- 1Division of Internal Medicine, Department of Medicine, University Hospital Basel, Basel, Switzerland
- 2Division of Hematology, Department of Medicine, University Hospital Basel, Basel, Switzerland
Aplastic anemia (AA) is characterized by bone marrow (BM) hypocellularity, resulting in peripheral cytopenias. An antigen-driven and likely auto-immune dysregulated T-cell homeostasis results in hematopoietic stem cell injury, which ultimately leads to the pathogenesis of the acquired form of this disease. Auto-immune and inflammatory processes further influence the disease course as well as response rate to therapy, mainly consisting of intensive immunosuppressive therapy and allogeneic hematopoietic cell transplantation. Bone marrow hematopoietic stem and progenitor cells are strongly regulated by the crosstalk with the surrounding microenvironment and its components like mesenchymal stromal cells, also consistently altered in AA. Whether latter is a contributing cause or rather consequence of the disease remains an open question. Overall, niche disruption may contribute to disease progression, sustain pancytopenia and promote clonal evolution. Here we review the existing knowledge on BM microenvironmental changes in acquired AA and discuss their relevance for the pathogenesis and therapy.
Introduction
Acquired aplastic anemia (AA) is characterized by a hypoplastic, fatty bone marrow (BM) with profound reductions in hematopoietic stem/progenitor cells (HSCs/HPCs) that lead to defective mature blood cell production and peripheral pancytopenia (1–3). Diagnosis of AA requires per definition at least two of the following criteria: Hemoglobin <100 g/L, platelets <50 G/L and neutrophils <1.5 G/L, together with a hypocellular BM and in the absence of abnormal infiltrates or fibrosis (4). The description of AA as an “empty” BM in which hematopoietic cells have been replaced by fat cells was first made by Paul Ehrlich (5). Nowadays, AA is defined by decreased numbers of BM HSCs and HPCs resulting in a hypo- or aplastic BM with precocious fat replacement (1, 4).
AA can be inherited or acquired (1, 6). All patients—regardless of etiology—typically present with anemia symptoms, bleeding, and infections (7, 8). Onset at young age, additional pathologies and/or positive family history may indicate congenital or inherited syndromes driven by genetic alterations. Several inherited bone marrow failure (BMF) syndromes have been described, among which the most common are Fanconi anemia, Dyskeratosis Congenita, Diamond Blackfan anemia, and Shwachman-Diamond syndrome. The pathogenesis of these genetic conditions has been extensively reviewed elsewhere (6) and is different from that of acquired AA, which is of considered of immunological nature and is the focus of the current review.
Given the deficit in HSCs/HPCs observed in patients with acquired AA or BMF, replacing these by allogeneic hematopoietic cell transplantation (allo-HCT) is an obvious treatment option (8–10) (Figure 1). Interestingly, graft rejection followed by autologous reconstitution was occasionally observed in patients with acquired AA (11) and latter attributed to immuno-suppressive effects of the conditioning therapy (12, 13). This led to the concept of treating AA patients with intensive immuno-suppressive therapies (IST) such as anti-lymphocyte globulin (14, 15) and cyclosporine (16), which nowadays represent the treatment backbone for acquired AA.
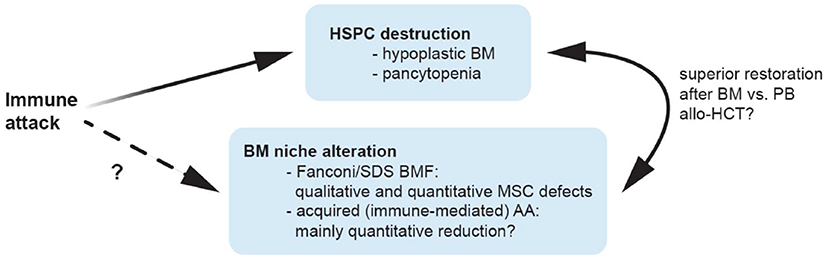
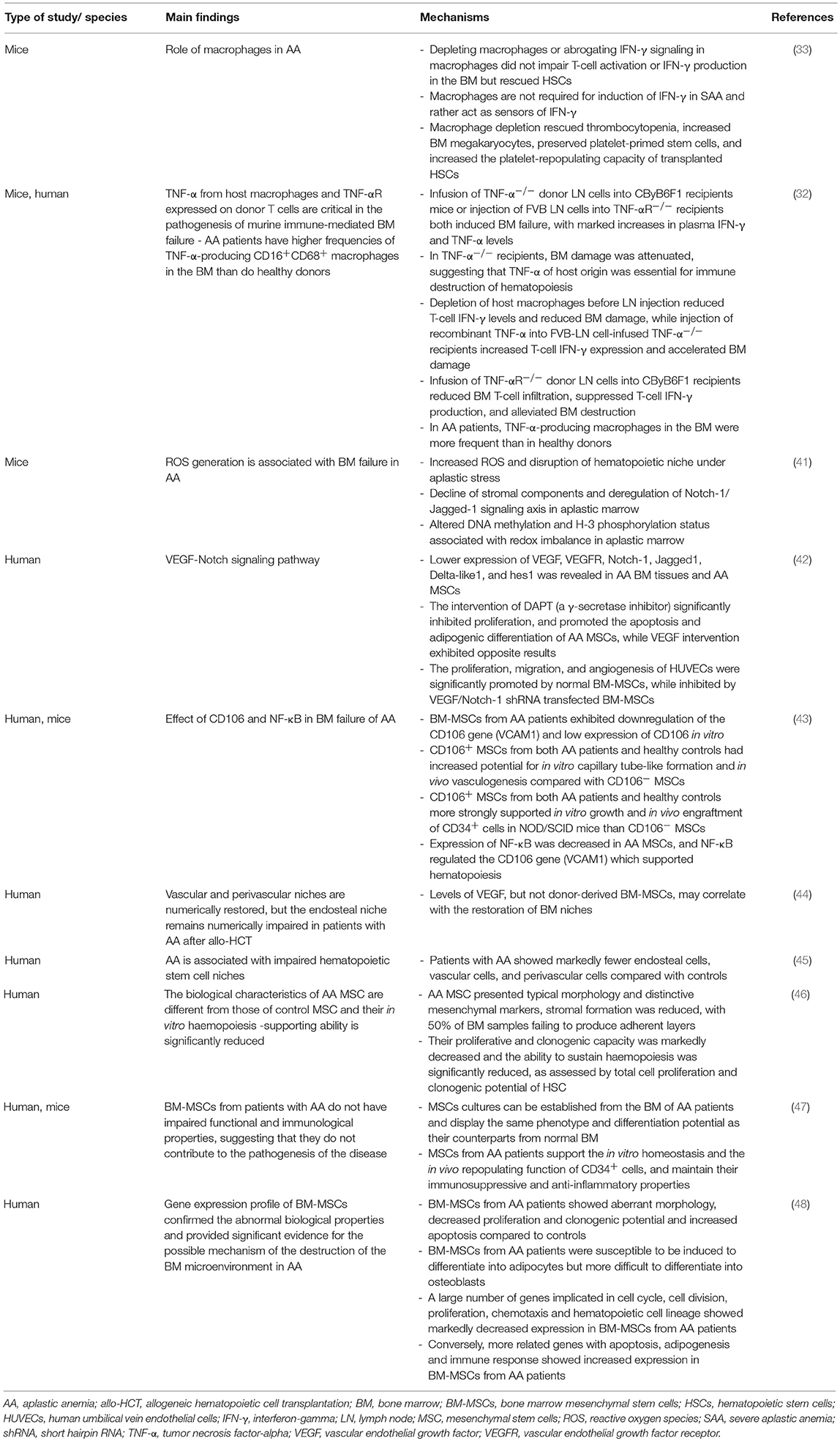
Figure 1. Possible mechanisms contributing to bone marrow niche modulation in aplastic anemia. Patients with aplastic anemia display not only low numbers of hematopoietic stem/progenitor cells (HSPC) but also an altered hematopoietic niche. This might result from immunologic attack and/or genetic defects impairing proliferation and survival in niche cells, or alternatively, from perturbed interactions of these with an unphysiologically diminished HSPC pool. Because of a quantitative MSC impairment in patients with acquired AA, it is tempting to speculate that bone marrow transplantations may yield better results compared to peripheral blood as stem cell source because they provide higher numbers of co-transplanted MSCs and supporting non-hematopoietic cell populations, which may promote niche reconstitution and thereby indirectly support nascent hematopoiesis in AA patients treated with allogeneic transplantations. allo-HCT, allogeneic hematopoietic cell transplantation; AA, aplastic anemia; BM, bone marrow; BMF, bone marrow failure; HSPC, hematopoietic stem and progenitor cells; MSC, mesenchymal stem cells; PB, peripheral blood; SDS, Shwachman-Diamond syndrome.
Next to the disease cause, disease severity and patient's age influence treatment choices. In symptomatic acquired AA, supportive treatment with erythrocyte and platelet transfusions and infection prevention is provided (4, 7). For severe cases, the first-line treatment is allo-HCT from a matched sibling donor in young patients (7–10) and IST in older patients without a well-matched donor (15, 17, 18). The success of allo-HCT is limited due to early and late complications, such as graft rejection, relapse due to resurgent autoimmune attack and development of graft-vs. -host-disease (GvHD), whereas disease persistence, relapse, and clonal evolution limit the success of IST. Inherited BMF are usually not responsive to IST, and besides supportive therapy, allo-HCT is the mainstay of the treatment.
The high overall response rate of about 70–80% observed in patients with acquired AA treated with IST suggests that indeed in most cases the primary mechanism inducing BM hypoplasia is of auto-immune nature (e.g., cytotoxic T cells triggering apoptosis in BM cells) (17–19). Alternative mechanisms include exposure to radiation or toxic agents such as pesticides or benzol, treatment with antineoplastic drugs, antibiotics, non-steroidal anti-inflammatory drugs, as well as active infections (e.g., with viruses such as Epstein Barr, hepatitis virus, human immunodeficiency virus, and parvovirus) (1, 2, 4). Rarely, AA is associated with lymphoproliferative neoplasms (20–22). In these cases, common denominators like particular (immuno-) genetic background or exposure to viruses and toxic environmental factors may in fact increase the risk for both diseases. On the other hand, treatment of lymphoproliferative processes may trigger auto-immunity. Possibly in a HLA-DR restricted manner, AA can co-occur as a “collateral damage” of an auto-immune process directed against the malignant lymphoid clone (21).
Immune Dysregulation in Acquired AA
The cause of acquired AA was not clear for many years. While initially toxic effects were postulated as the reason of a quantitative HSC defect, nowadays autoimmune processes are considered mainly responsible for acquired AA occurring in the absence of a positive medical history of predisposing drugs, toxic agents or infections (1–4) (Figure 1). In fact, while several pathomechanisms have been proposed, the greatest proportion of cases is likely due to uniform T-cell mediated auto-immunity and marrow destruction leading to defective, nearly absent hematopoiesis. Consistently, activated T lymphocytes were observed to induce apoptosis in HSCs (1–4) and oligoclonal expansion of dysregulated CD8+ T-cell populations demonstrated in ex vivo BM models of AA patients (Figure 2) (19, 23, 24). Furthermore, increases in T-helper 17 (Th17) cells, the effector cells which produce the pro-inflammatory cytokine interleukin-17 (IL-17), were found in peripheral (PB) and BM of AA patients (1, 3, 25). Disease activity associated positively with enhanced numbers of Th17 and interferon (IFN)-γ-producing cells, and negatively with regulatory T cells (Treg) populations known to suppress auto-reactivity of other T-cell populations to normal tissue including the BM environment and HSCs. Indeed, especially Tregs from the BM of patients with AA were found to show pronounced quantitative as well as qualitative defects (25).
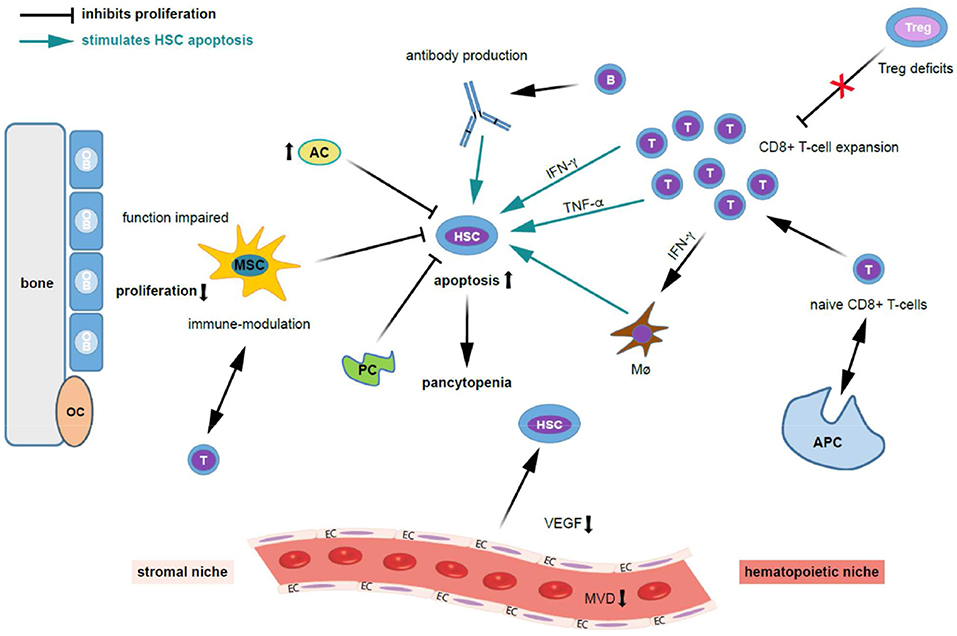
Figure 2. Possible mechanisms contributing to bone marrow niche modulation and immune destruction of hematopoiesis in acquired aplastic anemia. Patients with acquired aplastic anemia (AA) display not only low numbers of hematopoietic stem cells (HSC) but also an altered hematopoietic niche. On the left side of the figure the effect of stromal cells (“stromal niche”) and its interaction with HSC and on the right side the effects of the immune cells on HSC (“hematopoietic niche”) are shown. Regarding the auto-immune pathophysiology in acquired AA, antigens are presented to naive CD8+ T cells by antigen presenting cells (APCs), which trigger T cells to activate and proliferate. Cytotoxic T cells (a polyclonal expansion of dysregulated CD4+ T-cells) triggering apoptosis in bone marrow (BM) cells. Further, activated T lymphocytes induce apoptosis in HSCs and oligoclonal expansion of dysregulated CD8+ T-cell populations. Besides that, there is abnormal production of cytokines including interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF- α), and transforming growth factor (TGF) which induces HSC apoptosis through Fas and the Fas ligand. These events ultimately lead to reduced cell cycling and HSC cell death by apoptosis. Quantitative and qualitative deficits of regulatory T cells (Tregs), which normally suppress auto-reactivity of other T cell populations, further stimulates T cell expansion. TNF-α-producing macrophages (Mø) in the BM were more frequent in AA patients. Further, IFN-γ-mediated HSC loss was shown to require the presence of Mø. INF-γ increases BM Mø which drives loss of megakaryocytes and HSC. The potential for IFN-γ to both directly exhaust and deplete HSCs, as well as to indirectly reduce HSC function through microenvironmental niche cells, particularly Mø, and mesenchymal stem cells (MSCs), adds complexity to the study of AA pathogenesis. Possibly, B cells, which are increased in AA patients, produce auto-antibodies against HSC. Regarding the stromal niche, impairments in osteoblastic, vascular, and perivascular HSC niches might contribute to defective hematopoiesis in patients with AA. MSC function is impaired in AA, HSCs cannot adequately proliferate, and activated T-cells are not suppressed. MSC aberrant alteration impair the maintaining of the immune homeostasis. Adipocytes (AC) are increased and pericytes are decreased (PC) and suppress hematopoiesis. Further, the microvessel density (MVD) and vascular endothelial growth factor (VEGF) expression is decreased in AA. Given the close interaction and regulatory feedback loops between resident hematopoietic and niche cells, it is not surprising that besides immune destruction, AA also associates with defects in non-hematopoietic BM microenvironment components. AC, adipocytes; APC, antigen-presenting cell; HSC, hematopoietic stem cell; EC, endothelial cells; INF-γ, interferon-gamma; MVD, microvessel density; Mø, macrophages; MSC, mesenchymal stem cells; OB, osteoblasts; OC, osteoclasts; PC, pericytes; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor.
Further, BM lymphocytes from AA patients were shown to effectively inhibit hematopoietic cells from healthy donors in co-culture experiments (23). While the antigenic exposure leading to a polyclonal expansion of dysregulated CD4+ T-cells and, respectively, the antigens targeted by T-cells on HSCs remain unknown, the subsequent overproduction of pro-inflammatory cytokines such as IFN-γ as well as tumor necrosis factor (TNF)-α are likely involved in disease pathogenesis (26–29) (Figure 2). In experimental models, in vitro addition of anti-IFN-γ to BM cells from AA patients enhanced the amount of hematopoietic colonies, while the same treatment did not affect cultures from healthy BM cells (26–30). Mechanistically, IFN-γ binds to IFN-γ receptors to modulate the signal transducer and activator of transcription (STAT) and suppressor of cytokine signaling 2 (SOCS2) pathways and influence proliferation and stemness (31). In a very recent study by Sun et al. in a mouse model infusion of TNF-α−/− donor lymph node (LN) cells into CByB6F1 recipients or injection of FVB LN cells into TNF-αR−/− recipients both induced BM failure, with concurrent marked increases in plasma IFN-γ and TNF-α levels (32). In TNF-α−/− recipients, BM damage was attenuated, suggesting that TNF-α of host origin was essential for immune destruction of hematopoiesis. Depletion of host macrophages before LN injection reduced T-cell IFN-γ levels and reduced BM damage, while injection of recombinant TNF-α into FVB-LN cell-infused TNF-α−/− recipients increased T-cell IFN-γ expression and accelerated BM damage. Compared to healthy donors, TNF-α-producing macrophages in the BM were more frequent in AA patients. They concluded that TNF-α from host macrophages and TNF-αR expressed on donor T cells are critical in the pathogenesis of murine immune-mediated BM failure. Further, compared to healthy donors the frequency of TNF-α-producing CD16+CD68+ macrophages in the BM is higher in AA patients (32).
Interestingly, in a recently published mouse model of severe AA, IFN-γ-mediated hematopoietic stem cell loss was shown to require the presence of macrophages (33). Despite loss of other myeloid cells and HSCs, IFN-γ was also required for BM macrophage persistence. T cell activation or IFN-γ production in the BM was not impaired by depleting macrophages or terminating IFN-γ signaling specifically in macrophages, but instead rescued HSC numbers and reduced mortality (33). Thus, macrophages rather act as sensors of IFN-γ and are not required for induction of IFN-γ in AA. Macrophage depletion rescues thrombocytopenia, increases BM megakaryopoiesis, preserves platelet-primed stem cells, and increases the platelet-repopulating capacity of transplanted HSCs.
The Bone Marrow Microenvironment in AA
In murine models, acquired AA associates with a shortage of HSCs (34). In patients with AA, PB and BM show drastically reduced levels of CD34+ cells and, respectively, of so-called long-term culture initiating cells (LT-CIC), which are the functional equivalent of populations enriched for HSCs in humans (35–38). A critical factor sustaining a healthy HSCs/HPCs production is the BM microenvironment (39, 40) (Table 1). Important components thereof are BM stromal cells, the extracellular matrix and local cytokine gradients (40, 49). The hematopoietic and non-hematopoietic elements of the BM closely interact with each other thereby sustaining and balancing hematopoiesis and hematopoietic output (Figure 2). There is clear evidence that the BM microenvironment contains specialized HSC niches that include endothelial, perivascular and endosteal cells and provide key signals that regulate survival, quiescence, self-renewal and differentiation in HSCs (7).
Given the close interaction and regulatory feedback loops between resident hematopoietic and niche cells, it is not surprising that AA also associates with defects in non-hematopoietic BM microenvironment components. As such, patients with AA showed markedly fewer endosteal cells, vascular cells, and perivascular cells compared with controls and loss of non-hematopoietic podoplanin-positive stromal cells was reported in a SAA mouse model (33). In a further study, Chatterjee et al. examined the mechanisms underlying chemotherapeutics mediated BM aplasia (41). They demonstrated that ROS (reactive oxygen species) generated in response to chemotherapy treatment indeed negatively impact the hematopoietic niche (by deregulation of microenvironment related Notch-1 signaling) and thereby alter the epigenetic status of the HSCs leading to devastating HSC damage. They thus proposed that anti-oxidant based therapeutic strategies may be used to circumvent such adverse effects on hematopoiesis.
Impairments in osteoblastic, vascular, and perivascular HSC niches might contribute to defective hematopoiesis in patients with AA (8, 44). Several studies have reported abnormal function and disordered components of the BM microenvironment in patients with AA and BMF (50–53). For example, long-term cultures of BM stromal cells from patients with AA were shown to less robustly support hematopoiesis (44, 51). Mesenchymal stem cells (MSCs) are multipotent stromal cells that can differentiate into a variety of cell types: Namely adipocytes (fat cells which give rise to marrow adipose tissue), chondrocytes (cartilage cells), osteoblasts (bone cells), and myocytes (muscle cells) (3). Compared to healthy donors, MSCs derived from patients with AA showed aberrant morphology, decreased proliferation and clonogenic potential, increased apoptosis and a propensity to differentiate into adipogenic at the expense of osteogenic lineages (46, 48, 54–57), even if significant heterogeneity among individual patient samples was observed. Consistently, transcriptome analyses performed on these cells revealed altered expression especially of genes involved in cell proliferation, cell division, cell cycling, chemotaxis, hematopoietic cell interactions adipogenesis, and immune response in AA vs. healthy controls (43, 48).
In contrast, more recent studies reported MSCs derived from patients with acquired AA to not differ from those collected from healthy controls (47, 58). In particular, MSCs from acquired AA patients were shown to form adequate HSC niches in vivo, comparable to those resulting from healthy control MSCs (58). These controversial results might be due to (a) heterogeneity among individual patients (since in all studies a limited number of patients was analyzed), (b) technical differences among the employed assays and/or (c) potentially inclusion of patients with unrecognized congenital genetic defects in the analysis, since—especially in the older studies—advanced genetic testing was perhaps not uniformously used to distinguish between patients with immune-mediated acquired AA and aplasia resulting from congenital genetic syndromes. In latter, stromal and other non-hematopoietic microenvironmental cells carry also themselves the congenital genetic lesion and might be thus affected by it. Indeed a comprehensive comparison of MSCs derived from 18 patients with Fanconi anemia (n = 18) and age-matched healthy controls (n = 15) revealed increased spontaneous chromosomal fragility leading to precocious senescence and significantly reduced survival when compared to MSCs derived from age-matched healthy donors (59). These data were further confirmed in a subsequent study from Cagnan et al. which documented decreased proliferation, increased ROS levels and an arrest in G2 following DEB (Diepoxybutane) treatment in MSCs from 10 Fanconi anemia patients, with especially absent transforming growth factor (TGF)- β secretion and elevated senescence levels in the FANCD2 mutated cases (60). Analysis of MSCs from Shwachman-Diamond syndrome (SDS) patients, another major subgroup of congenital BMF, also revealed profound functional defects and failed to recreate a BM niche in an in vivo heterotopic ossicle model (61). When compared to healthy donor derived MSCs, these SDS-MSCs especially displayed a marked decrease in vascular endothelial growth factor (VEGF) expression and defective ability to form correct vascular networks, capillary tubes and vessels (61). In fact, using a genetic model of pre-leukemic SDS Zambetti et al. recently showed that perturbation of the MSC compartment induced mitochondrial dysfunction, oxidative stress and activation of the DNA damage responses in HSCs/HPCs (62), and suggested inflammation mediated niche-dependent genotoxic stress in HSCs/HPCs as mechanism promoting leukemogenesis in SDS. Together, these data suggest that the observed MSC impairment observed in acquired immune-mediated AA may be predominantly of quantitative nature while patients with BMF associated with Fanconi or SDS also display important qualitative MSC defects (Figure 1). It remains to be determined whether the enhanced risk of progression to malignant hematopoietic diseases (which is much higher in Fanconi or SDS syndromes, when compared to acquired immune-mediated AA) is in part also mediated by such qualitative changes in microenvironment cells.
Superiority of BM vs. Peripheral Blood Stem Cells (PBSC) Allogeneic Transplantation in Patients with Acquired AA
BM or mobilized PB derived HSCs/HPCs can both be used for allogeneic HCT therapies. Interestingly, unlike in most other conditions routinely treated by allo-HCT, unmanipulated BM is recommended as stem cell source in AA patients referred to allo-HCT. In a study by Bacigalupo et al. (63), BM vs. PB was examined as stem cell source in 1886 patients with AA. Patients receiving BM had a significant survival advantage compared to patients receiving PB as stem cell source. This advantage was statistically significant in both, patients aged 1–19 years (90% vs. 76% P < 0.00001) as well as in patients aged over 20 years (74% vs. 64%, P = 0.001) (63). The advantage for BM as stem cell source compared to PBSC was maintained above the age of 50 years (69% vs. 39%, P = 0.01). Patients with PB as stem cell source had a higher incidence of acute and chronic graft-vs. -host disease. This study reinforced that BM should be the preferred stem cell source for matched sibling transplants in acquired AA, irrespectively of the patients' age. A main reason for this recommendation is considered the increased risk of chronic GvHD after use of PB stem cells (which should be especially avoided in patients transplanted for AA as a non-malignant disease). Despite an earlier engraftment was observed with the use of PB stem cells, a joint EBMT/CIBMTR retrospective analysis suggests inferior outcome with the use of PBSC in this disease, particularly in younger patients (64). More recently, the survival advantage for BM grafts was confirmed across all age groups (65). A BM stem cell dose of at least 3 × 108 MNC/kg or 2 × 106 CD34/kg should be given, as a low stem cell dose increases the risk of graft failure (66). PB is an alternative stem cell source only in case of contraindications to a BM harvest, unwillingness of the donor to donate BM, or in case of second transplants after graft failure.
Given the above mentioned quantitative MSC impairment in patients with acquired AA, it is tempting to speculate that BM transplantations may yield better results because they provide higher numbers of co-transplanted MSCs and supporting non-hematopoietic cell populations, which may promote niche reconstitution and thereby indirectly support nascent hematopoiesis in AA patients treated with allogeneic transplantations (Figure 1). Indeed, as shown by De Felice et al. (67) in contrast to BM grafts which contained mesenchymal progenitors that could be further stimulated by granulocyte colony-stimulating factor (G-SCF) treatment, no mesenchymal progenitors activity was detected using the same functional assays among cells collected from the peripheral blood of healthy donors, regardless of G-CSF stimulation (67). The authors concluded, that BM could be a more useful HSC source for transplant as well as an MSC source because MSC progenitors were undetectable in PB before and after G-CSF stimulation. Several mechanisms may influence the restoration of the BM niche in case functional non-hematopoietic progenitor cells are transplanted next to HSCs/HPCs with BM vs. PB transplants (68–70): (1) Donor-derived BM-MSCs (71) may engraft and then differentiate to niche cells, vascular endothelial cells and perivascular cells after HCT; (2) CD34+ progenitor cells from the transplanted graft may generate endothelial cells in an appropriate host environment (72); (3) Numerous cytokines secreted by HSCs/HPCs and transplanted niche cells may influence the microenvironment and enhance BM stem cell homing and subsequent hematopoiesis (69, 71, 73).
Interestingly, data from AA patients receiving allo-HCT (most patients received BM together with PBSCs as stem cell source) showed that in spite of successful hematopoietic engraftment after allo-HCT, BM-MSCs remained host-derived (44). However, co-transplanted donor MSCs and non-hematopoietic populations contained in BM allo-transplants could perhaps transiently support the regeneration of the niche (and therefore of the hematopoietic compartment) in AA patients with quantitative niche defects. Alternatively, they might provide additional immune suppressive effects that improve the course of the disease (3).
AA can be cured or, respectively, ameliorated by allo-HCT or IST (9, 10, 15, 18). How an allo-HCT is influencing the BM microenvironment and especially the HSC niche, is not understood in detail. Wu et al. (44) analyzed the HSC niche components changes by immunohistochemistry (CD34, CD146, and osteopontin) before allo-HCT and at 1, 2, 3, 6, and 12 months after allo-HCT (44). A significant increase in the number of cellular elements in the BM niche, including vascular and perivascular cells could be shown in patients with acquired AA (n = 52) after allo-HCT (44). Regarding endosteal cells, no relevant changes could be found. To get more insight into the restoration of the BM niche after allo-HCT, the origin of BM-MSCs and the expression of cytokines in BM plasma were examined. At 1–12 months after transplantation, BM-MSCs were indeed derived from the host, not the donor. Further, after allo-HCT levels of VEGF significantly rose (44). Taken together, in patients with AA after allo-HCT vascular and perivascular niches are numerically restored, but the endosteal niche remains numerically (quantitatively) impaired. Allo-HCT from BM cells thus improves reconstitution of some (perhaps VEGF-dependent) but not all niches. Of note, a systematic comparison between BM and PB allo-transplants was not yet performed, thus the role of co-transplanted non-hematopoietic BM cells for these effects remains elusive. The restoration of BM niches may be the result of high VEGF levels, but not of donor-derived BM-MSCs.
Postnatal vasculogenesis during physiological and pathological neovascularization occurs by endothelial progenitor cells with BM origin (70). CD34+ endothelial/hematopoietic BM derived progenitors were shown to promote vasculogenesis and osteogenesis during bone healing (74), suggesting that also in the allo-HCT transplant setting they might promote niche reconstruction. However, as shown by Wu et al. (45) the osteoblastic niche-forming cells remained at low numbers after allo-HCT. The most likely explanation for the specific differences among niche cells is that transplanted CD34+ cells are localized to distinct locations and influence niche restoration. Cell-intrinsic and niche intrinsic variables influences the relationship of transplanted primitive hematopoietic cells to anatomical components of the trabecular niche (75).
When BM microvessel density (MVD) was compared using immunohistochemical staining for CD34, patients with severe AA showed significantly lower MVD compared with patients with non-severe AA, and, respectively, healthy controls (50) (Figure 2). The formation of the vasculature is mediated through VEGF secretion by CD34+ cells (76–78). A further study confirmed the lower BM MVD in patients with newly diagnosed AA and additionally demonstrated reduced VEGF serum levels in these cases compared to healthy controls (50). Moreover, response to therapy (IST or allo-HCT) was shown to associate with increase of both VEGF and MVD (50). In summary, AA is associated with reduced angiogenesis and reduced VEGF expression, which might contribute to disease pathogenesis. The secretion of VEGF from successfully engrafted hematopoietic - and perhaps also non-hematopoietic—BM cells after allo-HCT is likely to support the regeneration of the impaired BM environment in AA patients. In a study by Deng et al. the role of the VEGF-Notch signaling pathway on MSC was examined in AA (42). They found a significantly lower expression of VEGF, VEGFR, Notch-1, Jagged1, Delta-like1, and hes1 in AA BM tissues and AA MSCs compared to healthy controls. The activation of VEGF-Notch signaling promoted the proliferation and adipogenic differentiation, and inhibited the apoptosis of AA MSCs. They concluded that the activation of VEGF-Notch signaling pathway may be a potential therapeutic target for AA. In the embryo, HSCs are formed from endothelial cells upon activation of the VEGF-Notch pathway (79–81). Regulatory loops by which VEGF controls the survival of HSCs have been described (78), as well as cross-talk between hematopoietic, endothelial and mesenchymal cells in embryonic as well as adult hematopoietic environments. It remains unclear whether the observed decreased BM vascularization in AA is instrumental in the bone marrow failure process or rather another consequence of the profoundly disturbed hematopoiesis with reduced content of cytokine producing cells. Nevertheless, such a strongly impaired BM microenvironment is likely to sustain hematopoietic defects and furthermore delay hematopoietic regeneration even after the causative agent was removed (e.g., by IST or replacement of the altered immune system by allogeneic transplantation).
Taken together, a disturbed microenvironment with abnormal functioning MSCs is, next to BM hypoplasia, a further hallmark in patients with acquired AA. MSCs have been utilized in the settings of therapy for other disorders due to their immunomodulatory and proliferative functions; e.g., steroid-refractory gastrointestinal graft-vs. -host disease. Translation of MSC therapy to AA has been relatively limited. Preliminary studies have attempted to use MSCs as an adjunct to allo-HCT to improve engraftment or as primary, monotherapy of AA (82, 83). However, larger studies are needed to evaluate the utility of MSCs further. In a recent study by Yue et al. in patients with SAA, haploidentical-HCT from related donors was performed together with culture-expanded allogeneic bone marrow-derived MSCs, which were infused on day 0 and day +14 (84). All patients achieved sustained, full donor chimerism, and the median time of myeloid recovery and platelet engraftment was 13 days, respectively. MSCs and other non-hematopoietic cells present in the BM vs. PB as stem cell source, may contribute to niche regeneration post-transplantation by (a) providing transient VEGF and cytokine support, (b) enhancing immune suppression and possibly (but less probable) by (c) providing stable niche engraftment of donor-derived niche cells. Considering that multiple progenitor cells, as well as HSCs are enriched in transplanted PBSC and BM (68–70, 85) there may be several possible mechanisms to explain the restoration of the BM niche which include transient engraftment of cytokine producing non-hematopoietic BM cells that promote niche restoration and thereby facilitate hematopoietic regeneration (69–72).
Conclusion
The cross-talk between the microenvironment and the defective hematopoietic compartment may significantly contribute to the disrupted hematopoiesis and delayed hematopoietic regeneration commonly observed in AA patients. There are several in vitro and in vivo studies indicating quantitative as well as qualitative mesenchymal stromal alterations in patients with AA and, respectively, genetic BMF, which may be pathogenetically involved in the clinical evolution of the disease. Further, studies are needed to examine the importance of microenvironment changes in the acquired AA pathogenesis, progression and response to therapy. A better understanding of these processes may unravel novel niche-based therapeutic intervention possibilities that ameliorate the clinical course of AA and/or improve response to therapy by augmenting niche support.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Martina Konantz, University of Basel and University Hospital Basel, Department of Biomedicine Switzerland, for help with the figures.
References
1. Luzzatto L, Risitano AM. Advances in understanding the pathogenesis of acquired aplastic anaemia. Br J Haematol. (2018) 182:758–77. doi: 10.1111/bjh.15443
2. Balderman SR, Calvi LM. Biology of BM failure syndromes: role of microenvironment and niches. Hematol Am Soc Hematol Educ Program (2014) 2014:71–6. doi: 10.1182/asheducation-2014.1.71
3. Gonzaga VF, Wenceslau CV, Lisboa GS, Frare EO, Kerkis I. Mesenchymal stem cell benefits observed in bone marrow failure and acquired aplastic anemia. Stem Cells Int. (2017) 2017:8076529. doi: 10.1155/2017/8076529
4. Clucas D, Fox LC, Wood EM, Hong FS, Gibson J, Bajel A, et al. Australian aplastic anaemia registry steering committee. revisiting acquired aplastic anaemia: current concepts in diagnosis and management. Intern Med J. (2018). doi: 10.1111/imj.14140. [Epub ahead of print].
5. Ehrlich P. Ueber einen Fall von Anämie mit Bemerkungen über regenerative Veränderungen des Knochenmarks. Charité-Annalen (1888) 13:300–9.
6. Alter BP. Inherited bone marrow failure syndromes: considerations pre- and posttransplant. Blood (2017) 130:2257–64. doi: 10.1182/blood-2017-05-781799
8. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood (2006) 108:2509–19. doi: 10.1182/blood-2006-03-010777
9. Giammarco S, Peffault de Latour R, Sica S, Dufour C, Socie G, Passweg J, et al. European group for blood and marrow transplantation severe aplastic anemia working party. transplant outcome for patients with acquired aplastic anemia over the age of 40: has the outcome improved? Blood (2018) 131:1989–92. doi: 10.1182/blood-2017-09-807859
10. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program (2010) 2010:36–42. doi: 10.1182/asheducation-2010.1.36
11. Mathe G, Amiel JL, Schwarzenberg L, Choay J, Trolard P, Schneider M, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. (1970) 2:131–6. doi: 10.1136/bmj.2.5702.131
12. Speck B, Cornu P, Jeannet M, Nissen C, Burri HP, Groff P, et al. Autologous marrow recovery following allogeneic marrow transplantation in a patient with severe aplastic anemia. Exp Hematol. (1976) 4:131–7.
13. Speck B, Gluckman E, Haak HL, van Rood JJ. Treatment of aplastic anaemia by antilymphocyte globulin with or without marrow infusion. Clin Haematol. (1978) 7:611–21.
14. Speck B, Buckner CD, Cornu P, Jeannet B. Rationale for the use of ALG as sole immunosuppressant in allogeneic bone marrow transplantation for aplastic anemia. Transl Proc. (1976) 8:617–22.
15. Tichelli A, Marsh JC. Treatment of aplastic anaemia in elderly patients aged >60 years. Bone Marrow Transl. (2013) 48:180–2. doi: 10.1038/bmt.2012.224
16. Frickhofen N, Kaltwasser JP, Schrezenmeier H, Raghavachar A, Vogt HG, Herrmann F, et al. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. the german aplastic anemia study group. N Engl J Med. (1991) 324:1297–304. doi: 10.1056/NEJM199105093241901
17. Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. (2011) 365:430–8. doi: 10.1056/NEJMoa1103975
18. Tichelli A, Schrezenmeier H, Socié G, Marsh J, Bacigalupo A, Dührsen U, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA working party of the european group for blood and marrow transplantation. Blood (2011) 117:4434–41. doi: 10.1182/blood-2010-08-304071
19. Young NS, Maciejewski J. Mechanisms of disease – the pathophysiology of acquired aplastic anemia. N Engl J Med. (1997) 336:1365–72. doi: 10.1056/NEJM199705083361906
20. Rovó A, Kulasekararaj A, Medinger M, Chevallier P, Ribera JM, Peffault de Latour R, et al. Association of aplastic anaemia and lymphoma: a report from the severe aplastic anaemia working party of the European society of blood and bone marrow transplantation. Br J Haematol. (2017). doi: 10.1111/bjh.15074. [Epub ahead of print].
21. Tzankov A, Medinger M. Aplastic anemia: possible associations with lymphoproliferative neoplasms. Int J Lab Hematol. (2014) 36:382–7. doi: 10.1111/ijlh.12224
22. Medinger M, Buser A, Stern M, Heim D, Halter J, Rovo A, et al. Aplastic anemia in association with a lymphoproliferative neoplasm: coincidence or causality? Leuk Res. (2012) 36:250–1. doi: 10.1016/j.leukres.2011.09.008
23. Hoffman R, Zanjani ED, Lutton JD, Zalusky R, Wasserman LR. Suppression of erythroid-colony formation by lymphocytes from patients with aplastic anemia. N Engl J Med. (1977) 296:10–3. doi: 10.1056/NEJM197701062960103
24. Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet (2004) 364:355–64. doi: 10.1016/S0140-6736(04)16724-X
25. Shallis RM, Ahmad R, Zeidan AM. Aplastic anemia: etiology, molecular pathogenesis and emerging concepts. Eur J Haematol. (2018) 101:711–20. doi: 10.1111/ejh.13153
26. Zoumbos NC, Gascon P, Djeu JY, Young NS. Interferon is a mediator of hematopoietic suppression in aplastic-anemia in vitro and possibly in vivo. Proc Natl Acad Sci USA. (1985) 82:188–92. doi: 10.1073/pnas.82.1.188
27. Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-γ in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood (2002) 100:1185–91. doi: 10.1182/blood-2002-01-0035
28. Dubey S, Shukla P, Nityanand S. Expression of interferon-γ and tumor necrosis factor-α in bone marrow T cells and their levels in bone marrow plasma in patients with aplastic anemia. Ann Hematol. (2005) 84:572–7. doi: 10.1007/s00277-005-1022-8
29. Zeng WH, Miyazato A, Chen GB, Kajigaya S, Young NS, Maciejewski JP. Interferon-γ-induced gene expression in CD34 cells: identification of pathologic cytokine-specific signature profiles. Blood (2006) 107:167–75. doi: 10.1182/blood-2005-05-1884
30. Li JP, Zheng CL, Han ZC. Abnormal immunity and stem/progenitor cells in acquired aplastic anemia. Crit Rev Oncol Hematol. (2010) 75:79–93. doi: 10.1016/j.critrevonc.2009.12.001
31. Krebs DL, Hilton DJ. SOCS proteins: negative regulators of cytokine signaling. Stem Cells (2001) 19:378–87. doi: 10.1634/stemcells.19-5-378
32. Sun W, Wu Z, Lin Z, Hollinger M, Chen J, Feng X, et al. Macrophage TNF-α licenses donor T cells in murine bone marrow failure and can be implicated in human aplastic anemia. Blood (2018) blood-2018-05-844928. doi: 10.1182/blood-2018-05-844928
33. McCabe A, Smith JNP, Costello A, Maloney J, Katikaneni D, MacNamara KC. Hematopoietic stem cell loss and hematopoietic failure in severe aplastic anemia is driven by macrophages and aberrant podoplanin expression. Haematologica (2018) 103:1451–61. doi: 10.3324/haematol.2018.189449
34. Scheinberg P, Chen JC. Aplastic anemia: what have we learned from animal models and from the clinic. Semi Hematol. (2013) 50:156–64. doi: 10.1053/j.seminhematol.2013.03.028
35. Marsh JC, Chang J, Testa NG, Hows JM, Dexter TM. The hematopoietic defect in aplastic anemia assessed by long-term marrow culture. Blood (1990) 76:1748–57.
36. Marsh JC, Chang J, Testa NG, Hows JM, Dexter TM. In vitro assessment of marrow 'stem cell' and stromal cell function in aplastic anaemia. Br J Haematol. (1991) 78:258–67. doi: 10.1111/j.1365-2141.1991.tb04426.x
37. Maciejewski JP, Selleri C, Sato T, Anderson S, Young NS. A severe and consistent deficit in marrow and circulating primitive hematopoietic cells (long-term culture-initiating cells) in acquired aplastic anemia. Blood (1996) 88:1983–91.
38. Manz CY, Nissen C, Wodnar-Filipowicz A. Deficiency of CD34+ c-kit+ and CD34+38- hematopoietic precursors in aplastic anemia after immunosuppressive treatment. Am J Hematol. (1996) 52:264–74. doi: 10.1002/(SICI)1096-8652(199608)52:4 < 264::AID-AJH5>3.0.CO;2-Q
39. Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature (2003) 425:836–841. doi: 10.1038/nature02041
40. Crane GM, Jeffery E, Morrison SJ. Adult haematopoietic stem cell niches. Nat Rev Immunol. (2017) 17:573–90. doi: 10.1038/nri.2017.53
41. Chatterjee R, Law S. Epigenetic and microenvironmental alterations in bone marrow associated with ROS in experimental aplastic anemia. Eur J Cell Biol. (2018) 97:32–43. doi: 10.1016/j.ejcb.2017.11.003
42. Deng S, Zeng Y, Wu L, Hu Z, Shen J, Shen Y, et al. The regulatory roles of VEGF-Notch signaling pathway on aplastic anemia with kidney deficiency and blood stasis. J Cell Biochem. (2018). doi: 10.1002/jcb.27516. [Epub ahead of print].
43. Lu S, Ge M, Zheng Y, Li J, Feng X, Feng S, et al. CD106 is a novel mediator of bone marrow mesenchymal stem cells via NF-κB in the bone marrow failure of acquired aplastic anemia. Stem Cell Res Ther. (2017) 8:178. doi: 10.1186/s13287-017-0620-4
44. Wu L, Mo W, Zhang Y, Zhou M, Li Y, Zhou R, et al. Vascular and perivascular niches, but not the osteoblastic niche, are numerically restored following allogeneic hematopoietic stem cell transplantation in patients with aplastic anemia. Int J Hematol. (2017) 106:71–81. doi: 10.1007/s12185-017-2217-1
45. Wu L, Mo W, Zhang Y, Deng H, Li Y, Zhou R, et al. Impairment of hematopoietic stem cell niches in patients with aplastic anemia. Int J Hematol. (2015) 102:645–53. doi: 10.1007/s12185-015-1881-2
46. Hamzic E, Whiting K, Gordon Smith E, Pettengell R. Characterization of bone marrow mesenchymal stromal cells in aplastic anaemia. Br J Haematol. (2015) 169:804–13. doi: 10.1111/bjh.13364
47. Bueno C, Roldan M, Anguita E, Romero-Moya D, Martín-Antonio B, Rosu-Myles M, et al. Bone marrow mesenchymal stem cells from patients with aplastic anemia maintain functional and immune properties and do not contribute to the pathogenesis of the disease. Haematologica (2014) 99:1168–75. doi: 10.3324/haematol.2014.103580
48. Li J, Yang S, Lu S, Zhao H, Feng J, Li W, et al. Differential gene expression profile associated with the abnormality of bone marrow mesenchymal stem cells in aplastic anemia. PLoS ONE (2012) 7:e47764. doi: 10.1371/journal.pone.0047764
49. Riether C, Schürch CM, Ochsenbein AF. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell Death Differ. (2015) 22:187–98. doi: 10.1038/cdd.2014.89
50. Fureder W, Krauth MT, Sperr WR, Sonneck K, Simonitsch-Klupp I, Mullauer L, et al. Evaluation of angiogenesis and vascular endothelial growth factor expression in the bone marrow of patients with aplastic anemia. Am J Pathol. (2006) 168:123–30. doi: 10.2353/ajpath.2006.050034
51. Holmberg LA, Seidel K, Leisenring W, Torok-Storb B. Aplastic anemia: analysis of stromal cell function in long-term marrow cultures. Blood (1994) 84:3685–90.
52. Shipounova IN, Petrova TV, Svinareva DA, Momotuk KS, Mikhailova EA, Drize NI. Alterations in hematopoietic microenvironment in patients with aplastic anemia. Clin Transl Sci. (2009) 2:67–74. doi: 10.1111/j.1752-8062.2008.00074.x
53. Park M, Park CJ, Jang S, Kim DY, Lee JH, Lee JH, et al. Reduced expression of osteonectin and increased natural killer cells may contribute to the pathophysiology of aplastic anemia. Appl Immunohistochem Mol Morphol. (2015) 23:139–45. doi: 10.1097/PAI.0000000000000023
54. Caplan AI. All MSCs are pericytes? Cell Stem Cell. (2008) 3:229–30. doi: 10.1016/j.stem.2008.08.008
55. Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. (2008) 3:301–313. doi: 10.1016/j.stem.2008.07.003
56. Li WX, Fu JX, Wang Y, Shi WB, Zhang XG. Expression of membrane-bound IL-15 by bone marrow fibroblast-like stromal cells in aplastic anemia. Int Immunol. (2005) 17:429–37. doi: 10.1093/intimm/dxh223
57. Chao YH, Peng CT, Harn HJ, Chan CK, Wu KH. Poor potential of proliferation and differentiation in bone marrow mesenchymal stem cells derived from children with severe aplastic anemia. Ann Hematol. (2010) 89:715–23. doi: 10.1007/s00277-009-0892-6
58. Michelozzi IM, Pievani A, Pagni F, Antolini L, Verna M, Corti P, et al. Human aplastic anaemia-derived mesenchymal stromal cells form functional haematopoietic stem cell niche in vivo. Br J Haematol. (2017) 179:669–73. doi: 10.1111/bjh.14234
59. Mantelli M, Avanzini MA, Rosti V, Ingo DM, Conforti A, Novara F, et al. Comprehensive characterization of mesenchymal stromal cells from patients with Fanconi anaemia. Br J Haematol. (2015) 170:826–36. doi: 10.1111/bjh.13504
60. Cagnan I, Gunel-Ozcan A, Aerts-Kaya F, Ameziane N, Kuskonmaz B, Dorsman J, et al. Bone marrow mesenchymal stem cells carrying fancd2 mutation differ from the other fanconi anemia complementation groups in terms of TGF-β1 Production. Stem Cell Rev. (2018) 14:425–37. doi: 10.1007/s12015-017-9794-5
61. Bardelli D, Dander E, Bugarin C, Cappuzzello C, Pievani A, Fazio G, et al. Mesenchymal stromal cells from Shwachman-Diamond syndrome patients fail to recreate a bone marrow niche in vivo and exhibit impaired angiogenesis. Br J Haematol. (2018) 182:114–24. doi: 10.1111/bjh.15388
62. Zambetti NA, Ping Z, Chen S, Kenswil KJG, Mylona MA, Sanders MA, et al. Mesenchymal inflammation drives genotoxic stress in hematopoietic stem cells and predicts disease evolution in human pre-leukemia. Cell Stem Cell. (2016) 19:613–27. doi: 10.1016/j.stem.2016.08.021
63. Bacigalupo A, Socié G, Schrezenmeier H, Tichelli A, Locasciulli A, Fuehrer M, et al. Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (WPSAA-EBMT). Bone marrow versus peripheral blood as the stem cell source for sibling transplants in acquired aplastic anemia: survival advantage for bone marrow in all age groups. Haematologica (2012) 97:1142–8. doi: 10.3324/haematol.2011.054841
64. Schrezenmeier H, Passweg JR, Marsh JC, Bacigalupo A, Bredeson CN, Bullorsky E, et al. Worse outcome and more chronic GVHD with peripheral blood progenitor cells than bone marrow in HLA-matched sibling donor transplants for young patients with severe acquired aplastic anemia. Blood (2007) 110:1397–400. doi: 10.1182/blood-2007-03-081596
65. Locatelli F, Bruno B, Zecca M, Van-Lint MT, McCann S, Arcese W, et al. Cyclosporin A and short-term methotrexate versus cyclosporin A as graft versus host disease prophylaxis in patients with severe aplastic anemia given allogeneic bone marrow transplantation from an HLA-identical sibling: results of a GITMO/EBMT randomized trial. Blood (2000) 96:1690–7.
66. Islam MS, Anoop P, Datta-Nemdharry P, Sage D, Gordon-Smith EC, Turner D, et al. Implications of CD34+ cell dose on clinical and haematological outcome of allo-SCT for acquired aplastic anaemia. Bone Marrow Transl. (2010) 45:886–94. doi: 10.1038/bmt.2009.267
67. De Felice L, Agostini F, Suriano C, Fraboni D, Gregorj C, Tirindelli MC, et al. Hematopoietic, mesenchymal, and immune cells are more enhanced in bone marrow than in peripheral blood from granulocyte colony-stimulating factor primed healthy donors. Biol Blood Marrow Transl. (2016) 22:1758–64. doi: 10.1016/j.bbmt.2016.06.024
68. Villaron EM, Almeida J, López-Holgado N, Alcoceba M, Sánchez-Abarca LI, Sanchez-Guijo FM, et al. Mesenchymal stem cells are present in peripheral blood and can engraft after allogeneic hematopoietic stem cell transplantation. Haematologica (2004) 89:1421–7.
69. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science (1999) 284:143–7. doi: 10.1126/science.284.5411.143
70. Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. (1999) 85:221–8. doi: 10.1161/01.RES.85.3.221
71. Wei X, Liu C, Wang H, Wang L, Xiao F, Guo Z, et al. Surface phosphatidylserine is responsible for the internalization on microvesicles derived from hypoxia-induced human bone marrow mesenchymal stem cells into human endothelial cells. PLoS ONE (2016) 11:e0147360. doi: 10.1371/journal.pone.0147360
72. Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. (1999) 5:434–8. doi: 10.1038/7434
73. Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. (2015) 16:239–53. doi: 10.1016/j.stem.2015.02.019
74. Matsumoto T, Kawamoto A, Kuroda R, Ishikawa M, Mifune Y, Iwasaki H, et al. Therapeutic potential of vasculogenesis and osteogenesis promoted by peripheral blood CD34-positive cells for functional bone healing. Am J Pathol. (2006) 169:1440–57. doi: 10.2353/ajpath.2006.060064
75. Lo Celso C, Fleming HE, Wu JW, Zhao CX, Miake-Lye S, Fujisaki J, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature (2009) 457:92–6. doi: 10.1038/nature07434
76. Gerber HP, Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. J Mol Med. (2003) 81:20–31. doi: 10.1007/s00109-002-0397-4
77. Gianelli U, Vener C, Raviele PR, Savi F, Somalvico F, Calori R, et al. VEGF expression correlates with microvessel density in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol. (2007) 128:966–73. doi: 10.1309/FP0N3LC8MBJUFFA6
78. Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, Meng G, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature (2002) 417:954–8. doi: 10.1038/nature00821
79. Gordon-Keylock S, Medvinsky A. Endothelio–hematopoietic relationship: getting closer to the beginnings. BMC Biol (2011) 9:88. doi: 10.1186/1741-7007-9-88
80. Park C, Ma YD, Choi K. Evidence for the hemangioblast. Exp Hematol. (2005) 33:965–70. doi: 10.1016/j.exphem.2005.06.003
81. Konantz M, Alghisi E, Müller JS, Lenard A, Esain V, Carroll KJ, et al. Evi1 regulates Notch activation to induce zebrafish hematopoietic stem cell emergence. EMBO J. (2016) 35:2315–31. doi: 10.15252/embj.201593454
82. Liu Z, Zhang Y, Xiao H, Yao Z, Zhang H, Liu Q, et al. Cotransplantation of bone marrow-derived mesenchymal stem cells in haploidentical hematopoietic stem cell transplantation in patients with severe aplastic anemia: an interim summary for a multicenter phase II trial results. Bone Marrow Transl. (2017) 52:1080. doi: 10.1038/bmt.2017.85
83. Pang Y, Xiao HW, Zhang H, Liu ZH, Li L, Gao Y, et al. allogeneic bone marrow-derived mesenchymal stromal cells expanded in vitro for treatment of aplastic anemia: a multicenter phase ii trial. Stem Cells Transl Med. (2017) 6:1569–75. doi: 10.1002/sctm.16-0227
84. Yue C, Ding Y, Gao Y, Li L, Pang Y, Liu Z, et al. Cotransplantation of haploidentical hematopoietic stem cells and allogeneic bone marrow-derived mesenchymal stromal cells as a first-line treatment in very severe aplastic anemia patients with refractory infections. Eur J Haematol. (2018) 100:624–9. doi: 10.1111/ejh.13060
Keywords: aplastic anemia, microenvironment, microvessel density, mesenchymal stem cells, stem cell niche
Citation: Medinger M, Drexler B, Lengerke C and Passweg J (2018) Pathogenesis of Acquired Aplastic Anemia and the Role of the Bone Marrow Microenvironment. Front. Oncol. 8:587. doi: 10.3389/fonc.2018.00587
Received: 20 July 2018; Accepted: 22 November 2018;
Published: 05 December 2018.
Edited by:
Andreas Pircher, Innsbruck Medical University, AustriaReviewed by:
Markus G. SEIDEL, Medical University of Graz, AustriaJ. Luis Espinoza, Kindai University, Japan
Copyright © 2018 Medinger, Drexler, Lengerke and Passweg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Medinger, bWljaGFlbC5tZWRpbmdlckB1c2IuY2g=
 Michael Medinger
Michael Medinger Beatrice Drexler2
Beatrice Drexler2