- 1Office of Cancer Screening, National Cancer Center/ National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Oncology, Kailuan General Hospital, Tangshan, China
- 3Health Department of Kailuan (Group), Tangshan, China
- 4Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: Waist circumference, as an indicator of central adiposity, has been identified as an important predictor of several specific cancers such as colorectal cancer and gastroesophageal cancer risk, however, a consensus regarding the association between waist circumference and primary liver cancer (PLC) risk has not been reached.
Methods: A total of 104,825 males participating in the health checkup were included in the Kailuan male cohort study (2006–2015). Information on demographic and socioeconomic characteristics, lifestyle, medical records, and anthropometric measures were collected. Restricted cubic spline (RCS) and Cox proportional hazards regression models were used to estimate the hazard ratio (HR) and 95% confidence interval (CI) of association between waist circumference and the risk of PLC in males.
Results: During a median of 8.9 years of follow-up, 346 PLC cases were newly diagnosed in the cohort. The RCS model showed a U-shaped association between waist circumference and PLC risk (P-overall = 0.019, P-non-linear = 0.017). Overally, males with both high waist circumference (HRQ5vs.Q3 = 1.98, 95%CI: 1.39–2.82) and low waist circumference (HRQ1vs.Q3 = 1.52, 95%CI: 1.02–2.27) had an increased risk of PLC. Especially, the U-shaped association between waist circumference and PLC risk tended to be strengthened among subjects with hepatitis B surface antigen (HBsAg) negativity (HRQ5vs.Q3 = 2.39, 95%CI: 1.43–3.98; HRQ1vs.Q3 = 2.27, 95%CI = 1.29–4.01).
Conclusions: Waist circumference might be an independent predictor of PLC risk in males, especially for subjects with HBsAg negativity. Controlling waist circumference in an appropriate range might be an effective primary prevention to decrease PLC risk.
Introduction
Primary liver cancer (PLC) is one of the most common cancers. According to the estimation of GLOBOCAN 2012 by the International Agency for Research on Cancer (IARC), approximately 83% of all liver cancer occurred in less developed regions, with China accounting for over 50% of the world's burden (1).
It has been established that chronic infection with hepatitis B virus (HBV), causing chronic hepatic inflammation that may lead to fibrosis and cirrhosis, is the leading cause of PLC (2). With the successful introduction of hepatitis B vaccine into the national immunization program in China, the prevalence of hepatitis B surface antigen (HBsAg) among children under 5 years of age has dramatically declined from 9.67% in 1992 to 0.96% in 2006 (3). Hence, HBV, the dominant risk factor of PLC, is unlikely to be the main risk factor of PLC in the future. Thus, it is necessary to explore other important and potentially modifiable risk factors.
Several meta-analyses based on prospective cohort studies have identified increasing body mass index (BMI), the indicator of general adiposity which is often assumed to represent the degree of body fat, was related to higher risks of PLC (4, 5). However, abdominal fat may vary distinctly within a narrow range of BMI (6). In addition, current evidence suggests that visceral adipose is primarily found in the abdominal cavity, which had been confirmed more metabolically active than subcutaneous adipocytes (7–9). Previous study have suggested that waist circumference was a better predictor of abdominal fat compared with BMI in males (10). Hence, waist circumference, as the index considering both the amount and distribution of adipose, could be an appropriate measurement of abdominal obesity compared with BMI (11).
The recent study reported that the abdominal obesity (waist circumference ≥90 cm for male and ≥80 cm for female) prevalence was approximately quadrupled from 9.53% in 1993 to 36.7% in 2011 among Chinese males (12). Although waist circumference has been identified as an important predictor of several specific cancers such as colorectal cancer (13) and gastroesophageal cancer risk (14) in general, the association between high waist circumference and PLC risk in males has not reached a consensus (15–19). In addition, the effect of low waist circumference has rarely been investigated, leaving evidences to be further strengthened. Therefore, we conducted a large prospective cohort study based on the Kailuan Group to investigate the association between waist circumference and risk of PLC incidence in Chinese males, which might be helpful for identifying a potentially preventable risk factor of PLC.
Methods
Study Design and Population
The Kailuan male study, a large and dynamic prospective cohort study, was initiated in May 2006 and based on Kailuan Group in Tangshan city, Hebei province, northern China. The Kailuan Group is a functional community managing coal industry, machine manufacturing, coking, chemical engineering, transportation, new building materials, and health care institutions (including 11 affiliated hospitals) (20).
Participants were enrolled in the present study if they met the following criteria: (1) males with age>18, (2) providing informed consent, (3) completing the questionnaire interview. Participants without baseline waist circumference (n = 3,786), with waist circumference lower than 1st percentile (< 68 cm, n = 991), and with waist circumference higher than 99th percentile (>112 cm, n = 1,010) were excluded. Ultimately, a total of 104,825 male subjects were enrolled in the present study. This study was carried out in accordance with the recommendations of the ethical review committee of the Kailuan General Hospital. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Exposure Assessment
Standardized questionnaire and health examination for all participants were conducted by trained doctors and nurses at baseline entry. Information on demographic and socioeconomic characteristics, lifestyle, medical records, and anthropometric measures were collected. Smoking was defined as someone has tobacco smoking at least one cigarette per week for more than 6 consecutive months and was categorize as “non-smoker,” “ex-smoker,” or “current smoker” according to questionnaire information. Alcohol drinking was defined as drinking at least once per month for more than 6 consecutive months and was classified into “non-drinker,” “ex-drinker,” “<1 time per day” or “≥1 time per day” using self-reported information. The subjects' weights and heights were measured using standardized stadiometers and scales while wearing light clothes, and the BMI was calculated based on the formula that BMI = weight (kg)/height2 (m2). Waist circumference was measured at the midpoint between the lower border of the rib and the supra margin of iliac crest plane. Diabetes history was categorized as “yes” or “no” on the basis of fasting blood glucose (FBG) level according to diabetes diagnostic criteria recommended by International Diabetes Federation (FBG ≥ 7.0 mmol/L) (21) and history for antidiabetic medication use. Measurement of FBG was performed using the Hexokinase method (BioSino Bio-Technology & Science Inc., China.). The HBsAg was detected quantitatively by the enzyme-linked immunosorbent assay for HBsAg (SHANGHAI KEHUA BIO-ENGINEERING, KHB, Shanghai, China) with standard operating procedure.
Outcome Assessment
The follow-up of each participant terminated at diagnosis of cancer, death, or administrative censoring (December 31, 2015), whichever occurred first. During the study period, new cases were obtained through self-report when they took part in routine questionnaires and health examinations every 2 years until 31 December 2015. In addition, incident PLC cases were checked yearly by the diagnosis and medical records linkage with the Tangshan medical insurance system and Kailuan social security system. Moreover, discharge lists from the 11 affiliated hospitals and death certificates from state vital statistics offices were also tracked yearly to ascertain the outcome information (22).
The diagnosis of incident PLC cases was confirmed by medical records review by clinical experts. Information on pathological diagnosis, imaging diagnoses (including ultrasonography, computerized tomography, and magnetic resonance imaging), blood biochemical and alpha fetoprotein test was collected to assess the incident PLC cases (22). All PLC events were coded as C22 according to the International Classification of Diseases, Tenth Revision (ICD−10). Other details relating to Kailuan Cohort has been described previously (22–24).
Statistical Analyses
Subjects were grouped into quintiles according to the baseline waist circumference (< 80.0, 80.0–84.9, 85.0–89.9, 90.0–94.9, or ≥95.0 cm), and the third quintile of waist circumference (85.0–89.9 cm) served as the reference. Proportions and chi-square tests were used to describe the categorical variables. A restricted cubic spline (RCS) analysis was conducted to explore the potential non-linear relationship between continuous waist circumference and the risk of PLC in the study (25).
Furthermore, Cox's proportional hazards regression models were constructed to estimate the hazard ratio (HR) and 95% confidence interval (CI) of PLC risk according to waist circumference quintiles. In model 1, only waist circumference was included in this univariate model. In model 2, age (continuous) was added as the underlying time metric. In model 3, multiple factors including education level (illiterate/primary school, junior high school, senior high school, or college and above), dust exposure (no or yes), frequency of alcohol drinking (non-drinker, ex-drinker, < 1 time per day, or ≥1 time per day), and smoking status (non-smoker, ex-smoker, or current smoker) were further adjusted. In model 4, disease history including diabetes (yes or no), and HBV infection status (HBsAg negative or positive) served as additional adjustments. In model 5, the main model, BMI (continuous) was added in this multivariate model for exploring whether waist circumference is independent of BMI for PLC prediction.
Subgroup analyses were performed by alcohol drinking status (non-drinker vs. drinker), smoking status (non-smoker vs. smoker), and HBsAg status (negative vs. positive). And the multiplicative models were applied to test for the interaction between waist circumference and these variables.
Sensitivity analyses were conducted to examine the consistency of our findings. Firstly, the PLC cases occurred in the initial 3 years of follow-up were excluded from the analyses to evaluate whether potential preexisting disease influenced the association between waist circumference and PLC risk. Secondly, main models were repeated with exclusion of subjects with BMI < 18.5 kg/m2 in consideration of the effect of preclinical cancers that may cause weight loss and waist circumference decrement and thus result in overestimation of the association between lower waist circumference and PLC risk.
The data management and all analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). All statistical test presented were two-side, and P < 0.05 was considered statistically significant.
Results
Baseline Participant Characteristics
A total of 104,825 males were included in this study with a mean age of 51.4 years, for a total of 827,352.43 person-years. During a median follow-up time of 8.9 years, 346 members of the cohort were diagnosed with PLC. We compared the characteristics at baseline according to waist circumference quintiles of all subjects. As shown in Table 1, compared with subjects with low waist circumference, those with higher waist circumference were more likely to be older and have lower education level. Males in the higher waist circumference categories were more likely to be non-smokers and ex-drinkers. Negative HBsAg and diabetes were more common among males with higher waist circumference (Table 1).
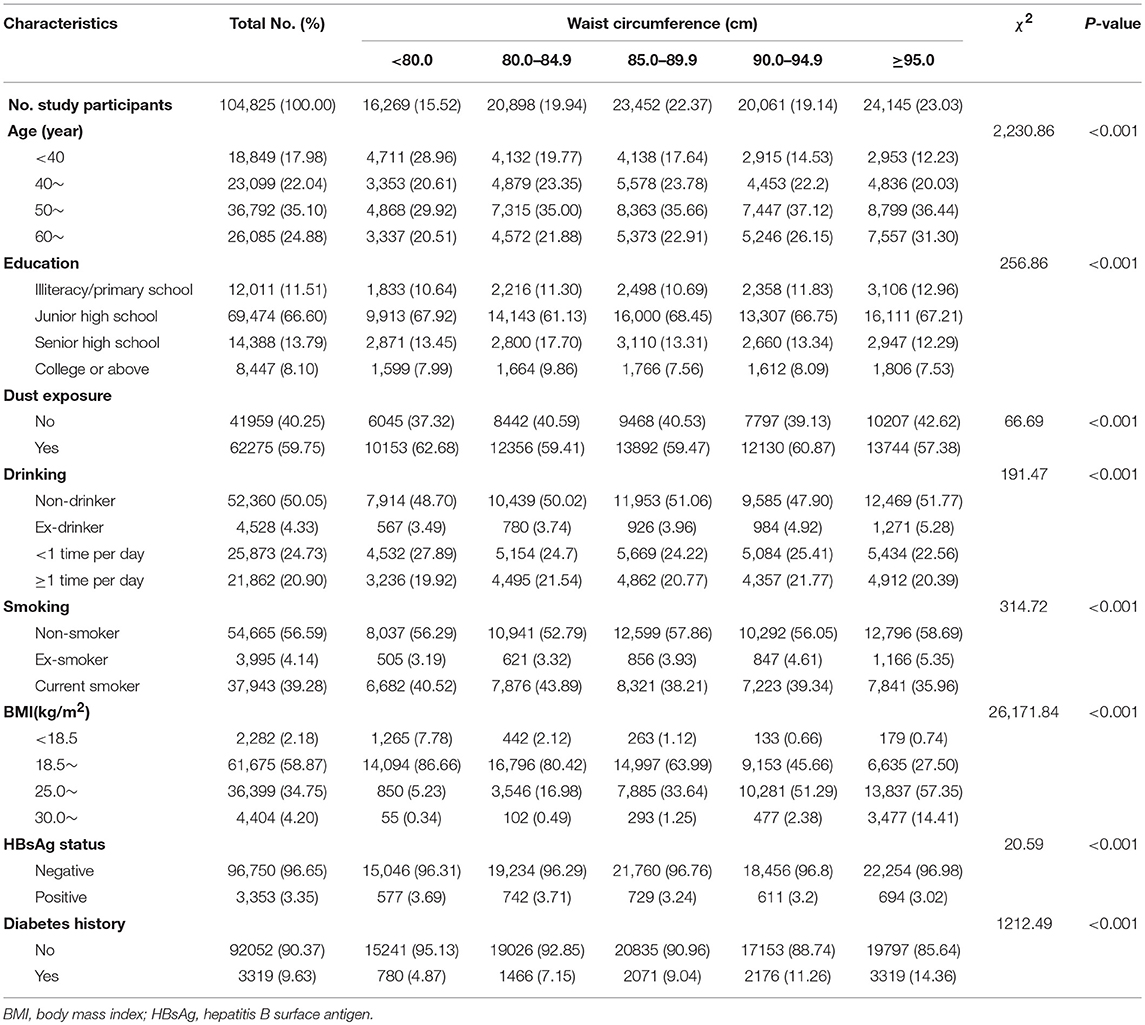
Table 1. Baseline characteristics of males stratified by waist circumference, Kailuan male cohort, 2006–2015.
The Association Between Waist Circumference and PLC Risk
The RCS model showed a significantly U-shaped association of waist circumference with the risk of PLC among the participants (P-overall = 0.019, P-non-linear = 0.017) (Figure 1). As the 40th quintile of waist circumference (85.0 cm) was chosen to be the reference, the HRs of PLC related to waist circumference rise obviously when waist circumference was over 95.0 cm or lower than 75.0 cm.
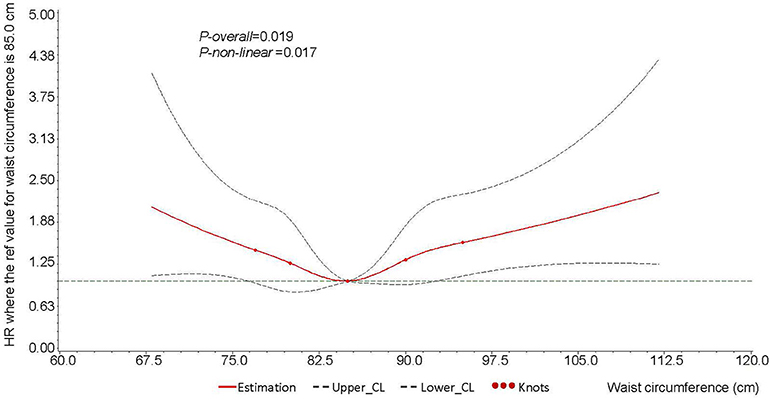
Figure 1. Cubic spline graph of the adjusted HR (represented by solid line) and 95%CI (represented by the dotted lines) for the association between waist circumference and risk of male liver cancer in Kailuan male cohort, 2006–2015.
Knots: 77.0, 80.0, 85.0, 90.0, 95.0 of the distribution of waist circumference (cm).
Referent: 85.0 cm, 40th of the distribution of waist circumference.
Adjusted for age (continuous), education level (illiteracy/primary school, junior high school, senior high school, or college and above), dust exposure (no or yes), smoking (non-smoker, ex-smoker, or current smoker), drinking (non-drinker, ex-drinker, < 1 time per day, or ≥1 time per day), diabetes (no or yes), HBsAg (negative or positive), and BMI (continuous). HR, hazard ratio; HBsAg, hepatitis B surface antigen; BMI, body mass index.
Furthermore, subjects were grouped into quintiles according to the baseline waist circumference, the crude PLC incidence rates for males according to waist circumference quintiles were 44.93/105, 35.26/105, 32.25/105, 35.94/105, and 59.75/105, respectively. Compared with the third quintile waist circumference (85.0–89.9 cm), the HRs were 1.98 (95% CI: 1.39–2.82) for highest quintile waist circumference (≥95.0 cm) and 1.52 (95% CI: 1.02–2.27) for lowest quintile waist circumference (< 80.0 cm), respectively, after adjusting for age, education, dust exposure, status of tobacco smoking and alcohol drinking, diabetes history, HBsAg status and BMI (Table 2).
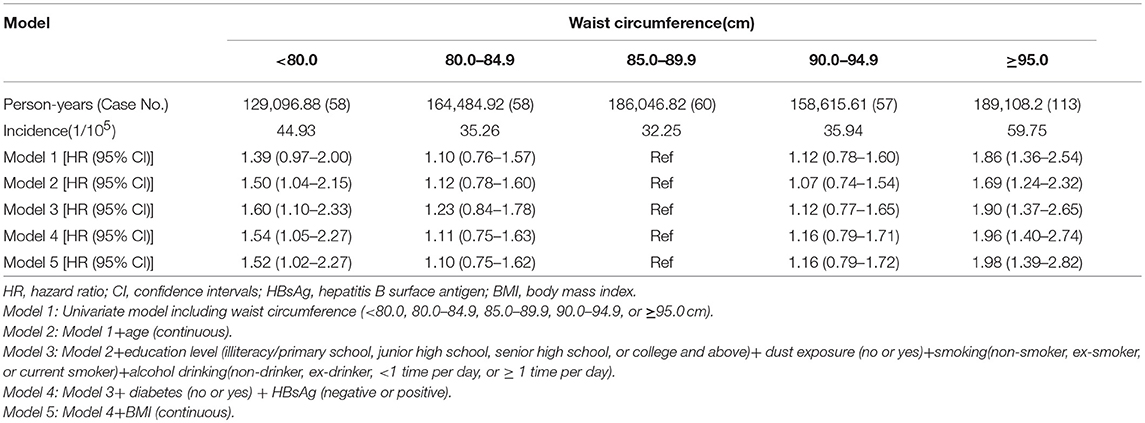
Table 2. The association between waist circumference and primary liver cancer in males, Kailuan male cohort, 2006–2015.
Population attributable fractions (PAFs) for categorical exposure variables were calculated to reveal the common risk factors' contribution to PLC incidence. As shown in the Supplementary Table S1, in addition to HBsAg status (45.64%), the waist circumference (23.01%) also account the main attributable proportions of PLC incidence (Supplementary Table S1 in Supplementary Material).
Subgroup Analyses Between the Waist Circumference and PLC Risk
Subgroup analyses showed that the statistically significant U-shaped association between waist circumference and PLC risk tended to be strengthened among subjects with hepatitis B surface antigen (HBsAg) negativity (HRQ5vs.Q3 = 2.39, 95%CI: 1.43–3.98; HRQ1vs.Q3 = 2.27, 95%CI = 1.29–4.01). In addition, high waist circumference (≥95.0 cm) among non-drinkers (HR = 2.14, 95%CI = 1.34–3.41) and non-smokers (HR = 1.80, 95%CI = 1.13–2.86) also indicated a positive association with PLC risk in present study. Interaction analyses were conducted between the waist circumference and these confounders. However, there was no evidence of interaction effect (all P > 0.05) between waist circumference and alcohol drinking, tobacco smoking and HBsAg status (Table 3).
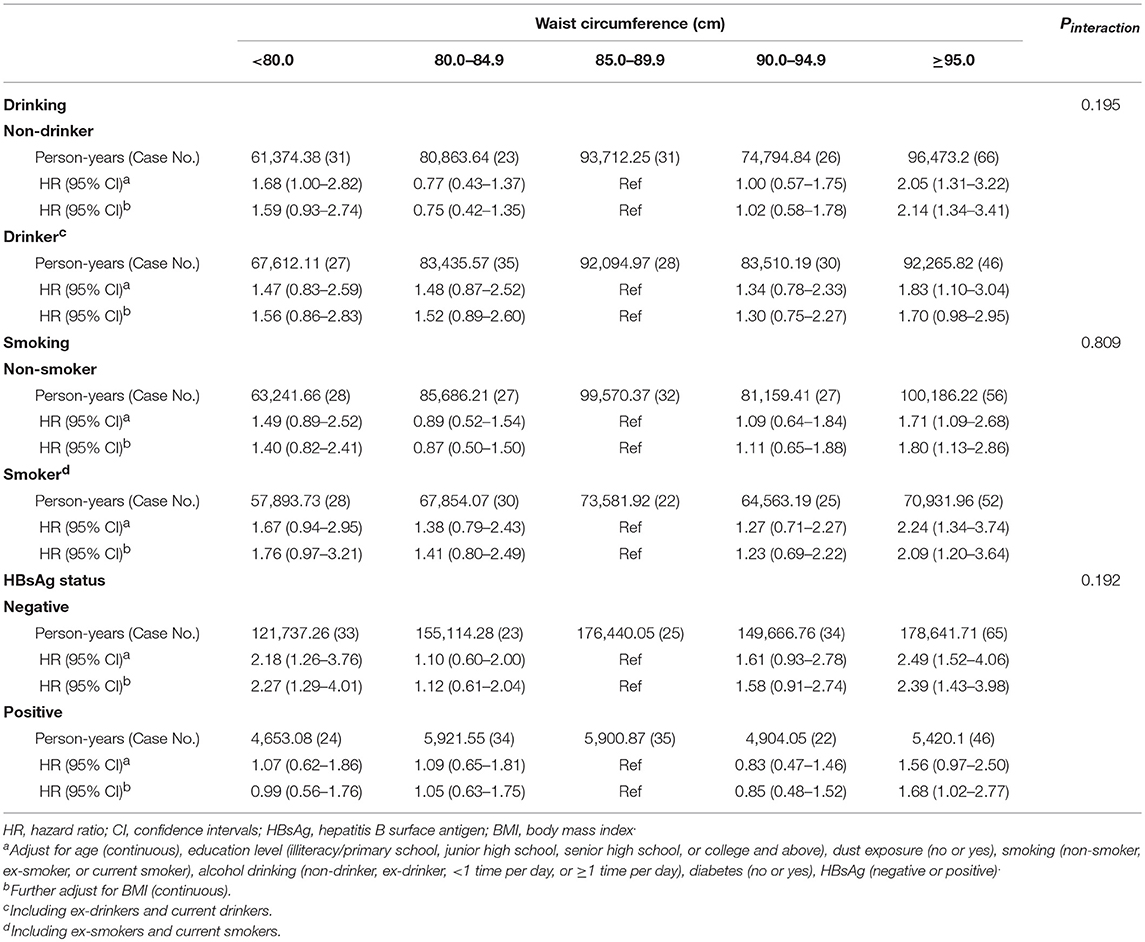
Table 3. Stratified analysis of the association between waist circumference and risk of primary liver cancer in Kailuan male cohort, 2006–2015.
Sensitivity Analysis
As shown in Table 4, after excluding PLC cases (case No. = 127) having occurred during the first 3 years of follow-up, there was still a positive association of the risk of PLC related to high waist circumference (HR = 1.78, 95% CI: 1.18–2.69). When excluding individuals without or with BMI < 18.5 kg/m2 (n = 2,347, case No. = 11), the results did not change substantially (HRQ5vs.Q3 = 1.90, 95%CI: 1.32–2.72; HRQ1vs.Q3 = 1.51, 95%CI = 1.00–2.28).
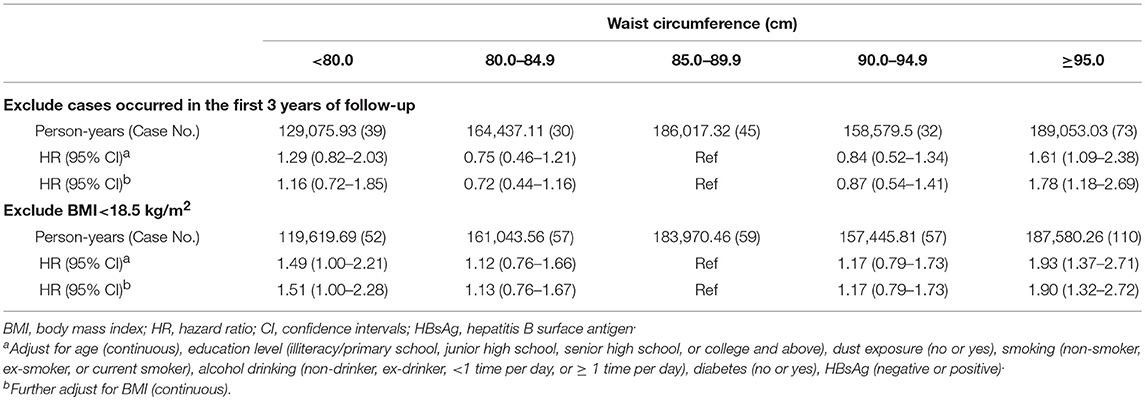
Table 4. Sensitivity analysis of the association between waist circumference and primary liver cancer risk in Kailuan male cohort, 2006–2015.
Discussion
In this large prospective cohort study among Chinese males, we found a significant U-shaped association between waist circumference and PLC risk. The association was robust even after including BMI in the statistical models, supporting the hypothesis that waist circumference is an independent predictor for PLC. In addition, the subgroup analyses showed that the association between waist circumference and risk of PLC differed across categories of alcohol drinking, tobacco smoking, and status of HBV infection, as there were discrepancies among subgroups. To our knowledge, this is the first prospective cohort study to report on the association of both high and low waist circumference with PLC risk in mainland Chinese males which could be a strong evidence suggesting waist circumference is an independent predictor of PLC.
Waist circumference was one of the earliest means of quantifying body fat distribution, as an approximation of central adiposity (26). Results from several prospective cohort studies have examined the association between high waist circumference and risk of PLC (15–19). European Prospective Investigation into Cancer and Nutrition study identified 177 liver cancer cases and reported that high waist circumference was related to higher risk of liver cancer (highest tertile VS. lowest tertile, HR = 2.60, 95% CI: 1.66–4.07) (17). Similarly, the Liver Cancer Pooling Project also found waist circumference to be an independent risk factor for liver cancer risk in males (waist circumference ≥110 cm VS. < 90 cm, HR = 1.88, 95% CI: 1.42–2.49) (15). A study from Taiwan reported that the association between central obesity (waist circumference >90 cm for men and < 80 cm for women) and PLC was only restricted in subjects with HBsAg negative and antibody to hepatitis C virus (HCV) positive (HR = 2.16, 95% CI: 1.19–3.92) (18). Our results on waist circumference were in line with the previous findings, whereby we consistently observed a significant association between high waist circumference and high liver cancer risk, even after further adjustment for BMI.
However, few studies have explored the association between low waist circumference and risk of PLC. Our study added a new perspective that the statistically U-shaped association between waist circumference and PLC risk, in which that low waist circumference might also play a potential role in PLC incidence. The RCS model showed that PLC risk increased obviously when waist circumference was lower than 75.0 cm. In addition, the present study also found a significant relationship when the first quintile (< 80.0 cm) compared to third quintile (85.0–89.9 cm) in subjects with HBsAg negativity, which support an association between low waist circumference and PLC risk. Perhaps males with low waist circumference were prone to accompany with preclinical disease that can cause weight loss and also increase risk of PLC, which may confuse the association. However, the association between low waist (< 80.0 cm) circumference and PLC risk remained robust with exclusion of participants with BMI < 18.5 kg/m2 (HR = 1.51, 95% CI: 1.00–2.28). In addition, for subjects with HBsAg negativity, the association was stronger (HR = 2.29, 95% CI: 1.28–4.08, data was not shown) when excluded the underweight participants (BMI < 18.5 kg/m2). Therefore, the robust findings indicated that low waist circumference might be a risk factor of PLC.
The previous study suggested that waist circumference was a better predictor of abdominal adiposity in males when compared with body weight or BMI (11). In our study, waist circumference conveyed statistically significant association with PLC risk, even after adjusting BMI in the statistical models, supporting the hypothesis that waist circumference is an independent predictor for PLC. Subjects with high waist circumference tend to be diagnosed with PLC maybe due to the following possible mechanisms. Increased release from metabolically active abdominal fat of substantial adipokines, such as tumor-necrosis factor-α, free fatty acids, leptin and inflammatory markers, and reduced release of adiponectin, contribute to development of insulin resistance, compensatory and chronic hyperinsulinaemia (8, 9, 26–28). Increased insulin levels, in turn, lead to reduced insulin-like growth factor (IGF) binding protein 1 synthesis in liver and other tissues, additionally, generally accompany with reduced levels of IGF binding protein 2 in the blood. Both the reduced IGF binding protein 1 and IGF binding protein 2 give rise to facilitate the biological activity of IGF1. Ultimately, insulin and IGF1 signal through the insulin receptors and IGF1 receptor, respectively, to promote cellular proliferation, inhibit apoptosis, and then contribute to tumorigenesis (8, 17). The mechanisms for subjects with low waist circumference also related to high PLC risk is still inconclusive, hence further research to better understand the underlying mechanisms are needed.
In the present study, the association between high waist circumference and risk of PLC differed by status of drinking and smoking. The association was statistically significant in non-drinkers or non-smokers but negative in drinkers or smokers. It is possible owing to the competing risks of tobacco smoking and alcohol drinking. Previous studies have suggested that alcohol drinking may increase 179% (95%CI: 2.00-3.87) risk of liver cancer incidence (29) via the induction of cytochrome P-450 2E1, which potentially leads to activation of procarcinogen (30) and inhibition of phase II enzymes (31), thus affecting the clearance of carcinogens (32). And tobacco smoking (HR = 1.51, 95% CI: 1.37–1.67) was also found to be an independent risk factor for liver cancer (33). Therefore, in the presence of a competing risk, the association may be attenuated among drinkers and smokers. However, for non-drinkers, or non-smokers, high waist circumference showed a significant effect on PLC development which could have key scientific and clinical importance for preventing PLC.
The prevalence of HBsAg was 3.30% in the present study, which was similar to the previous study on HBsAg prevalence (< 4%) among northern Chinese population (34). As it was estimated that hepatitis B viral infections accounts for more than 60% of liver cancer cases in Asia area (2), our study also proved that HBsAg positivity increased 30.74 (95%CI: 24.51–38.55) fold higher risk of PLC when compared with HBsAg negative. Therefore, the effect of waist circumference may be weakened, which explain the finding that increased PLC risk related to higher and lower waist circumference was restricted in subjects with HBsAg negativity. Although the HBV infection currently plays a leading role in the development of PLC (35), it is unlikely to be the main risk factor in the future, as the prevalence of HBsAg among the children under 5 years of age decreasing from 9.67% in 1992 to 0.96% in 2006 (3) with the successful massive hepatitis B vaccination implementation. Whereas, the abdominal obesity prevalence was approximately quadrupled from 9.53% in 1993 to 36.7% in 2011 among Chinese males (13). Thus, the findings of abdominal obesity, related to PLC risk differently depending on HBsAg status may shed some light on preventing PLC in the condition of hepatitis B vaccination application.
One of the main strengths of our study is its prospective design and inclusion of a large population, which gave us high power to detect quite modest associations as well as minimize the potential bias caused by preclinical disease. Furthermore, in our study, anthropometric factors (e.g., waist circumference, weight, and height) at baseline were measured by trained personnel rather than relied on self-reported, which avoid misclassification in analyses. However, there are several limitations that should be discussed when interpreting the results. Firstly, the lack of information on HCV infection is a major limitation of the current study. However, the HCV prevalence rate was only 0.43% in Chinese general population according to a national survey carried in 2006 (36), which attribute little to PLC incidence in China. In subsequent questionnaire interview and health examination, we will complement the HCV infection information to provide more comprehensive results in the future. Secondly, the follow-up time (Median, 8.9 years) was relatively short, which precluded stratified analyses by subtypes of PLC, such as hepatocellular carcinoma and intrahepatic cholangiocarcinoma, owing to limited number of cases. In addition, the subjects focused on male employees from Kailuan Group, it may be difficult in extrapolating to females or general population. So for other population, more studies are warranted to confirm these findings.
In conclusion, our analyses provided convincing evidence that waist circumference might be one of the scientific and important predictor of PLC based on the large-scale prospective study. The findings indicated that both high and low waist circumference could increase the risk of PLC in males, especially for subjects with HBsAg negative. Therefore, controlling waist circumference in an appropriate range might be an effective primary prevention to decrease PLC risk.
Data Availability
The datasets for this manuscript are not publicly available because all our data are under regulation of both the National Cancer Center of China and Kailuan Group. Requests to access the datasets should be directed to Jie He, aGVqaWVAY2ljYW1zLmFjLmNu and Shouling Wu, ZHJ3dXNsQDE2My5jb20=.
Author Contributions
NL, MD, SW, and JH did the study concept and design. GW, XF, YC, HC, and SC carried out the acquisition and quality control of data. LW, ZL, XL, and YW performed statistical analysis, or interpretation of data. LW performed the writing and drafting of the manuscript. NL and MD did the critical revision of the manuscript for important intellectual content. All authors agreed to be accountable for the content of the work.
Funding
This study was funded by National Key R&D Program of China (No. 2016YFC1302500 and 2016YFC1302503), Training Programme Foundation for the Talents in Beijing City (No. 2017000021223TD05), National Natural Science Foundation of China (No. 81673265), State Key Projects Specialized on Infectious Diseases (No. 2017ZX10201201-008-002).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank all participants, the principal investigators and their institutions for their contributions to this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00607/full#supplementary-material
Abbreviations
PLC, primary liver cancer; RCS, restricted cubic spline; HR, hazard ratio; CI, confidence interval; IARC, International Agency for Research on Cancer; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; BMI, body mass index; HCV, hepatitis C virus; IGF, insulin-like growth factor.
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer (2015) 136:E359–86. doi: 10.1002/ijc.29210
2. Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology (2004) 127:S5–16. doi: 10.1053/j.gastro.2004.09.011
3. Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis. (2009) 200:39–47. doi: 10.1086/599332
4. Berentzen TL, Gamborg M, Holst C, Sorensen TI, Baker JL. Body mass index in childhood and adult risk of primary liver cancer. J Hepatol. (2014) 60:325–30. doi: 10.1016/j.jhep.2013.09.015
5. Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer (2012) 48:2137–45. doi: 10.1016/j.ejca.2012.02.063
6. World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. World Health Organization Technical Report Series (2000).
7. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer (2004) 4:579–91. doi: 10.1038/nrc1408
8. Freedland ES. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: implications for controlling dietary carbohydrates: a review. Nutr Metab. (2004) 1:12. doi: 10.1186/1743-7075-1-12
9. Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, et al. Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (2013) 21:E439–47. doi: 10.1002/oby.20135
10. Chan DC, Watts GF, Barrett PH, Burke V. Waist circumference, waist-to-hip ratio and body mass index as predictors of adipose tissue compartments in men. QJM-Mon J Assoc Phys. (2003) 96:441–7. doi: 10.1093/qjmed/hcg069
11. Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. (2006) 98:920–31. doi: 10.1093/jnci/djj246
12. Du P, Zhang B, Wang HJ, Qi SF, Mi YJ, Yao JC, et al. The prevalence and secular trends of abdominal obesity among Chinese adults, 1993-2011. Ann Epidemiol. (2015) 25:797–9. doi: 10.1016/j.annepidem.2015.06.082
13. Freisling H, Arnold M, Soerjomataram I, O'Doherty MG, Ordonez-Mena JM, Bamia C, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer (2017) 116:1486–97. doi: 10.1038/bjc.2017.106
14. Du X, Hidayat K, Shi BM. Abdominal obesity and gastroesophageal cancer risk: systematic review and meta-analysis of prospective studies. Biosci Rep. (2017) 37:1–12. doi: 10.1042/BSR20160474
15. Campbell PT, Newton CC, Freedman ND, Koshiol J, Alavanja MC, Beane Freeman LE, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. Adults. Cancer Res. (2016) 76:6076–83. doi: 10.1158/0008-5472.CAN-16-0787
16. Chiang CH, Lee LT, Hung SH, Lin WY, Hung HF, Yang WS, et al. Opposite association between diabetes, dyslipidemia, and hepatocellular carcinoma mortality in the middle-aged and elderly. Hepatology (2014) 59:2207–15. doi: 10.1002/hep.27014
17. Schlesinger S, Aleksandrova K, Pischon T, Fedirko V, Jenab M, Trepo E, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. Int J Cancer (2013) 132:645–57. doi: 10.1002/ijc.27645
18. Chen CL, Yang HI, Yang WS, Liu CJ, Chen PJ, You SL, et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology (2008) 135:111–21. doi: 10.1053/j.gastro.2008.03.073
19. Åberg F, Helenius-Hietala J, Puukka P, Färkkilä M, Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology (2018) 67:2141–9. doi: 10.1002/hep.29631
20. Wang G, Li N, Chang S, Bassig BA, Guo L, Ren J, et al. A prospective follow-up study of the relationship between C-reactive protein and human cancer risk in the Chinese Kailuan Female Cohort. Cancer Epidemiol Biomarkers Prev. (2015) 24:459–65. doi: 10.1158/1055-9965.EPI-14-1112
21. International Diabetes Federation (IDF). IDF Diabetes Atlas. Avaliable online at: https://www.idf.org/e-library/welcome.html (2017).
22. Feng X, Wang G, Li N, Lyu Z, Chen S, Wei L, et al. The association between fasting blood glucose and the risk of primary liver cancer in Chinese males: a population-based prospective study. Br J Cancer (2017) 117:1405–11. doi: 10.1038/bjc.2017.296
23. Wang F, Wu S, Song Y, Tang X, Marshall R, Liang M, et al. Waist circumference, body mass index and waist to hip ratio for prediction of the metabolic syndrome in Chinese. Nutr Metab Cardiovacs Dis. (2009) 19:542–7. doi: 10.1016/j.numecd.2008.11.006
24. Wu S, Huang Z, Yang X, Zhou Y, Wang A, Chen L, et al. Prevalence of ideal cardiovascular health and its relationship with the 4-year cardiovascular events in a northern Chinese industrial city. Circ Cardiovasc Qual Outcomes (2012) 5:487–93. doi: 10.1161/CIRCOUTCOMES.111.963694
25. Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. (2010) 29:1037–57. doi: 10.1002/sim.3841
26. Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer (2015) 15:484–98. doi: 10.1038/nrc3967
27. Vansaun MN. Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res. (2013) 19:1926–32. doi: 10.1158/1078-0432.CCR-12-0930
28. Hanley AJ, McKeown-Eyssen G, Harris SB, Hegele RA, Wolever TM, Kwan J, et al. Cross-sectional and prospective associations between abdominal adiposity and proinsulin concentration. J Clin Endocr Metab. (2002) 87:77–83. doi: 10.1210/jcem.87.1.8139
29. Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer (2015) 112:580–93. doi: 10.1038/bjc.2014.579
30. Anderson LM, Chhabra SK, Nerurkar PV, Souliotis VL, Kyrtopoulos SA. Alcohol-related cancer risk: a toxicokinetic hypothesis. Alcohol (1995) 12:97–104. doi: 10.1016/0741-8329(94)00089-1
31. Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA (2001) 286:2143–51. doi: 10.1001/jama.286.17.2143
32. Shimazu T, Sasazuki S, Wakai K, Tamakoshi A, Tsuji I, Sugawara Y, et al. Alcohol drinking and primary liver cancer: a pooled analysis of four Japanese cohort studies. Int J Cancer (2012) 130:2645–53. doi: 10.1002/ijc.26255
33. Lee YC, Cohet C, Yang YC, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. (2009) 38:1497–511. doi: 10.1093/ije/dyp280
34. Yin J, Zhang H, He Y, Xie J, Liu S, Chang W, et al. Distribution and hepatocellular carcinoma-related viral properties of hepatitis B virus genotypes in Mainland China: a community-based study. Cancer Epidemiol Biomarkers Prev. (2010) 19:777–86. doi: 10.1158/1055-9965.EPI-09-1001
35. Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. (2016) 64:S84–101. doi: 10.1016/j.jhep.2016.02.021
Keywords: waist circumference, primary liver cancer, cohort studies, Chinese males, restricted cubic spline
Citation: Wei L, Li N, Wang G, Feng X, Lyu Z, Li X, Wen Y, Chen Y, Chen H, Chen S, Wu S, Dai M and He J (2018) Waist Circumference Might Be a Predictor of Primary Liver Cancer: A Population-Based Cohort Study. Front. Oncol. 8:607. doi: 10.3389/fonc.2018.00607
Received: 05 September 2018; Accepted: 28 November 2018;
Published: 12 December 2018.
Edited by:
Tianhui Chen, Zhejiang Academy of Medical Sciences, ChinaReviewed by:
Yimin Zhu, Zhejiang University, ChinaAnn Crispo, Istituto Nazionale Tumori Fondazione G. Pascale (IRCCS), Italy
Copyright © 2018 Wei, Li, Wang, Feng, Lyu, Li, Wen, Chen, Chen, Chen, Wu, Dai and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ni Li, bmxpQGNpY2Ftcy5hYy5jbg==
Min Dai, ZGFpbWluMjAwMkBob3RtYWlsLmNvbQ==
 Luopei Wei
Luopei Wei Ni Li
Ni Li Gang Wang2
Gang Wang2 Xiaoshuang Feng
Xiaoshuang Feng Zhangyan Lyu
Zhangyan Lyu