- 1Department of Colorectal Surgery, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Department of Urology, Fudan University Shanghai Cancer Center, Shanghai, China
Objective: This study was to investigate guiding role of elevated pretreatment serum carcinoembryonic antigen (CEA) levels for ACT receipt in stage IIA colon cancer.
Methods: Eligible patients diagnosed with stage IIA colon cancer (N = 21848) were identified from the Surveillance, Epidemiology, and End Results (SEER) database between January 2004 and December 2010. Pearson's chi-squared tests, Cox proportional hazards regression models, and Kaplan-Meier methods were performed. Propensity score matching (PSM) was used to decrease the risk of biased estimates of treatment effect.
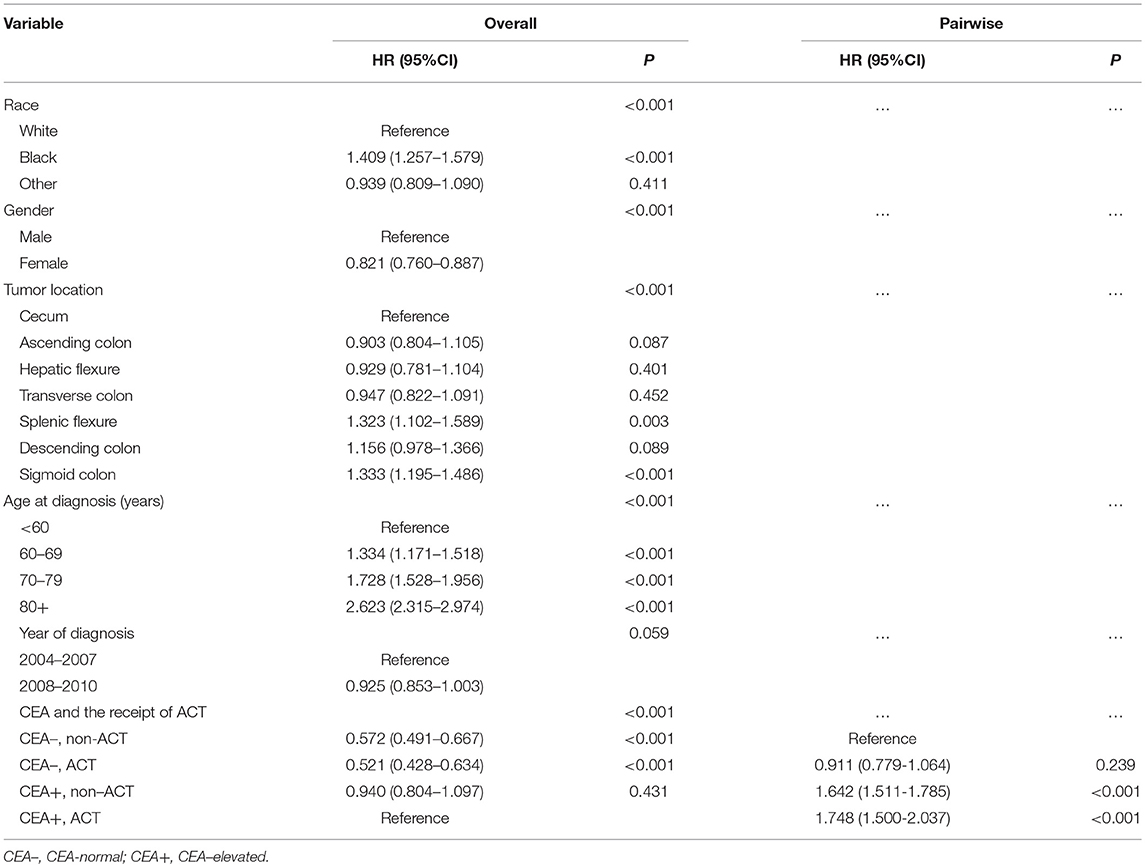
Results: Multivariate Cox analysis indicated that, in CEA-elevated group, receiving or not receiving ACT did not presented statistically CSS difference [hazard ratio (HR) = 0.940, 95% confidence interval (CI) = 0.804–1.097, P = 0.431]; in CEA-normal group, receiving or not receiving ACT also did not presented statistically CSS difference (HR = 0.911, 95% CI = 0.779–1.064, P = 0.239). After PSM, Kaplan-Meier analyses showed that there was no statistical CSS difference between receiving or not receiving ACT (P = 0.64).
Conclusion: ACT did not show substantial survival benefit in stage IIA colon cancer with elevated pretreatment serum CEA levels. Stage IIA disease with elevated pretreatment serum CEA should not be treated with ACT.
Introduction
Colon cancer is one of the most commonly diagnosed cancers in men and women (1). It was reported that stage II disease accounted for ~36% of new colon cancer diagnoses (2). The use of adjuvant chemotherapy (ACT) in stage II colon cancer was still controversial though it was widely accepted as standard treatment for patients with stage III colon cancer (3–5).
Although lack of enough direct evidence regarding the efficiency of ACT in stage II colon cancer, the American Society of Clinical Oncology (ASCO) clinical guidelines still had recommendations of ACT the so-called high-risk stage II disease (6). Also, the European Society for Medical Oncology (ESMO) had the similar recommendation for high-risk stage II colon cancer (7). In spite of a little different from each other regarding the definition of “high-risk factors,” both of ASCO and ESMO did not rank elevated serum carcinoembryonic antigen (CEA) levels as one of the so-called high-risk factors. And the ASCO Tumor Marker Panel had suggested that there was not sufficient data to support the use of preoperative CEA levels to guide the receipt of ACT stage II tumor (8). Later however, some reports supported the use of serum CEA levels to guide ACT (8–12) and regarded elevated preoperative CEA levels as one of the high-risk factors in stage II disease (13–16).
In fact, the efficacy of adjuvant ACT among high-risk stage II colon cancer had long been controversial (17–19). In spite of this, recently, the efficacy of ACT in T4 (stage IIB and IIC) disease was confirmed (18, 20, 21). In the present study, therefore, we have evaluated the guiding role of elevated pretreatment serum CEA levels for ACT receipt in stage IIA (T3N0M0) colon cancer using the large Surveillance, Epidemiology, and End Results (SEER) database.
Patients and Methods
Patient Selection From SEER Database
Data from the SEER Program of the United States National Cancer Institute were used. As an authoritative source, SEER collects patient demographic information, cancer diagnostic information, and outcomes from 18 cancer registries in the United States, thus including ~28% of the US population. The SEER database does not contain any identifiers and is publicly available for the studies of cancer-based epidemiology. In the present study, the National Cancer Institute's SEER-Stat software (version 8.3.5) was used to get access to SEER database.
Shown as Figure 1, at first, 40,968 patients, diagnosed with stage IIA (T3N0M0) colon cancer between January 1, 2004 and December 31, 2010, were identified. We identified patients diagnosed within these years as pretreatment serum CEA information was recorded starting from 2004 and we wanted to allow for 5 years of follow-up (SEER follow-up ended in 2015).
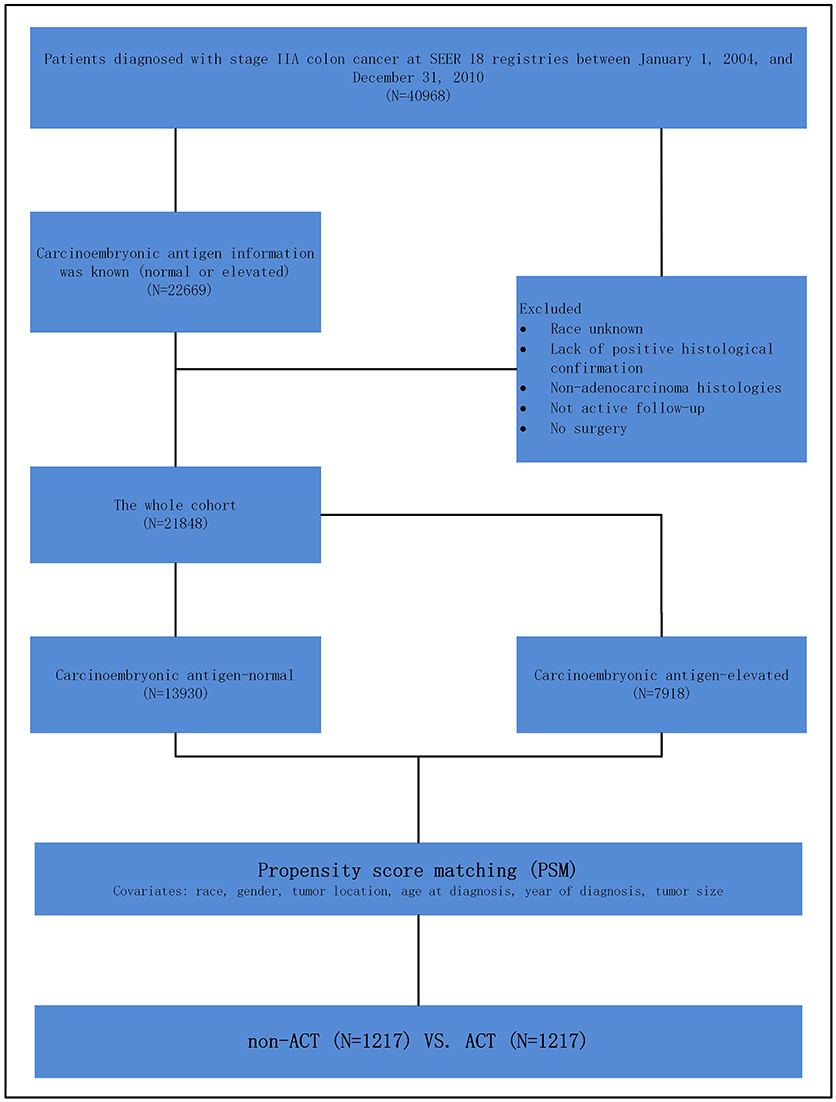
Figure 1. Schematic representation of patient population selected from Surveillance, Epidemiology, and End Results (SEER) database.
Those with known CEA information were included in our study. The exclusion criteria were followed: race unknown, lack of positive histological confirmation, non-adenocarcinoma histology, not active follow-up, no surgery. In this study, we stratified the “patient had chemotherapy” as ACT group and “no evidence of chemotherapy was found in the medical records examined” as a non-ACT group in the variable “chemotherapy recode” in SEER cohort.
Statistical Analyses
In the present study, we compared different clinicopathologic factors between the ACT and non-ACT groups using Pearson's chi-squared test for different variables. The outcome of interest in our study was cause-specific survival (CSS). The cause of death was categorized as colon cancer specific or non-colon cancer related. The CSS was calculated from the date of diagnosis to the date of colon cancer death. Patients who died of other causes were censored at the date of death. To determine whether there was a significant interaction between the level of serum CEA and ACT in predicting CSS, we defined a variable combined with serum CEA level and ACT. The Kaplan-Meier method with a log-rank test was used to analyze CSS. Then, several multivariate Cox proportional hazard models were constructed to identify independent prognostic factors along with hazard ratio (HR) for CSS. Variables that showed prognostic significance (log-rank, P < 0.20) in univariate analysis were included in the final multivariable analysis. Pair-wise comparisons were performed between different combinations of tumor grade and ACT to determine the presence of significant CSS differences.
However, as an observational one, there could be significant bias introduced by inherent differences between patients based on the receipt of ACT in the present study. Then, to decrease the risk of biased estimates of treatment effect, we defined the logit of predicted probability of treatment as a propensity score using the following patient and tumor characteristics: race, gender, tumor location, age at diagnosis, year of diagnosis and tumor size. Patients receiving ACT were matched on a one-to-one basis with patients not receiving ACT. Patients with and without receiving ACT were matched within their respective risk groups, matching was performed based on nearest-neighbor matching. Propensity scores reflect the probability that patients received or not received ACT based on their baseline characteristics. The details of propensity score matching (PSM) process are shown as Figure 1.
Statistical analysis was mainly performed with SPSS version 22 (IBM Corporation, Armonk, NY, USA); and two-sided P < 0.05 was considered statistically significant.
Results
Baseline Characteristics
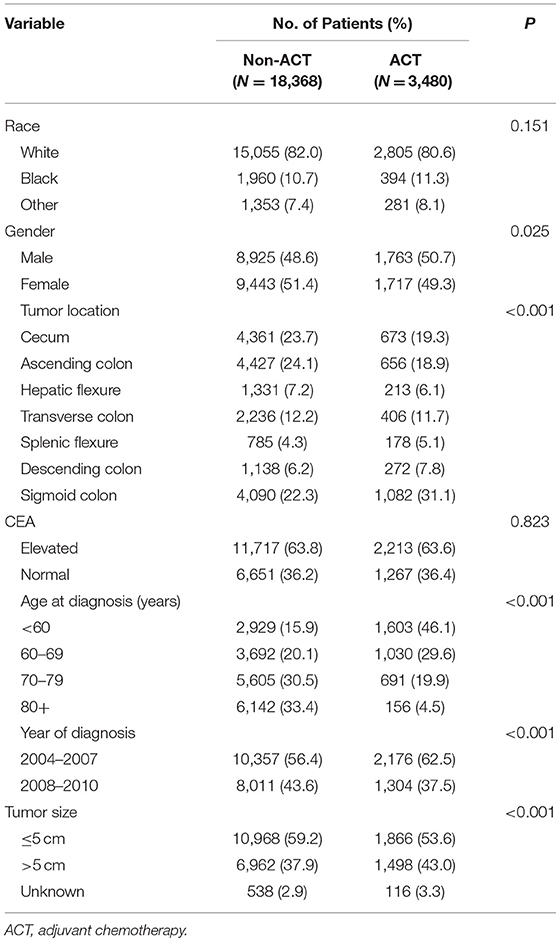
Twenty one thousand eight hundred and forty-eight patients diagnosed with stage IIA colon cancer were identified from SEER database with a median follow-up time of 75 months. And 2,666 (12.2%) patients died of colon cancer at the end of the follow-up time. Of all, 13,930 patients (63.8%) were stratified into the CEA-normal group, and 7,918 patients (36.2%) were stratified into the CEA-elevated group. The patients' baseline demographic characteristics are shown in Table 1. The results of Pearson's chi-squared test indicated that the level of serum carcinoembryonic antigen was not corrected with the receipt of ACT (P = 0.823, Table 1).
Associations of the Level of Serum CEA and ACT in Predicting CSS
Kaplan-Meier analysis showed that the receipt of ACT had better 6-year CSS (median follow-up time was 75 months) rate compared with not receiving ACT with serum CEA elevated (84.8 vs. 83.3%, P = 0.026, Figure 2). Similarly, in the context of normal serum CEA, the receipt of ACT had better 6-year CSS rate compared with not receiving ACT (93.0 vs. 89.6%, P < 0.001, Figure 2). After adjusting for other prognostic factors, such as race, gender, tumor location, age at diagnosis, and year of diagnosis, however, the results of multivariate Cox analysis indicated that, in CEA-elevated group, receiving or not receiving ACT did not presented statistically CSS difference (HR = 0.940, 95% CI = 0.804–1.097, P = 0.431). Similarly, in the pair-wise comparison, receiving or not receiving ACT also did not presented statistically CSS difference with normal serum CEA (HR = 0.911, 95% CI = 0.779–1.064, P = 0.239; Table 2).
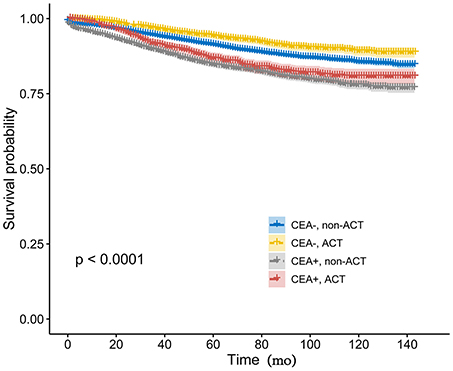
Figure 2. Kaplan-Meier CSS curves stratified by the combination of pretreatment serum CEA levels and receipt of ACT.
CSS of ACT With Elevated Serum CEA After PSM
PSM produced 1,217 patients in the non-ACT group and 1,217 patients in the ACT group, all the tumor and patient characteristics showed no statistically differences between the two groups (Table 3). The Kaplan-Meier method with a log-rank test was used to compare the CSS difference of receiving and not receiving ACT after PSM, and the result indicated there was no statistical CSS difference between receiving or not receiving ACT (P = 0.64, Figure 3).
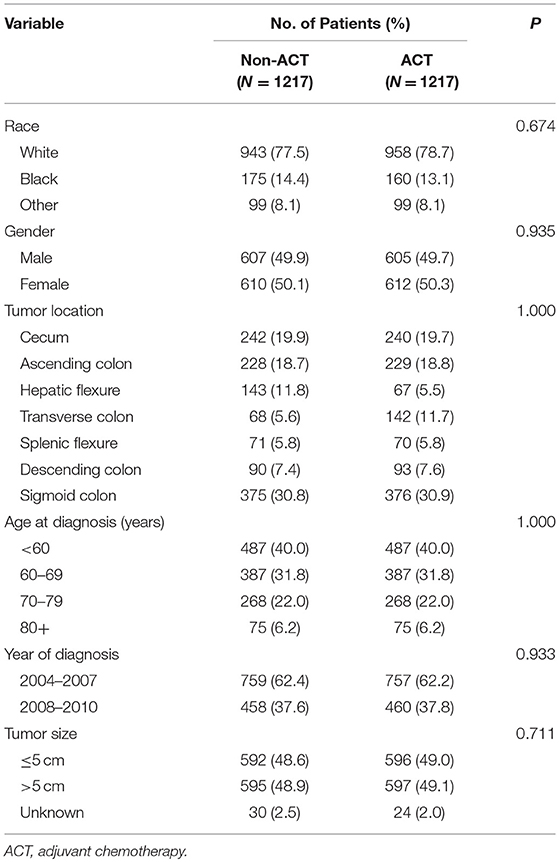
Table 3. Comparison of baseline characteristics of CEA-elevated stage IIA colon cancer by receipt of ACT after PSM.
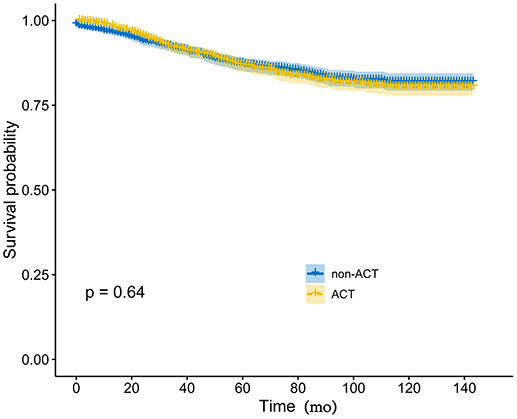
Figure 3. Kaplan-Meier CSS curves by the receipt of ACT in elevated pretreatment serum CEA group after PSM.
Discussion
Stage II colon cancer had a relatively good prognosis, however, it was reported that ~15–30% of patients with stage II disease would eventually develop distant metastases or locoregional recurrent disease, resulting in poor outcomes even after resection of the primary tumor (2, 22). Furthermore, to avoid the potential of excessive treatment, identification of candidates in stage II colon cancer for additional therapy was imperative.
Although both ESMO and ASCO had the recommendation for high-risk stage II colon cancer to receive ACT, the efficacy of ACT in high-risk disease had long been controversial and was suspected by many studies (15–19, 21, 23). In spite of this, recently, the definite survival benefit of ACT had been reported in T4 disease (stage IIB and IIC) among stage II colon cancer patients (18, 20, 21). In 2014, Aalok and his colleges (20) reported that the recurrence-free survival (RFS), disease-specific survival (DSS), and overall survival (OS) benefit of adjuvant CT was mainly observed in the T4 disease. Two later studies from the USA (20) and Netherlands (21) revealed the same findings, thus proving that the maximum survival benefit in T4 can be obtained with adjuvant therapy.
As a 201 kDa highly glycosylated antigen, serum CEA is the single most important tumor marker and elevated preoperative CEA correlate with poorer prognosis in rectal cancer (24–26). In 2,000, the Colorectal Working Group of the American Joint Committee on Cancer (AJCC) even proposed the inclusion of serum level of CEA (C-stage) into conventional TNM staging of rectal cancer (27). Furthermore, the ASCO (25) and the European Group on Tumor Markers (24) have both recommended the use of preoperative serum CEA as a prognostic tool in rectal cancer. However, we noted that both ESMO (including lymph nodes sampling <12; poorly differentiated tumor; vascular or lymphatic or perineural invasion; tumor presentation with obstruction or tumor perforation and pT4 stage) and ASCO (including patients with inadequately sampled nodes, T4 lesions, perforation, or poorly differentiated histology) did not regard elevated serum CEA levels as one of high-risk factors of stage II colon cancer (6, 7). Recently, however, many reports supported the use of serum CEA to guide ACT in stage II colon cancer. We then conducted this large propensity score-matched study to evaluate the value of elevated serum CEA levels in a subset (stage IIA colon cancer, non-T4 disease) of stage II colon cancer which, as far as we knew, had never been reported before.
In this present study, univariate analyses showed little survival benefit offered by ACT in stage IIA colon cancer both with and without elevated pretreatment serum CEA levels. After controlling for other known prognostic factors, however, the survival differences of receiving and not receiving ACT were not statistically significant in both CEA-elevated and CEA-normal groups. Furthermore, PSM was used to consolidated our finding, and analyses after PSM once again demonstrated ACT did not substantially improve survival in stage IIA colon cancer with elevated serum CEA levels and elevated serum CEA should not be used as one of high-risk factors to guide ACT, which was consistent with the ASCO and ESMO recommendations (6, 7) and added a strong evidence to the view of the ASCO Tumor Marker Panel that there was not sufficient data to support the use of preoperative CEA levels to guide the receipt of ACT stage II tumor (8). And we also noted that, in our study, the level of serum CEA was not corrected with the receipt of ACT, indicating that elevated serum CEA was not used for guiding ACE in clinical practice of stage II colon cancer in US, which was consistent with the ASCO and ESMO recommendations.
Considering the definite efficacy of ACT in T4 disease, we then assumed that the primary reason that the finding of our study was conflicted with previous researches (8, 9) which showed the survival benefit of ACT in stage II colon cancer with elevated serum CEA was because the studies did not exclusively focus on stage IIA colon cancer and their study cohorts were mixed with the subset of stage IIB (T4aN0M0) and IIC (T4bN0M0) colon cancers.
The main strength of our study was that to the best of our knowledge, it was the first to evaluate the value of serum CEA levels for guiding ACT in stage IIA colon cancer. By using a large population-based registry, it was possible to detect the absolute survival difference between CEA-elevated and CEA-normal patients. Furthermore, we also used PSM to demonstrated our finding that ACT did not show statistically survival difference in stage IIA colon cancer with elevated serum CEA levels, which provided information to guide ACT in stage II colon cancer.
There are some limitations in our study. First, this study did not include some prognostic factors of colon cancer. Nowadays, molecular biomarkers that could affect the prognosis of stage IIA colon cancer such as microsatellite instability (MSI) and BRAF V600E mutation that have been intensively studies, were not included into our analyses, which might result into bias to some extent (28). Second, it was not possible to differentiate the type of CT, preoperative CT or postoperative CT in SEER database. Yet, as the preoperative CT is not the standard treatment for stage IIA disease, we could cautiously describe the “patient had chemotherapy” in “chemotherapy recode” variable as “ACT” receipt. Finally, the present study was retrospective rather than prospective, thus our conclusions still need to be validated in other cohorts, especially in large RCTs.
In conclusion, ACT did not show substantial survival benefit in stage IIA colon cancer with elevated pretreatment serum CEA levels. Our study provided a strong evidence that serum CEA did not has a guiding role for ACT in stage IIA colon cancer and stage IIA disease with elevated pretreatment serum CEA should not be treated with ACT.
Ethics Statement
The study was approved by the Ethical Committee and Institutional Review Board of the Fudan University Shanghai Cancer Center. The data did not include the use of human subjects or personal identifying information and no informed consent was required for this study.
Author Contributions
XL and YM conceived this study. QiL and DL improved the study design and contributed to the interpretation of results. SZ collected the data. QinL performed data processing and statistical analysis. QiL and DL wrote the manuscript. YH revised the manuscript. XL, YM, and SC approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81702353 and 81772599) and Shanghai Municipal Natural Science Foundation (17ZR1406400). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
1. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. Ca A Cancer J Clin. (2017) 67:104–17. doi: 10.3322/caac.21395
2. O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new american joint committee on cancer sixth edition staging. J Natl Cancer Instit. (2004) 96:1420–5. doi: 10.1093/jnci/djh275
3. André T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. (2004) 350:2343–51. doi: 10.1056/NEJMoa032709
4. Gray R, Barnwell J, McConkey C, Hills RK, Williams NS. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet (2007) 370:2020. doi: 10.1016/S0140-6736(07)61866-2
5. Sargent D, Sobrero A, Grothey A, O'Connell MJ, Buyse M, Andre T, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. (2009) 27:872–7. doi: 10.1200/JCO.2008.19.5362
6. Benson AB, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American society of clinical oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. (2004) 22:3408–19. doi: 10.1200/JCO.2004.05.063
7. Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol Offic J Eur Soc Med Oncol. (2013) 24(Suppl. 6):vi64. doi: 10.1093/annonc/mdt354
8. Ogata Y, Murakami H, Sasatomi T, Ishibashi N, Mori S, Ushijima M, et al. Elevated preoperative serum carcinoembrionic antigen level may be an effective indicator for needing adjuvant chemotherapy after potentially curative resection of stage II colon cancer. J Surg Oncol. (2009) 99:65–70. doi: 10.1002/jso.21161
9. Lin HH, Chang YY, Lin JK, Jiang JK, Lin CC, Lan YT, et al. The role of adjuvant chemotherapy in stage II colorectal cancer patients. Int J Colorectal Dis. (2014) 29:1237–43. doi: 10.1007/s00384-014-1943-6
10. Margalit O, Mamtani R, Yang YX, Reiss KA, Golan T, Halpern N, et al. Assessing the prognostic value of carcinoembryonic antigen levels in stage I and II colon cancer. Eur J Cancer (2018) 94:1–5. doi: 10.1016/j.ejca.2018.01.112
11. Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Landmann RG, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum (2008) 51:503–7. doi: 10.1007/s10350-008-9246-z
12. Spindler BA, Bergquist JR, Thiels CA, Habermann EB, Kelley SR, Larson DW, et al. Incorporation of CEA improves risk stratification in stage II colon cancer. J Gastro Surg. (2017) 21:770–7. doi: 10.1007/s11605-017-3391-4
13. Amri R, England J, Bordeianou LG, Berger DL. Risk stratification in patients with stage II colon cancer. Ann Surg Oncol. (2016) 23:1–8. doi: 10.1245/s10434-016-5387-9
14. Becerra AZ, Probst CP, Tejani MA, Aquina CT, González MG, Hensley BJ, et al. Evaluating the prognostic role of elevated preoperative carcinoembryonic antigen levels in colon cancer patients: results from the national cancer database. Ann Surg Oncol. (2016) 23:1554–61. doi: 10.1245/s10434-015-5014-1
15. Matsuda C, Ishiguro M, Teramukai S, Kajiwara Y, Fujii S, Kinugasa Y, et al. A randomised-controlled trial of 1-year adjuvant chemotherapy with oral tegafur-uracil versus surgery alone in stage II colon cancer: SACURA trial. Eur J Cancer (2018) 96:54–63. doi: 10.1016/j.ejca.2018.03.009
16. O'Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. (2011) 29:3381–8. doi: 10.1200/JCO.2010.34.3426
17. Babaei M, Balavarca Y, Jansen L, van Erning FN, van Eycken L, et al. Administration of adjuvant chemotherapy for stage II-III colon cancer patients: an european population-based study. Int J Cancer (2017) 42:1480–9. doi: 10.1002/ijc.31168
18. Babcock BD, Aljehani MA, Jabo B, Choi AH, Morgan JW, Selleck MJ, et al. High-risk stage II colon cancer: not all risks are created equal. Ann Surg Oncol. (2018) 25:1–6. doi: 10.1245/s10434-018-6484-8
19. Kim MK, Won DD, Park SM, Kim T, Kim SR, Oh ST, et al. Effect of adjuvant chemotherapy on stage II colon cancer: analysis of korean national data. Cancer Res Treat. (2017) 50:1149–63. doi: 10.4143/crt.2017.194
20. Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gill S, Woods R, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer (2015) 121:527–34. doi: 10.1002/cncr.29072
21. Verhoeff SR, Van Erning FN, Lemmens VEPP, De Wilt JHW, Pruijt JFM. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer (2016) 139:187–93. doi: 10.1002/ijc.30053
22. Ju JH, Chang SC, Wang HS, Yang SH, Jiang JK, Chen WC, et al. Changes in disease pattern and treatment outcome of colorectal cancer: a review of 5,474 cases in 20 years. Int J Colorect Dis. (2007) 22:855–62. doi: 10.1007/s00384-007-0293-z
23. Casadaban L, Rauscher G, Aklilu M, Villenes D, Freels S, Maker AV. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer (2016) 122:3277–87. doi: 10.1002/cncr.30181
24. Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer (2007) 43:1348–60. doi: 10.1016/j.ejca.2007.03.021
25. Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. (2006) 24:5313–27. doi: 10.1200/JCO.2006.08.2644
26. Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. (1999) 9:67–81. doi: 10.1006/scbi.1998.0119
27. Yarbro JW, Page DL, Fielding LP, Partridge EE, Murphy GP. American Joint Committee on Cancer prognostic factors consensus conference. Cancer (1999) 86:2436.
Keywords: carcinoembryonic antigen, adjuvant chemotherapy, stage IIA colon cancer, PSM, SEER
Citation: Liu Q, Huang Y, Luo D, Zhang S, Cai S, Li Q, Ma Y and Li X (2019) Evaluating the Guiding Role of Elevated Pretreatment Serum Carcinoembryonic Antigen Levels for Adjuvant Chemotherapy in Stage IIA Colon Cancer: A Large Population-Based and Propensity Score-Matched Study. Front. Oncol. 9:37. doi: 10.3389/fonc.2019.00037
Received: 06 August 2018; Accepted: 14 January 2019;
Published: 13 February 2019.
Edited by:
John James Tentler, University of Colorado, United StatesReviewed by:
Mirko Omejc, University of Ljubljana, SloveniaQingfeng Zhu, Johns Hopkins Medicine, United States
Copyright © 2019 Liu, Huang, Luo, Zhang, Cai, Li, Ma and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxiang Li, MTE0OWx4eEBzaW5hLmNvbQ==
Yanlei Ma, eWFubGVpbWFAZnVkYW4uZWR1LmNu
†These authors have contributed equally to this work
 Qi Liu
Qi Liu Yongqiang Huang2,3†
Yongqiang Huang2,3† Xinxiang Li
Xinxiang Li