- 1Division of Radiation Oncology, Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 2Division of Diagnostic Imaging, Department of Nuclear Medicine, University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 3Division of Diagnostic Imaging, Department of Diagnostic Radiology, University of Texas MD Anderson Cancer Center, Houston, TX, United States
We hereby report the case of a patient with optic nerve sheath meningioma (ONSM), whose diagnosis and multidisciplinary management was guided by the use of Gallium-68 (68Ga)-labeled dodecanetetraacetic acid-tyrosine-3-octreotate (DOTATATE) positron emission tomography (PET)/computed tomography (CT) scan. We briefly review the diagnosis and management of ONSM, and review the literature on the role and current status of nuclear imaging with somatostatin receptor ligands in the non-invasive diagnosis and management of meningiomas.
Introduction
Orbital space-occupying lesions include a wide variety of benign and malignant diseases, including orbital inflammatory pseudotumor, optic nerve sheath meningioma (ONSM), orbital metastases, orbital lymphoma, and optic pathway gliomas (1). ONSMs represent 2% of orbital tumors (2, 3) and 1–2% of all intracranial meningiomas (3). Different diagnostic strategies have been employed to aid in the diagnosis of orbital tumors. The main imaging modalities used currently to aid in diagnosis include contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) (4). However, in certain circumstances, these imaging modalities are not sufficient to make a definitive diagnosis, and alternative methods are needed to avoid an invasive open orbital biopsy.
This is one of the few reported cases on the use of Gallium-68 (68Ga)-labeled dodecanetetraacetic acid-tyrosine-3-octreotate (DOTATATE) positron emission tomography (PET)/CT scan for non-invasive diagnosis of an orbital space-occupying tumor.
Case Presentation
A 28-year old female presented with a history of progressive left-sided temporal vision loss over a year. She noticed that she was running into objects and people on the left side of her field of vision. The patient also complained of a dull ache in her left eye but denied any other focal neurological symptoms.
On physical examination, the only pertinent finding was left temporal hemianopia.
MRI of the orbits with contrast revealed a heterogeneously enhancing large mass lesion occupying the mid- and posterior thirds of the optic nerve pathway. The typical “tram-track” appearance of sheath enhancement around the central optic nerve expected for an optic nerve meningioma was absent. Rather, the lesion essentially replaced the optic nerve and appeared to demonstrate infiltration into the nerve, which raised the suspicion for an optic nerve glioma (Figure 1). The lesion extended through the optic canal with a component extending superiorly onto the left side of the planum sphenoidale rather than remaining intrinsic to the nerve, as it would be expected for an optic nerve glioma. By virtue of dural involvement of the planum sphenoidale, this mass was suggestive of an atypical left ONSM, which had on some images apparently replaced the nerve (Figure 1). Given this unusual clinical presentation and the lack of typical findings on MRI, decision was taken in a multidisciplinary tumor board to proceed with a 68Ga-DOTATATE PET/CT scan, particularly to rule out an optic nerve glioma. Biopsy was deemed too morbid in this context.
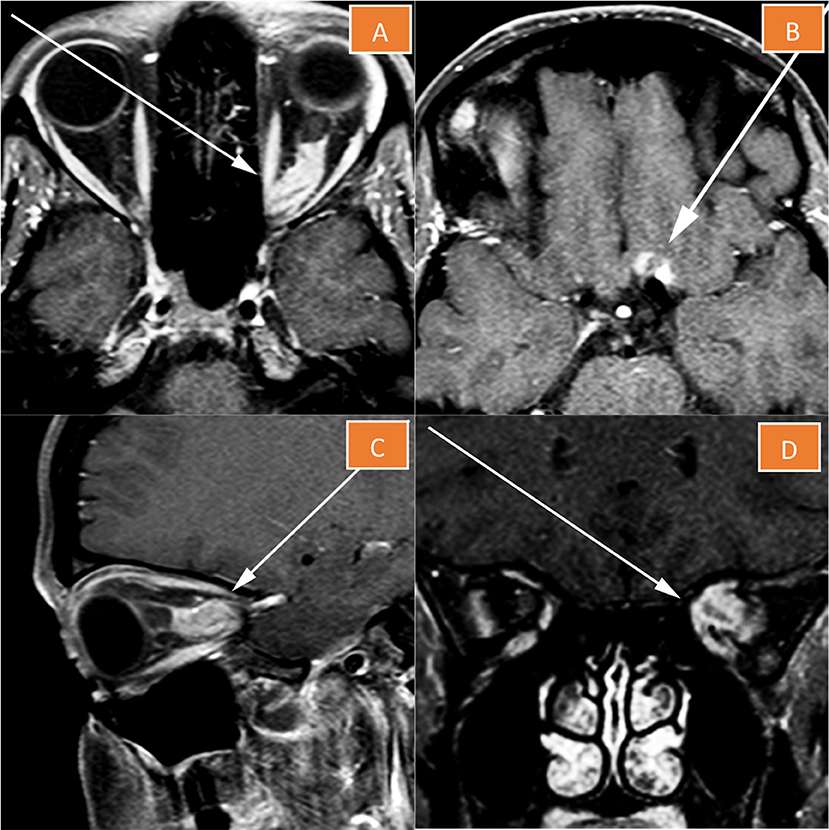
Figure 1. Magnetic resonance imaging of the left orbit. The lesion essentially replaces the optic nerve (A,C,D), and has a component extending superiorly onto the left side of the planum sphenoidale (B).
68Ga-DOTATATE PET/CT scan revealed an asymmetric fusiform enlargement of the left optic nerve with associated conspicuous diffuse radiotracer uptake and maximum standardized uptake value (SUVmax) of 10.8. Portions of the lesion showed increased attenuation on non-contrast CT, suggesting calcification. There was a nearby but separate focus of activity more superoposteriorly, which localized to the left aspect of the planum sphenoidale (Figure 2). The combination of anatomic and metabolic findings was compatible with an optic sheath meningioma, as an optic nerve glioma, similarly to a pilocytic astrocytoma, would not be expected to demonstrate significant uptake on 68Ga-DOTATATE PET (5).
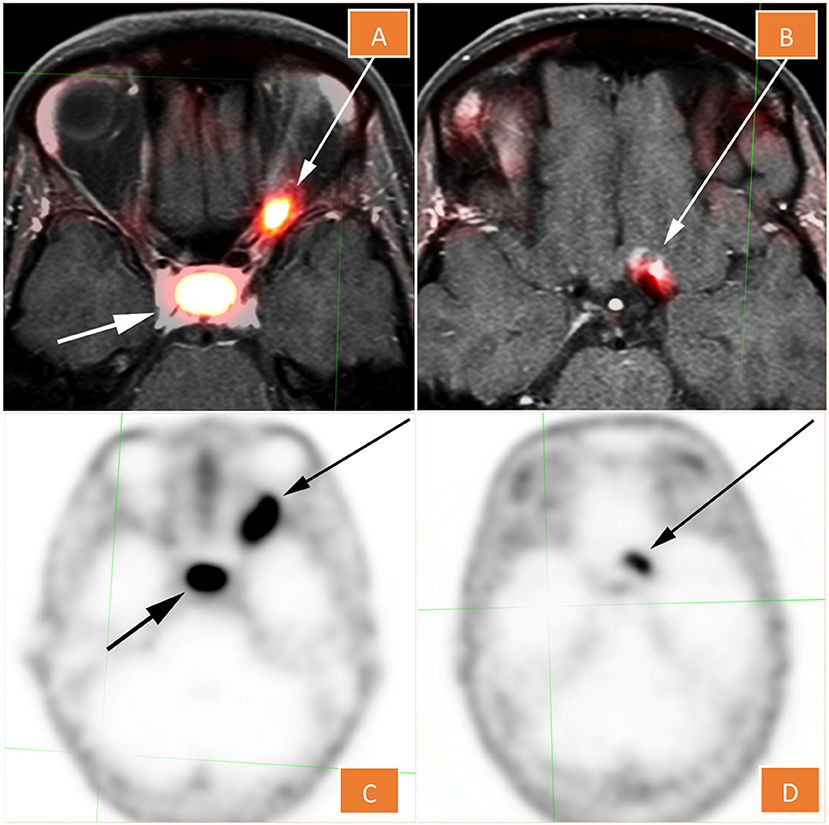
Figure 2. 68Ga -DOTATATE PET (C,D), fused with MRI in (A,D). The lesion shows conspicuous diffuse radiotracer uptake and maximum standardized uptake value of 10.8. There is a nearby but separate focus that localizes to the left aspect of the planum sphenoidale (B,D).
Based on this non-invasive diagnosis, volumetric-modulated arc therapy (VMAT) to a total dose of 50.4 Gray (Gy) in 28 fractions was delivered. A VMAT radiation plan (Figure 3A) was chosen over a proton therapy plan (Figure 3B) because of improved dose conformity and target coverage in the former. The patient had no major complications. She developed Common Terminology for Adverse events (CTCAE) Grade 1 fatigue and Grade 1 headaches during treatment. Five months after treatment completion, the patient had significant improvement in her left temporal hemianopia based on subjective report and on objective assessment through a formal visual field examination performed by her ophthalmologist. The meningioma on the 2- and 5-month follow-up MRIs was found to be less enhancing and its size was slightly decreased to stable (Figure 4), indicating good local control.
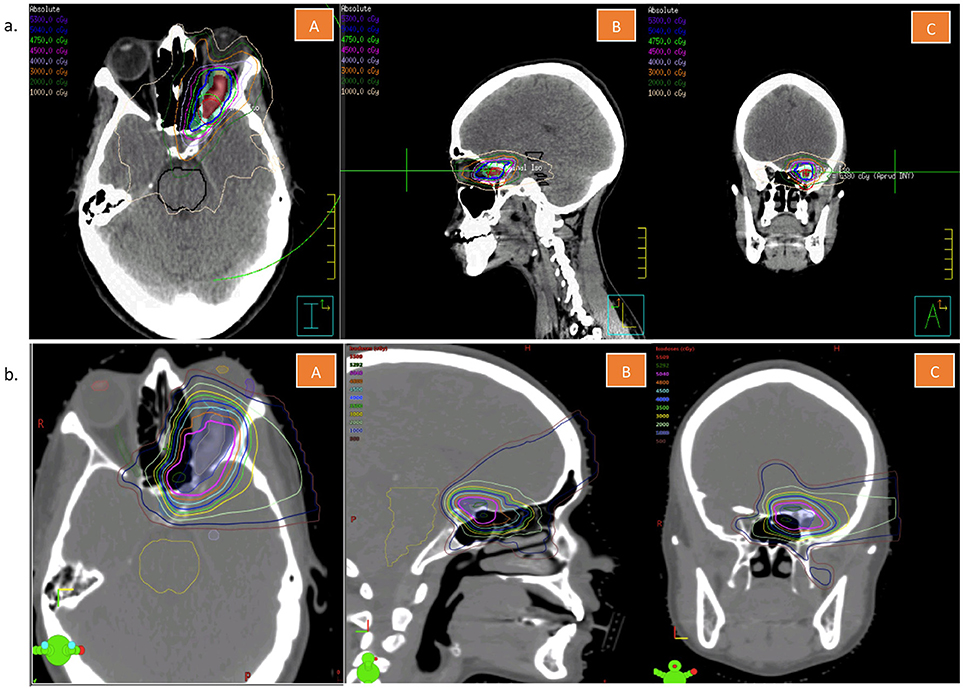
Figure 3. (a) Volumetric arc treatment plan of the patient described in this case report. This figure is showing representative cuts in the transverse (A), sagittal (B), and coronal (C) planes. (b) Tentative proton treatment plan of the patient described in this case report. This figure is showing representative cuts in the transverse (A), sagittal (B), and coronal (C) planes.
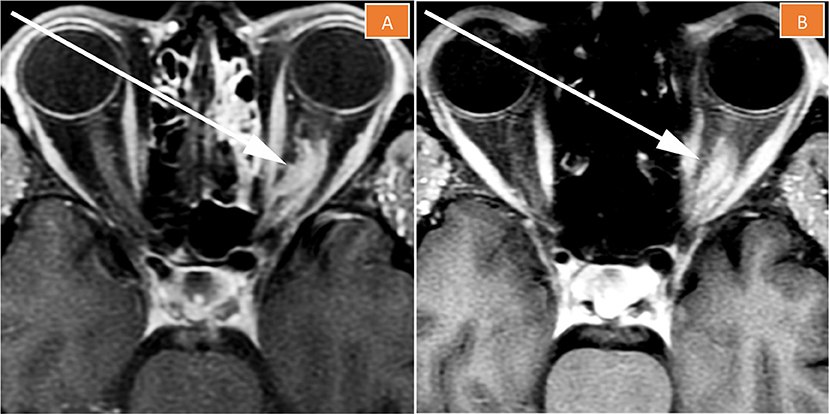
Figure 4. Magnetic resonance imaging of the left orbit 2 months (A) and 5 months (B) after treatment completion. The tumor is less enhancing than pre-treatment, its size is stable to slightly decreased, and “tram-tracking” is more apparent.
Discussion
ONSM: Diagnosis and Management
MRI and CT are currently the most commonly used imaging modalities in the workup of orbital space-occupying lesions (4). In a study by Sepahdari et al. evaluating 47 patients with orbital lesions, diffusion-weighted (DW) imaging was used to detect malignant lesions with a sensitivity of 63%, a specificity of up to 90%, and an accuracy of 81% (1). The incorporation of DWI to conventional MR imaging has helped improve radiological diagnoses and characterization of indeterminate orbital lesions. For the diagnosis of ONSM, MRI is considered the gold standard for determining a radiological diagnosis (6). Typically, ONSM displays intense enhancement with gadolinium on T1 fat-suppressed MR images of the orbit, and is surrounding a non-enhancing optic nerve (negative defect), creating the classic “tram-track” sign (7, 8). When the MR characteristics do not show all the hallmark features, the detection of calcification on CT can provide complementary imaging evidence to support the diagnosis of ONSM as calcification is better demonstrated on CT than MRI (9).
In certain instances, as in our case report, MRI and CT scan may not provide sufficient diagnostic information to differentiate ONSM from other optic tumors, such as optic pathway gliomas. An open orbital biopsy can provide definitive pathological diagnosis, but this procedure is associated with significant risks, including postoperative diplopia, orbital hemorrhage, and symblepharon (10). Albeit rare, these potential risks are compelling physicians to find an alternative non-invasive diagnostic modality for accurately identifying these tumors based on clinical information and imaging. We however acknowledge that, short of the gold standard of biopsy, the diagnosis of optic meningioma could not be confirmed with absolute certainty for the patient presented in this case report.
Role of 68Ga-DOTATATE PET/CT and 68Ga-DOTATOC PET/CT in the Diagnosis and Management of ONSM
Meningioma cells have been shown to strongly express somatostatin receptor subtype 2 (SSTR2) (11), unlike pilocytic astrocytomas (including optic pathway gliomas) (5). This characteristic of meningioma cells is the basis of using molecular imaging with somatostatin receptor ligands, which is being proposed as an excellent tool to discriminate between these two medical entities.
The use of somatostatin receptor ligands in meningiomas, and even ONSM, is not novel. Some studies used 111Indium (In)-octreotide scintigraphy (OctreoscanTM) to differentiate meningiomas from other dural-based tumors, increasing the diagnostic accuracy of MRI (12), and others used this same technique, as a complement to serial MRIs, to follow-up ONSMs after treatment completion (13). However, OctreoscanTM was found to lack specificity (12). More recently, two somatostatin analogs, DOTA (0)-D-Phe (1)-Tyr (3)-Octreotide (DOTATOC) and DOTATATE, were radiolabeled with 68Ga to be used in 68Ga-DOTATOC PET/CT and 68Ga-DOTATATE PET/CT. They have been found to have better lesion detectability and spatial resolution as compared to 111In-labeled peptides (14, 15), with significantly lower radiation exposure (16). Both DOTATOC and DOTATATE have been shown to have similar diagnostic accuracy in the setting of neuroendocrine tumors, with marginal superiority of DOTATOC, which shows higher tumor uptake (17, 18); that being said, they have not yet been compared in the setting of meningiomas.
A study by Rachinger et al. compared the diagnostic accuracy of 68Ga-DOTATATE PET/CT and MRI in 21 patients with primary or recurrent meningiomas. The former was shown to delineate meningioma from normal tissue better than the latter. Sensitivity to detect tumor tissue was 90.1% for 68Ga-DOTATATE PET/CT vs. 79% for MRI both in the primary and recurrent settings. Specificities (73 vs. 63%) and positive predictive values (89 vs. 84%) were, however, similar between the two imaging modalities (19).
A study by Klingenstein et al. on 13 patients with ambiguous lesions of the anterior optic pathway demonstrated excellent sensitivity (100%) and specificity (100%) for 68Ga-DOTATATE PET/CT. It allowed differentiation of meningioma (10 patients) from other entities (intracerebral metastasis, inflammatory connective tissue disease, and leukemic infiltrate), as meningiomas demonstrated high 68Ga-DOTATATE uptake (mean SUVmax = 14.3 ± 15.4), whereas the other three lesions did not (SUVmax = 2.1 ± 1.0) (20). The sample size of that study is too small to draw meaningful conclusions, but it is rather hypothesis-generating. In our case report, we are providing fused 68Ga-DOTATATE PET-MR imaging, not provided in Klingenstein's case series, thus allowing direct correlation between the anatomical and metabolic information from 68Ga-DOTATATE-PET and MRI, respectively.
Despite the fact that ONSM are indolent benign tumors, they can eventually lead to proptosis, progressive loss of visual acuity, and if left untreated, to blindness (3, 21). The optimal timing of intervention has not yet been fully elucidated, and observation with strict regular follow-up visits is considered a reasonable option as a first step if the patient has no or minimal visual symptoms (22, 23). Some experts have advocated that treatment becomes warranted when a new decline in acuity and/or visual fields has been documented (24), but the highly variable and unpredictable natural history of ONSM makes that strategy sometimes risky. Beyond its strength in diagnosis and discriminating between meningiomas and other tumors, 68Ga-DOTATATE PET/CT has shown the potential to predict tumor growth rate. Higher expression of SSTR2 resulting in higher SUVmax on 68Ga-DOTATATE PET imaging has been shown to predict for faster growth rates in World Health Organization (WHO) grade I and II meningiomas (25). As such, this imaging modality may provide guidance with respect to the initiation of therapy.
Treatment options include surgery in rare instances, and radiation therapy in most scenarios. Because of significant functional morbidity, surgery for ONSM has fallen out of favor, except in cases of rapid visual decline in which quick surgical decompression is needed, or in case of intracranial extension (26, 27). Stereotactic fractionated radiation therapy (RT) is now recommended as the treatment of choice in ONSM to stop tumor growth and preserve vision (28, 29). A retrospective study by Turbin et al. comparing observation, surgery, RT, or surgery and RT, showed that RT alone was associated with half the complication rates of surgery (33.3 vs. 66.7%), and with better visual preservation outcomes (30). RT for ONSM consists of 50.4–54 Gy at 1.8 Gy per fraction, typically using three-dimensional (3D) conformal plans, or intensity modulated radiation therapy (IMRT) plans (28, 31–34). Plans with VMAT as used in our case might allow for more conformal dose distribution as well as better tumor coverage and normal tissue sparing than conventional static field IMRT, while also allowing for reduced treatment delivery time (35). The use of proton therapy for ONSM is not well documented in the literature, but seems to offer comparable local control and vision preservation rates as photon radiation (28, 36). Proton plans may include the use of passive scatter with collimators or pencil beam scanning with intensity modulated proton therapy (IMPT). There may be range uncertainty with the use of protons, which can be an important consideration when evaluating treatment plans involving critical structures such as the optic nerves (37). Secondly, the relative biological effectiveness (RBE) may also be higher with protons, which is estimated to ~1.1 within a wide range of values (38). For the particular case and anatomy, our group favored VMAT/IMRT for the conformity and uniformity in tumor coverage it provided while maintaining the dose below organ-at-risk tolerance. In addition to its diagnostic utility, there is potential for 68Ga-DOTATOC PET to serve as a complementary modality to MRI (39, 40) in terms of tumor delineation for radiotherapy.
Conclusion
Imaging with somatostatin receptor ligands seems to be a valuable tool in the non-invasive diagnosis of orbital space-occupying lesions. Further studies are needed to better establish the potential applications of 68Ga-DOTATATE PET/CT and 68Ga-DOTATOC PET/CT in the diagnosis, management, and follow-up of ONSMs, and in other intracranial meningioma types.
Ethics Statement
The patient described in this case report consented to have her case described in this manuscript. Written informed consent was obtained from the patient for publication of this case report. The patient's personal identifiers were not included in this manuscript.
Author Contributions
KA, CC, DY, and JJ contributed to the conception and design of the work. BC, MG, and JJ assembled and interpreted the patient's imaging data. KA and CC wrote the first draft of the manuscript. All authors contributed to manuscript revision, and provided approval for publication of the content.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sepahdari AR, Aakalu VK, Setabutr P, Shiehmorteza M, Naheedy JH, Mafee MF. Indeterminate orbital masses: restricted diffusion at MR imaging with echo-planar diffusion-weighted imaging predicts malignancy. Radiology (2010) 256:554–64. doi: 10.1148/radiol.10091956
2. Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 montgomery lecture, part 1. Ophthalmology (2004) 111:997–1008. doi: 10.1016/j.ophtha.2003.01.002
4. Goh PS, Gi MT, Charlton A, Tan C, Gangadhara Sundar JK, et al. Review of orbital imaging. Eur J Radiol. (2008) 66:387–95. doi: 10.1016/j.ejrad.2008.03.031
5. Kiviniemi A, Gardberg M, Kivinen K, Posti JP, Vuorinen V, Sipilä J, et al. Somatostatin receptor 2A in gliomas: association with oligodendrogliomas and favourable outcome. Oncotarget (2017) 8:49123–32. doi: 10.18632/oncotarget.17097
6. Mafee MF, Goodwin J, Dorodi S. Optic nerve sheath meningiomas: role of MR imaging. Radiol Clin North Am. (1999) 37:37–58. doi: 10.1016/S0033-8389(05)70077-4
7. Kanamalla US. The optic nerve tram-track sign. Radiology (2003) 227:718–9. doi: 10.1148/radiol.2273010758
9. Saeed P, Rootman J, Nugent RA, White VA, Mackenzie IR, Koornneef L. Optic nerve sheath meningiomas. Ophthalmology (2003) 110:2019–30. doi: 10.1016/S0161-6420(03)00787-5
10. Ting DSJ, Perez-Lopez M, Chew NJ, Clarke L, Dickinson AJ, Neoh C. A 10-year review of orbital biopsy: the newcastle eye centre study. Eye (2015) 29:1162–6. doi: 10.1038/eye.2015.95
11. Dutour A, Kumar U, Panetta R, Ouafik L, Fina F, Sasi R, et al. Expression of somatostatin receptor subtypes in human brain tumors. Int J Cancer (1998) 76:620–7.
12. Nathoo N, Ugokwe K, Chang AS, Li L, Ross J, Suh JH, et al. The role of111indium-octreotide brain scintigraphy in the diagnosis of cranial, dural-based meningiomas. J Neurooncol. (2007) 81:167–74. doi: 10.1007/s11060-006-9210-5
13. Andrews DW, Faroozan R, Yang BP, Hudes RS, Werner-Wasik M, Kim SM, et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery (2002) 51:890–4. doi: 10.1016/S0002-9394(03)00042-4
14. Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging (2007) 34:982–93. doi: 10.1007/s00259-006-0317-x
15. Hofmann M, Maecke H, Börner A, Weckesser E, Schöffski P, Oei M, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: preliminary data. Eur J Nucl Med. (2001) 28:1751–7. doi: 10.1007/s002590100639
16. Walker RC, Smith GT, Liu E, Moore B, Clanton J, Stabin M. Measured human dosimetry of 68Ga-DOTATATE. J Nucl Med. (2013) 54:855–60. doi: 10.2967/jnumed.112.114165
17. Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. (2011) 52:1864–70. doi: 10.2967/jnumed.111.091165
18. Johnbeck CB, Knigge U, Loft A, Berthelsen AK, Mortensen J, Oturai P, et al. Head-to-Head Comparison of 64 Cu-DOTATATE and 68 Ga-DOTATOC PET/CT: a prospective study of 59 patients with neuroendocrine tumors. J Nucl Med. (2017) 58:451–7. doi: 10.2967/jnumed.116.180430
19. Rachinger W, Stoecklein VM, Terpolilli NA, Haug AR, Ertl L, Poschl J, et al. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. (2015) 56:347–53. doi: 10.2967/jnumed.114.149120
20. Klingenstein A, Haug AR, Miller C, Hintschich C. Ga-68-DOTA-TATE PET/CT for discrimination of tumors of the optic pathway. Orbit (2015) 34:16–22. doi: 10.3109/01676830.2014.959185
21. Shapey J, Sabin HI, Danesh-Meyer HV, Kaye AH. Diagnosis and management of optic nerve sheath meningiomas. J Clin Neurosci. (2013) 20:1045–56. doi: 10.1016/j.jocn.2013.03.008
22. Kennerdell JS, Maroon JC, Malton M, Warren FA. The management of optic nerve sheath meningiomas. Am J Ophthalmol. (1988) 106:450–7.
23. Kim JW, Rizzo JF, Lessell S. Controversies in the management of optic nerve sheath meningiomas. Int Ophthalmol Clin. (2005) 45:15–23. doi: 10.1097/01.iio.0000176367.16758.f4
24. Egan RA, Lessell S. A contribution ot the natural history of optic nerve sheath meningiomas. Arch Ophthalmol. (2002) 120:1505–8. doi: 10.1001/archopht.120.11.1505
25. Sommerauer M, Burkhardt JK, Frontzek K, Rushing E, Buck A, Krayenbuehl N, et al. 68Gallium-DOTATATE PET in meningioma: a reliable predictor of tumor growth rate? Neuro Oncol. (2016) 18:1021–7. doi: 10.1093/neuonc/now001
26. Roser F, Nakamura M, Martini-Thomas R, Samii M, Tatagiba M. The role of surgery in meningiomas involving the optic nerve sheath. Clin Neurol Neurosurg. (2006) 108:470–6. doi: 10.1016/j.clineuro.2005.08.001
27. Cristante L. Surgical treatment of meningiomas of the orbit and optic canal: a retrospective study with particular attention to the visual outcome. Acta Neurochir. (1994) 126:27–32. doi: 10.1007/BF01476490
28. Arvold ND, Lessell S, Bussiere M, Beaudette K, Rizzo JF, Loeffler JS, et al. Visual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. (2009) 75:1166–72. doi: 10.1016/j.ijrobp.2008.12.056
29. Bloch O, Sun M, Kaur G, Barani IJ, Parsa AT. Fractionated radiotherapy for optic nerve sheath meningiomas. J Clin Neurosci. (2012) 19:1210–5. doi: 10.1016/j.jocn.2012.02.010
30. Turbin RE, Thompson CR, Kennerdell JS, Cockerham KP, Kupersmith MJ. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapya aNo author has any commercial interest in any of the opinions expressed in this article. Ophthalmology (2002) 109:890–9. doi: 10.1016/S0161-6420(02)01017-5
31. Brower JV, Amdur RJ, Kirwan J, Mendenhall WM, Friedman W. Radiation therapy for optic nerve sheath meningioma. Pract Radiat Oncol. (2013) 3:223–8. doi: 10.1016/j.prro.2012.06.010
32. Liu JK, Forman S, Hershewe GL, Moorthy CR, Benzil DL. Optic nerve sheath meningiomas: visual improvement after stereotactic radiotherapy. Neurosurgery (2002) 50:950–7. doi: 10.1097/00006123-200205000-00006
33. Narayan S, Cornblath WT, Sandler HM, Elner V, Hayman JA. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. (2003) 56:537–43. doi: 10.1016/S0360-3016(02)03483-1
34. Metellus P, Kapoor S, Kharkar S, Batra S, Jackson JF, Kleinberg L, et al. Fractionated conformal radiotherapy for management of optic nerve sheath meningiomas: long-term outcomes of tumor control and visual function at a single institution. Int J Radiat Oncol Biol Phys. (2011) 80:185–92. doi: 10.1016/j.ijrobp.2010.01.034
35. Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. (2011) 84:967–96. doi: 10.1259/bjr/22373346
36. Moyal L, Vignal-Clermont C, Boissonnet H, Alapetite C. [Results of fractionated targeted proton beam therapy in the treatment of primary optic nerve sheath meningioma]. J Fr Ophtalmol. (2014) 37:288–95. doi: 10.1016/j.jfo.2013.09.006
37. Zheng MH, Sun HT, Xu JG, Yang G, Huo LM, Zhang P, et al. Combining whole-brain radiotherapy with Gefitinib/Erlotinib for brain metastases from non-small-cell lung cancer: a meta-analysis. Biomed Res Int. (2016) 2016:5807346. doi: 10.1155/2016/5807346
38. Paganetti H, Niemierko A, Ancukiewicz M, Gerweck LE, Goitein M, Loeffler JS, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. (2002) 53:407–21. doi: 10.1088/0031-9155/59/22/R419
39. Gehler B, Paulsen F, Öksüz MT, Hauser TK, Eschmann SM, Bares R, et al. [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat Oncol. (2009) 4:1–8. doi: 10.1186/1748-717X-4-56
40. Milker-Zabel S, Zabel-du Bois A, Henze M, Huber P, Schulz-Ertner D, Hoess A, et al. Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int J Radiat Oncol Biol Phys. (2006) 65:222–7. doi: 10.1016/j.ijrobp.2005.12.006
Keywords: optic nerve sheath meningioma positron-emission tomography and computed tomography, 68Ga-DOTATATE PET/CT, orbital neoplasms, meningioma, somatostatin (analogs and derivatives)
Citation: Al Feghali KA, Yeboa DN, Chasen B, Gule MK, Johnson JM and Chung C (2018) The Use of 68Ga-DOTATATE PET/CT in the Non-invasive Diagnosis of Optic Nerve Sheath Meningioma: A Case Report Front. Oncol. 8:454. doi: 10.3389/fonc.2018.00454
Received: 03 August 2018; Accepted: 26 September 2018;
Published: 16 October 2018.
Edited by:
Youssef Zeidan, American University of Beirut, LebanonReviewed by:
Osama Mohamad, University of Texas Southwestern Medical Center, United StatesPaul Stephen Rava, UMass Memorial Medical Center, United States
Copyright © 2018 Al Feghali, Yeboa, Chasen, Gule, Johnson and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline Chung, CChung3@mdanderson.org
 Karine A. Al Feghali
Karine A. Al Feghali