- 1Department of Radiation Oncology, Cancer Hospital, The First Affiliated Hospital of Xiamen University, Teaching Hospital of Fujian Medical University, Xiamen, China
- 2Department of Radiation Oncology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China
- 3Department of Oncology, Dongguan Third People's Hospital, Affiliated Dongguan Shilong People's Hospital of Southern Medical University, Dongguan, China
- 4Eye Institute of Xiamen University, Fujian Provincial Key Laboratory of Ophthalmology and Visual Science, Medical College, Xiamen University, Xiamen, China
Introduction: To assess the role of the 21-gene recurrence score (RS) assay on decision-making of postoperative radiotherapy (RT) following breast-conserving surgery (BCS) in elderly women with early-stage breast cancer.
Methods: The 21-gene RS for elderly (≥65 years) women with stage T1–2N0M0 estrogen receptor-positive breast cancer who underwent BCS from 2004 to 2015 was obtained from the Surveillance, Epidemiology, and End Results program. We estimated the association of 21-gene RS and adjuvant RT related to breast cancer-specific survival (BCSS) using propensity score matching (PSM).
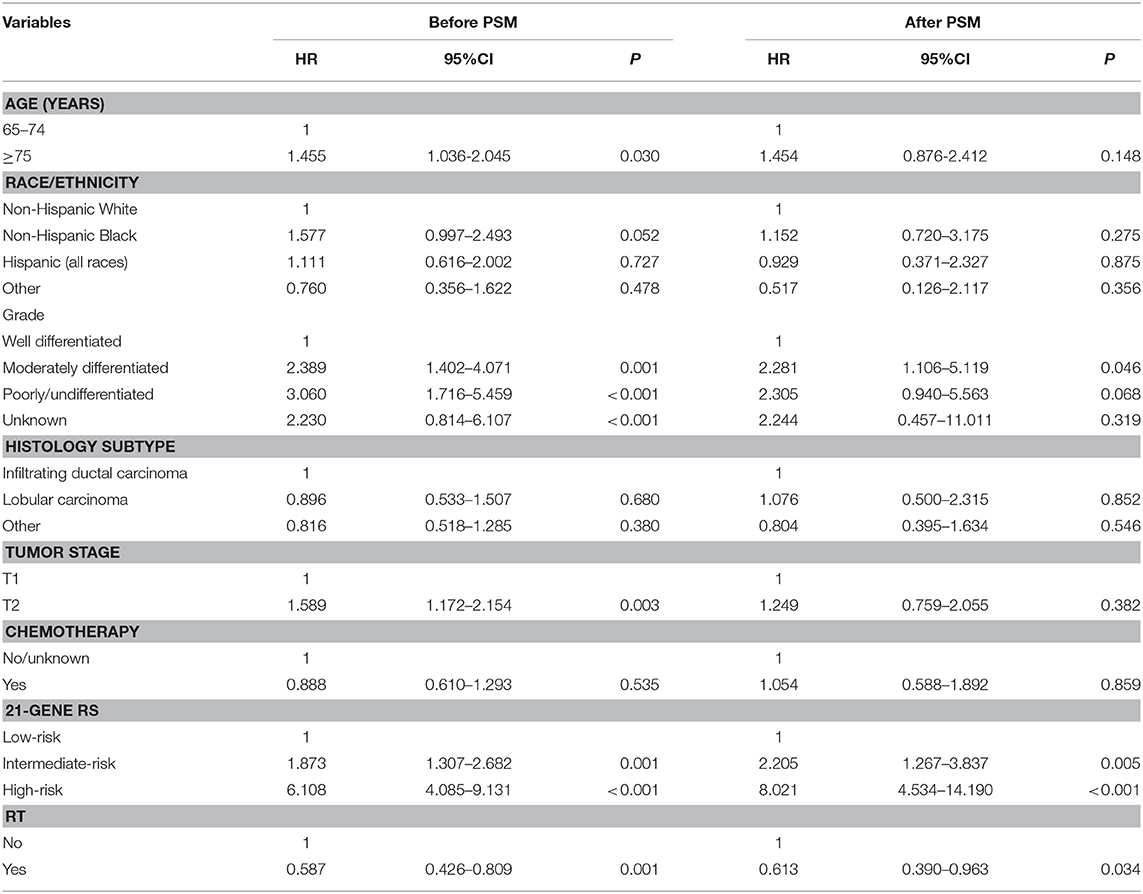
Results: We identified 18,456 patients, of which 15,326 (83.0%) received postoperative RT. Of identified patients, 58.9, 34.0, and 7.1% of patients had a low-, intermediate-, and high-risk RS, respectively. Receipt of postoperative RT was not related to the year of diagnosis according to the 21-gene RS groups. Multivariate analysis suggested that receipt of postoperative RT was an independent predictor of better BCSS before (hazard ratio [HR] 0.587, 95% confidence interval [CI] 0.426–0.809, P = 0.001) and after (HR 0.613, 95%CI 0.390–0.963, P = 0.034) PSM. However, subgroups analyses indicated that receipt of postoperative RT was related to better BCSS in women with intermediate-risk RS before (HR 0.467, 95%CI 0.283–0.772, P = 0.003) and after (HR 0.389, 95%CI 0.179–0.846, P = 0.017) PSM, but not in women with low- and high-risk RS groups before and after PSM.
Conclusions: Although causation cannot be implied, adjuvant RT in elderly women was associated with a greater effect size in patients with an intermediate-risk RS.
Background
Several prospective studies have shown that radiotherapy (RT) following breast-conserving surgery (BCS) can improve locoregional control in elderly women with low-risk breast cancer, but this regimen has no effect on distant recurrence or overall survival (OS) (1–4). Nevertheless, more than two-thirds of elderly patients undergo RT after BCS (5, 6), which suggests that the results of prospective studies have not decreased use of postoperative RT significantly in this population. Comorbidities are common among elderly patients, but life expectancy could be increased with better management of such comorbidities (7–10). Therefore, valuable decision-making tools to identify elderly patients with breast cancer who may benefit from postoperative RT are required. However, authoritative decision-making tools in this population are lacking.
The 21-gene recurrence score (RS) assay (Oncotype DX; Genomic Health, Redwood City, CA, USA) is used to assess the breast-cancer genes related to proliferation and invasion. The 21-gene RS assay can also provide information on the risk of distant recurrence as well as the benefit of adjuvant chemotherapy (11–13). In addition, several recent studies have shown that the 21-gene RS is correlated with the risk of locoregional recurrence (LRR). That is, a high RS is associated with a high risk of LRR in patients with node-negative or node-positive estrogen receptor (ER)-positive breast cancer (14, 15).
Studies focusing on whether the 21-gene RS assay can be used to identify a subgroup of elderly women with breast cancer for whom RT following BCS is not indicated are lacking. We hypothesized that postoperative RT could provide greater survival benefit for patients with a high RS. Therefore, we investigated the potential role of the 21-gene RS assay on prediction of RT outcome in elderly women with breast cancer after BCS.
Materials and Methods
Database and Patients
This retrospective study included data derived from Surveillance, Epidemiology, and End Results Program (SEER) database, the SEER database contains anonymized data of cancer incidence, patient demographics, first course of treatment, and survival outcomes of ~28% of the USA population. We linked to the National Cancer Institute's SEER 18 Regs (Excl AK) Custom Data Malignant Breast (with Oncotype DX and Additional Treatment Fields) to include breast cancer patients with available 21-gene RS testing between 2004 and 2015 (16). The results of 21-gene RS assay were provided by Genomic Health Clinical Laboratory, and were released in 2018 in the SEER database. The permission to access this database for the present study was authorized.
We identified women with 21-gene RS data who: (i) were aged ≥65 years with invasive breast cancer; (ii) had or did not have adjuvant RT following BCS; (iii) had stage tumor size ≤ 5 cm and node-negative disease (T1–T2N0); (iv) had ER-positive disease. Patients with no positive pathology diagnosis, receipt of preoperative RT, or an unknown sequence of RT and surgery were excluded. We did not need to obtain written informed consent from our institution because SEER data are anonymized.
Variables
We were interested in the following variables: 21-gene RS, RT, age, tumor grade, race/ethnicity, histology subtype, tumor stage, and chemotherapy. The groups of 21-gene RS were classified as having a “low-risk” (RS < 18), “intermediate-risk” (17–29), or “high-risk” (>30) (30). Breast cancer-specific survival (BCSS) was the primary endpoint of this study and was defined as the time from diagnosis to death from breast cancer.
Statistical Analyses
The variables of patient demographic, clinicopathological, and treatment variables, were compared using the chi-squared test or Fisher's exact test upon receipt of postoperative RT. To reduce the potential confounding of retrospective studies, a 1:1 match including the variables mentioned above was conducted using propensity score matching (PSM) to create matched cohorts (17, 18). BCSS curves were plotted using the Kaplan–Meier method and then compared with the log-rank test. Multivariate Cox proportional hazards models with the backward Wald method were used to assess the independent prognostic indicators related to BCSS. The hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) were calculated. Statistical analyses were carried out using SPSS 22.0 (IBM, Armonk, NY, USA). P < 0.05 was considered statistically significant.
Results
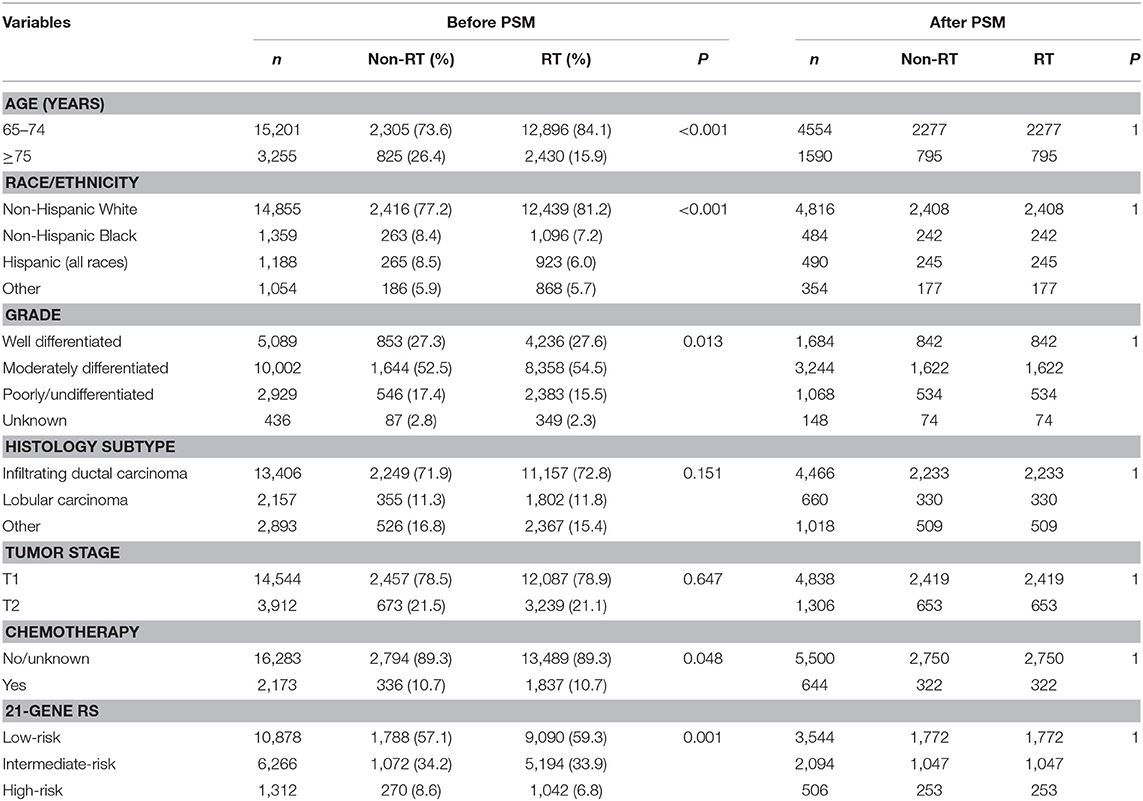
We identified 18,456 patients (median age, 69 years; range, 65–93 years). The baseline characteristics of RT and non-RT groups are listed and compared in Table 1. A total of 15,326 patients (83.0%) received postoperative RT. Patients with younger age (P < 0.001), non-Hispanic White (P < 0.001), and moderately differentiated (P = 0.013) were more likely to receive postoperative RT. We found that 11.8% of patients had chemotherapy, and patients who received postoperative RT were more likely to receive chemotherapy (P = 0.048). Of the identified patients, 58.9, 34.0, and 7.1% of patients were classified as having a low-, intermediate-, and high-risk RS, respectively. In addition, patients with low-risk RS group were more likely to receive postoperative RT (P = 0.001). Moreover, patients with a higher RS were more likely to receive chemotherapy (P < 0.001)
In the entire cohort, receipt of postoperative RT was not associated with the year of diagnosis (P = 0.275). In addition, in the low- (P = 0.302) (Figure 1A), intermediate- (P = 0.512) (Figure 1B), and high-risk RS groups (P = 0.849) (Figure 1C), receipt of postoperative RT was also not related to the year of diagnosis. Five-year BCSS was 98.5% with a median follow-up of 38 (range, 0–142) months. Postoperative RT was related to better BCSS. Five-year BCSS was 97.4 and 98.7% in non-RT and RT groups, respectively (P < 0.001) (Figure 2A). Multivariate analysis suggested that receipt of adjuvant RT was an independent prognostic factor related to better BCSS (HR 0.587, 95%CI 0.426–0.809, P = 0.001). Age, tumor grade, tumor stage, and 21-gene RS were also the independent prognostic indicators for BCSS (Table 2).
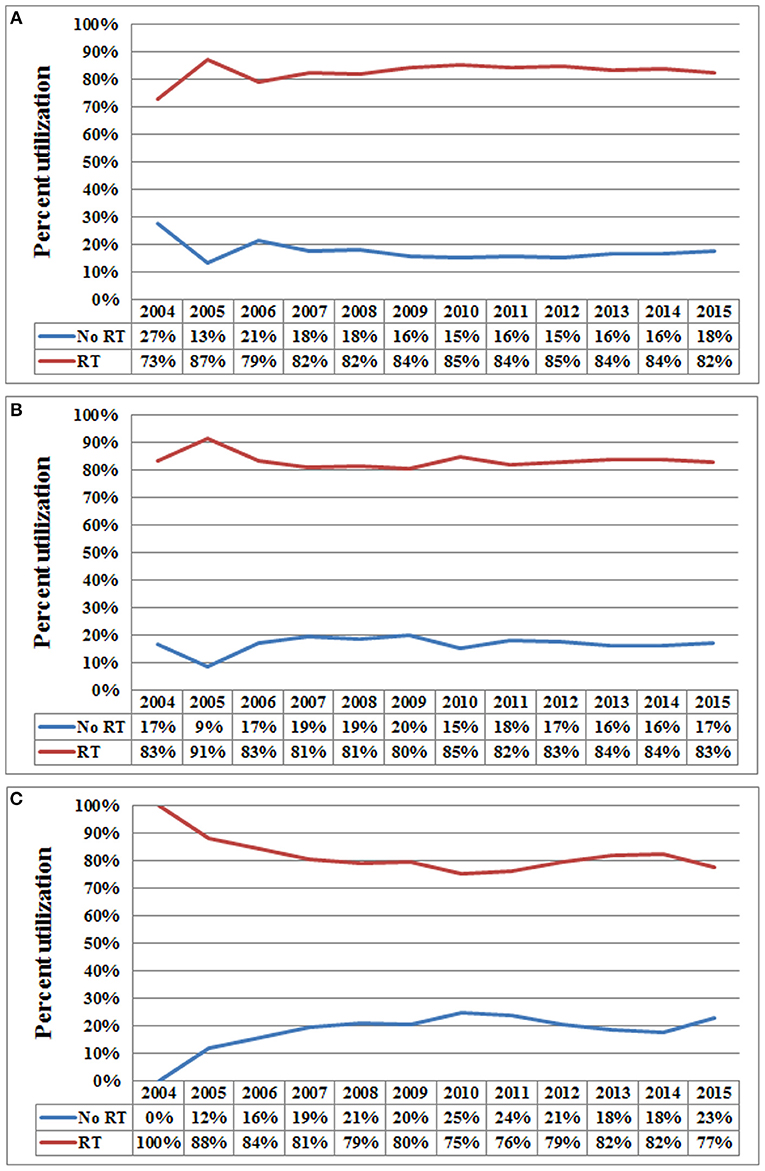
Figure 1. Use of postoperative radiotherapy vs. non-use of postoperative radiotherapy over time (A, low-risk RS group; B, intermediate-risk RS group; C, high-risk RS group).
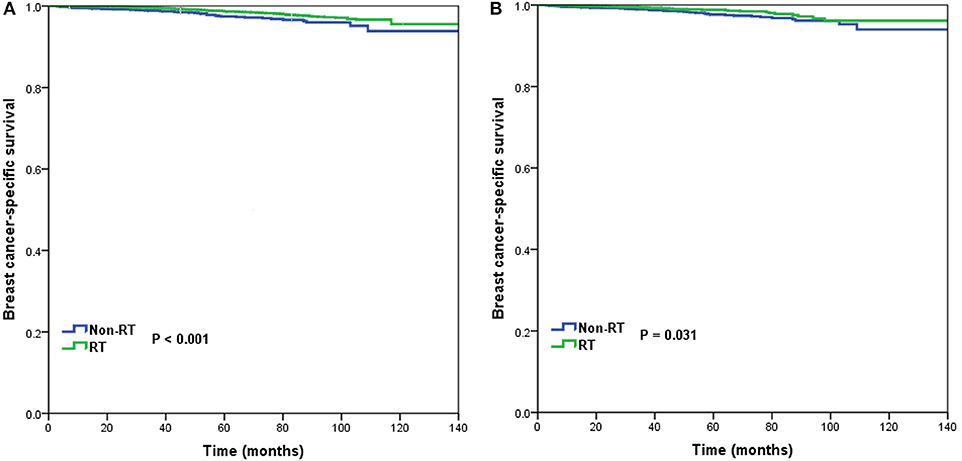
Figure 2. Breast cancer-specific survival in patients who had or did not have postoperative radiotherapy in the entire cohort before (A) and after (B) propensity score matching.
A total of 3,072 pairs of patients were matched completely. Patient characteristics after PSM are listed in Table 1. Receipt of postoperative RT was related to better BCSS. Five-year OS was 97.7 and 98.8% in non-RT and RT groups, respectively (P = 0.031) (Figure 2B). Receipt of postoperative RT was an independent prognostic indicator related to better BCSS (HR 0.613, 95%CI 0.390–0.963, P = 0.034) in multivariate analysis after PSM (Table 2).
In 10,878 patients of the low-risk RS group, 9,090 (83.6%) patients had postoperative RT. A total of 1,772 pairs of patients were matched completely. Patient characteristics before and after PSM are listed in Supplemental Table 1. The administration of postoperative RT was not associated with better BCSS before (5-year BCSS was 98.9 and 99.3% in non-RT and RT groups, respectively, P = 0.080) (Figure 3A) and after (5-year BCSS was 99.1 and 99.2% in non-RT and RT groups, respectively, P = 0.712) (Figure 3B) PSM. Multivariate analysis also indicated that receipt of postoperative RT was not related to better BCSS before (HR 0.653, 95%CI 0.347–1.227, P = 0.186) and after (HR 0.836, 95%CI 0.354–1.973, P = 0.683) PSM (Table 3).
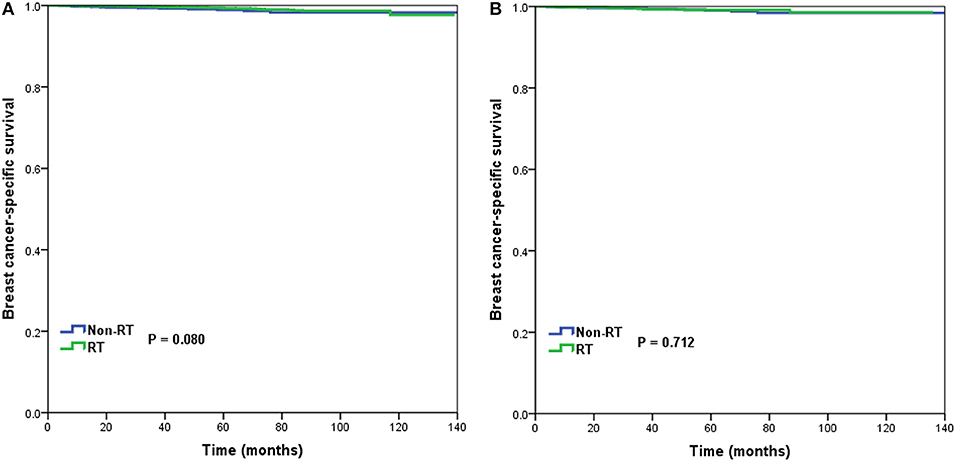
Figure 3. Breast cancer-specific survival in patients who had or did not have postoperative radiotherapy in the low-risk group before (A) and after (B) propensity score matching.
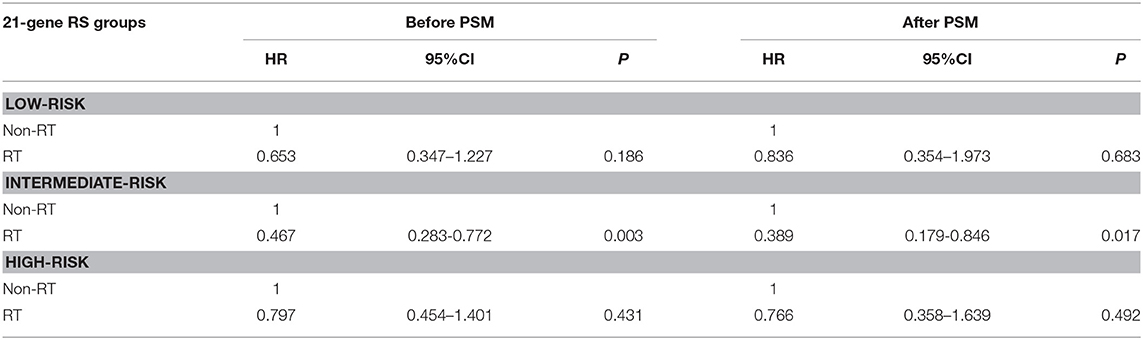
Table 3. Multivariate prognostic analyses according to RS before and after propensity score matching.
In 6,266 patients of the intermediate-risk RS group, 6,194 (82.9%) patients received postoperative RT. A total of 1,047 pairs of patients were matched completely. Patient characteristics before and after PSM are listed in Supplemental Table 2. Before PSM, receipt of postoperative RT was correlated with better BCSS. Five-year BCSS was 97.4 and 98.8% in non-RT and RT groups, respectively (P = 0.002) (Figure 4A). There was an absolute BCSS benefit of 2.3% in the RT group compared with the non-RT group after PSM (99.6 vs. 97.3%, P = 0.012) (Figure 4B). Multivariate analysis showed that receipt of postoperative RT was independently related to better BCSS before (HR 0.467, 95%CI 0.283–0.772, P = 0.003) and after (HR 0.389, 95%CI 0.179–0.846, P = 0.017) PSM (Table 3).
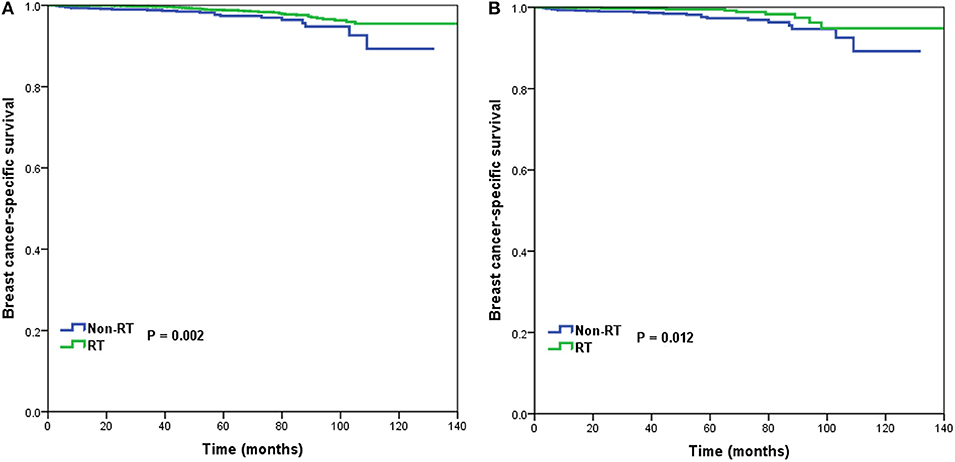
Figure 4. Breast cancer-specific survival in patients who had or did not have postoperative radiotherapy in the intermediate-risk group before (A) and after (B) propensity score matching.
In 1,312 patients of the high-risk RS group, 1,042 (79.4%) patients received postoperative RT. A total of 253 pairs of patients were matched completely. Patients characteristics before and after PSM are listed in Supplemental Table 3. Before PSM, receipt of postoperative RT was not associated with better BCSS. Five-year BCSS was 89.5 and 93.1% in non-RT and RT groups, respectively (P = 0.209) (Figure 5A). There was also comparable BCSS between non-RT and RT groups after PSM (90.7 vs. 93.9%, P = 0.477) (Figure 5B). Multivariate analysis showed that receipt of postoperative RT did not independently impact BCSS before (HR 0.797, 95%CI 0.454–1.401, P = 0.431) and after (HR 0.766, 95%CI 0.358–1.639, P = 0.492) PSM (Table 3).
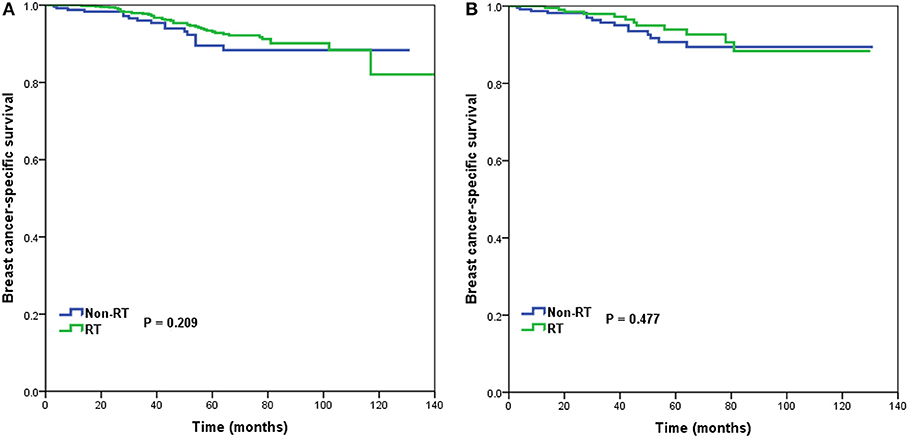
Figure 5. Breast cancer-specific survival in patients who had or did not have postoperative radiotherapy in the high-risk group before (A) and after (B) propensity score matching.
Discussion
We undertook a population-based study to assess the role of the RS in predicting the benefit of RT following BCS in elderly women with breast cancer. Our results suggested that postoperative RT was related to better BCSS in intermediate-risk RS group, whereas women with low- and high-risk RS groups who received RT after BCS had no BCSS benefit compared with women who did not receive postoperative RT.
The results from Early Breast Cancer Trialists' Collaborative Group have indicated that the administration of postoperative RT after BCS was associated with improving locoregional control and OS even in the younger age groups (19, 20). In addition, several randomized trials have demonstrated that receipt of RT following BCS in elderly patients is associated with better locoregional control. However, the improvement in LRR did not translate into an advantage in distant disease-free survival and OS (1–4). The results of those studies have a minimal impact on the use of postoperative RT among elderly women with a low risk of breast cancer after BCS. There were 67.5–89% of patients still receipt of adjuvant RT after the Cancer and Leukemia Group B (CALGB) 9343 publication (5, 6), which was similar to our study. In addition, we found that receipt of postoperative RT improved BCSS compared with patients who did not have postoperative RT, data that are similar to results from two National Cancer Database (NCDB) studies (21, 22). Therefore, elderly patients for whom postoperative RT can be omitted safely should be investigated further.
The results of 21-gene RS testing could predict the risk of distant recurrence. There were several studies also indicated that the 21-gene RS was related to the risk of LRR in patients with node-negative or node-positive disease (14, 15, 23). The 10-year LRR was 3.3–12.5, 5.1–27.7, and 12.0–26.5% in patients with low-, intermediate-, and high-risk RS groups, respectively (14, 15, 23). Therefore, it is hypothesized that adjuvant RT after BCS may provide the greatest survival benefit in patients with a higher RS.
No studies have assessed the role of the 21-gene RS assay in predicting the benefit of RT following BCS in elderly women with breast cancer. A recent study by Goodman et al. included a large cohort of T1–2N1 patients who underwent mastectomy from NCDB and SEER databases. Their results suggested that longer OS associated with postmastectomy RT was limited to women with a low-risk RS, but not for women with an intermediate- or high-risk RS. Also, the 5-year OS benefit in the low-risk RS group who received postmastectomy RT was ~2–3% (24). However, they did not analyze the results of BCSS. Since there were tons of confounding factors associated with OS. The analysis of BCSS would be much more robust than OS in the SEER database. A recent study by Jayasekera et al. included 1,778 patients aged 40–74 years from seven trials, they showed that the omission of RT in low-risk RS group who treated with hormonal therapy may lead to small absolute differences in LRR (5-year LRR 6.3 and 1.4% in non-RT and RT groups, respectively), but does not appear to increase the risk of distant recurrence or death (25). In our study, we found that receipt of postoperative RT was related to better BCSS in patients with intermediate-risk RS, the BCSS advantage among women with intermediate-risk RS who received postoperative RT was 2.3% at 5 years. However, receipt of postoperative RT was not related to better BCSS in patients with low- and high-risk RS before and after PSM. Therefore, in the modern clinical management of breast cancer, the multigene assays maybe available to determine the subgroups who can safely omit RT after BCS.
There were several potentially reasonable reasons to explain our findings. First, the risk of LRR and distant recurrence was extremely low in low-risk RS group (11–15, 23), even in patients who did not receive adjuvant RT (25). However, patients in the high-risk RS cohort who are at highest risk for subclinical micrometastatic disease may not be able to benefit from adjuvant RT because of the competitive risk of distant recurrence (24). Therefore, postoperative RT may sterilization of potential residual disease and finally has the greatest survival benefit to patients with intermediate-risk RS. The Danish Breast Cancer Cooperative Group 82 b&c trials also showed that patients with intermediate-risk of LRR were associated with the significantly benefit in LRR, disease specific survival, and OS after receiving postmastectomy RT. However, the benefit in LRR did not translate into disease specific survival and OS benefit in the low- and high-risk groups (26). Second, this effect may be related to differences in radiosensitivity between molecular subtypes; patients with intermediate-risk of LRR may have better outcomes due to higher radiosensitivity (27). In addition, the translation from LRR benefit to BCSS benefit appears to be heterogeneous and varies between subpopulations on the basis of distant-recurrence risk and/or intrinsic radiosensitivity. Finally, it is hypothesized that adjuvant RT may provide the greatest benefit in women with a high-risk RS. The opposite result of our study maybe due to the higher percentage of chemotherapy receipt in high-risk RS group, and thus the effect of adjuvant RT was diluted.
Validated tools to help clinicians determine which elderly women will benefit from adjuvant RT are lacking (28, 29). The RS assay can quantify the risk of distant metastasis and predict the benefit from adjuvant chemotherapy (31, 32). However, in our study, receipt of postoperative RT was not related to the year of diagnosis according to the RS assay. Nevertheless, our results suggest that the RS may be useful for identifying elderly patients who may benefit from RT after BCS.
Our study had several limitations. First, although we used PSM to reduce potential confounding effects, there are potential inherent biases in retrospective studies. Second, patients who did not receive RT may have had more complications that affect the receipt of radiotherapy. However, the SEER database does not contain information on patients' complications. Third, the details of the target volume, dose, and technology of RT were lacking, and the patterns of LRR or distant metastasis are not in the SEER database. Moreover, it has been shown that there are several inaccuracies in the SEER database, with a high prevalence of under-reporting of receipt of radiotherapy and chemotherapy (33, 34).
Conclusion
In conclusion, although causation cannot be implied, adjuvant RT in elderly women was associated with a greater effect size in patients with an intermediate-risk RS. Our results caution against omission of RT after BCS for elderly women with a low risk of LRR. More prospective studies are required to confirm our findings.
Ethics Statement
The study was exempt from the approval processes of the Institutional Review Boards because the SEER database patient information is de-identified.
Author Contributions
S-GW and W-WZ are lead authors who participated in data collection, manuscript drafting, table and figure creation, and manuscript revision. JW, YD, and J-YS are senior authors who aided in drafting the manuscript and manuscript revision. Y-XC and Z-YH is the corresponding author who initially developed the concept and drafted and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was partly supported by the National Natural Science Foundation of China (Nos. 81872459, 81803050), Natural Science Foundation of Fujian Province (No. 2016J01635), Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20174070), and Natural Science Foundation of Guangdong Province (No. 2018A030313666).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00001/full#supplementary-material
References
1. Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. (2004) 351:971–7. doi: 10.1056/NEJMoa040587
2. Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. (2013) 31:2382–7. doi: 10.1200/JCO.2012.45.2615
3. Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. (2004) 351:963–70. doi: 10.1056/NEJMoa040595
4. Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM, PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. (2015) 16:266–73. doi: 10.1016/S1470-2045(14)71221-5
5. Chu QD, Zhou M, Medeiros KL, Peddi P, Wu XC. Impact of CALGB 9343 trial and sociodemographic variation on patterns of adjuvant radiation therapy practice for elderly women (≥70 Years) with Stage I, estrogen receptor-positive breast cancer: analysis of the national cancer data base. Anticancer Res. (2017) 37:5585–94. doi: 10.21873/anticanres.11992
6. Nichol AM, Chan EK, Lucas S, Smith SL, Gondara L, Speers C, et al. The use of hormone therapy alone versus hormone therapy and radiation therapy for breast cancer in elderly women: a population-based study. Int J Radiat Oncol Biol Phys. (2017) 98:829–39. doi: 10.1016/j.ijrobp.2017.02.094
7. Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. (2016) 66:337–50. doi: 10.3322/caac.21342
8. Janssen-Heijnen ML, Houterman S, Lemmens VE, Louwman MW, Maas HA, Coebergh JW. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol. (2005) 55:231–40. doi: 10.1016/j.critrevonc.2005.04.008
9. Van Leeuwen BL, Rosenkranz KM, Feng LL, Bedrosian I, Hartmann K, Hunt KK, et al. The effect of under-treatment of breast cancer in women 80 years of age and older. Crit Rev Oncol Hematol. (2011) 79:315–20. doi: 10.1016/j.critrevonc.2010.05.010
10. Biganzoli L, Wildiers H, Oakman C, Marotti L, Loibl S, Kunkler I, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA). Lancet Oncol. (2012) 13:e148–60. doi: 10.1016/S1470-2045(11)70383-7
11. Nguyen MT, Stessin A, Nagar H, D'Alfonso TM, Chen Z, Cigler T, et al. Impact of oncotype DX recurrence score in the management of breast cancer cases. Clin Breast Cancer (2014) 14:182–90. doi: 10.1016/j.clbc.2013.12.002
12. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast Cancer Version 2.2015. J Natl Compr Canc Netw. (2015) 13:448–75. doi: 10.6004/jnccn.2015.0060
13. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, et al. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. (2007) 25:5287–312. doi: 10.1200/JCO.2007.14.2364
14. Mamounas EP, Liu Q, Paik S, Baehner FL, Tang G, Jeong JH, et al. 21-Gene recurrence score and locoregional recurrence in Node-Positive/ER-Positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst. (2017) 109:djw259. doi: 10.1093/jnci/djw259
15. Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABPB-20. J Clin Oncol. (2010) 28:1677–83. doi: 10.1200/JCO.2009.23.7610
16. Surveillance Epidemiology and, End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs (Excl AK) Custom Data Malignant Breast (with Oncotype DX and Additional Treatment Fields). Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program.
17. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. (1985) 39:33–8.
18. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multiv Behav Res. (2011) 46:399–24. doi: 10.1080/00273171.2011.568786
19. Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet (2011) 378:1707–16. doi: 10.1016/S0140-6736(11)61629-2
20. Speers C, Pierce LJ. Postoperative radiotherapy after breast-conserving surgery for early-stage breast cancer: a review. JAMA Oncol. (2016) 2:1075–82. doi: 10.1001/jamaoncol.2015.5805
21. Herskovic AC, Wu X, Christos PJ, Nagar H. Omission of adjuvant radiotherapy in the elderly breast cancer patient: missed opportunity? Clin Breast Cancer (2018) 18:418–31. doi: 10.1016/j.clbc.2018.02.006
22. Efird JT, Hunter S, Chan S, Jeong S, Thomas SL, Jindal C, et al. The association between age, comorbidities and use of radiotherapy in women with breast cancer: implications for survival. Medicines (2018) 5:E62. doi: 10.3390/medicines5030062
23. Solin LJ, Gray R, Goldstein LJ, Recht A, Baehner FL, Shak S, et al. Prognostic value of biologic subtype and the 21-gene recurrence score relative to local recurrence after breast conservation treatment with radiation for early stage breast carcinoma: results from the Eastern Cooperative Oncology Group E2197 study. Breast Cancer Res Treat. (2012) 134:683–92. doi: 10.1007/s10549-012-2072-y
24. Goodman CR, Seagle BL, Kocherginsky M, Donnelly ED, Shahabi S, Strauss JB. 21-gene recurrence score assay predicts benefit of post-mastectomy radiotherapy in T1–2 N1 breast cancer. Clin Cancer Res. (2018) 24:3878–87. doi: 10.1158/1078-0432.CCR-17-3169
25. Jayasekera J, Schechter CB, Sparano JA, Jagsi R, White J, Chapman JW, et al. Effects of radiotherapy in early-stage, low-recurrence risk, hormone-sensitive breast cancer. J Natl Cancer Inst. (2018) 110:1370–9. doi: 10.1093/jnci/djy128
26. Kyndi M, Overgaard M, Nielsen HM, Sørensen FB, Knudsen H, Overgaard J. High local recurrence risk is not associated with large survival reduction after postmastectomy radiotherapy in high-risk breast cancer: a subgroup analysis of DBCG 82 b&c. Radiother Oncol. (2009) 90:74–9. doi: 10.1016/j.radonc.2008.04.014
27. Wu SG, He ZY, Li Q, Li FY, Lin Q, Lin HX, et al. Predictive value of breast cancer molecular subtypes in Chinese patients with four or more positive nodes after postmastectomy radiotherapy. Breast (2012) 21:657–61. doi: 10.1016/j.breast.2012.07.004
28. Thavarajah N, Menjak I, Trudeau M, Mehta R, Wright F, Leahey A, et al. Towards an optimal multidisciplinary approach to breast cancer treatment for older women. [Article in English, French] Can Oncol Nurs J. (2015) 25:384–408. doi: 10.5737/23688076254384395
29. D'Alimonte L, Angus J, Wong J, Paszat L, Soren B, Szumacher E. Working toward a decision: The development and first impressions of a decision aid for older women with early-stage breast cancer. J Med Imaging Radiat Sci. (2012) 43:60–5. doi: 10.1016/j.jmir.2011.08.005
30. Wong WB, Ramsey SD, Barlow WE, Garrison LP Jr, Veenstra DL. The value of comparative effectiveness research: projected return on investment of the RxPONDER trial (SWOG S1007). Contemp Clin Trials (2012) 33:1117–23. doi: 10.1016/j.cct.2012.08.006
31. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. (2004) 351:2817–26. doi: 10.1056/NEJMoa041588
32. Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. (2006) 24:3726–34. doi: 10.1200/JCO.2005.04.7985
33. Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, et al. Comparison of SEER treatment data with medicare claims. Med Care (2016) 54:e55–64. doi: 10.1097/MLR.0000000000000073
Keywords: breast cancer, elderly, 21-gene recurrence score, radiotherapy, breast-conserving surgery
Citation: Wu S-G, Zhang W-W, Wang J, Dong Y, Sun J-Y, Chen Y-X and He Z-Y (2019) 21-Gene Recurrence Score Assay and Outcomes of Adjuvant Radiotherapy in Elderly Women With Early-Stage Breast Cancer After Breast-Conserving Surgery. Front. Oncol. 9:1. doi: 10.3389/fonc.2019.00001
Received: 28 November 2018; Accepted: 02 January 2019;
Published: 29 January 2019.
Edited by:
Minesh P. Mehta, Baptist Health South Florida, United StatesReviewed by:
Vivek Verma, Allegheny General Hospital, United StatesCorrado Spatola, Università degli Studi di Catania, Italy
Copyright © 2019 Wu, Zhang, Wang, Dong, Sun, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Xiong Chen, eXhjaGVuMTk2MkB4bXUuZWR1LmNu
Zhen-Yu He, aGV6aHlAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work
 San-Gang Wu
San-Gang Wu Wen-Wen Zhang
Wen-Wen Zhang Jun Wang
Jun Wang Yong Dong3
Yong Dong3 Zhen-Yu He
Zhen-Yu He