- 1Pain Management, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Graduate School of Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: CD44 is widely used as a putative cancer stem cells (CSCs) marker for colorectal cancer (CRC). However, the prognostic role of CD44 in CRC remains controversial.
Methods: We performed a systematic review and meta-analysis to evaluate the association of various CD44 isoforms and overall survival (OS) and clinicopathological features of CRC patients.
Results: A total of 48 studies were included in the meta-analysis. Total CD44 isoforms overexpression was significantly correlated with worse OS of patients with CRC (HR = 1.32, 95% CI = 1.08–1.61, P = 0.007). In a stratified analysis, a higher level of either CD44v6 or CD44v2 had an unfavorable impact on OS (HRCD44v6 = 1.50, 95% CI = 1.10–2.14, P = 0.010; HRCD44v2 = 2.93, 95% CI = 1.49–5.77, P = 0.002). Additionally, CD44 was shown to be associated with some clinicopathological features, such as lymph node metastasis (ORCD44 = 1.56, 95% CI = 1.01–2.41, P = 0.044; ORCD44v6 = 1.97, 95% CI = 1.19–3.26, P = 0.008; ORTotal CD44 isoforms = 1.57, 95% CI = 1.15–2.14, P = 0.004), distant metastasis (ORCD44 = 2.90, 95% CI = 1.08–7.83, P = 0.035; ORTotal CD44 isoforms = 1.89, 95% CI = 1.02–3.53, P = 0.044). Moreover, a high level of CD44 showed a possible correlation with poor differentiation (ORTotal CD44 isoforms = 1.44, 95% CI = 1.00–2.08, P = 0.051), elevated level of CD44v6 tend to be correlated with tumor size (OR = 1.71, 95% CI = 0.99–2.96, P = 0.056).
Conclusions: This meta-analysis demonstrated that CD44 overexpression might be an unfavorable prognostic factor for CRC patients and could be used to predict poor differentiation, lymph node metastasis and distant metastasis.
Introduction
Although therapies for colorectal cancer (CRC) has improved in recent years, colorectal cancer is still the third most common cause of cancer related death worldwide (1). Metastasis are observed in 25% of patients at initial diagnosis and approximately 50% of patients will develop metastasis (2). Presently, the outcome prediction and the therapy schedule determination of CRC patients is based on the TNM classification (3). However, TNM classification cannot precisely predict the prognosis of CRC patients at an early stage, therefore, finding the bio-markers in CRC patients is very important for diagnosis and prognosis prediction.
Currently, accumulating evidence supports a hypothesis that a subpopulation of cancer cells, called cancer stem cells (CSCs) exist, which contribute to tumor initiation, recurrence and resistance to radio-or chemotherapy (4–7). Although CSCs play crucial roles in cancer initiation and progression, there is no normalized CSCs marker. It has recently been reported that CSCs markers, such as CD133, CD44, EpCAM, and ALDH1, are potential prognostic markers for various cancers (7–9). Among them, CD44 is the most common reported CSCs marker in CRC (8, 9).
CD44 is an important membrane receptor for hyaluronic acid (HA), which has been reported to activate various tumor biological behaviors, including proliferation, differentiation, invasion and motility (10–14). The alternative splicing of variable exons in the mRNA of CD44 bring about plentiful variants, including CD44v2, CD44v3, CD44v5, CD44v6 and so forth, and CD44v is only detected in some epithelial cells. Additionally, the isoform with no variable exons of CD44 is called CD44s (15), which is the smallest CD44 molecular (85–95 kDa) and expressed on vertebrate cells (15, 16). CD44s and its isoforms play a different role in cancer (16). Previous studies have reported that CD44 activates a number of signaling pathways, including the MAPK, PI3K/Akt and Wnt pathways. The activation of these pathways is linked to tumor growth, migration, EMT, chemoresistance and apoptosis resistance (17–21). Additionally, CD44 has been shown to localize MMP-9 activity to the cell surface and then enhance tumor growth and metastasis (22).
Several reports have demonstrated that the overexpression of CD44s and CD44 variants was associated with prognosis and clinicopathological features in some tumors, including CRC (23–25). However, some published studies concluded that loss of CD44 is a poor prognostic factor for CRC patients (26–28). Currently, a series of meta-analyses and published studies have proved that CD44 is a promising prognostic biomarker for head and neck cancer (23), gastric cancer (29), hepatocellular cancer (30), and other cancers (31, 32). However, there is no systematic review and meta-analysis for assessing the prognostic value of CD44 in CRC. Thus, we performed the present meta-analysis to evaluate the prognostic value of CD44 and to clarify the relationship between CD44 and clinicopathological features in patients with CRC.
Materials and Methods
Search Strategy
The search strategy used in the present meta-analysis was in accordance with the PRISMA statement (33). Relative studies were searched in PubMed, Embase and the Cochrane library using combination terms: (“Colorectal Neoplasm” OR “Colorectal Tumor” OR “Colorectal Carcinoma” OR “Colorectal Cancer” OR” Colonic Neoplasm” OR” Colon Cancers” OR “Colonic Cancer” OR “Rectum Cancers” OR “Rectal Tumor” OR” Rectal Cancer” OR “CRC”) [Title/abstract] AND “CD44” [Title/abstract]. In addition, we read relative review articles and manually searched relevant studies. The last search was performed on 7 November 2018.
Inclusion Criteria
Primary studies were included under the following conditions:
(1) The study evaluated the expression level of CD44 in primary tumor tissues after surgical resection; (2) The sample size was more than 45 in the overall survival analysis; (3) A definite stage was reported; (4) The most recent and complete study was selected when the same author published several papers in the field of CD44; (5) Studies were written in English and (6) published in a peer-review journal; (7) Hazard ratio (HR) and 95% confidence intervals (CIs) were reported, or the data was sufficient to estimate the HR and 95% CIs from the survival analysis.
Data Extraction and Management
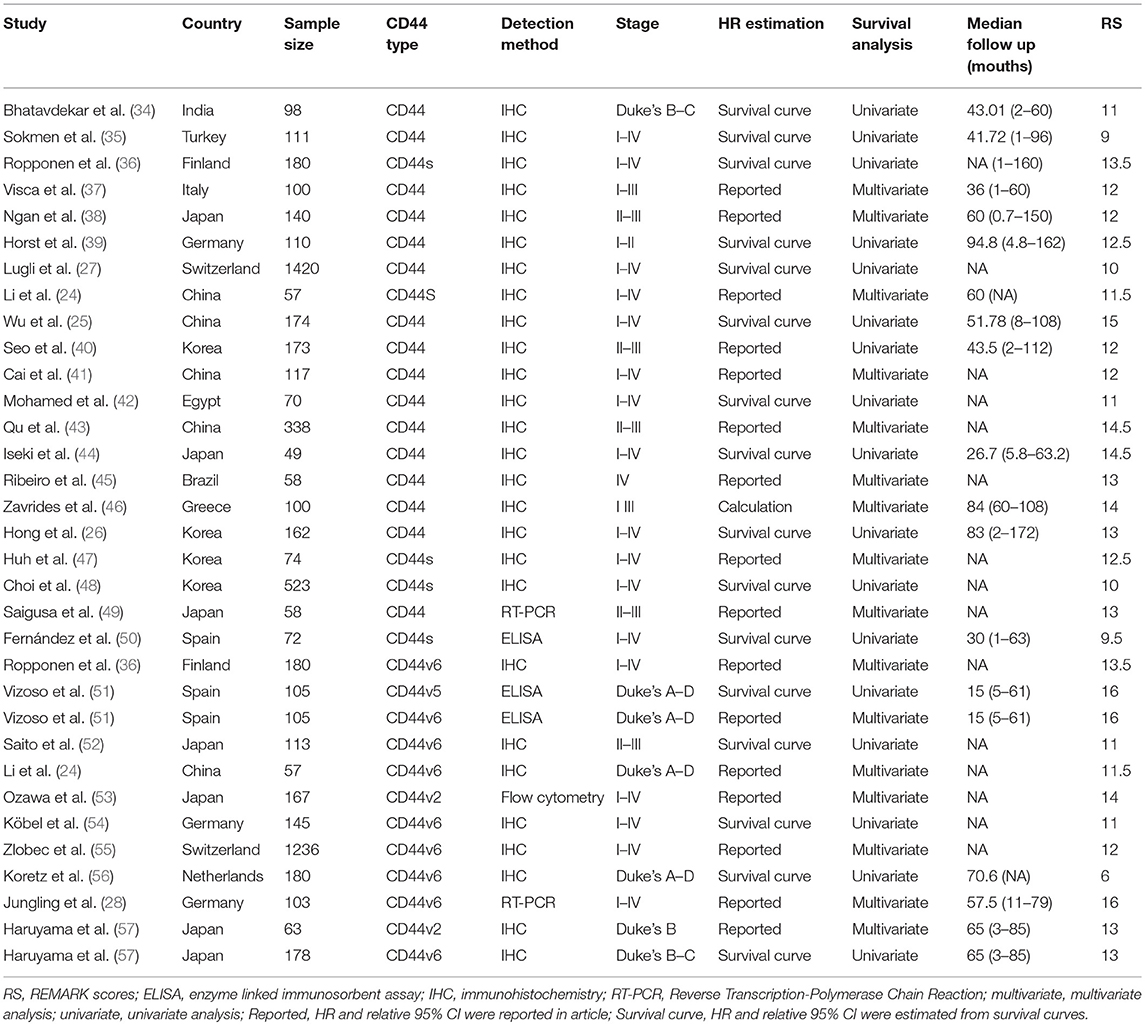
Two authors (ZSQ and WZP) independently extracted data from each eligible article. The predefined table was used to record the baseline characteristics including the first author's name, year of publication, CD44 isoform type, nationality, sample size, detection method, HRs and 95% CIs, and method to estimate HRs (univariate and multivariate analysis), median follow-up years, and REMARK scores (Table 1). If HRs and 95% CIs were not directly reported in eligible studies, we extracted and estimated HR and 95% CIs using a method reported by Parmar et al (58, 59).
Methodological Assessment
The quality of included studies was assessed using REMARK guidelines (60). Two reviewers (TYF, WZP) evaluated the study quality and reported scores independently. Finally, all authors discussed together to reach a consensus value.
Statistical Analysis
The individual HRs and relative 95% CIs were pooled into a summary HR and 95% CIs to assess the impact of CD44 on overall survival (OS) (61). For the measurement of the correlation of CD44 with clinicopathological parameters, odds ratios (ORs) and 95% CIs were used to estimate the effect. If HR or OR > 1 it represented a worse prognostic value of CD44 or a significant correlation between CD44 and clinicopathological features, respectively. If the 95% CI did not include the value 1, the pooled result was considered statistically significant. The heterogeneity across studies was detected using the Q test and I2 test, and a random-effect model (DerSimonian-Laird method) was used to calculate ORs and HRs when I2 was more than 50%; otherwise, a fixed-effects model (Mantel-Haenszel method) was used (62, 63). A subgroup analyses of the association between CD44 expression and prognosis were performed by CD44 types (Total CD44 isoforms, CD44, CD44v2, CD44v5, CD44v6); detection method (IHC, ELISA, RT-PCR, Flow cytometry); race (Caucasian, Asian, Black); Publication year (<2010 or ≥2010); tumor stage (I-III, I-IV, IV); univariate or multivariate analysis; REMARK scores (≤12, >12). Publication bias was assessed using Egger's test and Begg's funnel plot (64, 65). All statistical analyses were conducted using STATA version 12.0 (STATA, College Station, TX).
Results
Search Results
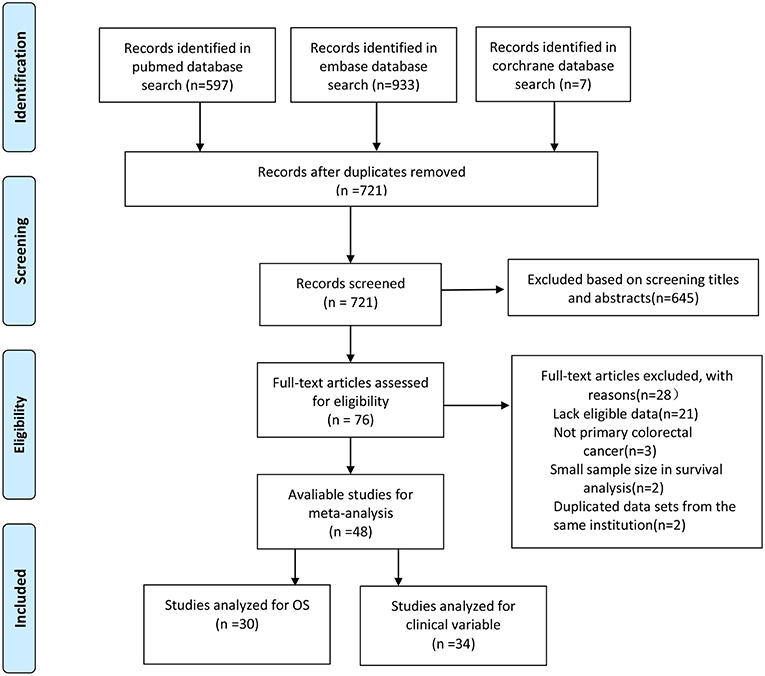
The literature search strategy is shown in Figure 1. A total of 48 studies were included in the final analysis. Of these studies, 30 studies that evaluated the prognostic role of CD44 in CRC patients were in accordance with the inclusion criteria (24–28, 34–57, 66). Thirty four studies reported the association of CD44 expression with clinicopathological features (24, 26, 27, 35, 39–42, 44, 46, 47, 49, 52, 54, 57, 67–84).
Quality of Studies
Study quality was evaluated using the REMARK guidelines, the results showed that 17 studies had scores of more than 12, and the remaining 16 studies had quality scores of 12 or less.
Study Results Report
The individual HRs and 95% CIs were obtained using a previous method (58). Sixteen studies directly reported HRs and 95% CIs. One study provided the total number of events, P-value and coefficient statistical value. In the remaining studies, HRs and 95% CIs were estimated using graphical survival plots.
Study Characteristics
The characteristics of the 30 studies that evaluated the prognostic role of CD44 in patients with CRC are summarized in Table 1. Studies were mainly from Asian and European nations including Japan (n = 7), China (n = 5), Korea (n = 4), India (n = 1), Turkey (n = 1), Germany (n = 3), Greece (n = 1), Finland (n = 1), Spain (n = 2), Switzerland (n = 2), Netherlands (n = 1), and the remaining two studies from Egypt (n = 1) and Brazil (n = 1). The studies included mainly focused on the total CD44 (n = 16) more than on CD44v6 (n = 9) and other isoform CD44s (n = 5), CD44v2 (n = 2), CD44v5 (n = 1), and these studies were published between 1994 and 2018. Four detection methods including immunohistochemistry (IHC, n = 27), fluorescence Quantitative PCR (RT-PCR, n = 2), enzyme-linked immunosorbent assay (ELISA, n = 3) and flow cytometry (FC, n = 1) were applied to detect the level of CD44 (Table 1).
Overall Survival
A total of 30 studies with 6816 patients were selected for survival analysis. CD44 overexpression was significantly associated with a worse OS (HR = 1.32, 95% CI = 1.08–1.61, I2 = 75.9%; Figure S1). The subgroup and meta-regression analyses did not find the source of heterogeneity (Table 2). However, we observed that worse OS was significantly associated with the overexpression of CD44v2 (HR = 2.93, 95% CI = 1.49–5.77, P = 0.002), CD44v6 (HR = 1.50, 95% CI = 1.10–2.14, P = 0.010) and total CD44 isoforms (HR = 1.32, 95% CI = 1.08–1.61, P = 0.007) (Figure 2). Additionally, a high level of CD44 was associated with worse OS for CRC patients of stage I-IV (HR = 1.42, 95% CI = 1.12–1.80, P = 0.001) (Figure S2). CD44 overexpression was also related significantly with OS in Caucasian patients (HR = 1.44, 95% CI = 1.13–1.85, P = 0.004) (Figure S3). Furthermore, the pooled HR estimate by IHC was 1.28 (95% CI = 1.03–1.60, P = 0.025) (Figure S4). Additionally, studies published before 2010 also showed a significant association between CD44 overexpression and OS (HR = 1.47, 95% CI = 1.13–1.91, P = 0.005) (Figure S5). Of note, the studies with REMARK scores of more than 12 showed worse OS (HR = 1.46, 95% CI = 1.05–2.02, P = 0.022) (Figure S6). We also observed that CD44 overexpression significantly correlated with OS in studies where HR was estimated by multivariate analysis (HR = 1.81, 95% CI = 1.13–2.91, P = 0.014) (Figure S7). These results suggested that CD44 overexpression might be a poor prognosis factor for patients of CRC.
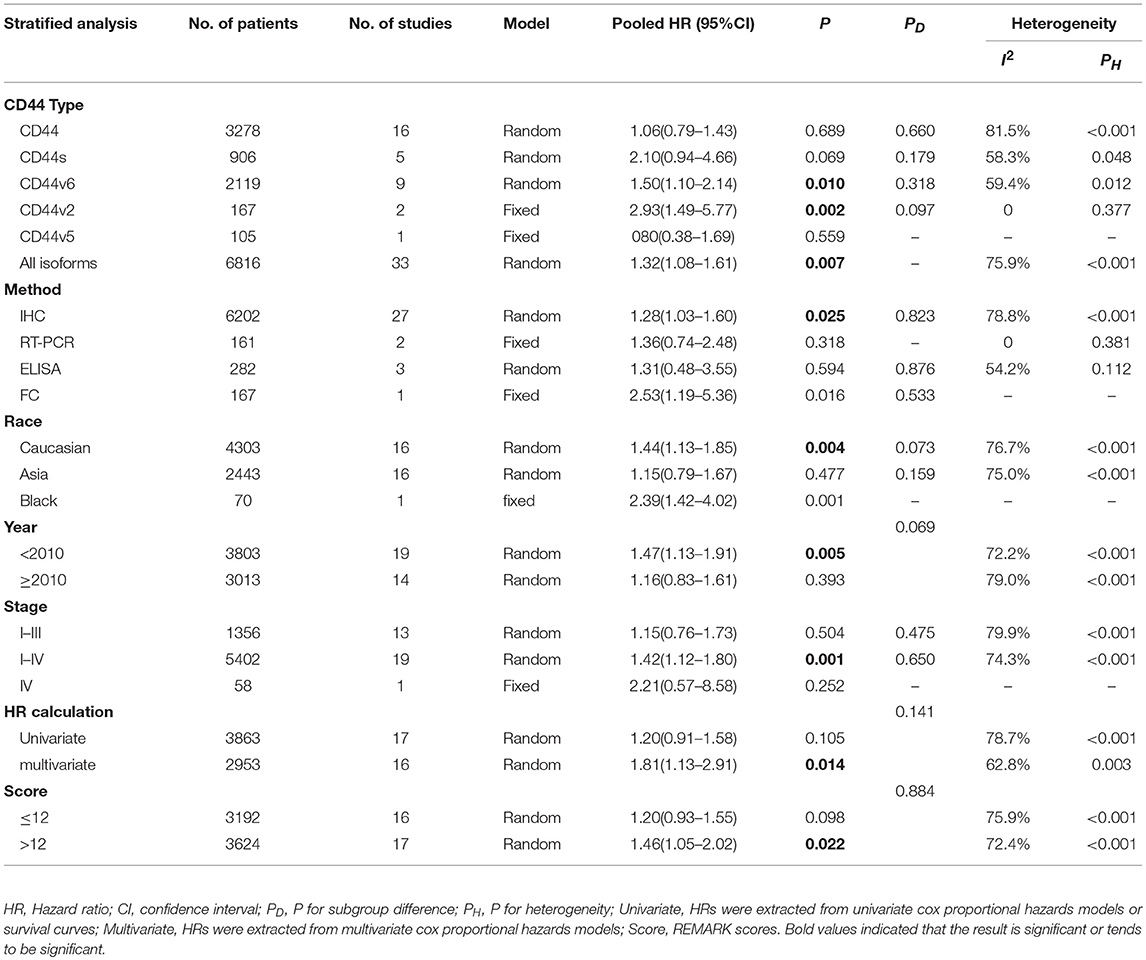
Table 2. Subgroup analysis and meta-regression of the association of CD44 and overall survival of colorectal cancer patients.
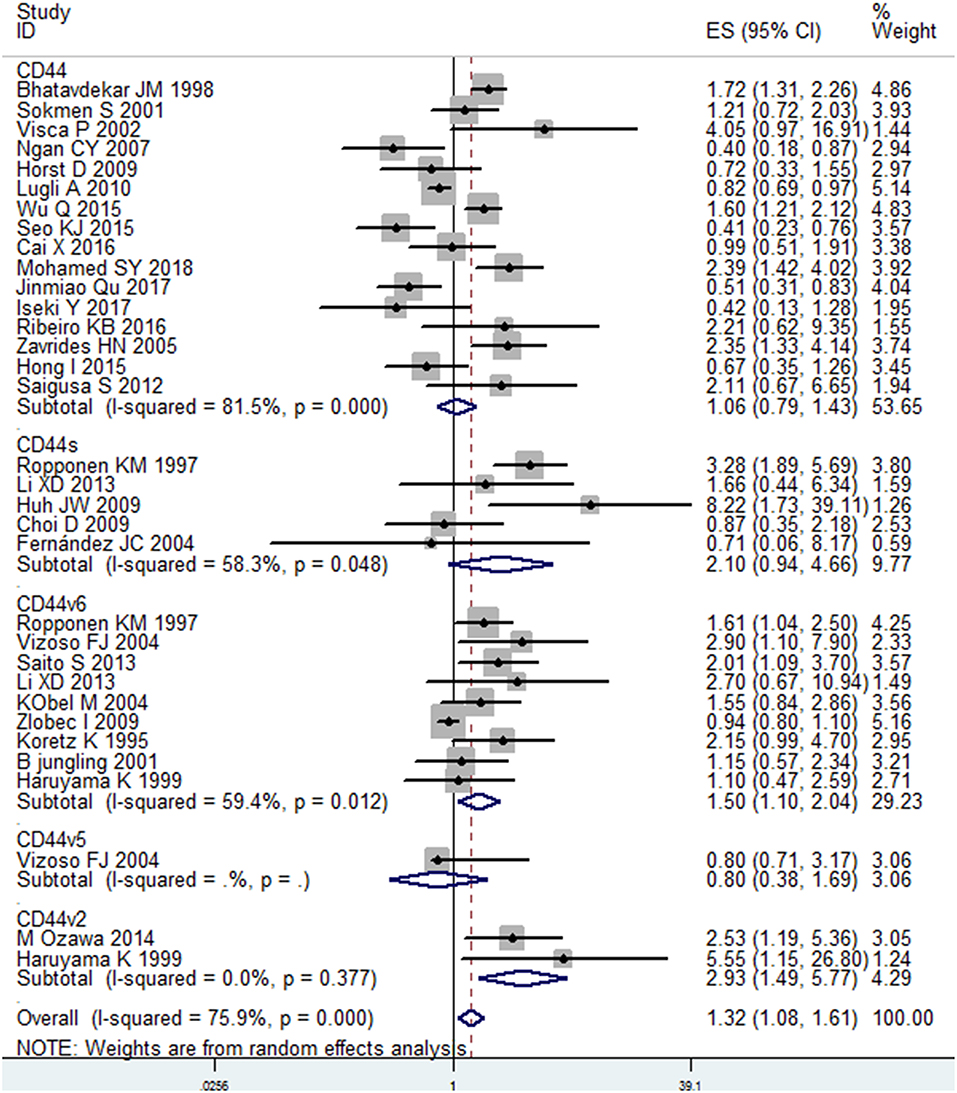
Figure 2. Subgroup analysis of the association between different isoforms of CD44 and OS in patients with colorectal cancer.
Clinicopathological Features
We evaluated the correlation between the overexpression of CD44 and clinicopathological features of CRC patients. The results were shown in Table 3, a higher level CD44, CD44V6, and total CD44 isoforms had a significant association with lymph node metastasis (ORCD44 = 1.56, 95% CI = 1.01–2.41, P = 0.044; ORCD44v6 = 1.97, 95% CI = 1.19–3.26, P = 0.008; OR Total CD44 isoforms = 1.57, 95% CI = 1.15–2.14, P = 0.004) (Figure S10). In addition, a high level of Total CD44 isoforms tend to be associated with poor differentiation (OR = 1.44, 95% CI = 1.00–2.08, P = 0.051) (Figure S11). Additionally, CD44, and total CD44 isoforms overexpression was significantly correlated with distant metastasis (ORCD44 = 2.90, 95% CI = 1.08–7.83, P = 0.035; OR Total CD44 isoforms = 1.89, 95% CI = 1.02–3.53, P = 0.044) (Figure S12). However, no significant correlation between other clinical features and CD44 exists (Table 3; Figures S8, S9, S14, S15).
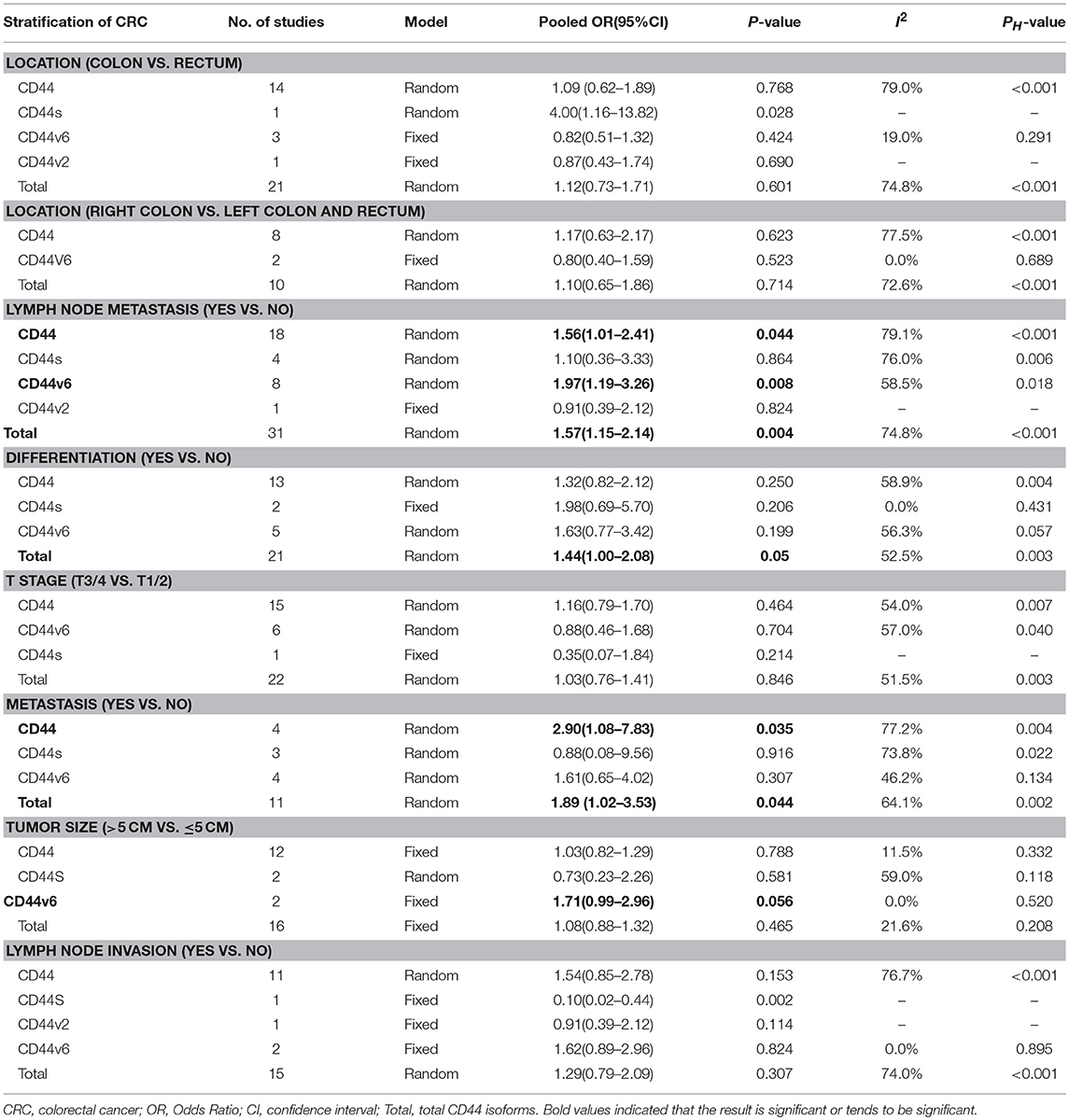
Table 3. Meta-analysis of the association between CD44 and clinicopathological features of colorectal cancer.
Publication Bias
Egger's test and Begg's test were performed to evaluate the publication bias of OS analysis. The study of the impact CD44 overexpression on OS suggested a Begg's test score of P = 0.722, however, an Egger's test score of P = 0.062 reflected slight evidence of publication bias in the analysis. The funnel plot also revealed slight evidence of publication bias (Figure S13).
Discussion
CD44, is a transmembrane glycoprotein and an adhesion molecule, which was first discovered on lymphocytes in 1982 (85), and is commonly accepted as a CSC marker in solid tumors (8, 86–89). In previous studies, it has been reported that CD44 is associated with various tumor biological behaviors, including proliferation, metastasis, recurrence, resistance to radio- and chemotherapy (10, 90, 91). However, a pan-CD44 antibody was used to detect the level of CD44 expression in many studies, which recognizes total CD44 variants, and therefore, these reports reveal limited knowledge about the association between specific CD44 variants and tumor progression. Currently, several studies show a different role between CD44s and CD44v. Recently, CD44s has been reported to promote EMT process, and Brown RL et al. found a shift in CD44 isoforms from CD44v to CD44s during EMT (92, 93). Additionally, Mashita et al. (94) showed that high CD44s/CD44v9 expression ratios was an independent prognosis factor in CRC. However, the CD44 variants (CD44v6 and v7/8) were up-regulated by hypoxia inducible factor (HIF) under a hypoxic condition (95). Additionally, CD44 variants were found to regulate ROS defense by stabilizing the xCT and promoting tumor growth (96). These studies showed the distinct role of CD44s and CD44v in tumor initiation and progression.
The expression of CD44 standard, v2, v3, v6, and v9 have been demonstrated in CRC patients (36, 51, 53, 55, 57). However, there are still controversies about the prognostic role of CD44s and CD44v in patients of CRC. Here, we performed a comprehensive meta-analysis to evaluate the prognostic role of various CD44 isoforms in patients with CRC. In total, 48 studies were included in our study. The results showed that a higher level of total CD44 isoforms was significantly associated with worse OS. However, a significant heterogeneity in these studies existed, thus a subgroup analysis and meta-regression analysis was performed to explore the source of heterogeneity.
Considering that various CD44 isoforms have different roles in tumor initiation and development, total CD44 isoforms, CD44s, CD44v2, CD44v6, and CD44v9 were separately analyzed, and the results showed that CD44v2 and CD44v6 was significantly associated with worse OS in CRC patients. Previous research showed that the metastatic phenotype was associated with CD44v2-10 isoform expression, especially with CD44v6 (97, 98). Additionally, a higher expression level of CD44v6 was detected in colorectal carcinoma (77, 99). However, the certain mechanism of CD44 variant isoforms in cancer development is not well-understood and further studies were needed to explore the role of CD44s and CD44v.
In addition, subgroup analysis indicated that CD44 overexpression is a poor prognosis factor in Caucasian patients (HR = 1.44; 95% CI = 1.13–1.85), while not in another race. The genetic background and environmental factors are varied in different regions, which may cause the particular characteristics of colorectal cancer in a relative human race. Moreover, a higher level of CD44 expression was significantly associated with worse OS in studies where CD44 expression was detected using immunohistochemistry staining (IHC) (HR = 1.28; 95% CI = 1.03–1.60). IHC, which is characterized by high sensibility and specificity is widely used in clinic. ELISA is used to detect CD44 protein levels in serum, however the substantial quantities of blood CD44 is not only influenced by tumor growth, but also by immune system activity (100). Additionally, RT-PCR is just used to detect the gene expression level. Therefore, the result of IHC is more convincing than other detection methods. Furthermore, subgroup analysis showed that a higher level of CD44 significantly correlated with poor survival in studies that estimated HRs using multivariate analysis (HR = 1.65; 95% CI = 1.17–2.32). These results further prove the conclusion that CD44 overexpression is a poor prognosis factor for CRC. Moreover, we stratified the variables by clinicopathological features, a higher level of total CD44 isoforms showed a significant correlation with poor differentiation (P = 0.048). As we know, one of the characteristics of CSCs is undifferentiated, which is in accordance with the theory that CD44 is a CSCs marker for colorectal cancer. A higher level of CD44, CD44v6 and total CD44 isoforms significantly correlated with lymph node metastasis, overexpression of CD44 and all CD44 isoforms, was found to be markedly related with distant metastasis and these results might be the reason why CD44 overexpression contributes to a poor prognosis.
There are some limitations in our meta-analysis. First, the CD44s and CD44v were identified by various methods and antibodies in different studies. Thus, CD44s and its variants were strictly defined according to the exact reactivity of antibodies. Second, we calculated HRs and 95% CI from survival curves. Third, there was significant heterogeneity in our meta-analysis, however, we minimized heterogeneity using a random effects and subgroup analysis based on various variables, such as race, publication year, detection method etc. In addition, we performed a meta-regression analysis to explore the source of heterogeneity.
A certain degree of publication bias exists in our meta-analysis. As known, studies that possess negative results and small samples are difficult to accept for publication. In addition, negative studies are more often published in a native language, however, positive studies are more likely to be published in English journals. Therefore, “negative” results should be encouraged to publish in the future. Furthermore, only fully published articles in English journals were included, while conference abstracts and unpublished studies were excluded.
Conclusions
In summary, our meta-analysis revealed the prognostic value of CD44 expression in CRC. Specifically, positive CD44 expression was significantly correlated with poor overall survival (OS). High CD44 expression was also associated with poor differentiation, lymph node metastasis and distant metastasis, and well-designed studies are needed to confirm the findings of our meta-analysis in the future.
Author Contributions
SZ and KW designed the study. ZW and YT performed the literature searches and assessed the quality of included studies. LX, ZG, AH, and CX analyzed the data. SZ wrote the manuscript. All authors commented on the manuscript and made the decision to submit.
Funding
This work is supported by the Special Research Project of Shanghai TCM University (No. JXDXSCXJH11), and the National Natural Science Foundation of China (No. 8160141675).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00309/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2014) 25 (Suppl. 3):iii1–9. doi: 10.1093/annonc/mdu260
3. Weiser MR. AJCC 8th Edition: colorectal cancer. Ann Surg Oncol. (2018) 25:1454–5. doi: 10.1245/s10434-018-6462-1
4. Pardal R, Clarke MF, Morrison SJ. Morrison: applying the principles of stem-cell biology to cancer. Nat Rev Cancer. (2003) 3:895–902. doi: 10.1038/nrc1232
5. Liu Y, Burness ML, Martin-Trevino R, Guy J, Bai S, Harouaka R, et al. RAD51 mediates resistance of cancer stem cells to PARP inhibition in triple-negative breast cancer. Clin Cancer Res. (2017) 23:514–22. doi: 10.1158/1078-0432.ccr-15-1348
6. Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. (2004) 432:396–401. doi: 10.1038/nature03128
7. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. (2007) 445:106–10. doi: 10.1038/nature05372
8. Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. (2007) 104:10158–63. doi: 10.1073/pnas.0703478104
9. Du L, Wang H, He L, Zhang J, Ni B, Wang X, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. (2008) 14:6751–60. doi: 10.1158/1078-0432.ccr-08-1034
10. Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. (2004) 95:930–5. doi: 10.1111/j.1349-7006.2004.tb03179.x
11. Cheng C, Sharp PA. Regulation of CD44 alternative splicing by SRm160 and its potential role in tumor cell invasion. Mol Cell Biol. (2006) 26:362–70. doi: 10.1128/mcb.26.1.362-370.2006
12. Vigetti D, Viola M, Karousou E, Rizzi M, Moretto P, Genasetti A, et al. Hyaluronan-CD44-ERK1/2 regulate human aortic smooth muscle cell motility during aging. J Biol Chem. (2008) 283:4448–58. doi: 10.1074/jbc.M709051200
13. Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. (1997) 71:241–319.
14. Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. (2003) 4:33–45. doi: 10.1038/nrm1004
15. Negi LM, Talegaonkar S, Jaggi M, Ahmad FJ, Iqbal Z, et al. Role of CD44 in tumour progression and strategies for targeting. J Drug Target. (2012) 20:561–73. doi: 10.3109/1061186x.2012.702767
16. Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. (2011) 11:254–67. doi: 10.1038/nrc3023
17. Bates RC, Edwards NS, Burns GF, Fisher DE. A CD44 survival pathway triggers chemoresistance via lyn kinase and phosphoinositide 3-kinase/Akt in colon carcinoma cells. Cancer Res. (2001) 61:5275–83.
18. Fujita Y, Kitagawa M, Nakamura S, Azuma K, Ishii G, Higashi M, et al. CD44 signaling through focal adhesion kinase and its anti-apoptotic effect. FEBS Lett. (2002) 528:101–108. doi: 10.1016/s0014-5793(02)03262-3
19. Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg. (2006) 132:771–8. doi: 10.1001/archotol.132.7.771
20. Miletti-Gonzalez KE, Chen S, Muthukumaran N, Saglimbeni GN, Wu X, Yang J, et al. The CD44 receptor interacts with P-glycoprotein to promote cell migration and invasion in cancer. Cancer Res. (2005) 65:6660–7. doi: 10.1158/0008-5472.can-04-3478
21. Kim MS, Park MJ, Moon EJ, Kim SJ, Lee CH, Yoo H, et al. Hyaluronic acid induces osteopontin via the phosphatidylinositol 3-kinase/Akt pathway to enhance the motility of human glioma cells. Cancer Res. (2005) 65:686–91.
22. Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. (1999) 13:35–48.
23. Chen J, Zhou J, Lu J, Xiong H, Shi X, Gong L. Significance of CD44 expression in head and neck cancer: a systemic review and meta-analysis. BMC Cancer. (2014) 14:15. doi: 10.1186/1471-2407-14-15
24. Li XD, Ji M, Wu J, Jiang JT, Wu CP. Clinical significance of CD44 variants expression in colorectal cancer. Tumori. (2013) 99:88–92. doi: 10.1700/1248.13794
25. Wu Q, Yang Y, Wu S, Li W, Zhang N, Dong X, et al. Evaluation of the correlation of KAI1/CD82, CD44, MMP7 and beta-catenin in the prediction of prognosis and metastasis in colorectal carcinoma. Diagn Pathol. (2015) 10:176. doi: 10.1186/s13000-015-0411-0
26. Hong I, Hong SW, Chang YG, Lee WY, Lee B, Kang YK, et al. Expression of the cancer stem cell markers CD44 and CD133 in colorectal cancer: an immunohistochemical staining analysis. Ann Coloproctol. (2015) 31:84–91. doi: 10.3393/ac.2015.31.3.84
27. Lugli A, Iezzi G, Hostettler I, Muraro MG, Mele V, Tornillo L, et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. (2010) 103:382–90. doi: 10.1038/sj.bjc.6605762
28. Jungling B, Menges M, Goebel R, Wittig BM, Weg-Remers S, Pistorius G, et al. Expression of CD44v6 has no prognostic value in patients with colorectal cancer. Z Gastroenterol. (2002) 40:229–33. doi: 10.1055/s-2002-25152
29. Chen Y, Fu Z, Xu S, Xu Y, Xu P. The prognostic value of CD44 expression in gastric cancer: a meta-analysis. Biomed Pharmacother. (2014) 68:693–7. doi: 10.1016/j.biopha.2014.08.001
30. Luo Y, Tan Y. Prognostic value of CD44 expression in patients with hepatocellular carcinoma: meta-analysis. Cancer Cell Int. (2016) 16:47. doi: 10.1186/s12935-016-0325-2
31. Li X, Ma X, Chen L, Gu L, Zhang Y, Zhang F, et al. Prognostic value of CD44 expression in renal cell carcinoma: a systematic review and meta-analysis. Sci Rep. (2015) 5:13157. doi: 10.1038/srep13157
32. Lin J, Ding D. The prognostic role of the cancer stem cell marker CD44 in ovarian cancer: a meta-analysis. Cancer Cell Int. (2017) 17:8. doi: 10.1186/s12935-016-0376-4
33. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
34. Bhatavdekar JM, Patel DD, Chikhlikar PR, Trivedi TI, Gosalia NM, Ghosh N, et al. Overexpression of CD44: a useful independent predictor of prognosis in patients with colorectal carcinomas. Ann Surg Oncol. (1998) 5:495–501.
35. Sokmen S, Lebe B, Sarioglu S, Fuzun M, Terzi C, Kupelioglu A, et al. Prognostic value of CD44 expression in colorectal carcinomas. Anticancer Res. (2001) 21:4121–6.
36. Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Expression of CD44 and variant proteins in human colorectal cancer and its relevance for prognosis. Scand J Gastroenterol. (1998) 33:301–9.
37. Visca P, Del Nonno F, Botti C, Marandino F, Sebastiani V, Di Tondo U, et al. Role and prognostic significance of CD44s expression in colorectal cancer. Anticancer Res. (2002) 22:2671–5.
38. Ngan CY, Yamamoto H, Seshimo I, Ezumi K, Terayama M, Hemmi H, et al. A multivariate analysis of adhesion molecules expression in assessment of colorectal cancer. J Surg Oncol. (2007) 95:652–62. doi: 10.1002/jso.20638
39. Horst D, Kriegl L, Engel J, Kirchner T, Jung A. Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest. (2009) 27:844–50. doi: 10.1080/07357900902744502
40. Seo KJ, Kim M, Kim J. Prognostic implications of adhesion molecule expression in colorectal cancer. Int J Clin Exp Pathol. (2015) 8:4148–57.
41. Cai X, Qi WX, Wang L, Zhang Z. Correlation of multiple proteins with clinic-pathological features and its prognostic significance in colorectal cancer with signet-ring cell component. Eur Rev Med Pharmacol Sci. (2016) 20:3358–67.
42. Mohamed SY, Kaf RM, Ahmed MM, Elwan A, Ashour HR, Ibrahim A. The prognostic value of cancer stem cell markers (Notch1, ALDH1, and CD44) in primary colorectal carcinoma. J Gastrointest Cancer. (2018). doi: 10.1007/s12029-018-0156-6. [Epub ahead of print]
43. Qu J, Jiang Y, Liu H, Deng H, Yu J, Qi X, et al. Prognostic value of E-cadherin-, CD44-, and MSH2-associated nomograms in patients with stage II and III colorectal cancer. Transl Oncol. (2017) 10:121–31. doi: 10.1016/j.tranon.2016.12.005
44. Iseki Y, Shibutani M, Maeda K, Nagahara H, Ikeya T, Hirakawa K. Significance of E-cadherin and CD44 expression in patients with unresectable metastatic colorectal cancer. Oncol Lett. (2017) 14:1025–34. doi: 10.3892/ol.2017.6269
45. Ribeiro KB, da Silva Zanetti J, Ribeiro-Silva A, Rapatoni L, de Oliveira HF., et al. KRAS mutation associated with CD44/CD166 immunoexpression as predictors of worse outcome in metastatic colon cancer. Cancer Biomark. (2016) 16:513–21. doi: 10.3233/cbm-160592
46. Zavrides HN, Zizi-Sermpetzoglou A, Panousopoulos D, Athanasas G, Elemenoglou I, Peros G. Prognostic evaluation of CD44 expression in correlation with bcl-2 and p53 in colorectal cancer. Folia Histochem Cytobiol. (2005) 43:31–6.
47. Huh JW, Kim HR, Kim YJ, Lee JH, Park YS, Cho SH, et al. Expression of standard CD44 in human colorectal carcinoma: association with prognosis. Pathol Int. (2009) 59:241–6. doi: 10.1111/j.1440-1827.2009.02357.x
48. Choi D, Lee HW, Hur KY, Kim JJ, Park GS, Jang SH, et al. Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World J Gastroenterol. (2009) 15:2258–2264. doi: 10.3748/wjg.15.2258
49. Saigusa S, Inoue Y, Tanaka K, Toiyama Y, Matsushita K, Kawamura M, et al. Clinical significance of LGR5 and CD44 expression in locally advanced rectal cancer after preoperative chemoradiotherapy. Int J Oncol. (2012) 41:1643–52. doi: 10.3892/ijo.2012.1598
50. Fernandez JC, Vizoso FJ, Corte MD, Gava RR, Corte MG, Suarez JP, et al. CD44s expression in resectable colorectal carcinomas and surrounding mucosa. Cancer Invest. (2004) 22:878–85. doi: 10.1081/cnv-200039658
51. Vizoso FJ, Fernandez JC, Corte MD, Bongera M, Gava R, Allende MT, et al. Expression and clinical significance of CD44V5 and CD44V6 in resectable colorectal cancer. J Cancer Res Clin Oncol. (2004) 130:679–86. doi: 10.1007/s00432-004-0596-1
52. Saito S, Okabe H, Watanabe M, Ishimoto T, Iwatsuki M, Baba Y, et al. CD44v6 expression is related to mesenchymal phenotype and poor prognosis in patients with colorectal cancer. Oncol Rep. (2013) 29:1570–8. doi: 10.3892/or.2013.2273
53. Ozawa M, Ichikawa Y, Zheng YW, Oshima T, Miyata H, Nakazawa K, et al. Prognostic significance of CD44 variant 2 upregulation in colorectal cancer. Br J Cancer. (2014) 111:365–74. doi: 10.1038/bjc.2014.253
54. Kobel M, Weichert W, Cruwell K, Schmitt WD, Lautenschlager C, Hauptmann S. Epithelial hyaluronic acid and CD44v6 are mutually involved in invasion of colorectal adenocarcinomas and linked to patient prognosis. Virchows Arch. (2004) 445:456–64. doi: 10.1007/s00428-004-1095-0
55. Zlobec I, Gunthert U, Tornillo L, Iezzi G, Baumhoer D, Terracciano L, et al. Systematic assessment of the prognostic impact of membranous CD44v6 protein expression in colorectal cancer. Histopathology. (2009) 55:564–75. doi: 10.1111/j.1365-2559.2009.03421.x
56. Koretz K, Moller P, Lehnert T, Hinz U, Otto HF, Herfarth C. Effect of CD44v6 on survival in colorectal carcinoma. Lancet. (1995) 345:327–8.
57. Haruyama K, Matsumura Y, Moriya Y, Kakizoe T, Ochiai A, Kawaguchi M, et al. Clinicopathological significance of the expression of CD44v2 in colorectal cancer. Anticancer Res. (1999) 19:4421–8.
58. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34.
59. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. (2011) 343:d2090. doi: 10.1136/bmj.d2090
60. Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG. Reporting recommendations for tumor marker prognostic studies (REMARK): an abridged explanation and elaboration. J Natl Cancer Inst. (2018) 110:803–11. doi: 10.1093/jnci/djy088
61. Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. (1985) 27:335–71.
62. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
64. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101.
65. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34.
66. Katoh S, Goi T, Naruse T, Ueda Y, Kurebayashi H, Nakazawa T, et al. Cancer stem cell marker in circulating tumor cells: expression of CD44 variant exon 9 is strongly correlated to treatment refractoriness, recurrence and prognosis of human colorectal cancer. Anticancer Res. (2015) 35:239–44.
67. Ichikawa W. Positive relationship between expression of CD44 and hepatic metastases in colorectal cancer. Pathobiology. (1994) 62:172–9. doi: 10.1159/000163907
68. Kunimura T, Yoshida T, Sugiyama T, Morohoshi T. The relationships between loss of standard CD44 expression and lymph node, liver metastasis in T3 colorectal carcinoma. J Gastrointest Cancer. (2009) 40:115–8. doi: 10.1007/s12029-009-9100-0
69. Wang XF, Zhang XL, Xu LP, Shi GG, Zheng HY, Sun BC. [Expression of stem cell markers CD44 and Lgr5 in colorectal cancer and its relationship with lymph node and liver metastasis]. Zhonghua Yi Xue Za Zhi. (2018) 98:2899–904. doi: 10.3760/cma.j.issn.0376-2491.2018.36.005
70. Isozaki H, Ohyama T, Mabuchi H. Expression of cell adhesion molecule CD44 and sialyl Lewis A in gastric carcinoma and colorectal carcinoma in association with hepatic metastasis. Int J Oncol. (1998) 13:935–42.
71. Zhao LH, Lin QL, Wei J, Huai YL, Wang KJ, Yan HY. CD44v6 expression in patients with stage II or stage III sporadic colorectal cancer is superior to CD44 expression for predicting progression. Int J Clin Exp Pathol. (2015) 8:692–701.
72. Gotley DC, Fawcett J, Walsh MD, Reeder JA, Simmons DL, Antalis TM. Alternatively spliced variants of the cell adhesion molecule CD44 and tumour progression in colorectal cancer. Br J Cancer. (1996) 74:342–51.
73. Gunther K, Dworak O, Remke S, Pfluger R, Merkel S, Hohenberger W, et al. Prediction of distant metastases after curative surgery for rectal cancer. J Surg Res. (2002) 103:68–78. doi: 10.1006/jsre.2001.6312
74. Avoranta ST, Korkeila EA, Syrjanen KJ, Pyrhonen SO, Sundstrom JT. Lack of CD44 variant 6 expression in rectal cancer invasive front associates with early recurrence. World J Gastroenterol. (2012) 18:4549–56. doi: 10.3748/wjg.v18.i33.4549
75. Ismaiel NE, Sharaf WM, Helmy DO, Zaki MM, Badawi MA, Soliman AS. Detection of cancer stem cells in colorectal cancer: histopathological and immunohistochemical study. Open Access Maced J Med Sci. (2016) 4:543–7. doi: 10.3889/oamjms.2016.126
76. Amirghofran Z, Jalali SA, Hosseini SV, Vasei M, Sabayan B, Ghaderi A. Evaluation of CD44 and CD44v6 in colorectal carcinoma patients: soluble forms in relation to tumor tissue expression and metastasis. J Gastrointest Cancer. (2008) 39:73–8. doi: 10.1007/s12029-009-9062-2
77. Ishida T. Immunohistochemical expression of the CD44 variant 6 in colorectal adenocarcinoma. Surg Today. (2000) 30:28–32. doi: 10.1007/pl00010042
78. Yamane N, Tsujitani S, Makino M, Maeta M, Kaibara N. Soluble CD44 variant 6 as a prognostic indicator in patients with colorectal cancer. Oncology. (1999) 56:232–8. doi: 10.1159/000011970
79. Michl M, Heinemann V, Jung A, Engel J, Kirchner T, Neumann J. Expression of cancer stem cell markers in metastatic colorectal cancer correlates with liver metastasis, but not with metastasis to the central nervous system. Pathol Res Pract. (2015) 211:601–9. doi: 10.1016/j.prp.2015.05.006
80. Choi JE, Bae JS, Kang MJ, Chung MJ, Jang KY, Park HS, et al. Expression of epithelial-mesenchymal transition and cancer stem cell markers in colorectal adenocarcinoma: clinicopathological significance. Oncol Rep. (2017) 38:1695–705. doi: 10.3892/or.2017.5790
81. Neumann J, Lohrs L, Albertsmeier M, Reu S, Guba M, Werner J, et al. Cancer stem cell markers are associated with distant hematogenous liver metastases but not with peritoneal carcinomatosis in colorectal cancer. Cancer Invest. (2015) 33:354–60. doi: 10.3109/07357907.2015.1047507
82. Huh JW, Lee JH, Kim HR. Pretreatment expression of 13 molecular markers as a predictor of tumor responses after neoadjuvant chemoradiation in rectal cancer. Ann Surg. (2014) 259:508–15. doi: 10.1097/SLA.0b013e31829b3916
83. Jing F, Kim HJ, Kim CH, Kim YJ, Lee JH, Kim HR. Colon cancer stem cell markers CD44 and CD133 in patients with colorectal cancer and synchronous hepatic metastases. Int J Oncol. (2015) 46:1582–8. doi: 10.3892/ijo.2015.2844
84. Rohani P, Noroozinia F, Modarresi P, Abbasi A. CD44 standard isoform; not a good marker for colon cancer. Int J Cancer Manag. (2017) 10:5. doi: 10.5812/ijcm.9166
85. Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. (1983) 304:30–4.
86. Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. (2007) 104:973–8. doi: 10.1073/pnas.0610117104
87. Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. (2008) 98:756–65. doi: 10.1038/sj.bjc.6604242
88. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. (2003) 100:3983–8. doi: 10.1073/pnas.0530291100
89. Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. (2008) 68:4311–20. doi: 10.1158/0008-5472.can-08-0364
90. Inoue K, Fry EA. Aberrant splicing of estrogen receptor, HER2, and CD44 genes in breast cancer. Genet Epigenet. (2015) 7:19–32. doi: 10.4137/geg.s35500
91. Xiaoping L, Xiaowei Z, Leizhen Z, Weijian G. Expression and significance of CD44 and p-AKT in pancreatic head cancer. World J Surg Oncol. (2015) 13:334. doi: 10.1186/s12957-015-0746-8
92. Biddle A, Gammon L, Fazil B, Mackenzie IC. CD44 staining of cancer stem-like cells is influenced by down-regulation of CD44 variant isoforms and up-regulation of the standard CD44 isoform in the population of cells that have undergone epithelial-to-mesenchymal transition. PLoS ONE. (2013) 8:e57314. doi: 10.1371/journal.pone.0057314
93. Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest. (2011) 121:1064–74. doi: 10.1172/jci44540
94. Mashita N, Yamada S, Nakayama G, Tanaka C, Iwata N, Kanda M, et al. Epithelial to mesenchymal transition might be induced via CD44 isoform switching in colorectal cancer. J Surg Oncol. (2014) 110:745–51. doi: 10.1002/jso.23705
95. Krishnamachary B, Penet MF, Nimmagadda S, Mironchik Y, Raman V, Solaiyappan M, et al. Hypoxia regulates CD44 and its variant isoforms through HIF-1alpha in triple negative breast cancer. PLoS ONE. (2012) 7:e44078. doi: 10.1371/journal.pone.0044078
96. Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, et al. CD44 variant regulatesredox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. (2011) 19:387–400. doi: 10.1016/j.ccr.2011.01.038
97. Barbour P, Reeder JA, Walsh MD, Fawcett J, Antalis TM, Gotley DC. Expression of the CD44v2-10 isoform confers a metastatic phenotype: importance of the heparan sulfate attachment site CD44v3. Cancer Res. (2003) 63:887–92.
98. Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. (1991) 65:13–24.
99. Weg-Remers S, Anders M, von Lampe B, Riecken EO, Schuder G, Feifel G, et al. Decreased expression of CD44 splicing variants in advanced colorectal carcinomas. Eur J Cancer. (1998) 34:1607–11.
Keywords: CD44, variant, colorectal cancer, survival, prognosis, meta-analysis
Citation: Wang Z, Tang Y, Xie L, Huang A, Xue C, Gu Z, Wang K and Zong S (2019) The Prognostic and Clinical Value of CD44 in Colorectal Cancer: A Meta-Analysis. Front. Oncol. 9:309. doi: 10.3389/fonc.2019.00309
Received: 04 February 2019; Accepted: 05 April 2019;
Published: 30 April 2019.
Edited by:
Aga Syed Sameer, King Saud bin Abdulaziz University for Health Sciences, Saudi ArabiaReviewed by:
Mujeeb Zaffar Banday, United Arab Emirates University, United Arab EmiratesNarasimha Reddy Parine, King Saud University, Saudi Arabia
Copyright © 2019 Wang, Tang, Xie, Huang, Xue, Gu, Wang and Zong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaiqiang Wang, MTEwMEBzenkuc2guY24=
Shaoqi Zong, dmlnaWx1Y2t5QDEyNi5jb20=
†These authors have contributed equally to this work
 Zhenpeng Wang1†
Zhenpeng Wang1† Shaoqi Zong
Shaoqi Zong