
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Oncol. , 28 November 2018
Sec. Genitourinary Oncology
Volume 8 - 2018 | https://doi.org/10.3389/fonc.2018.00571
This article is part of the Research Topic Emerging Biomarkers in Genitourinary Tumors View all 10 articles
 Michal Chovanec1,2
Michal Chovanec1,2 Costantine Albany2
Costantine Albany2 Michal Mego1
Michal Mego1 Rodolfo Montironi3
Rodolfo Montironi3 Alessia Cimadamore3
Alessia Cimadamore3 Liang Cheng4,5*
Liang Cheng4,5*Testicular germ cell tumors are unique among solid cancers. Historically, this disease was deadly if progressed beyond the stage I. The implementation of cisplatin-based chemotherapy regimens has drastically changed the clinical outcome of metastatic testicular cancer. Several biomarkers were established to refine the prognosis by International Germ Cell Collaborative Group in 1997. Among these, the most significant were primary tumor site; metastatic sites, such as non-pulmonary visceral metastases; and the amplitude of serum tumor markers α-fetoprotein, β-chorionic gonadotropin, and lactate dehydrogenase. Since then, oncology has experienced discoveries of various molecular biomarkers to further refine the prognosis and treatment of malignancies. However, the ability to predict the prognosis and treatment response in germ cell tumors did not improve for many years. Clinical trials with novel targeting agents that were conducted in refractory germ cell tumor patients have proven to have negative outcomes. With the recent advances and developments, novel biomarkers emerge in the field of germ cell tumor oncology. This review article aims to summarize the current knowledge in the research of novel prognostic biomarkers in testicular germ cell tumors.
Testicular germ cell tumors (GCT) are unique in terms of molecular landscape, pathogenesis, clinical presentation, and response to chemotherapy (1). The exceptional position of GCT among the solid cancers can be perhaps attributed to their developmental origin in primordial germ cells. While the cure rate of patients with metastatic disease exceeds 80% (2), the ones failing the initial and salvage chemotherapy die of their disease in young age. About 40–80% of patients with relapsed GCT fail the salvage chemotherapy, resulting in the loss of 35 years of life on average (3–5). The utility of biomarkers to risk-stratify the treatment is well-established in GCT. Markers of the risk of relapse in the stage I disease, such as tumor size of >4 cm and rete testis invasion for seminoma, and lymphovascular invasion and predominance of embryonal carcinoma for non-seminoma, are currently used to risk-stratify the patients for surveillance or adjuvant treatment (6–9). International Germ Cell Cancer Collaborative Group (IGCCCG) presented the risk-stratification model for metastatic disease in 1997 using biomarkers such as primary tumor site, metastatic sites, the amplitude of serum α-fetoprotein (AFP), β-chorionic gonadotropin (HCG), and lactate dehydrogenase (LDH) (10). These criteria are based on patient series collected retrospectively between 1975 and 1990. Since then, the treatment strategy was optimized, and outcomes improved as reported from high volume centers (2, 11, 12). Further refining of IGCCCG criteria is expected soon in the updated version of the IGCCCG classification (Figure 1).
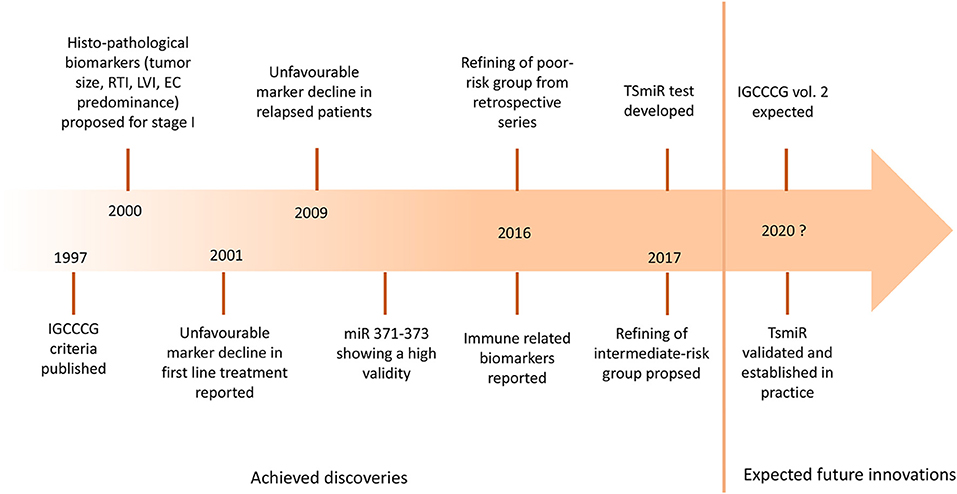
Figure 1. The landmarks of prognostic biomarkers in germ cell tumors. IGCCCG, International Germ Cell Cancer Consensus Group; RTI, rete testis invasion; LVI, lymphovascular invasion; EC, embryonal carcinoma; miR, microRNA; TSmiR, targeted serum microRNA test.
New reports on novel biomarkers are scarce since the introduction of the commonly used GCT biomarkers over three decades ago. The utility of novel molecular biomarkers in numerous solid cancers has significantly moved the advancement of oncology. Malignancies, such as lung cancer, melanoma, and kidney cancer, were previously considered untreatable, but now the array of molecular markers renders these diseases treatable with targeting agents ultimately prolonging lives of patients with incurable cancer (13, 14). Such advancement seemingly evades testicular GCT due to lack of known drugable targets. While the overall cure rate of GCT patients is excellent, ones refractory to standard chemotherapy lack the possibility to receive novel effective treatments and their prognosis is dismal. The biology of GCTs is unique, therefore translational research to uncover the biological implications is essential in the pursuit of treatment targets that may improve the prognosis of platinum refractory GCT patients. This article aims to summarize the current knowledge on the emerging biomarkers in GCT.
Understanding why we lack a significant predictive biomarker in GCT requires a look into their molecular landscape and developmental origins. The origin of GCT particularly show how different their biology is compared to other solid cancers. The data from The Cancer Genome Atlas (TCGA) show a rather quiet mutational landscape in GCT compared to other solid tumors (15).
Several genomewide studies suggested driver mutations in only three genes (KIT, KRAS, and NRAS) in 4–31% of seminoma, and up to 14% of non-seminoma patients (16–19). Since these mutations were discovered in a minority of patients, a single universal mutational driver is not a feasible explanation in the development of GCT. Rather, a polygenic nature of testicular cancer was proposed, where the number of low frequency susceptibility genes (up to 50 risk loci reported until present) seems to produce an increased risk for the GCT (20). A recent paper by Shen et al. conducted a comprehensive molecular characterization of available tissue from 137 GCT patients. The authors confirmed findings of previously known mutated genes (KIT, KRAS, and NRAS) and provided yet additional evidence of low mutational burden with frequency of 0.5 mutations per megabase (15).
Despite the unimpressive mutational characteristics, GCT share a unique epigenetic landscape. GCT subtypes are an example of developmental processes from pluripotent embryonic stem cells toward certain degrees of differentiation to somatic tissues. The mapping of GCT methylome is perhaps the most comprehensively assessed to this date. The global DNA-methylation status clearly correlates with the state of differentiation in the histological GCT subtypes. Seminomas, which show the lowest degree of differentiation are typically unmethylated or severely hypomethylated tumors. Embryonal carcinomas show low to intermediate levels of global DNA methylation and well-differentiated yolk sac tumors, and teratomas show high levels of DNA methylation. Thus, the significant histological variability complies with the epigenetic heterogeneity. These findings also comply with the epigenetic landscape of healthy tissues where differentiated somatic tissues show hypermethylated pattern (21–23). Non-CpG methylation, acetylation, and methylation of histones are also mechanisms likely involved in the biology of GCT. They are, however, poorly understood in present time. microRNA (miR) signaling research on the other hand seems to provide promising results toward increasing the knowledge about molecular biology of GCT. While the miR signaling is generally complex and is a subject of innumerous interactions, the clusters of miR discussed later in this paper provide a significant biomarker potency to further refine the management of GCT.
The unique germline origin of GCT is underlined with the overexpression of markers of pluripotency such as NANOG, OCT3/4 or a tissue stem cell factor KIT and its' ligand (24–30). The expressions of these markers have been linked to epigenetic regulation with DNA methylation and histone acetylation (30–34).
IGCCCG vol. 2 will bring a long-awaited update for risk stratification of treatment of GCT based on clinical characteristics. The advent of clinical biomarkers is rather slow since the original publication of the IGCCCG criteria. Several other risk assessment criteria were proposed that considered a more detailed look into clinical characteristics in GCT patients. Adra et al. published results of their retrospective analysis of 273 patients with a poor risk disease treated at Indiana University (35). Primary mediastinal non-seminoma (PMNSGCT), brain metastases and increasing age were significant predictors of mortality (HR = 4.63, 3.30, and 1.06, respectively). Multiple criteria for a poor risk disease carried a significantly worse prognosis compared to a single criterion (35).
Necchi et al. proposed an improved model for intermediate risk patients in the two-institutional initiative using PMNSGCT, brain metastases, pulmonary metastases, and age at diagnosis as risk factors. According to the results, a number of intermediate risk patients would suffice from treatment with BEPx3, whereas the current standard remains BEPx4 (11). While the refining of prognosis based on clinical criteria may have reached its limits, authors from Memorial Sloan Kettering Cancer Center have proposed a novel prognostic marker based on a marker decline after the first course of chemotherapy (36). Patients who had unfavorable (slower) marker decline after the initiation if chemotherapy had reportedly worse outcomes compared to patients with favorable marker decline (72 vs. 95% for 2-year overall survival; P < 0.01) (36). These findings were subsequently replicated in independent studies (37, 38).
Furthermore, the prognostic significance of tumor marker decline was reported also in patients with relapse (39–41). Fizazi et al. conducted a randomized phase III study in poor risk GCT. Patients receiving first cycle of BEP had an assessment of serum markers prior to second cycle and ones with an unfavorable decline were randomized to receive either remaining three cycles of standard BEP or dose-intensified chemotherapy regimen. Based on this biomarker-based strategy, a significant advantage was reported for 5-year progression-free survival (PFS) (60 vs. 48%, P = 0.037), but not for 5-year overall survival (OS) (70 vs. 61%, P = 0.012) (42, 43). Interestingly, in cases of progression, patients from this study relapsed predominantly in brain (54% of all relapses) (44).
Immunohistochemistry studies have started to emerge in recent years to supplement the clinical biomarkers in predicting the prognosis of GCT. The higher expression of DNA repair enzyme poly (ADP-ribose) polymerase (PARP) was reported in GCT tissue compared to normal testicular tissue. However, no association with clinical characteristics nor the survival difference was reported in regard to levels of expression (45).
Kalavska et al. published two studies examining the prognostic value of carbonic anhydrase nine assessed from plasma and from tumor tissue (46, 47). Levels of this marker of hypoxia and aggressive tumor behavior correlated in plasma and in tumor tissue. High expression in tumor was associated with shorter PFS; however, the clinically more useful utility of plasmatic assessment failed to be prognostic in GCT (46, 47). The hepatocyte growth factor (HGF) and its receptor c-MET were investigated by immunohistochemistry in tumors and in cell-line culture. c-MET is a known proto-oncogene involved in tumor progression and metastasis. Authors of this study reported an abundant immunohistochemical expression in both seminomas and non-seminomas, particularly in epithelial structures of well-differentiated subtypes such as teratomas, yolk sac tumors, and choriocarcinomas. Upon the activation of c-MET in an NT2 cell line (embryonal carcinoma), the cells acquired a more robust ability to proliferate, migrate, and invade. This may create the rationale for further research; however, the clinical significance of this finding is currently unknown (48).
The discovery of novel immune-related biomarkers, programmed-death receptor and its ligand (PD-1 and PD-L1) in various cancers, led to a confirmation of active PD-1/PD-L1 signaling also in GCT by Fankhauser et al. (49). The authors conducted an immunohistochemistry study and showed a frequent PD-L1 expression in 479 GCT tissue samples. Both seminomas and non-seminomas exhibited a significant expression of PD-L1 (in 73% and 64% of patients, respectively) (49).
Another research team led by Mardiak et al. performed a similar study and scored the PD-L1 expression semi-quantitatively with multiplicative quick score. The scores were correlated with clinical outcome. Patients with low levels of PD-L1 expression had significantly better PFS (HR = 0.40; P = 0.008) and OS (HR = 0.43; P = 0.040) (50). Furthermore, the expression of PD-L1 on tumor infiltrating lymphocytes (TIL) proved to be highly predictive of outcome in a reverse manner. Patients with high PD-L1 expression on TIL had significantly better prognosis than patients with low PD-L1 TIL (51). The prognostic significance of TIL was earlier reported by Bols et al., who also performed the phenotyping of immune-cell infiltrates (52). However, the abundant expression of PD-L1 does not seem to be predictive of response to treatment with immune-checkpoint inhibitors.
A phase II study with anti-PD1 agent pembrolizumab provided data about insufficient anti-tumor activity in refractory patients with GCT (53). While several case reports documented possible responses to immune-check point inhibitor, these are likely due to concomitant treatment with chemotherapy (54–56). Another phase II study with anti-PD-L1 agent avelumab is currently ongoing, which will shed more light on single agent immunotherapy in refractory GCT (NCT03403777).
Currently, there is a level of uncertainty in predicting response according to PD-L1 expression levels. While several cancer types have proven to be sensitive to PD-1/PD-L1 blockade based on PD-L1 expression, PD-L1 negative tumors were described to respond to such treatment as well. On the other hand, the expression of PD-L1 in tumor and TIL in GCT signifies a vivid immunogenic microenvironment but fails to respond to immunotherapy according to our present knowledge. As such, PD-1/PD-L1 axis seems to be only a part of the involved immune machinery and we are lacking a deeper understanding. Shen et al. recently published findings of comprehensive molecular characterization of GCT and did not discover a significant neoantigen signal in GCT, thus the insufficient activity of immune check-point inhibitors in GCT may be partly explained by this fact and the presence of very low mutational load (15).
Two independent studies published simultaneously examined the role of a simple marker of proinflammatory macroenvironment, a systemic-immune infiltration index (SII) (57, 58). SII is calculated from total counts of neutrophils, lymphocytes, and platelets. Fankhauser et al. reported numerous markers associated with poor prognosis in GCT, including low hemoglobin and albumin, high leukocytes, neutrophils, CRP, neutrophil to lymphocyte ratio, and SII (58). At the same time, our study showed that high SII was associated with poor prognosis in two independent cohorts of GCT patients. We also evaluated a combined prognostic value of SII and PD-L1 expression on TIL. As a result, we identified patients who never experienced death nor a relapse if they exhibited low SII and high PD-L1 on TIL (57). Both studies reported the prognostic significance of SII being independent from the standard IGCCCG risk criteria. SII can be easily calculated from complete blood count performed prior to treatment and offers a simple tool to predict outcome in metastatic GCT. Poor prognosis in patients exhibiting high levels of SII also suggests that proinflammatory pathways likely unleashed by an aggressive tumor microenvironment may point to an unsuccessful struggle of the host immune system to overcome the tumor growth. Furthermore, signaling of proinflammatory cytokines, such as IFN-α2, IL-2Rα, or IL-16, was reported to be associated with poor risk clinical characteristics and inferior survival in GCT patients (59).
Nilius et al. recently reported that high expression of β-1,4-galactosyltransferase-I (B4GALT1) in peripheral T-lymphocytes is a marker of lower risk of relapse in GCT patients treated with salvage high-dose chemotherapy and peripheral stem cell transplant (HR = 0.66; 95% CI 0.45–0.97; P = 0.02) (60). T-cells were collected before the high-dose chemotherapy using the non-myeloablative chemotherapy and granulocyte growth factor (60). B4GALT1 is important for interaction and adhesion of immune cells and its role in disease control in stage I lung cancer has been established (61). This study supported their hypothesis of the importance of activated peripheral T cells in in vitro experiments by lectin stimulation of mononuclear cells with Concavalin A. As a result, B4GALT1 was upregulated, particularly in CD4+ cells and an antiinflammatory cytokine IL10 was significantly expressed. Interestingly, higher levels of IL10 from patient T cells were also associated with better outcome in GCT (60). Activated T cells, thus, seem to play an important role in cancer control.
Sensitive and specific biomarkers indicating the presence of cancer that are assessed from peripheral blood represent an attractive and convenient approach in the diagnosis malignancies. Researchers recently published an array of articles showing that certain clusters of miR are highly informative of the presence of viable cancer in GCT patients (62–70). Serum examination for miR371-373 showed sensitivity of 98–100%, exceeding the sensitivity of the commonly used serum tumor markers AFP and HCG (71, 72). The targeted serum miRNA test (TSmiR) was developed and it seems to be very effective in predicting viable GCT after orchiectomy in clinical stage I patients or after chemotherapy in metastatic disease (72). The clinical utility of the TSmiR test is therefore very promising and clinicians may be expecting this novel biomarker to be implemented in the common practice in the near future (73). One possible utility of these highly sensitive miRNAs seems to be predicting the presence of a microscopic disease in clinical stage I GCTs. As such, these are likely to change the outlook over adjuvant treatment vs. surveillance. Another valuable input would be predicting the presence of viable cancer in post-chemotherapy residual masses, thus refining the need to perform often difficult surgeries in this setting. However, TSmiR does not identify teratoma components which still represent a diagnostic dilemma in the residual disease. Establishing the novel clinical practice stems from our ability to validate the utility of TSmiR in larger prospective cohorts of patients.
Majewski et al. assessed five patients with stage I seminoma and evaluated a possible role of liquid biopsy in identifying the presence of the tumor. The study showed promising results and identified candidate genes in whole blood prior to orchiectomy. This series is, however, too small to draw any conclusions and a larger study is suggested for validation (74).
A global DNA hypermethylation was proposed as one of the acting mechanisms in cisplatin resistance, the most frustrating challenge for oncologists treating GCT patients. In vitro epigenetic studies suggested that treatment with DNA demethylating agents may restore the sensitivity to cisplatin (75–77). In a study by Beyrouthy et al., a GCT cell-line treated with decitabine was resensitized to cisplatin (78). Based on these findings, Albany et al. performed a series of experiments in cell-line culture and patient-derived xenograft mouse model using a second-generation inhibitor of DNA-methyltransferase guadecitabine. Upon treatment of platinum resistant xenografts, a significant growth inhibition and even complete tumor regression was registered (79). An ongoing phase I trial using guadecitabine in combination with cisplatin in refractory GCT will shed more light on clinical significance of these promising findings (NCT02429466).
The investigation for biomarkers in testicular cancer has been insufficient in the past, but with emerging data our knowledge it is built up with an increasing consistency. Such consistency is essential to generate experimental data and perform laboratory research which will ultimately lead to development of novel drugs with a promise to overcome the resistance to cisplatin.
MC and LC contributed to conception and design. MC drafted the manuscript. CA, MM, RM, and AC contributed critical revision of the manuscript.
This work was supported by the Slovak Research and Development Agency under contract No. APVV-15-0086 and Scientific Grant Agency under contract number VEGA 1/0043/18 for MC.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Cheng L, Albers P, Berney DM, Feldman DR, Daugaard G, Gilligan T, et al. Testicular cancer. Nat Revs Dis Primers (2018) 4:29. doi: 10.1038/s41572-018-0029-0
2. Albany C, Adra N, Snavely AC, Cary C, Masterson TA, Foster RS. Multidisciplinary clinic approach improves overall survival outcomes of patients with metastatic germ-cell tumors. Ann Oncol. (2018) 29:341–6. doi: 10.1093/annonc/mdx731
3. Mardiak J, Sálek T, Sycová-Milá Z, Obertová J, Hlavatá Z, Mego M. Paclitaxel plus ifosfamide and cisplatin in second-line treatment of germ cell tumors: a phase II study. Neoplasma (2005) 52:497–501.
4. Kondagunta GV, Bacik J, Donadio A, Bajorin D, Marion S, Sheinfeld J. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. (2005) 23:6549–55. doi: 10.1200/JCO.2005.19.638
5. Adra N, Abonour R, Althouse SK, Albany C, Hanna NH, Einhorn LH. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: the indiana university experience. J Clin Oncol. (2017) 35:1096–102. doi: 10.1200/JCO.2016.69.5395
6. Warde P, Specht L, Horwich A, Oliver T, Panzarella T, Gospodarowicz M. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. (2002) 20:4448–52. doi: 10.1200/JCO.2002.01.038
7. Mortensen MS, Lauritsen J, Gundgaard MG, Agerbæk M, Holm NV, Christensen IJ. A nationwide cohort study of stage I seminoma patients followed on a surveillance program. Eur Urol. (2014) 66:1172–8. doi: 10.1016/j.eururo.2014.07.001
8. Albers P, Siener R, Kliesch S, Weissbach L, Krege S, Sparwasser C. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German testicular cancer study group trial. J Clin Oncol. (2003) 21:1505–12. doi: 10.1200/JCO.2003.07.169
9. Sweeney CJ, Hermans BP, Heilman DK, Foster RS, Donohue JP, Einhorn LH. Results and outcome of retroperitoneal lymph node dissection for clinical stage I embryonal carcinoma–predominant testis cancer. J Clin Oncol. (2000) 18:358–62. doi: 10.1200/JCO.2000.18.2.358
10. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International germ cell cancer collaborative group. J Clin Oncol. (1997) 15:594–603. doi: 10.1200/JCO.1997.15.2.594
11. Necchi A, Pond GR, Nicolai N, Giannatempo P, Raggi D, Adra N. A suggested prognostic reclassification of intermediate and poor-risk nonseminomatous germ cell tumors. Clin Genitourin Cancer (2017) 15:306–312 e303. doi: 10.1016/j.clgc.2016.07.022
12. Ku K, Ibrahim S, Adra N, Althouse S, Hanna NH, Einhorn LH, et al. A retrospective analysis of patients with metastatic germ cell tumor (GCT) treated at Indiana University (IU) from 2000 to 2012. J Clin Oncol. (2015) 33:4539. doi: 10.1200/jco.2015.33.15
13. Cheng L, Lopez-Beltran A, Massari F, MacLennan GT, Montironi R. Molecular testing for BRAF mutations to inform melanoma treatment decisions: a move toward precision medicine. Mod Pathol. (2018) 31:24–38. doi: 10.1038/modpathol.2017.104
14. Cheng L, Alexander RE, Maclennan GT, Cummings OW, Montironi R, Lopez-Beltran A. Molecular pathology of lung cancer: key to personalized medicine. Mod Pathol. (2012) 25:347–69. doi: 10.1038/modpathol.2011.215
15. Shen H, Shih J, Hollern DP, Wang L, Bowlby R, Tickoo SK. Integrated molecular characterization of testicular germ cell tumors. Cell Rep. (2018) 23:3392–406. doi: 10.1016/j.celrep.2018.05.039
16. McIntyre A, Summersgill B, Grygalewicz B, Gillis AJ, Stoop J, van Gurp RJ. Amplification and overexpression of the KIT gene is associated with progression in the seminoma subtype of testicular germ cell tumors of adolescents and adults. Cancer Res. (2005) 65:8085–9. doi: 10.1158/0008-5472.CAN-05-0471
17. Kemmer K, Corless CL, Fletcher JA, McGreevey L, Haley A, Griffith D. KIT mutations are common in testicular seminomas. Am J Pathol. (2004) 164:305–13. doi: 10.1016/S0002-9440(10)63120-3
18. Litchfield K, Summersgill B, Yost S, Sultana R, Labreche K, Dudakia D. Whole-exome sequencing reveals the mutational spectrum of testicular germ cell tumours. Nat Commun. (2015) 6:5973. doi: 10.1038/ncomms6973
19. Cheng L, Lyu B, Roth LM. Perspectives on testicular germ cell neoplasms. Hum Pathol. (2017) 59:10–25. doi: 10.1016/j.humpath.2016.08.002
20. Litchfield K, Levy M, Orlando G, Loveday C, Law PJ, Migliorini G. Identification of 19 new risk loci and potential regulatory mechanisms influencing susceptibility to testicular germ cell tumor. Nat Genet. (2017) 49:1133–40. doi: 10.1038/ng.3896
21. Liu J, Shi H, Li X, Chen G, Larsson C, Lui WO. miR2233p regulates cell growth and apoptosis via FBXW7 suggesting an oncogenic role in human testicular germ cell tumors. Int J Oncol. (2017) 50:356–64. doi: 10.3892/ijo.2016.3807
22. Kremenskoy M, Kremenska Y, Ohgane J, Hattori N, Tanaka S, Hashizume K. Genome-wide analysis of DNA methylation status of CpG islands in embryoid bodies, teratomas, and fetuses. Biochem Biophys Res Commun. (2003) 311:884–90. doi: 10.1016/j.bbrc.2003.10.078
23. Fukushima S, Yamashita S, Kobayashi H, Takami H, Fukuoka K, Nakamura T. Genome-wide methylation profiles in primary intracranial germ cell tumors indicate a primordial germ cell origin for germinomas. Acta Neuropathol. (2017) 133:445–62. doi: 10.1007/s00401-017-1673-2
24. Cheng L, Sung MT, Cossu-Rocca P, Jones TD, MacLennan GT, De Jong J. OCT4:biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. (2007) 211:1–9. doi: 10.1002/path.2105
25. Cheng L. Establishing a germ cell origin for metastatic tumors using OCT4 immunohistochemistry. Cancer (2004) 101:2006–10. doi: 10.1002/cncr.20566
26. Looijenga LH, Stoop H, de Leeuw HP, de Gouveia Brazao CA, Gillis AJ, van Roozendaal KE. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. (2003) 63:2244–50.
27. Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature (1988) 335:88–9. doi: 10.1038/335088a0
28. Huang E, Nocka K, Beier DR, Chu TY, Buck J, Lahm HW. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell (1990) 63:225–33. doi: 10.1016/0092-8674(90)90303-V
29. Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. (1987) 6:3341–51. doi: 10.1002/j.1460-2075.1987.tb02655.x
30. Mirabello L, Kratz CP, Savage SA, Greene MH. Promoter methylation of candidate genes associated with familial testicular cancer. Int J Mol Epidemiol Genet. (2012) 3:213–27.
31. Freberg CT, Dahl JA, Timoskainen S, Collas P. Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol Biol Cell (2007) 18:1543–53. doi: 10.1091/mbc.e07-01-0029
32. Almstrup K, Nielsen JE, Mlynarska O, Jansen MT, Jørgensen A, Skakkebæk NE. Carcinoma in situ testis displays permissive chromatin modifications similar to immature foetal germ cells. Br J Cancer (2010) 103:1269–76. doi: 10.1038/sj.bjc.6605880
33. Nettersheim D, Biermann K, Gillis AJ, Steger K, Looijenga LH, Schorle H. NANOG promoter methylation and expression correlation during normal and malignant human germ cell development. Epigenetics (2011) 6:114–22. doi: 10.4161/epi.6.1.13433
34. Villasante A, Piazzolla D, Li H, Gomez-Lopez G, Djabali M, Serrano M. Epigenetic regulation of Nanog expression by Ezh2 in pluripotent stem cells. Cell Cycle (2011) 10:1488–98. doi: 10.4161/cc.10.9.15658
35. Adra N, Althouse SK, Liu H, Brames MJ, Hanna NH, Einhorn LH. Prognostic factors in patients with poor-risk germ-cell tumors: a retrospective analysis of the Indiana University experience from 1990 to 2014. Ann Oncol. (2016) 27:875–9. doi: 10.1093/annonc/mdw045
36. Mazumdar M, Bajorin DF, Bacik J, Higgins G, Motzer RJ, Bosl GJ. Predicting outcome to chemotherapy in patients with germ cell tumors: the value of the rate of decline of human chorionic gonadotrophin and alpha-fetoprotein during therapy. J Clin Oncol. (2001) 19:2534–41. doi: 10.1200/JCO.2001.19.9.2534
37. Fizazi K, Culine S, Kramar A, Amato RJ, Bouzy J, Chen I. Early predicted time to normalization of tumor markers predicts outcome in poor-prognosis nonseminomatous germ cell tumors. J Clin Oncol. (2004) 22:3868–76. doi: 10.1200/JCO.2004.04.008
38. Motzer RJ, Nichols CJ, Margolin KA, Bacik J, Richardson PG, Vogelzang NJ. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol. (2007) 25:247–56. doi: 10.1200/JCO.2005.05.4528
39. Mego M, Rejlekova K, Reckova M, Sycova-Mila Z, Obertova J, Rajec J. Kinetics of tumor marker decline as an independent prognostic factor in patients with relapsed metastatic germ-cell tumors. Neoplasma (2009) 56:398–403. doi: 10.4149/neo_2009_05_398
40. Massard C, Kramar A, Beyer J, Hartmann JT, Lorch A, Pico JL. Tumor marker kinetics predict outcome in patients with relapsed disseminated non-seminomatous germ-cell tumors. Ann Oncol. (2013) 24:322–8. doi: 10.1093/annonc/mds504
41. Murphy BA, Motzer RJ, Mazumdar M, Vlamis V, Nisselbaum J, Bajorin D. Serum tumor marker decline is an early predictor of treatment outcome in germ cell tumor patients treated with cisplatin and ifosfamide salvage chemotherapy. Cancer (1994) 73:2520–6.
42. Fizazi K, Pagliaro L, Laplanche A, Fléchon A, Mardiak J, Geoffrois L. Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial. Lancet Oncol. (2014) 15:1442–50. doi: 10.1016/S1470-2045(14)70490-5
43. Fizazi K, Flechon A, Teuff GL, Mardiak J, Pagliaro LC, Geoffrois L, et al. Mature results of the GETUG 13 phase III trial in poor-prognosis germ-cell tumors (GCT). J Clin Oncol. (2016) 34:4504. doi: 10.1200/JCO.2016.34.15_suppl.4504
44. Loriot Y, Pagliaro L, Fléchon A, Mardiak J, Geoffrois L, Kerbrat P. Patterns of relapse in poor-prognosis germ-cell tumours in the GETUG 13 trial: Implications for assessment of brain metastases. Eur J Cancer (2017) 87:140–6. doi: 10.1016/j.ejca.2017.09.029
45. Mego M, Cierna Z, Svetlovska D, Macak D, Machalekova K, Miskovska V. PARP expression in germ cell tumours. J Clin Pathol. (2013) 66:607–12. doi: 10.1136/jclinpath-2012-201088
46. Kalavska K, Cierna Z, Chovanec M, Takacova M, Svetlovska D, Miskovska V. Prognostic value of intratumoral carbonic anhydrase IX expression in testicular germ cell tumors. Oncol Lett. (2017) 13:2177–85. doi: 10.3892/ol.2017.5745
47. Kalavska K, Chovanec M, Zatovicova M, Takacova M, Gronesova P, Svetlovska D. Prognostic value of serum carbonic anhydrase IX in testicular germ cell tumor patients. Oncol Lett. (2016) 12:2590–8. doi: 10.3892/ol.2016.5010
48. Scheri KC, Leonetti E, Laino L, Gigantino V, Gesualdi L, Grammatico P. c-MET receptor as potential biomarker and target molecule for malignant testicular germ cell tumors. Oncotarget (2018) 9:31842–31860. doi: 10.18632/oncotarget.25867
49. Fankhauser CD, Curioni-Fontecedro A, Allmann V, Beyer J, Tischler V, Sulser T. Frequent PD-L1 expression in testicular germ cell tumors. Br J Cancer (2015) 113:411–3. doi: 10.1038/bjc.2015.244
50. Cierna Z, Mego M, Miskovska V, Machalekova K, Chovanec M, Svetlovska D. Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann Oncol. (2016) 27:300–5. doi: 10.1093/annonc/mdv574
51. Chovanec M, Cierna Z, Miskovska V, Machalekova K, Svetlovska D, Kalavska K. Prognostic role of programmed-death ligand 1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular germ cell tumors. Oncotarget (2017) 8:21794–805. doi: 10.18632/oncotarget.15585
52. Bols B, Jensen L, Jensen A, Braendstrup O. Immunopathology of in situ seminoma. Int J Exp Pathol. (2000) 81:211–7. doi: 10.1046/j.1365-2613.2000.00151.x
53. Adra N, Einhorn LH, Althouse SK, Ammakkanavar NR, Musapatika D, Albany C. Phase II trial of pembrolizumab in patients with platinum refractory germ cell tumors: a hoosier cancer research network study GU14–206. Ann Oncol. (2017) 29:209–14. doi: 10.1093/annonc/mdx680
54. Zschäbitz S, Lasitschka F, Hadaschik B, Hofheinz RD, Jentsch-Ullrich K, Grüner M. Response to anti-programmed cell death protein-1 antibodies in men treated for platinum refractory germ cell cancer relapsed after high-dose chemotherapy and stem cell transplantation. Eur J Cancer (2017) 76:1–7. doi: 10.1016/j.ejca.2017.01.033
55. Zschabitz S, Lasitschka F, Jager D, Grullich C. Activity of immune checkpoint inhibition in platinum refractory germ-cell tumors. Ann Oncol. (2016) 27:1356–60. doi: 10.1093/annonc/mdw146
56. Shah S, Ward JE, Bao R, Hall CR, Brockstein BE, Luke JJ. Clinical response to anti-PD1 immunotherapy in a patient with non-seminomatous germ cell tumor and evaluation of the immune landscape in testicular cancer. J Clin Oncol. (2016) 34:e16040. doi: 10.1200/JCO.2016.34.15_suppl.e16040
57. Chovanec M, Cierna Z, Miskovska V, Machalekova K, Kalavska K, Rejlekova K, et al. Systemic immune-inflammation index in germ-cell tumours. Br J Cancer (2018) 118:831–8. doi: 10.1038/bjc.2017.460
58. Fankhauser CD, Sander S, Roth L, Gross O, Eberli D, Sulser T. Systemic inflammatory markers have independent prognostic value in patients with metastatic testicular germ cell tumours undergoing first-line chemotherapy. Br J Cancer (2018) 118:825–30. doi: 10.1038/bjc.2017.467
59. Svetlovska D, Miskovska V, Cholujova D, Gronesova P, Cingelova S, Chovanec M. Plasma cytokines correlated with disease characteristics, progression-free survival, and overall survival in testicular germ-cell tumor patients. Clin Genitourin Cancer (2017) 15:411–416 e412. doi: 10.1016/j.clgc.2017.01.027
60. Nilius V, Killer MC, Timmesfeld N, Schmitt M, Moll R, Lorch A. High beta-1,4-Galactosyltransferase-I expression in peripheral T-lymphocytes is associated with a low risk of relapse in germ-cell cancer patients receiving high-dose chemotherapy with autologous stem cell reinfusion. Oncoimmunology (2018) 7:e1423169. doi: 10.1080/2162402X.2017.1423169
61. Lu Y, Wang L, Liu P, Yang P, You M. Gene-expression signature predicts postoperative recurrence in stage I non-small cell lung cancer patients. PLoS ONE (2012) 7:e30880. doi: 10.1371/journal.pone.0030880
62. Voorhoeve PM, le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Adv Exp Med Biol. (2007) 604:17–46. doi: 10.1007/978-0-387-69116-9_2
63. Cheung HH, Davis AJ, Lee TL, Pang AL, Nagrani S, Rennert OM. Methylation of an intronic region regulates miR-199a in testicular tumor malignancy. Oncogene (2011) 30:3404–15. doi: 10.1038/onc.2011.60
64. Dieckmann KP, Spiekermann M, Balks T, Flor I, Löning T, Bullerdiek J. MicroRNAs miR-371–3 in serum as diagnostic tools in the management of testicular germ cell tumours. Br J Cancer (2012) 107:1754–60. doi: 10.1038/bjc.2012.469
65. Syring I, Bartels J, Holdenrieder S, Kristiansen G, Müller SC, Ellinger J. Circulating serum miRNA (miR-367–3p, miR-371a-3p, miR-372–3p and miR-373–3p) as biomarkers in patients with testicular germ cell cancer. J Urol (2015) 193:331–7. doi: 10.1016/j.juro.2014.07.010
66. Spiekermann M, Belge G, Winter N, Ikogho R, Balks T, Bullerdiek J. MicroRNA miR-371a-3p in serum of patients with germ cell tumours: evaluations for establishing a serum biomarker. Andrology (2015) 3:78–84. doi: 10.1111/j.2047-2927.2014.00269.x
67. Dieckmann KP, Spiekermann M, Balks T, Ikogho R, Anheuser P, Wosniok W. MicroRNA miR-371a-3p - a novel serum biomarker of testicular germ cell tumors: evidence for specificity from measurements in testicular vein blood and in neoplastic hydrocele fluid. Urol Int. (2016) 97:76–83. doi: 10.1159/000444303
68. Dieckmann KP, Radtke A, Spiekermann M, Balks T, Matthies C, Becker P, et al. Serum levels of MicroRNA miR-371a-3p: a sensitive and specific new biomarker for germ cell tumours. Eur Urol. (2017) 71:213–20. doi: 10.1016/j.eururo.2016.07.029
69. van Agthoven T, Eijkenboom WMH, Looijenga LHJ. microRNA-371a-3p as informative biomarker for the follow-up of testicular germ cell cancer patients. Cell Oncol. (2017) 40:379–88. doi: 10.1007/s13402-017-0333-9
70. Flor I, Spiekermann M, Löning T, Dieckmann KP, Belge G, Bullerdiek J. Expression of microRNAs of C19MC in different histological types of testicular germ cell tumour. Cancer Genom Proteom (2016) 13:281–9.
71. Gillis AJ, Rijlaarsdam MA, Eini R, Dorssers LC, Biermann K, Murray MJ. Targeted serum miRNA (TSmiR) test for diagnosis and follow-up of (testicular) germ cell cancer patients: a proof of principle. Mol Oncol. (2013) 7:1083–92. doi: 10.1016/j.molonc.2013.08.002
72. Nazario Leao RR, van Agthoven T, Figueiredo A, Jewett MAS, Fadaak K, Sweet J, et al. Serum miRNA to predict post-chemotherapy viable disease in testicular non-seminomatous germ cell tumor patients. J Clin Oncol. (2018) 36:abstr 546. doi: 10.1200/JCO.2018.36.6
73. Belge G, Dieckmann KP, Spiekermann M, Balks T, Bullerdiek J. Serum levels of microRNAs miR-371–3:a novel class of serum biomarkers for testicular germ cell tumors? Eur Urol. (2012) 61:1068–9. doi: 10.1016/j.eururo.2012.02.037
74. Majewski M, Nestler T, Kagler S, Richardsen I, Ruf CG, Matthies C, et al. Liquid biopsy using whole blood from testis tumor and colon cancer patients-a new and simple way? Health Phys. (2018) 115:114–20. doi: 10.1097/HP.0000000000000867
75. Koul S, Houldsworth J, Mansukhani MM, Donadio A, McKiernan JM, Reuter VE. Characteristic promoter hypermethylation signatures in male germ cell tumors. Mol Cancer (2002) 1:8. doi: 10.1186/1476-4598-1-8
76. Koul S, McKiernan JM, Narayan G, Houldsworth J, Bacik J, Dobrzynski DL. Role of promoter hypermethylation in Cisplatin treatment response of male germ cell tumors. Mol Cancer (2004) 3:16. doi: 10.1186/1476-4598-3-16
77. Honorio S, Agathanggelou A, Wernert N, Rothe M, Maher ER, Latif F. Frequent epigenetic inactivation of the RASSF1A tumour suppressor gene in testicular tumours and distinct methylation profiles of seminoma and nonseminoma testicular germ cell tumours. Oncogene (2003) 22:461–6. doi: 10.1038/sj.onc.1206119
78. Beyrouthy MJ, Garner KM, Hever MP, Freemantle SJ, Eastman A, Dmitrovsky E. High DNA methyltransferase 3B expression mediates 5-aza-deoxycytidine hypersensitivity in testicular germ cell tumors. Cancer Res. (2009) 69:9360–6. doi: 10.1158/0008-5472.CAN-09-1490
Keywords: testis, testicular germ cell tumors, molecular genetics, biomarkers, liquid biopsy
Citation: Chovanec M, Albany C, Mego M, Montironi R, Cimadamore A and Cheng L (2018) Emerging Prognostic Biomarkers in Testicular Germ Cell Tumors: Looking Beyond Established Practice. Front. Oncol. 8:571. doi: 10.3389/fonc.2018.00571
Received: 12 September 2018; Accepted: 14 November 2018;
Published: 28 November 2018.
Edited by:
Fabio Grizzi, Humanitas Research Hospital, ItalyReviewed by:
Riccardo Autorino, Virginia Commonwealth University, United StatesCopyright © 2018 Chovanec, Albany, Mego, Montironi, Cimadamore and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Cheng, bGlhbmdfY2hlbmdAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.