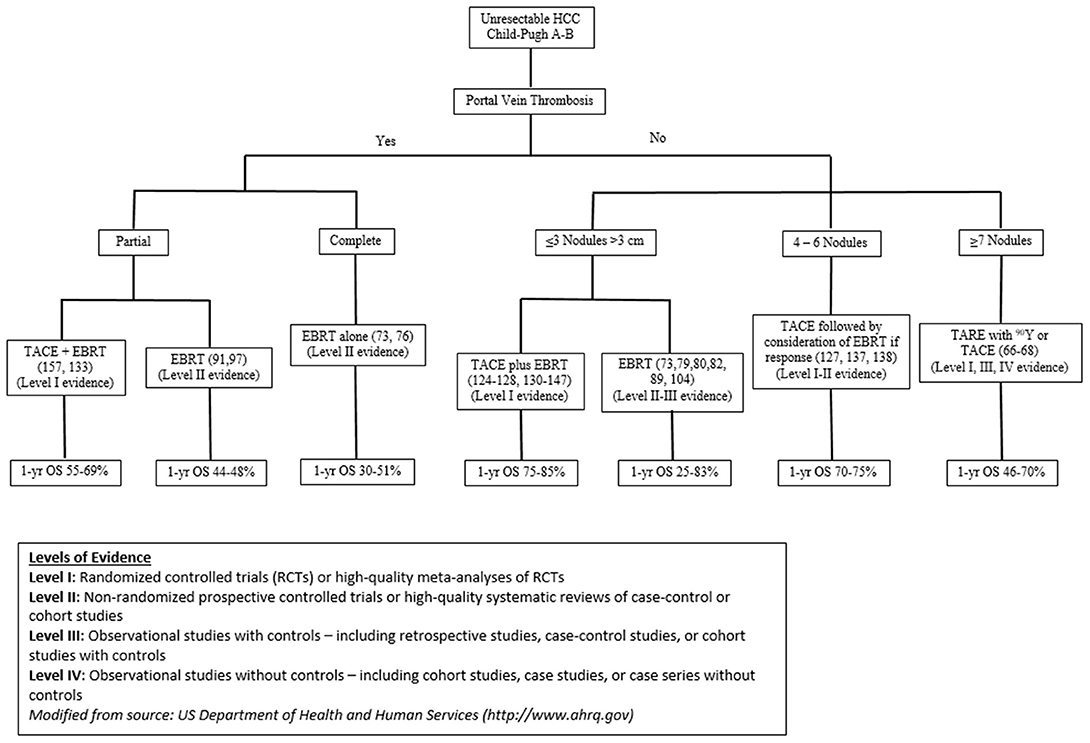
Figure 1. Data driven Rutgers treatment algorithm for optimizing outcomes for unresectable HCC patients.
TACE
TACE is a commonly used treatment modality in patients with HCC who are deemed poor candidates for curative treatment with surgery or RFA. According to a recent Surveillance, Epidemiology, and End Results (SEER) database analysis, TACE is the most common initial therapy utilized in the US (64%) among patients who receive treatment for HCC (17). This treatment involves the intra-arterial injection of chemotherapeutic agents followed by obstruction of selective hepatic arterial inflow with embolizing particles. The purpose of this procedure is to increase tumor cell exposure to cytotoxic agents and selectively tamponade the blood supply to the tumor or affected liver lobe. The resultant stasis of blood flow and hypoxia induces vascular endothelial growth factor (VEGF) secretion, which increases vessel permeability and results in higher intra-hepatic chemotherapy deposition (18). TACE with drug-eluting beads (DEB-TACE) utilizes embolizing particles to both embolize the hepatic artery and carry the cytotoxic agents. The most commonly employed chemotherapeutic agents are mitomycin-C and doxorubicin.
Patient selection for TACE is an important step to avoid treatment-related adverse events. Ideal candidates present with Eastern Cooperative Oncology Group (ECOG) performance status ≤2, preserved liver function (CP-A), and tumors measuring <10 cm without portal vein thrombosis (PVT). Select patients with impaired liver function (CP-B) and mildly impaired performance status can be treated with TACE; however, the incidence of treatment-related toxicities including abdominal pain, nausea, and vomiting increases in this patient population (19). Contraindications to TACE include an ECOG performance status >2, advanced cirrhosis (CP-C), >50% replacement of the liver by tumor, PVT, renal insufficiency (creatinine ≥2 mg/dl or creatinine clearance <30 ml/min), bilirubin levels >3 mg/dL, macroscopic vascular invasion, extrahepatic disease, bile duct occlusion, and comorbidities involving compromised organ function, such as active cardiovascular disease (20–25).
Multiple studies have demonstrated, when compared to best supportive care, TACE improves survival in HCC patients with a wide range of disease states including CP-A and B, tumor size measuring <14 cm, and multinodular disease (Table 2) (10, 11, 26–30). In order to further improve the response to TACE, combination treatments with systemic and/or other locoregional therapies have been investigated in recent years (31–40).
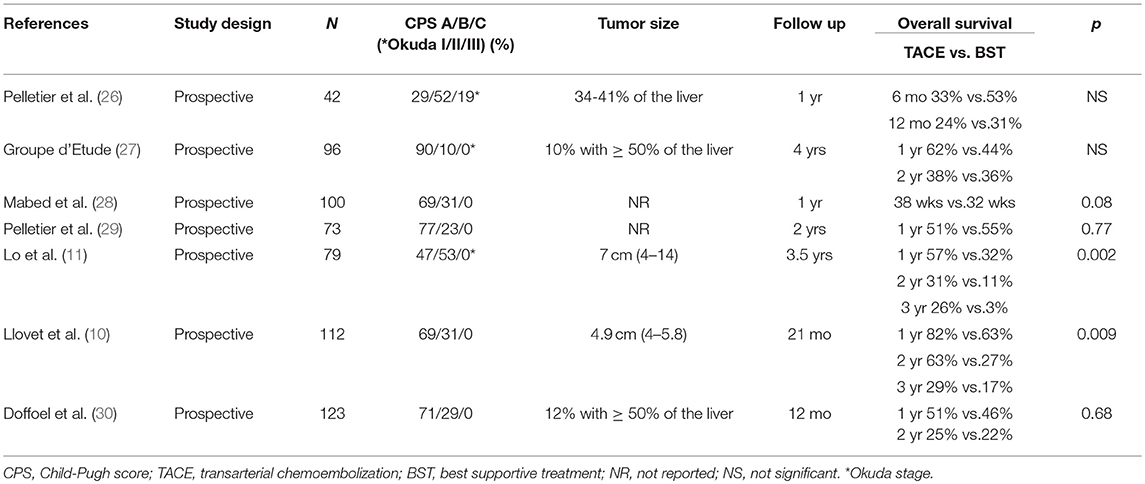
Table 2. Studies of transcatheter arterial chemoembolization vs. best supportive treatment for hepatocellular carcinoma.
TACE Plus Molecularly Targeted Therapy
Treatment with TACE can lead to the promotion of tumorigenesis and angiogenesis (41), which may partially explain the limited long-term benefit of this therapy. Thus, in an attempt to improve the efficacy of TACE, studies have combined this treatment with concurrent systemic targeted therapies (Table 3) (31–40). One such targeted therapy is sorafenib, a small molecule inhibitor of several tyrosine protein kinases (TKI) including vascular endothelial growth factor receptors (VEGFRs)-1, 2, and 3 and platelet-derived growth factor receptor β (PDGFR-β) (43, 44). In preclinical studies, sorafenib demonstrated antiproliferative activity in malignant hepatic cell lines by decreasing tumor angiogenesis and tumor-cell signaling as well as increasing tumor-cell apoptosis (45). Given these findings, in a multicenter randomized phase III trial, sorafenib demonstrated improved median overall survival (OS) when compared to placebo (10.7 months vs. 7.9 months; P < 0.001) in patients with CP-A advanced HCC (46). Furthermore, in cell culture models, sorafenib reduced the susceptibility of hepatocytes to HCV infection via anti-VEGF activity (47, 48) and directly inhibited HCV replication via non-structural HCV replicon protein NS5A interaction with C-Raf (49). In light of this preclinical information, sorafenib is also thought to be more effective in hepatitis-related HCC (50).
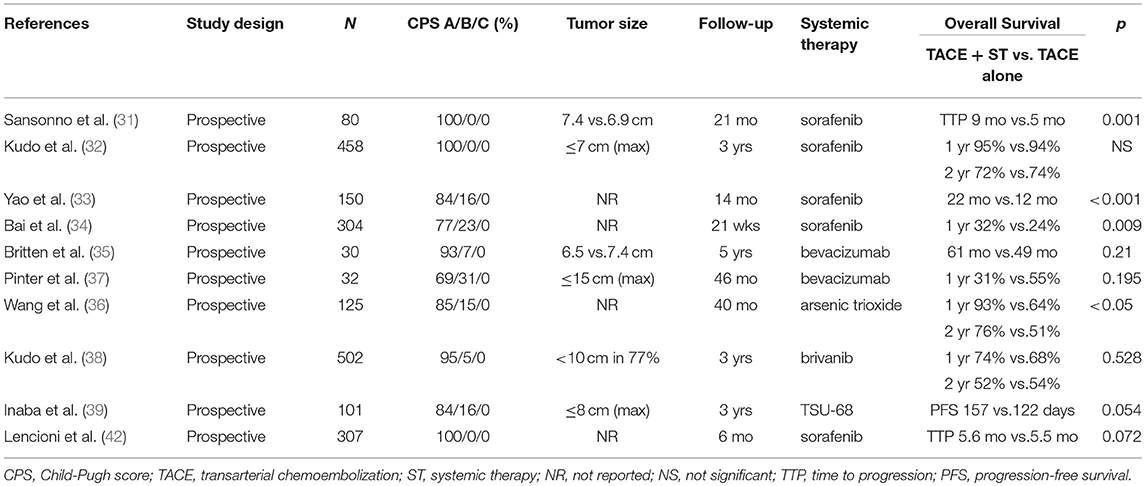
Table 3. Studies of transcatheter arterial chemoembolization plus systemic therapy vs. transcatheter arterial chemoembolization alone for hepatocellular carcinoma.
Given the mechanisms of action of both sorafenib and TACE, there is growing support for this therapeutic combination to take advantage of a possible synergistic effect. TACE increases the concentration of angiogenic growth factors such as VEGF and insulin-like growth factor-2 (IGF-2), which may contribute to disease progression (51), while sorafenib inhibits angiogenic growth factors to prevent progression. Based on this rationale, the combination of TACE with sorafenib has been investigated in a number of studies (32, 42, 52). Lencioni et al. randomized 307 intermediate stage HCC patients to sorafenib plus drug eluting bead (DEB)-TACE vs. placebo plus DEB-TACE (42). All patients had no evidence of macrovascular invasion or extrahepatic spread, were CP-A, and had an ECOG performance status of 0. There was no difference in median time-to-tumor progression (TTP) between the two groups (5.6 months vs. 5.5 months, hazard ratio (HR) 0.797, p = 0.072). Additionally, the overall response rates for patients receiving sorafenib vs. placebo were 55.9% and 41.3%, respectively, and the disease control rates were 89.2% and 76.1%, respectively. Kudo et al. randomized 458 HCC patients with CP-A and tumors ≤3 cm to sorafenib plus TACE or TACE alone. Similarly, there were no differences in OS at 1 year (95% vs. 94%) or 2 years (72% vs. 74%) (32). While Lencioni et al. and Kudo et al. reported combination therapy did not impact OS, smaller prospective studies evaluating the same combination therapy reported opposite results with improved survival (31, 33).
Therefore, TACE has been combined with multiple targeted agents including sorafenib, but to date, this combination therapy has not led to a meaningful increase in survival (Table 3) (35–38, 40). While sorafenib is considered first-line systemic therapy after failure of liver-directed therapies, a new multikinase inhibitor, lenvatinib, has emerged as a new alternative first-line treatment option (53). In the randomized phase III REFLECT trial, lenvatinib demonstrated a comparable OS (median, 13.6 vs. 12.3 months, HR 0.92, 95% CI 0.79–1.06) and improved TTP (median, 8.9 vs. 3.7 months, HR 0.63, 95% CI 0.53–0.73) over sorafenib. In the phase I/II CheckMate 040 trial, a PD-1 inhibitor, nivolumab, demonstrated a 20% objective response (HR 95% CI 15–26) in patients with advanced HCC and is now approved as second-line therapy following prior sorafenib (54). More recently, in the phase III CLESTIAL trial, cabozantinib has been shown to improve median OS compared with placebo after progression on sorafenib (10.2 vs. 8 months, P = 0.005) (55). Given the encouraging results of these studies, combination of these new agents with TACE should be investigated in prospective studies.
Radiosensitization with systemic therapy is the principle underlying many treatment regimens for solid malignancies and has gained interest for HCC in recent years. RT has multiple effects on the tumor microenvironment and the immune system, including cytokine and antigen release leading to increased immune cell infiltrate (56). The potential increase in toxicity with using combination therapy remains the main concern as several clinical studies (NCT03203304 and NCT03482102) are currently evaluating the optimal combination strategies with RT.
TACE Plus RFA
Radiofrequency ablation (RFA) plus TACE can also be utilized in HCC patients. RFA is considered an effective treatment for tumors <3 cm by conducting high-energy electrical current or microwaves into the target lesion which then leads to tumor tissue necrosis (57). Reported LC rates are as high as 90%; however, this decreases significantly with increasing tumor size as well as close proximity to major vessels due to a heat-sink effect (58, 59). The heat-sink effect is a phenomenon that occurs when flowing hepatic blood causes a cooling effect, thereby reducing the ablation volume. Based on the theory that performing TACE before RFA may allow retention of thermal energy within the tumor environment by decreasing blood flow, studies compared RFA alone to TACE plus RFA and demonstrated improved survival for the latter in patients with HCC measuring <3 cm (60). However, the survival benefit decreases in tumors >3 cm. Lin et al. randomized 62 patients with HCC to either TACE plus RFA or RFA alone from 2006 to 2010 (61). Patients were CP-A or B and had ≤ 3 tumors measuring 3–5 cm with no evidence of extrahepatic tumor metastasis or macrovascular invasion. The 1-, 2-, and 3-year local tumor progression rates in the TACE plus RFA group (12.5%, 18.75%, and 18.75%) were significantly lower than in the RFA alone group (16.7%, 30%, and 36.6%, P = 0.047). However, 1-, 2-, and 3- year OS rates remained similar between the two treatment groups (90.6% vs. 83.3%, 72% vs. 56.75%, and 53.1% vs. 23.3%, P = 0.176). Given the improved prognosis with combination therapy in patients with small HCCs, TACE plus RFA may be considered in CP-A or B patients with ≤3 tumors measuring <3 cm.
Transarterial Radioembolization (TARE) With Yttrium-90 (90Y)
TARE with 90Y involves the injection of β-emitting 90Y loaded glass matrices or resin microspheres into the hepatic artery which leads to delivery of concentrated radiation to the tumor. The radioisotope 90Y is a pure β-radiation emitter with a half-life of 64.2 h, an average energy of 0.94 MeV, and an average penetration range in tissue of 2.5 mm (62–64). Absolute contraindications for 90Y radioembolization include significant intractable clinical ascites, bleeding diathesis, severe portal hypertension with hepatofugal flow, or severe peripheral vascular disease that would preclude arterial catheterization (65). Moreno-Luna et al. compared unresectable HCC patients treated with TARE (n = 61, 87% CP-A, 69% multinodular, and mean tumor size 6 cm, range 2–9 cm) in a non-randomized study to those treated with TACE (n = 55, 80% CP-A, 42% multinodular, and mean tumor size 6 cm, range 2–10 cm) between 2005 and 2008 (66). While the complete tumor response rate was higher with TARE (12% vs. 4%, p = 0.17), there was no difference in median OS between the two groups (15.0 months for TARE vs. 14.4 months for TACE; p = 0.47). Furthermore, TARE was more likely to induce fatigue (p = 0.003) but less likely to cause fever (p = 0.02). Salem et al. also prospectively compared unresectable HCC patients treated with TARE (n = 123, 54% CP-A, 55% multinodular, and mean tumor size 5 cm, range 2–7 cm) to those treated with TACE (n = 122, 55% CP-A, 53% multinodular, and mean tumor size 3 cm, range 2–6 cm) (67). They found median TTP was longer following TARE (13.3 months vs. 8.4 months, p = 0.046) but median OS did not differ significantly between the two groups (17.4 months vs. 20.5 months, respectively, p = 0.232). Additionally, abdominal pain and increased transaminase activity were more common with TACE (p < 0.05). In the phase III SIRveNIB trial, Chow et al. randomized 360 HCC patients (90% CP-A and 24% with tumor size >50% of liver) to TARE or sorafenib (68). While there was no difference in OS (HR, 1.1; 95% CI, 0.9 to 1.4; p = 0.36), there was an improvement in response rate with TARE (16.5% vs. 1.7%, P < 0.001), as well as reduced grade ≥3 toxicity compared with sorafenib (27.7% vs. 50.6%, P < 0.001). In light of this data, 90Y radioembolization is considered a viable treatment option for patients with multinodular HCC, but sorafenib remains a standard of care.
Radiation Therapy
Technological advances in EBRT such as CT-based treatment planning, management of respiratory motion, understanding of treatment dose distributions, delineation of organs at risk (OAR), and the transition from whole liver irradiation (WLI) to more conformal/dose-escalated treatment regimens have provided the opportunity to offer EBRT safely and effectively. As such, the use of EBRT in patients with HCC has increased in recent years (69). RT techniques, including 3D-conformal radiotherapy (3D-CRT), intensity modulated radiotherapy (IMRT), stereotactic-body radiotherapy (SBRT), and proton beam radiotherapy (PBT), have allowed for the delivery of higher RT doses to tumor volumes compared to historical WLI, and in turn, may have resulted in improved outcomes when compared to other local therapies for HCC (58, 70, 71).
Different RT techniques have been used for a wide range of patients and within various HCC subgroups. Nevertheless, we must note that the use of RT for patients with CP-C disease is very limited. Furthermore, the data on the use of RT for patients with CP-B disease is still unsettled, as a smaller proportion of patients with CP-B disease were enrolled in clinical trials (Tables 4–7). Therefore, use of RT in this subgroup of patients should be carried out after multi-disciplinary evaluation with individualized treatment for each patient.
Conformal Radiotherapy (CRT)
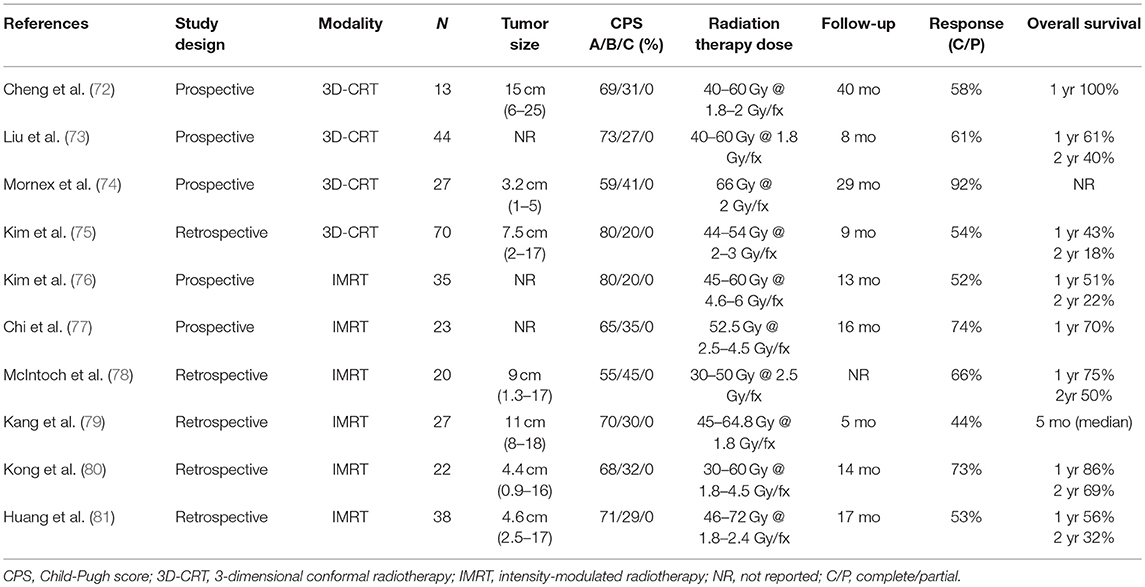
3D-CRT and IMRT improve the effectiveness of EBRT by increasing the RT dose to the tumor while simultaneously reducing the RT dose to the surrounding normal liver parenchyma when compared to WLI (Table 4) (72–81). Historically, EBRT techniques used for HCC consisted of 2-dimentional planning. Given the associated toxicities of this WLI technique, it was not considered clinically beneficial owing to the sub-therapeutic dose of RT delivered to the tumor.
With the advancement of RT delivery to 3D techniques, the normal liver parenchyma could be from spared the high dose exposure while the dose to the tumor itself could be increased. The ability to escalate dose has been a significant improvement since studies have demonstrated that higher RT dose to the tumor correlates with better OS. Seong et al. treated 158 patients with HCC (74% CP-A, 26% CP-B, 51% PVT, 75% tumor size <10 cm, and 25% tumor size >10 cm) with a dose of 25.2–60 Gy in 1.8 Gy per fraction (142). The median OS in patients treated with <40 Gy, 40–50 Gy, and >50 Gy were 6 months, 8 months, and 13 months, respectively. On multivariate analysis, greater RT dose to the tumor was the only significant factor associated with survival (p = 0.01). Other studies also demonstrated that a total dose of >40–50 Gy in standard fractionation led to a higher response or survival rate (73, 143, 144). Larger radiotherapy doses can often more readily be delivered to smaller tumors in locations where nearby organs are not abutting the tumor. However, the tolerance dose of the normal liver parenchyma, especially in the setting of poor baseline liver function, often limits the use of higher doses of EBRT in the setting of HCC.
IMRT is another conformal RT technique that allows delivery of a higher RT dose when compared to 3D-CRT which may further improve OS without increasing the risk of radiation-induced liver disease (RILD) in patients with ≤3 tumor nodules measuring >3 cm and/or PVT. IMRT uses inverse treatment planning which modulates the intensity of multiple beams to gain a desired target coverage while minimizing the dose to normal structures. Early dosimetric studies comparing IMRT to 3D-CRT suggested that IMRT improves planning target volume (PTV) coverage while maintaining normal tissue tolerances (145). Yoon et al. retrospectively reviewed 187 patients with HCC and CP-A treated with 3D-CRT (n = 122; median fractional and total dose: 1.8 Gy and 45 Gy, respectively) or IMRT (n = 65; median fractional and total dose: 2.5 Gy and 50 Gy, respectively) from 2006 to 2011. Median tumor size (9 cm vs. 10 cm, p = 0.779) and ECOG PS ≤1 (44% vs. 40%, p = 0.557) were similar in both groups and 74% had ≤3 tumor nodules. Patients treated with IMRT had significantly higher 3-year OS (33.4% vs. 13.5 %, P < 0.001), progression-free survival (PFS) (11.1% vs. 6.0%, P = 0.004), and in field-failure-free survival rates (46.8% vs. 28.2%, P = 0.007) when compared to patients treated with 3D-CRT; no difference in RILD was demonstrated (P = 0.716) (146). Similar results were reported by Hou et al. in 118 HCC patients with portal vein and/or inferior vena cava tumor thrombi (81% CP-A and 19% CP-B) (147). Higher RT doses were delivered when IMRT was utilized compared to 3D-CRT (average dose 57.86 ± 7.03 Gy vs. 50.88 ± 6.60 Gy, P ≤ 0.001). Additionally, median OS was significantly higher in patients treated with IMRT compared to 3D-CRT (15.47 months vs. 10.46 months, P = 0.005) while the overall toxicity was similar between the two groups (grade 3 toxicity 5% vs. 2%, P = 0.786). While robust prospective data are lacking, dose escalation with IMRT is considered as a treatment modality in HCC patients with CP-A/B and ≤3 tumor nodules measuring >3 cm and/or PVT.
SBRT
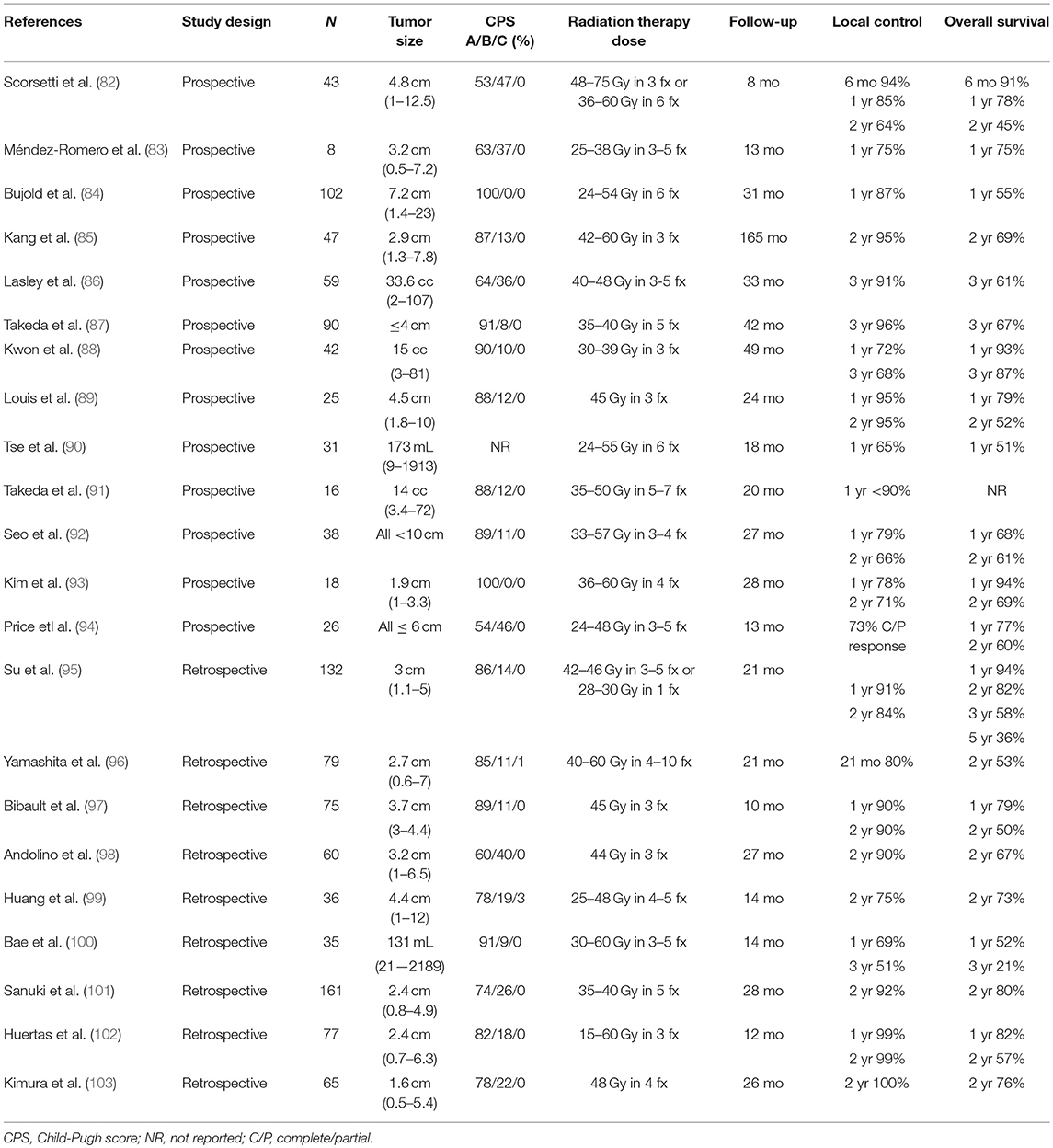
SBRT is a type of EBRT that delivers an highly conformal high dose of RT to a target in 1–5 fractions. One of the first series evaluating SBRT for HCC was described by Blomgren et al. in 1995 (148); since then, this technique has demonstrated excellent outcomes in numerous trials/studies despite often being utilized in patients unsuitable for other therapies believed to have a poor prognosis (Table 5) (82–103).
Although there are no phase III data yet, growing retrospective as well as prospective evidence support promising outcomes with SBRT and its use as an alternative HCC therapy. Yuan et al. retrospectively compared 48 patients with HCC treated with SBRT (n = 22, 88% CP-A, 12% CP-B, median tumor size 4.3 cm, median dose 45 Gy, range 39–54 Gy in 3–8 fractions) or microscopic complete resection (n = 26, CP-A, 26% CP-B median tumor size 4.6 cm) from 2006 to 2011 and found no significant difference in OS or PFS between the two cohorts (149). Sapir et al. reported the outcomes of 209 patients with HCC treated with SBRT (n = 125, ≤2 tumor nodules, median tumor size 2.4 cm, range 0–20.8 cm, median CP-score 6, range 5–9) vs. TACE (n = 84, ≤2 tumor nodules, median tumor size 2.7 cm, range 0.7–15 cm, median CP-score 6, range 5–9) (70). The 1- year and 2- year LC rates favored SBRT (97% and 91%, respectively) when compared to TACE (47% and 23%, respectively; HR 66.5, p < 0.001). Wahl et al. retrospectively reported the outcomes from 224 patients with HCC (median tumor size 2.2 cm; range 0–10 cm) treated with SBRT (n = 63, 69% CP-A, 29% CP-B, 92% ≤2 tumor nodules, and median dose 27 to 60 Gy in 3–5 fractions) or RFA (n = 161, 50% CP-A, 18% CP-B, and 89% ≤2 tumor nodules) from 2004 to 2012 (58). One-year liver specific PFS after SBRT compared to RFA was 97.4% vs. 83.6%, and 2-year OS rates were 46% vs. 53%, respectively. For tumors ≥2 cm, LC with RFA was significantly lower compared to SBRT (HR, 3.35; 95% CI, 1.17 to 9.62; p = 0.025). There was no difference in acute grade ≥3 toxicities between the RFA and SBRT groups (11% vs. 5%, p = 0.31). Mendez-Romero et al. reported the first prospective outcomes of SBRT for HCC in 2006. Eight HCC patients (median tumor size 3.2 cm and 63% with CP-A) who were ineligible for other local therapies received SBRT (83); tumors <4 cm received 37.5 Gy in 3 fractions and those ≥4 cm received 25 Gy in 5 fractions. One-year LC and OS rates were both 75%. Local failure was seen in 4 patients that were in the 25 Gy treatment arm. Kang et al. reported the efficacy of SBRT as a local salvage treatment after incomplete response to TACE (85). Forty-seven patients with HCC (median tumor size 2.9 cm, 87% CP-A, and 98% ≤2 tumor nodules) were treated with a RT dose up to 60 Gy in 3 fractions (42–60 Gy) 1 to 2 months post TACE. The 2-year LC, OS, and PFS rates were 95%, 69%, and 34%, respectively. SBRT was well tolerated, with CP-class worsening from A to B in 13% of patients following treatment. Given these results, currently SBRT can be considered in patients with CP-A/B and ≤2 tumor nodules measuring ≤10 cm and/or PVT (84); however, it should be used cautiously in the setting of lower platelet counts (OR, 0.90; median, 108 × 109/L vs. 150 × 109/L) or higher dose to 800 cc of liver (OR, 1.11; median, 14.3 Gy vs. 6.0 Gy) as these factors are strongly associated with liver function decline (150). Finally, multiple comparative trials are currently ongoing to define optimal sequencing of SBRT in combination with other treatment modalities (NCT02323360, NCT02182687, NCT02762266, NCT02470533, and RTOG 1112).
Proton Beam Therapy
Protons, unlike photons, exhibit a sharp dose falloff known as the Bragg peak (151). The lack of exit dose with PBT becomes important in the management of HCC as it allows the sparing of large volumes of normal liver parenchyma and other surrounding OAR, which may potentially decrease the risk of toxicity while permitting possible escalation of radiation doses.
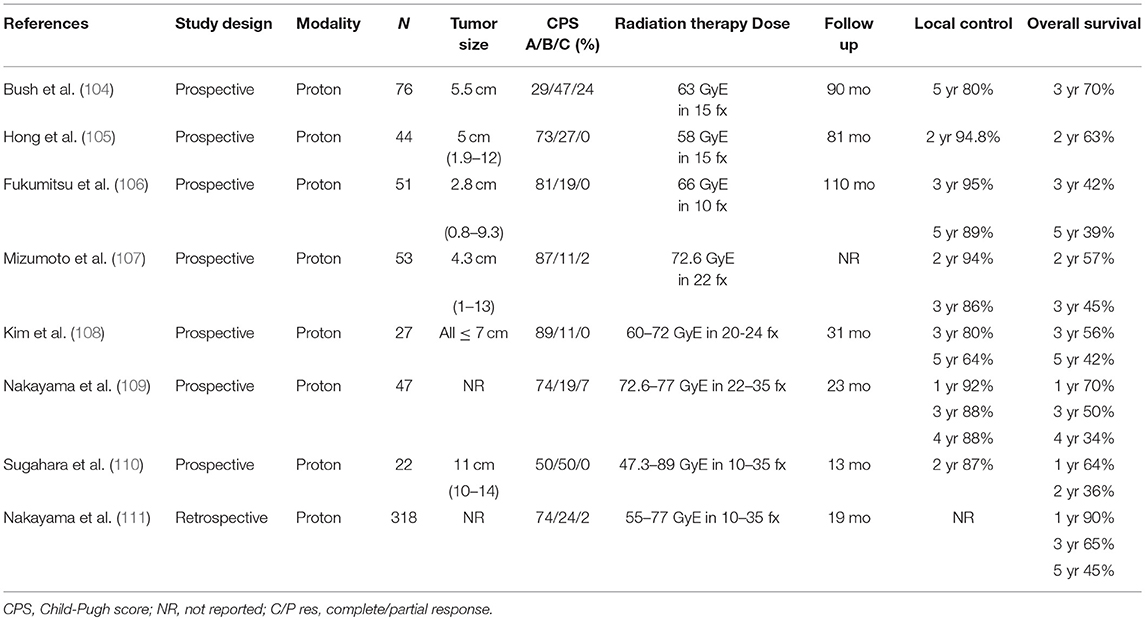
Numerous single-institutional series have evaluated the efficacy and toxicity of PBT for HCC (Table 6) (104–110). Nakayama et al. reported outcomes in a prospective study of 318 patients with HCC (74% CP-A, 24% CP-B, ≤3 tumor nodules, and 14% PVT) treated with 55–79.2 Cobalt Gy Equivalent (CGE) in 10–35 fractions from 2001 to 2007 (111). The 1-year and 5-year OS rates were 90% and 45%, respectively, and only 1.6% of patients experienced grade ≥3 toxicity. Fukuda et al. recently published 5-year clinical outcomes in a prospective study of 129 patients with HCC (78% CP-A, 22% CP-B, 92% ≤2 tumor nodules, and 61% >3 cm tumors) treated with 66–70 CGE in 10–35 fractions from 2002 to 2009 (152). The 5-year local tumor control and OS rates were 94% and 69% for patients with BCLC 0/A stage, 87% and 66% for patients with BCLC B stage, and 75% and 25% for patients with BCLC C stage disease. No grade ≥3 toxicity was observed. Based on these data, PBT is considered in CP-A/B patients with ≤3 tumor nodules measuring >3 cm and/or PVT.
For relatively large tumors located near OAR, delivering tumoricidal doses of RT while sparing normal tissue becomes more challenging. “Dose painting,” or simultaneous integrated boost (SIB), typically used in the setting of IMRT or PBT, allows for the delivery of different RT doses to different regions at the same time. The gross tumor volume can receive higher doses, while areas of subclinical disease abutting OAR (e.g., GI tract) can receive lower doses. Kim et al. retrospectively reported favorable outcomes among patients with inoperable HCC who underwent SIB-PBT. Forty-one patients with tumor vascular thrombosis (93% CP-A, 61% >5 cm tumor, range 2–16 cm) received 50 CGE, 60 CGE or 66 CGE in 10 fractions to planning target volume 1 (and 30 CGE to planning target volume 2) based on the distance between the gross tumor volume and GI tract (<1 cm [n = 27], 1–1.9 cm [n = 7], or ≥2 cm [n = 7]) (153). Two-year OS and local PFS rates were 51.1% and 88.1%, respectively. There were no grade ≥3 toxicities. This retrospective study provides promising results with a novel treatment delivery technique in patients with relatively large tumors. While dosimetric studies demonstrated that PBT can better spare the liver compared to photon therapy, it remains unclear if this translates to a clinically relevant decrease in hepatotoxicity.
TACE Plus RT
Historically, the use of RT for HCC was limited by the risk of RILD; however, as described above, with advances in EBRT delivery techniques, utilization of RT has increased and can be used in conjunction with other therapies including TACE. Thirty prospective trials, primarily from China, demonstrated that TACE plus RT significantly improves OS compared to TACE alone (Table 7) (112–141). A recent meta-analysis evaluated 25 trials involving 2,577 patients treated with TACE plus RT or TACE alone (154). This meta-analysis revealed TACE plus RT significantly improved 1-, 2-, 3-, 4-, and 5-year OS rates as well as complete and partial tumor response in patients with unresectable HCC (respectively: OR, 1.36 [95% CI, 1.19–1.54]; OR, 1.55 [95% CI, 1.31–1.85]; OR, 1.91 [95% CI, 1.55–2.35]; OR, 3.01 [95% CI, 1.38–6.55]; OR, 3.98 [95% CI, 1.86–8.51]). While TACE plus RT improved OS, it also significantly increased the risk of gastroduodenal ulcers (OR, 12.80 [95% CI, 1.57–104.33]), and caused elevations in ALT (OR, 2.46 [95%CI, 1.30–4.65]) as well as total bilirubin levels (OR, 2.16 [95% CI, 1.05–4.45]) when compared to TACE alone. Therefore, appropriate patient selection is important to avoid the possibility of added toxicity.
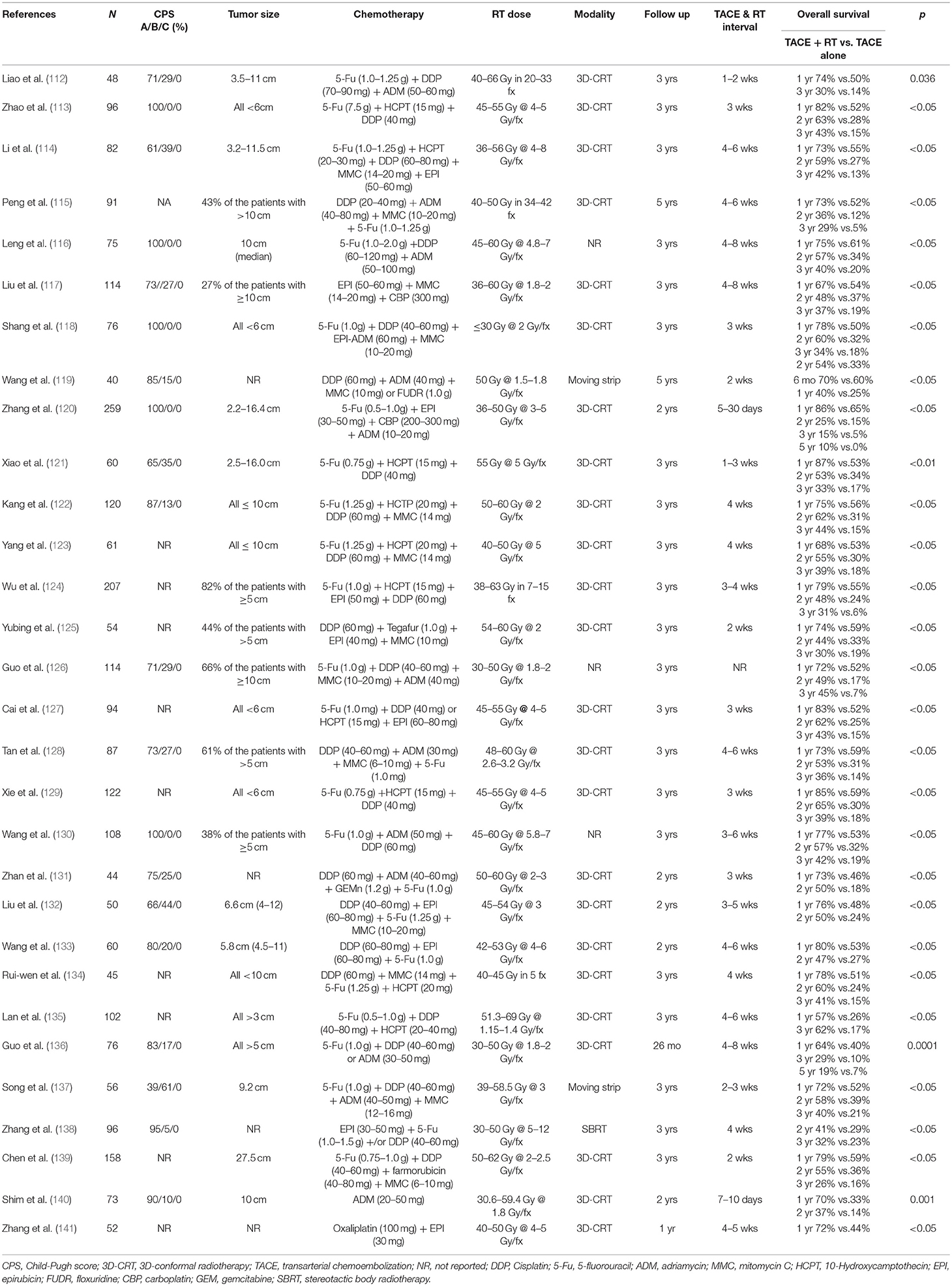
Table 7. Prospective studies comparing transcatheter arterial chemoembolization plus radiation therapy with transcatheter arterial chemoembolization alone for hepatocellular carcinoma.
TACE plus RT significantly improves survival compared to TACE alone or RT alone based on randomized studies detailed in Table 7. Leng et al. prospectively randomized 107 unresectable HCC patients with CP-A (median tumor size 10 cm) to RT alone, TACE alone, or TACE plus RT (116). The 1-, 2-, and 3- year OS rates were significantly higher with TACE plus RT (75%, 57%, and 40%) compared to TACE alone (61%, 34%, 20%) and RT alone (53%, 31%, and 19%) (p < 0.05). Liu et al. randomized 50 HCC patients with ≤2 tumor nodules (66% CP-A, 44% CP-B, and median tumor size 6.6 cm, range 4–12 cm) to TACE plus RT or TACE alone (132). The 1- and 2-year OS rates were significantly higher with TACE plus RT (76% and 56% vs. 48% and 24%, p < 0.05). Wang et al. randomized 60 HCC patients (80% CP-A, 20% CP-B, 92% ≤3 tumor nodules, and median tumor size 5.8 cm, range 4.5–11 cm) to TACE plus RT or TACE alone (133). The 1- and 2-year OS rates were significantly higher with TACE plus RT (80% and 47%) compared to TACE alone (53% and 27%) (p < 0.05). Finally, in the setting of large volume disease, a single nodule >15 cm or nodules totaling a maximum sum of <20 cm, TACE plus RT is a treatment option given the significant survival benefit compared to TACE alone (1-, 2-, 3- year OS 79% vs. 59%, 55% vs. 36%, and 26% vs. 16%, p = <0.05) (139). In light of this data, TACE plus RT must be considered in CP-A and B disease patients with ≤3 tumor nodules measuring ≥3 cm.
Non-randomized data suggests TACE plus RT improves outcomes for HCC patients with partial PVT (155, 156). More recently, Yoon et al. randomized 90 HCC patients with macroscopic vascular invasion to TACE plus RT (87% multinodular disease, measuring 9.8 cm, range 8–13 cm) or sorafenib (78.9% multiple lesions, median maximal tumor diameter 9.7 cm, range 7–12 cm) between 2013 and 2016 (157). All patients had tumor portal vein invasion and CP-A liver function. The TACE plus RT group experienced a significantly longer median TTP (31 weeks vs. 12 weeks, p < 0.01) and median OS (55 weeks vs. 43 weeks, p = 0.04) than the sorafenib group. Furthermore, 11% of patients treated with TACE plus RT had curative surgery due to downstaging. Therefore, the combination of TACE and RT can be considered a treatment option in patients with partial PVT.
In the setting (4–6 nodules) of multinodular HCC, TACE plus RT also improves survival in CP-A and B disease. Peng et al. randomized 91 patients to TACE plus RT (23% multinodular disease) or TACE alone (17% multinodular disease) (115). The 1- and 3-year OS rates were significantly higher with TACE plus RT (73% and 36%) compared to TACE alone (52% and 12%) (p < 0.05). Yubing et al. randomized 54 patients to TACE plus RT (63% multinodular disease) or TACE alone (56% multinodular disease) (125). The 1- and 3-year OS rates were significantly higher with TACE plus RT (74% and 30%) compared to TACE alone (60% and 19%) (p < 0.05). Guo et al. randomized 114 patients (71% CP-A, 29% CP-B, and 37% multinodular disease) to TACE plus RT or TACE alone (126). The 1- and 3-year OS rates were significantly higher with TACE plus RT (72% and 45%) compared to TACE alone (52% and 17%) (p < 0.05). There were no differences in toxicities between treatment groups, other than a more frequent elevation in alanine aminotransferase levels with TACE plus RT (Grade ≤ 2, 25% with combination vs. 7% with TACE alone). In light of this prospective data, TACE plus RT should be considered in patients with multinodular HCC.
Conclusions
While the BCLC classification provides a framework for treatment selection of patients with HCC, it may simplify the decision-making process and may not uniformly take into consideration recent studies, and detailed tumor volumes, which may better guide decisions about local therapies, including radiation therapy and combination treatments. We critically reviewed the literature and devised a data-driven treatment algorithm for optimizing outcomes for patients with unresectable BCLC Stage B or C HCC (Figure 1). This treatment algorithm captures modern data to guide treatment options for those with CP-A or B and ≤3 nodules measuring >3 cm, multinodular disease, or PVT, incorporating tumor volume considerations.
For unresectable, localized HCC patients with either partial PVT, ≤3 nodules >3 cm or multinodular disease, prospective, randomized data suggest that TACE plus RT provides improved survival outcomes and response rates compared to TACE alone; however, toxicities appear more frequent with combination therapy (154). Therefore, appropriate patient selection is important to minimize toxicity. The treatment algorithm used at our institution is shown in Figure 1. For patients with PVT (either partial or complete), RT alone (delivered via IMRT, SBRT or PBT) appears to be a viable option for those unfit to undergo TACE plus RT. One-year survival rates of 44–69% and 30–51% have been observed for those with partial and complete PVT, respectively. For patients without PVT who have ≤3 nodules measuring >3 cm, TACE plus RT results in 1-year survival rates of 75–85%. Lastly, for patients without PVT who have multinodular disease, TACE alone or TARE with 90Y is a reasonable option for those unfit for TACE plus RT, particularly those presenting with ≥7 nodules. One-year survival rates of 46–70% have been observed after either modality.
We acknowledge that the best treatment approach is determined through a multidisciplinary management approach in experienced cancer centers with a dedicated HCC program. This offers robust access to all the modalities discussed plus systemic therapy. We hope that this data driven treatment algorithm will aid clinicians in managing localized HCC.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA. (2016) 66:7–30. doi: 10.3322/caac.21332
2. Cancer Facts & Figures 2017 American Cancer Society (2017). Available305 online at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-factsfigures-2017.html (accessed December 2, 2017).
3. Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD. Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol. (2008) 14:4300–8. doi: 10.3748/wjg.14.4300
4. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md). (2018) 67:358–80. doi: 10.1002/hep.29086
5. Forner A, Vilana R, Ayuso C, Bianchi L, Sole M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology (Baltimore, Md). (2008) 47:97–104. doi: 10.1002/hep.21966
6. Couto OF, Dvorchik I, Carr BI. Causes of death in patients with unresectable hepatocellular carcinoma. Digestive Dis Sci. (2007) 52:3285–9. doi: 10.1007/s10620-007-9750-3
7. Kaczynski J, Hansson G, Wallerstedt S. Metastases in cases with hepatocellular carcinoma in relation to clinicopathologic features of the tumor. An autopsy study from a low endemic area. Acta Oncol. (1995) 34:43–8. doi: 10.3109/02841869509093637
8. Delis SG, Dervenis C. Selection criteria for liver resection in patients with hepatocellular carcinoma and chronic liver disease. World J Gastroenterol. (2008) 14:3452–60. doi: 10.3748/wjg.14.3452
9. Xu Q, Kobayashi S, Ye X, Meng X. Comparison of hepatic resection and radiofrequency ablation for small hepatocellular carcinoma: a meta-analysis of 16,103 patients. Sci Rep. (2014) 4:7252. doi: 10.1038/srep07252
10. Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. (2002) 359:1734–9. doi: 10.1016/S0140-6736(02)08649-X
11. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology (Baltimore, Md). (2002) 35:1164–71. doi: 10.1053/jhep.2002.33156
12. Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. (1995) 31:1237–48. doi: 10.1016/0360-3016(94)00418-K
13. Kalogeridi MA, Zygogianni A, Kyrgias G, Kouvaris J, Chatziioannou S, Kelekis N, et al. Role of radiotherapy in the management of hepatocellular carcinoma: a systematic review. World J Hepatol. (2015) 7:101–12. doi: 10.4254/wjh.v7.i1.101
14. Ohri N, Dawson LA, Krishnan S, Seong J, Cheng JC, Sarin SK, et al. Radiotherapy for Hepatocellular Carcinoma: new indications and DIRECTIONS FOR FUTURE STUDY. J Natl Cancer Inst. (2016) 108:1–10. doi: 10.1093/jnci/djw133
15. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. (2010) 30:61–74. doi: 10.1055/s-0030-1247133
16. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet (London, England). (2018) 391:1301–14. doi: 10.1016/S0140-6736(18)30010-2
17. Gray SH, White JA, Li P, Kilgore ML, Redden DT, Abdel Aal AK, et al. A SEER database analysis of the survival advantage of transarterial chemoembolization for hepatocellular carcinoma: an underutilized therapy. J Vasc Intervent Radiol. (2017) 28:231–7.e2. doi: 10.1016/j.jvir.2016.09.022
18. Ferrara N, Houck K, Jakeman L, Leung DW. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. (1992) 13:18–32. doi: 10.1210/edrv-13-1-18
19. Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Intervent Radiol. (2001) 12:321–6. doi: 10.1016/S1051-0443(07)61911-3
20. Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, et al. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2012) 56:908–43. doi: 10.1016/j.jhep.2011.12.001
21. Yamada R, Kishi K, Sato M, Sonomura T, Nishida N, Tanaka K, et al. Transcatheter arterial chemoembolization (TACE) in the treatment of unresectable liver cancer. World J Surg. (1995) 19:795–800. doi: 10.1007/BF00299773
22. Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. (2014) 11:525–35. doi: 10.1038/nrclinonc.2014.122
23. Salem R, Lewandowski RJ. Chemoembolization and radioembolization for hepatocellular carcinoma. Clin Gastroenterol Hepatol. (2013) 11:604–11. quiz:e 43-4. doi: 10.1016/j.cgh.2012.12.039
24. Di Costanzo GG, Tortora R. Intermediate hepatocellular carcinoma: how to choose the best treatment modality? World J Hepatol. (2015) 7:1184–91. doi: 10.4254/wjh.v7.i9.1184
25. Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. (2011) 37:212–20. doi: 10.1016/j.ctrv.2010.07.006
26. Pelletier G, Roche A, Ink O, Anciaux ML, Derhy S, Rougier P, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol. (1990) 11:181–4. doi: 10.1016/0168-8278(90)90110-D
27. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. New Engl J Med. (1995) 332:1256–61. doi: 10.1056/NEJM199505113321903
28. Mabed M, Esmaeel M, El-Khodary T, Awad M, Amer T. A randomized controlled trial of transcatheter arterial chemoembolization with lipiodol, doxorubicin and cisplatin versus intravenous doxorubicin for patients with unresectable hepatocellular carcinoma. Eur J Cancer Care. (2009) 18:492–9. doi: 10.1111/j.1365-2354.2008.00984.x
29. Pelletier G, Ducreux M, Gay F, Luboinski M, Hagege H, Dao T, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. (1998) 29:129–34. doi: 10.1016/S0168-8278(98)80187-6
30. Doffoel M, Bonnetain F, Bouche O, Vetter D, Abergel A, Fratte S, et al. Multicentre randomised phase III trial comparing Tamoxifen alone or with Transarterial Lipiodol Chemoembolisation for unresectable hepatocellular carcinoma in cirrhotic patients (Federation Francophone de Cancerologie Digestive 9402). Eur J Cancer (Oxford, England: 1990). (2008) 44:528–38. doi: 10.1016/j.ejca.2008.01.004
31. Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. oncologist. (2012) 17:359–66. doi: 10.1634/theoncologist.2011-0313
32. Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer (Oxford, England: 1990). (2011) 47:2117–27. doi: 10.1016/j.ejca.2011.05.007
33. Yao X, Yan D, Zeng H, Liu D, Li H. Concurrent sorafenib therapy extends the interval to subsequent TACE for patients with unresectable hepatocellular carcinoma. J Surg Oncol. (2016) 113:672–7. doi: 10.1002/jso.24215
34. Bai W, Wang YJ, Zhao Y, Qi XS, Yin ZX, He CY, et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Digest Dis. (2013) 14:181–90. doi: 10.1111/1751-2980.12038
35. Britten CD, Gomes AS, Wainberg ZA, Elashoff D, Amado R, Xin Y, et al. Transarterial chemoembolization plus or minus intravenous bevacizumab in the treatment of hepatocellular cancer: a pilot study. BMC Cancer. (2012) 12:16. doi: 10.1186/1471-2407-12-16
36. Wang H, Liu Y, Wang X, Liu D, Sun Z, Wang C, et al. Randomized clinical control study of locoregional therapy combined with arsenic trioxide for the treatment of hepatocellular carcinoma. Cancer. (2015) 121:2917–25. doi: 10.1002/cncr.29456
37. Pinter M, Ulbrich G, Sieghart W, Kolblinger C, Reiberger T, Li S, et al. Hepatocellular Carcinoma: A Phase II Randomized Controlled Double-Blind Trial of Transarterial Chemoembolization in Combination with Biweekly Intravenous Administration of Bevacizumab or a Placebo. Radiology. (2015) 277:903–12. doi: 10.1148/radiol.2015142140
38. Kudo M, Han G, Finn RS, Poon RT, Blanc JF, Yan L, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology (Baltimore, Md). (2014) 60:1697–707. doi: 10.1002/hep.27290
39. Inaba Y, Kanai F, Aramaki T, Yamamoto T, Tanaka T, Yamakado K, et al. A randomised phase II study of TSU-68 in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Eur J Cancer (Oxford, England: 1990). (2013) 49:2832–40. doi: 10.1016/j.ejca.2013.05.011
40. Li M, Lu C, Cheng J, Zhang J, Cao C, Xu J, et al. Combination therapy with transarterial chemoembolization and interferon-alpha compared with transarterial chemoembolization alone for hepatitis B virus related unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. (2009) 24:1437–44. doi: 10.1111/j.1440-1746.2009.05863.x
41. Rosmorduc O, Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis. (2010) 30:258–70. doi: 10.1055/s-0030-1255355
42. Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol. (2016) 64:1090–8. doi: 10.1016/j.jhep.2016.01.012
43. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. (2004) 64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443
44. Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. (2007) 59:561–74. doi: 10.1007/s00280-006-0393-4
45. Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. (2006) 66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377
46. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl. J. Med. (2008) 359:378–90. doi: 10.1056/NEJMoa0708857
47. Arrondeau J, Mir O, Boudou-Rouquette P, Coriat R, Ropert S, Dumas G, et al. Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Investigat New Drugs. (2012) 30:2046–9. doi: 10.1007/s10637-011-9764-8
48. Himmelsbach K, Sauter D, Baumert TF, Ludwig L, Blum HE, Hildt E. New aspects of an anti-tumour drug: sorafenib efficiently inhibits HCV replication. Gut. (2009) 58:1644–53. doi: 10.1136/gut.2009.182212
49. Mee CJ, Farquhar MJ, Harris HJ, Hu K, Ramma W, Ahmed A, et al. Hepatitis C virus infection reduces hepatocellular polarity in a vascular endothelial growth factor-dependent manner. Gastroenterology. (2010) 138:1134–42. doi: 10.1053/j.gastro.2009.11.047
50. Li Q, Zhang P, Yang Y, Wen F, Wheeler J. Efficacy and cost-effectiveness of antiviral therapy in patients with advanced hepatitis B virus-related hepatocellular carcinoma treated with sorafenib. J Clin Oncol. (2016) 34(15 Suppl.):e15622-e. doi: 10.1200/JCO.2016.34.15_suppl.e15622
51. Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma (HCC): the role of angiogenesis and invasiveness. Am J Gastroenterol. (2008) 103:914–21. doi: 10.1111/j.1572-0241.2007.01712.x
52. Pawlik TM, Reyes DK, Cosgrove D, Kamel IR, Bhagat N, Geschwind JF. Phase II trial of sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. (2011) 29:3960–7. doi: 10.1200/JCO.2011.37.1021
53. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. (2018) 391:1163–73. doi: 10.1016/S0140-6736(18)30207-1
54. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet (London, England). (2017) 389:2492–502. doi: 10.1016/S0140-6736(17)31046-2
55. Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Engl J Med. (2018) 379:54–63. doi: 10.1056/NEJMoa1717002
56. Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. (2015) 520:373–7. doi: 10.1038/nature14292
57. Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, Rajan DK. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Intervent Radiol. (2009) 20(7 Suppl.):S342–7. doi: 10.1016/j.jvir.2009.04.029
58. Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. (2016) 34:452–9. doi: 10.1200/JCO.2015.61.4925
59. McGahan JP, Browning PD, Brock JM, Tesluk H. Hepatic ablation using radiofrequency electrocautery. Investigat Radiol. (1990) 25:267–70. doi: 10.1097/00004424-199003000-00011
60. Tamai T, Oshige A, Tabu K, Tabu E, Ijyuin S, Sakae H, et al. Utility of percutaneous radiofrequency ablation alone or combined with transarterial chemoembolization for early hepatocellular carcinoma. Oncol Lett. (2017) 14:3199–206. doi: 10.3892/ol.2017.6476
61. Lin JJ, Wu W, Jiang XF, Jin XJ, Lu LJ, Bao LW. [Clinical outcomes of radiofrequency ablation combined with transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma: a single-center experience]. Chin J Oncol. (2013) 35:144–7. doi: 10.3760/cma.j.issn.0253-3766.2013.02.016
62. Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Nemcek AA Jr., Kulik L, et al. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol. (2007) 30:571–92. doi: 10.1007/s00270-007-9064-z
63. Hickey RM, Lewandowski RJ, Salem R. Yttrium-90 Radioembolization for Hepatocellular Carcinoma. Semin Nucl Med. (2016) 46:105–8. doi: 10.1053/j.semnuclmed.2015.10.011
64. Venkatanarasimha N, Gogna A, Tong KTA, Damodharan K, Chow PKH, Lo RHG, et al. Radioembolisation of hepatocellular carcinoma: a primer. Clin Radiol. (2017) 72:1002–13. doi: 10.1016/j.crad.2017.07.021
65. Tong AK, Kao YH, Too CW, Chin KF, Ng DC, Chow PK. Yttrium-90 hepatic radioembolization: clinical review and current techniques in interventional radiology and personalized dosimetry. Brit J Radiol. (2016) 89:20150943. doi: 10.1259/bjr.20150943
66. Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. (2013) 36:714–23. doi: 10.1007/s00270-012-0481-2
67. Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. (2011) 140:497–507.e2. doi: 10.1053/j.gastro.2010.10.049
68. Chow PKH, Gandhi M, Tan SB, Khin MW, Khasbazar A, Ong J, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in Asia-Pacific patients with hepatocellular carcinoma. J Clin Oncol. (2018) 36:1913–21. doi: 10.1200/jco.2017.76.0892
69. Wo JY, Dawson LA, Zhu AX, Hong TS. An emerging role for radiation therapy in the treatment of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Oncol Clin North Am. (2014) 23:353–68. doi: 10.1016/j.soc.2013.10.007
70. Sapir E, Tao Y, Schipper MJ, Bazzi L, Novelli PM, Devlin P, et al. Stereotactic body radiation therapy as an alternative to transarterial chemoembolization for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. (2018) 100:122–30. doi: 10.1016/j.ijrobp.2017.09.001
71. Yeung RH, Chapman TR, Bowen SR, Apisarnthanarax S. Proton beam therapy for hepatocellular carcinoma. Exp Rev Anticancer Ther. (2017) 17:911–24. doi: 10.1080/14737140.2017.1368392
72. Cheng SH, Lin YM, Chuang VP, Yang PS, Cheng JC, Huang AT, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. (1999) 14:1025–33. doi: 10.1046/j.1440-1746.1999.01994.x
73. Liu MT, Li SH, Chu TC, Hsieh CY, Wang AY, Chang TH, et al. Three-dimensional conformal radiation therapy for unresectable hepatocellular carcinoma patients who had failed with or were unsuited for transcatheter arterial chemoembolization. Japanese J Clin Oncol. (2004) 34:532–9. doi: 10.1093/jjco/hyh089
74. Mornex F, Girard N, Beziat C, Kubas A, Khodri M, Trepo C, et al. Feasibility and efficacy of high-dose three-dimensional-conformal radiotherapy in cirrhotic patients with small-size hepatocellular carcinoma non-eligible for curative therapies–mature results of the French Phase II RTF-1 trial. Int J Radiat Oncol Biol Phys. (2006) 66:1152–8. doi: 10.1016/j.ijrobp.2006.06.015
75. Kim TH, Kim DY, Park JW, Kim YI, Kim SH, Park HS, et al. Three-dimensional conformal radiotherapy of unresectable hepatocellular carcinoma patients for whom transcatheter arterial chemoembolization was ineffective or unsuitable. Amer J Clin Oncol. (2006) 29:568–75. doi: 10.1097/01.coc.0000239147.60196.11
76. Kim JY, Yoo EJ, Jang JW, Kwon JH, Kim KJ, Kay CS. Hypofractionated radiotheapy using helical tomotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Radiat Oncol. (2013) 8:15. doi: 10.1186/1748-717X-8-15
77. Chi KH, Liao CS, Chang CC, Ko HL, Tsang YW, Yang KC, et al. Angiogenic blockade and radiotherapy in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. (2010) 78:188–93. doi: 10.1016/j.ijrobp.2009.07.1725
78. McIntosh A, Hagspiel KD, Al-Osaimi AM, Northup P, Caldwell S, Berg C, et al. Accelerated treatment using intensity-modulated radiation therapy plus concurrent capecitabine for unresectable hepatocellular carcinoma. Cancer. (2009) 115:5117–25. doi: 10.1002/cncr.24552
79. Kang MK, Kim MS, Kim SK, Ye GW, Lee HJ, Kim TN, et al. High-dose radiotherapy with intensity-modulated radiation therapy for advanced hepatocellular carcinoma. Tumori. (2011) 97:724–31. doi: 10.1177/030089161109700608
80. Kong M, Hong SE, Choi WS, Choi J, Kim Y. Treatment outcomes of helical intensity-modulated radiotherapy for unresectable hepatocellular carcinoma. Gut Liver. (2013) 7:343–51. doi: 10.5009/gnl.2013.7.3.343
81. Huang CM, Huang MY, Tang JY, Chen SC, Wang LY, Lin ZY, et al. Feasibility and efficacy of helical tomotherapy in cirrhotic patients with unresectable hepatocellular carcinoma. World J Surg Oncol. (2015) 13:201. doi: 10.1186/s12957-015-0611-9
82. Scorsetti M, Comito T, Cozzi L, Clerici E, Tozzi A, Franzese C, et al. The challenge of inoperable hepatocellular carcinoma (HCC): results of a single-institutional experience on stereotactic body radiation therapy (SBRT). J Cancer Res Clin Oncol. (2015) 141:1301–9. doi: 10.1007/s00432-015-1929-y
83. Mendez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution phase i-ii study. Acta Oncol. (Stockholm, Sweden). (2006) 45:831–7. doi: 10.1080/02841860600897934
84. Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. (2013) 31:1631–9. doi: 10.1200/JCO.2012.44.1659
85. Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. (2012) 118:5424–31. doi: 10.1002/cncr.27533
86. Lasley FD, Mannina EM, Johnson CS, Perkins SM, Althouse S, Maluccio M, et al. Treatment variables related to liver toxicity in patients with hepatocellular carcinoma, Child-Pugh class A and B enrolled in a phase 1-2 trial of stereotactic body radiation therapy. Pract Radiat Oncol. (2015) 5:e443–9. doi: 10.1016/j.prro.2015.02.007
87. Takeda A, Sanuki N, Tsurugai Y, Iwabuchi S, Matsunaga K, Ebinuma H, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. (2016) 122:2041–9. doi: 10.1002/cncr.30008
88. Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. (2010) 10:475. doi: 10.1186/1471-2407-10-475
89. Louis C, Dewas S, Mirabel X, Lacornerie T, Adenis A, Bonodeau F, et al. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treatment. (2010) 9:479–87. doi: 10.1177/153303461000900506
90. Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. (2008) 26:657–64. doi: 10.1200/JCO.2007.14.3529
91. Takeda A, Takahashi M, Kunieda E, Takeda T, Sanuki N, Koike Y, et al. Hypofractionated stereotactic radiotherapy with and without transarterial chemoembolization for small hepatocellular carcinoma not eligible for other ablation therapies: preliminary results for efficacy and toxicity. Hepatology Res. (2008) 38:60–9. doi: 10.1111/j.1872-034X.2007.00084.x
92. Seo YS, Kim MS, Yoo SY, Cho CK, Choi CW, Kim JH, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol. (2010) 102:209–14. doi: 10.1002/jso.21593
93. Kim JW, Seong J, Lee IJ, Woo JY, Han KH. Phase I dose escalation study of helical intensity-modulated radiotherapy-based stereotactic body radiotherapy for hepatocellular carcinoma. Oncotarget. (2016) 7:40756–66. doi: 10.18632/oncotarget.9450
94. Price TR, Perkins SM, Sandrasegaran K, Henderson MA, Maluccio MA, Zook JE, et al. Evaluation of response after stereotactic body radiotherapy for hepatocellular carcinoma. Cancer. (2012) 118:3191–8. doi: 10.1002/cncr.26404
95. Su TS, Liang P, Lu HZ, Liang J, Gao YC, Zhou Y, et al. Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 Chinese patients. J Surg Oncol. (2016) 113:181–7. doi: 10.1002/jso.24128
96. Yamashita H, Onishi H, Murakami N, Matsumoto Y, Matsuo Y, Nomiya T, et al. Survival outcomes after stereotactic body radiotherapy for 79 Japanese patients with hepatocellular carcinoma. J Radiat Res. (2015) 56:561–7. doi: 10.1093/jrr/rru130
97. Bibault JE, Dewas S, Vautravers-Dewas C, Hollebecque A, Jarraya H, Lacornerie T, et al. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS ONE. (2013) 8:e77472. doi: 10.1371/journal.pone.0077472
98. Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J. Radiat. Oncol. Biol. Phys. (2011) 81:e447–53. doi: 10.1016/j.ijrobp.2011.04.011
99. Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol. Biol Phys. (2012) 84:355–61. doi: 10.1016/j.ijrobp.2011.11.058
100. Bae SH, Kim MS, Cho CK, Kim KB, Lee DH, Han CJ, et al. Feasibility and efficacy of stereotactic ablative radiotherapy for Barcelona Clinic Liver Cancer-C stage hepatocellular carcinoma. J Kor Med Sci. (2013) 28:213–9. doi: 10.3346/jkms.2013.28.2.213
101. Sanuki N, Takeda A, Oku Y, Eriguchi T, Nishimura S, Aoki Y, et al. Influence of liver toxicities on prognosis after stereotactic body radiation therapy for hepatocellular carcinoma. Hepatol Res. (2015) 45:540–7. doi: 10.1111/hepr.12383
102. Huertas A, Baumann AS, Saunier-Kubs F, Salleron J, Oldrini G, Croise-Laurent V, et al. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother. Oncol. (2015) 115:211–6. doi: 10.1016/j.radonc.2015.04.006
103. Kimura T, Aikata H, Takahashi S, Takahashi I, Nishibuchi I, Doi Y, et al. Stereotactic body radiotherapy for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Hepatol Res. (2015) 45:378–86. doi: 10.1111/hepr.12359
104. Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. (2011) 117:3053–9. doi: 10.1002/cncr.25809
105. Hong TS, Wo JY, Yeap BY, Ben-Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. (2016) 34:460–8. doi: 10.1200/JCO.2015.64.2710
106. Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. (2009) 74:831–6. doi: 10.1016/j.ijrobp.2008.10.073
107. Mizumoto M, Tokuuye K, Sugahara S, Nakayama H, Fukumitsu N, Ohara K, et al. Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys. (2008) 71:462–7. doi: 10.1016/j.ijrobp.2007.09.056
108. Kim TH, Park JW, Kim YJ, Kim BH, Woo SM, Moon SH, et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res Treatment. (2015) 47:34–45. doi: 10.4143/crt.2013.218
109. Nakayama H, Sugahara S, Fukuda K, Abei M, Shoda J, Sakurai H, et al. Proton beam therapy for hepatocellular carcinoma located adjacent to the alimentary tract. Int J Radiat Oncol Biol Phys. (2011) 80:992–5. doi: 10.1016/j.ijrobp.2010.03.015
110. Sugahara S, Oshiro Y, Nakayama H, Fukuda K, Mizumoto M, Abei M, et al. Proton beam therapy for large hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. (2010) 76:460–6. doi: 10.1016/j.ijrobp.2009.02.030
111. Nakayama H, Sugahara S, Tokita M, Fukuda K, Mizumoto M, Abei M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. (2009) 115:5499–506. doi: 10.1002/cncr.24619
112. Liao X-F, H-JH, Zhou Z-S. Three-dimensional Conformal Radiotherapy Combined with Interventional Therapy in Treatment of Primary Hepatocellular Carcinoma. J Pract Oncol. (2010) 25:681.
113. Zhao M, Lang F, QQian J, Jingjing MA, Yuxiu S. Three-dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for inoperable primary liver cancer. Chin J Radiat Oncol. (2006) 15:39–41. doi: 10.3760/j.issn:1004-4221.2006.01.010
114. Li Y, Yan Y, Zhang H, Guo Z, Yan Z, Li D. Three-dimensional conformal radiation combined with transarterial chemoembolization for unresectable primary liver cancer. Chin J Radiat Oncol. (2003) 12:30–2. doi: 10.3760/j.issn:1004-4221.2003.01.008
115. Peng K, Han F, Liu H. Clinical study of unresectable liver cancer treated by intraoperative hepatic arterial embolizat ion and postoperative hyperfractionation radiotherapy. Chin J Radiat Oncol. (2000) 9:11–3. doi: 10.3760/j.issn:1004-4221.2000.01.003
116. Leng Z, Liang Z, Shi S. Comparison of treatment results of interventional therapy alone, radiotherapy alone, and combined intervent ional therapy plus radiotherapy for primary hepatic cancer. Chin J Radiat Oncol. (2000) 9:99. doi: 10.3760/j.issn:1004-4221.2000.02.007
117. Liu MZ, Wang XS, Cai L, Gu MF, Liu H, Li Q, et al. External radiation and combined transcatheter arterial chemoembolization for unresectable primary liver cancer. Chin J Cancer. (2005) 24:82–6.
118. Shang Y, You G-X, Xu H-Y, Chen N-C. Prospective randomized clinical study of transcatheter arterial chemoembolization, combined with three dimensional conformal radiotherapy for primary liver cancer:an analysis of 40 cases. World Chin J Digestol. (2007) 15:3140–2. doi: 10.3969/j.issn.1009-3079.2007.29.016
119. Wang G, Shen W, Song M, Xu H. Results of combined treatment with transcatheter hepatic arterial chemoembolization and whole-liver irradiation with the moving strip technique in unresectable hepatocellular carcinoma. Int J Clin Oncol. (2000) 5:380–5. doi: 10.1007/PL00012067
120. Zhang Z, Yang X, Wena M, Wan J. Evaluation of TACE combined with gamma-knife radiotherapy for primary hepatocellular carcinoma. J Intervent Radiol (China). (2012) 7:596–9.
121. Xiao Z, Ouyang T, Yu R, Jiang X, Reng H, Wu Z. Transcatheter arterial chemoembolization combined with 3-dimensional conformal radiotherapy for patients with unresectable primary hepatic carcinoma. Chin J Clin Oncol. (2008) 35:18–21.
122. Kang J, Qi N, Wang T, Cheng Y, Yang Z, Li W, et al. Clinical outcome of three dimensional conformal radiation combined with transarterial chemoembolizaton for unresectable primary hepatic cancer. J Mod Oncol. (2013) 21:1834–6. doi: 10.3969/j.issn.1672-4992.2013.08.55
123. Yang Y, Wang T, Li S, Zhang Y. Three dimensional conformal radiation combined with transarterial chemoembolizaton for primary hepatic cancer. J Mod Oncol. (2007) 15:1646–8. doi: 10.3969/j.issn.1672-4992.2007.11.046
124. Wu N, Ma Y, Huang M, Ouyang X, Zhau C, Huang D. Transcatheter arterial chemoembolization combined with three-dimensional conformal radiotherapy in 77 patients with primany hepatic carcinoma. J Pract Med. (2008) 24:2573–5. doi: 10.3969/j.issn.1006-5725.2008.15.011
125. Yubing X, Haiying J, Lan W, Yuanhang Z, Minjie Z, Guoqing K. Comparison of therapeutic effects Of 3-DCRT combined with TACE and simple TACE in treating primary liver cancer(PLC). Contemp Med. (2010) 16:315–7. doi: 10.3969/j.issn.1009-4393.2010.17.019
126. Guo W, Song M, Yu E. Transcatheter arterial chemoembolization combined with external radiation for primary liver cancer. Chin J Oncol. (1999) 21:25–8.
127. Cai C, Zhang F, Duan C. Three dimensional conformal radiation therapy combined with interventional therapy for Primary hepatic carcinoma. Mod J Integr Trad Chin Western Med. (2007) 16:1600–1. doi: 10.3969/j.issn.1008-8849.2007.12.003
128. Tan X, Ren L, Liu J. Clinical observation of the effect of TACE combined with three dimensional conformal radiotherapy on patients with advanced hepatocellular carcinoma. J Clin Hepatol. (2011) 14:433–5. doi: 10.3969/j.issn.1672-5069.2011.06.013
129. Xie X, Zhang Z, Zhao D, Zhu J, Li T. Three dimentinal radiotherapy combined with tanscatheter arterial chemoebolization hyperthermia for primary liver cancer. J Mod Oncol. (2007) 15:1307–9. doi: 10.3969/j.issn.1672-4992.2007.09.040
130. Wang X, Li J, Gao K. Radiotherapy combined with hepatic cheoembolization in the treatment of 54 primary liver cancer. Shanxi Med J. (2006) 35:461–2. doi: 10.3969/j.issn.1000-7377.2006.04.030
131. Zhan Y, Huang C, Huang J. Combination of transcatheter arterial chemoembolization and three dimensional conformal radiotherapy in the treatment for primary hepatocellular carcinoma. J Oncol. (2008) 14:292–3.
132. Liu Y, Zhou S, Gao F. Clinical study of inresectable primary hepatic carcinoma treated by transartherial chemoembolization and three dimensional conformal radiotherapy. Mod Oncol. (2005) 13:645–6.
133. Wang X, Zheng L, Xiong J. A clinical study of transcatheter artheria chemoembolization combined with three-dimensional conformal radiotherapy in primary hepatocellular carcinoma. Pract Clin Med. (2006) 7:21–3.
134. Rui-wen Z. Clinical observation of interventional therapy combination with conformal radiotherapy used for the unsuitable surgery of primary liver cancer. Chin J Med Guide. (2009) 11:1268–70. doi: 10.3969/j.issn.1009-0959.2009.08.007
135. Lan D, Gong X, Wei X. The efficacy analysis of transcatheter hepatic arterial chemoembolization combined with radiotherapy for primary liver cancer. Chin J Radiat Oncol. (2005) 14:152–3.
136. Guo WJ, Yu EX, Liu LM, Li J, Chen Z, Lin JH, et al. Comparison between chemoembolization combined with radiotherapy and chemoembolization alone for large hepatocellular carcinoma. World J Gastroenterol. (2003) 9:1697–701. doi: 10.3748/wjg.v9.i8.1697
137. Song S, Kuang J, Xiong K. The efficacy of transcatheter arterial chemoembolization combined with or without radiotherapy for liver cancer. Chin J Clin Oncol. (2002) 29:141–2.
138. Zhang X, He X, Wang Y, Zhao C. Clinical effects of transcatheter arterial chemoembolization combined with stereotactic body radiation therapy on patients with unresectable hepatocellular carcinoma. Mod Oncol. (2016) 24:3437–40. doi: 10.3969/j.issn.1672-4992.2016.21.025
139. Chen WJ, Yuan SF, Zhu LJ, Sun XN, Zheng W. Three-dimensional conformal radiotherapy in combination with transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma. J f BUON. (2014) 19:692–7.
140. Shim SJ, Seong J, Han KH, Chon CY, Suh CO, Lee JT. Local radiotherapy as a complement to incomplete transcatheter arterial chemoembolization in locally advanced hepatocellular carcinoma. Liver Int. (2005) 25:1189–96. doi: 10.1111/j.1478-3231.2005.01170.x
141. Zhang K, Yu W, Chen S, Wu J, Shao L. Effects of transcatheter hepatic arterial chemoembolization combined with radiotherapy on the survival of primary hepatic carcinoma patients with portal vein tumor thrombus. Chin J Clin Oncol. (2012) 39:35–7.
142. Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. (2003) 55:329–36. doi: 10.1016/S0360-3016(02)03929-9
143. Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. (2002) 54:150–5. doi: 10.1016/S0360-3016(02)02864-X
144. Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. (2005) 61:1143–50. doi: 10.1016/j.ijrobp.2004.08.028
145. Eccles CL, Bissonnette JP, Craig T, Taremi M, Wu X, Dawson LA. Treatment planning study to determine potential benefit of intensity-modulated radiotherapy versus conformal radiotherapy for unresectable hepatic malignancies. Int J Radiat Oncol Biol Phys. (2008) 72:582–8. doi: 10.1016/j.ijrobp.2008.06.1496
146. Yoon HI, Lee IJ, Han KH, Seong J. Improved oncologic outcomes with image-guided intensity-modulated radiation therapy using helical tomotherapy in locally advanced hepatocellular carcinoma. J Cancer Res Clin Oncol. (2014) 140:1595–605. doi: 10.1007/s00432-014-1697-0
147. Hou JZ, Zeng ZC, Wang BL, Yang P, Zhang JY, Mo HF. High dose radiotherapy with image-guided hypo-IMRT for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombi is more feasible and efficacious than conventional 3D-CRT. Japanese J Clin Oncol. (2016) 46:357–62. doi: 10.1093/jjco/hyv205
148. Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta oncologica (Stockholm, Sweden). (1995) 34:861–70. doi: 10.3109/02841869509127197
149. Yuan Z, Tian L, Wang P, Song Y, Dong Y, Zhuang H. Comparative research on the efficacy of CyberKnife(R) and surgical excision for Stage I hepatocellular carcinoma. OncoTargets Ther. (2013) 6:1527–32. doi: 10.2147/OTT.S51452
150. Velec M, Haddad CR, Craig T, Wang L, Lindsay P, Brierley J, et al. Predictors of liver toxicity following stereotactic body radiation therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. (2017) 97:939–46. doi: 10.1016/j.ijrobp.2017.01.221
151. Bortfeld T, Schlegel W. An analytical approximation of depth-dose distributions for therapeutic proton beams. Phys Med Biol. (1996) 41:1331–9. doi: 10.1088/0031-9155/41/8/006
152. Fukuda K, Okumura T, Abei M, Fukumitsu N, Ishige K, Mizumoto M, et al. Long-term outcomes of proton beam therapy in patients with previously untreated hepatocellular carcinoma. Cancer Sci. (2017) 108:497–503. doi: 10.1111/cas.13145
153. Kim DY, Park JW, Kim TH, Kim BH, Moon SH, Kim SS, et al. Risk-adapted simultaneous integrated boost-proton beam therapy (SIB-PBT) for advanced hepatocellular carcinoma with tumour vascular thrombosis. Radiother Oncol. (2017) 122:122–9. doi: 10.1016/j.radonc.2016.12.014
154. Huo YR, Eslick GD. Transcatheter Arterial Chemoembolization Plus Radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. (2015) 1:756–65. doi: 10.1001/jamaoncol.2015.2189
155. Zhang XB, Wang JH, Yan ZP, Qian S, Du SS, Zeng ZC. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. (2009) 115:1245–52. doi: 10.1002/cncr.24139
156. Koo JE, Kim JH, Lim YS, Park SJ, Won HJ, Sung KB, et al. Combination of transarterial chemoembolization and three-dimensional conformal radiotherapy for hepatocellular carcinoma with inferior vena cava tumor thrombus. Int J Radiat Oncol Biol Phys. (2010) 78:180–7. doi: 10.1016/j.ijrobp.2009.07.1730
157. Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs. sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. (2018) 4:661–9. doi: 10.1001/jamaoncol.2017.5847
Keywords: hepatocellular carcinoma, radiation therapy, transcatheter arterial chemoembolization, transarterial embolization, systemic therapy
Citation: Sayan M, Yegya-Raman N, Greco SH, Gui B, Zhang A, Chundury A, Grandhi MS, Hochster HS, Kennedy TJ, Langan RC, Malhotra U, Rustgi VK, Shah MM, Spencer KR, Carpizo DR, Nosher JL and Jabbour SK (2019) Rethinking the Role of Radiation Therapy in the Treatment of Unresectable Hepatocellular Carcinoma: A Data Driven Treatment Algorithm for Optimizing Outcomes. Front. Oncol. 9:345. doi: 10.3389/fonc.2019.00345
Received: 05 December 2018; Accepted: 15 April 2019;
Published: 18 June 2019.
Edited by:
Sunil Krishnan, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Jason Chia-Hsien Cheng, National Taiwan University, TaiwanMaria A. Hawkins, University of Oxford, United Kingdom
Copyright © 2019 Sayan, Yegya-Raman, Greco, Gui, Zhang, Chundury, Grandhi, Hochster, Kennedy, Langan, Malhotra, Rustgi, Shah, Spencer, Carpizo, Nosher and Jabbour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Salma K. Jabbour, amFiYm91c2tAY2luai5ydXRnZXJzLmVkdQ==
 Mutlay Sayan
Mutlay Sayan Nikhil Yegya-Raman
Nikhil Yegya-Raman Stephanie H. Greco2
Stephanie H. Greco2