- Department of Pathology, Kanagawa Cancer Center, Kanagawa, Japan
Background: Although gangliocytic paraganglioma (GP) is considered a rare benign neuroendocrine tumor, cases of mortality have been reported. Occasionally, GP is misdiagnosed as neuroendocrine tumor G1, which is associated with a poorer prognosis than GP. To avoid such misdiagnoses, it is important to understand the clinicopathological characteristics of GP. Thus, herein, we discuss the current literature on the clinicopathological characteristics of GP.
Methods: We conducted a systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. PubMed and Japana Centra Revuo Medicina searches were used to identify papers describing GP. Inclusion criteria included confirmation of epithelioid, spindle-shaped, and ganglion-like cells in the main article and/or figures and whether the paper was cited in other studies of GP. Data were collected on age, sex, site of the primary lesion, tumor size, treatment, prognosis, lymph node metastasis (LNM), depth of tumor invasion, rate of preoperative diagnosis, and clinical symptoms.
Results: In total, 162 papers containing 263 cases of GP met the criteria. The mean age at diagnosis was 53.5 years. The male-to-female ratio was 157:104. The mean tumor size was 25.7 mm. The predominant site of the primary tumor was the duodenum (89.7%). The most common clinical sign of GP was gastrointestinal bleeding (47.9%). Other signs and symptoms of GP included abdominal pain (44.7%), anemia (20.3%), incidental findings (12.9%), nausea (6.9%), weight loss (5.5%), general fatigue (5.1%), jaundice (4.6%), and incidental autopsy findings (5.1%). LNM was observed in 11.4% of patients. Liver metastasis was observed in 1.1% of patients. Depth of tumor invasion (penetrating beyond the submucosal layer or sphincter of Oddi) was by far the most significant risk factor for LNM in patients with GP. This suggests, along with histological heterogeneity, that GP may have hamartomatous characteristics. Furthermore, immunohistochemical expression of progesterone receptor and pancreatic polypeptide were useful in distinguishing between GP and neuroendocrine tumor G1, even in small biopsy specimens.
Conclusions: We reveal the clinicopathological characteristics of GP, including risk factors for LNM, differential diagnostic approaches, and improvements in the clinical management of this tumor.In addition, GP may have hamartomatous characteristics.
Introduction
Rationale
Gangliocytic paraganglioma (GP) is considered a rare neuroendocrine tumor (NET) that typically arises in the second part of the duodenum (1). Although a few investigators have reported cases of liver metastasis (2–4) and, in one case, a fatal duodenal GP (4), this tumor is usually associated with an excellent prognosis (5). The first case report was published in 1957 (6). Two hundred and sixty-three cases have been reported to date. It is noteworthy that in our previous literature survey in 2011, we identified 192 cases of GP (7). Therefore, 71 new cases have been reported in the last seven years, suggesting that, more recently, GP may be attracting more attention from oncologists. However, GP is often misdiagnosed as NET G1 (8), despite their prognostic differences (GP is associated with a better prognosis) (9). In addition, few studies have reported on the epidemiological and clinicopathological characteristics of GP due to its rarity. Thus, we performed this systematic review.
Objectives
We wish to provide up-to-date information on the above mentioned topics by conducting a systematic review to help oncologists gain better insights into the diagnosis and clinical management of this rare NET.
Research Question
We aimed to determine more detailed clinicopathological characteristics of GP, including risk factors for lymph node metastasis (LNM), differential diagnostic approaches (e.g., immunohistochemical staining to differentiate GP from NET G1), and improvements in the clinical management of this tumor.
Methods
Study Design
A literature survey was performed on February 24, 2018 in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (10).
Participants, Interventions, Comparators
This systematic review involved a cumulative case series. Therefore, timing and effect measures are based on individual case reports/series.
Systematic Review Protocol
The primary outcome of this systematic review was obtaining data on age, sex, site of the primary lesion, tumor size, treatment, prognosis, presence of LNM, depth of tumor invasion, rate of preoperative diagnosis, clinical symptoms, and immunohistochemical findings. Statistical analysis was conducted using the primary extracted data.
Search Strategy
The protocol was approved by the Ethics Committee of Kanagawa Cancer Center (Kanagawa, Japan) (approval no.: 27-38). Because this study was a systematic review, the requirement for informed consent was waived.
Data Sources, Studies Sections, and Data Extraction
The term “gangliocytic paraganglioma” was entered into PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and Japana Centra Revuo Medicina (a medical database for Japanese papers; http://www.jamas.or.jp/) without limiting functions. The resultant Abstracts were screened to identify papers describing GP. After screening, the full-text articles were checked to identify papers describing GP following the inclusion and exclusion criteria outlined below.
Papers were considered to be case reports/series of GP if any of the following inclusion criteria were met: (1) epithelioid, spindle-shaped, and ganglion-like cells were reported in the main article and/or figures and (2) the paper was cited in other studies of GP. Papers were excluded if they were written in any language other than English or Japanese or were review articles.
Data Analysis
We collected data on age, sex, site of the primary lesion, tumor size, treatment, prognosis, presence of LNM, depth of tumor invasion, rate of preoperative diagnosis, clinical symptoms, and immunohistochemical findings. Statistical analyses (non-parametric Mann-Whitney U-tests, Chi-square tests, and multivariate logistic regression analysis) were performed using Statistical Package for the Social Sciences for Windows (software version 20; IBM Corp., Armonk, NY, USA). A P < 0.05 was considered statistically significant.
Results
Provide a Flow Diagram of the Studies Retrieved for the Review
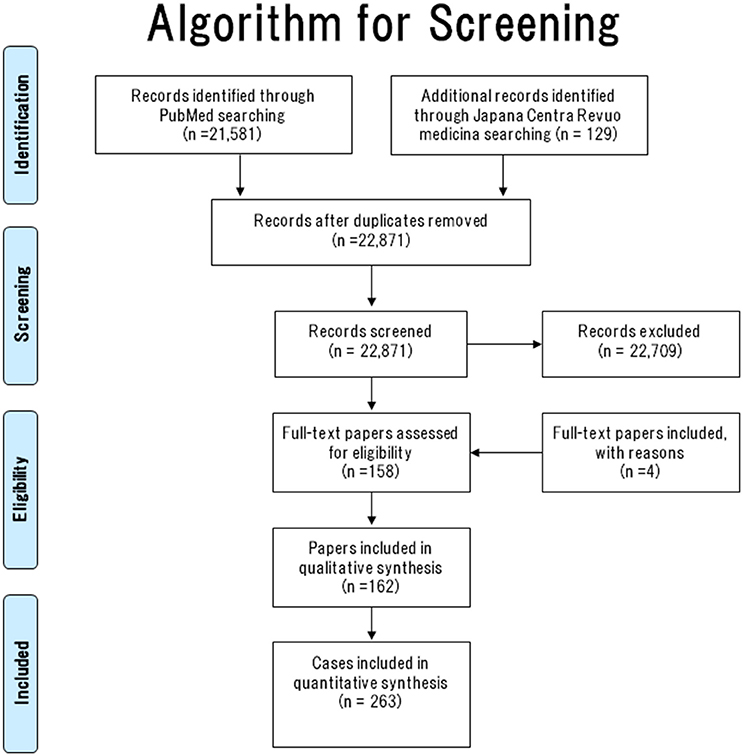
The literature search algorithm (flow diagram of the studies retrieved for this systematic review) is summarized in Figure 1.
Study Selection and Characteristics
In total, 22,871 papers were screened (PubMed [n = 22,742] and Japana Centra Revuo Medicina [n = 129]). Following Abstract screening, 22,709 papers were excluded because they did not describe GP or were written in a language other than English or Japanese (11–29). The full-text was checked to confirm that all papers had met the inclusion criteria. In total, 158 (English [n = 127] and Japanese [n = 31]) GP case reports were identified.
Until the term “gangliocytic paraganglioma” was coined by Kepes et al. (30) in 1971, became widely known or standardized globally, GP was still being reported under other names. Therefore, we checked the main article documents, figures, and references to the 158 papers.
As a result of this search, another four papers (31–34) that met the above inclusion criteria were added. Consequently, 162 papers (158 and 4 GP case reports/series) containing 263 cases of GP were collected. The literature search algorithm (flow diagram) is summarized in Figure 1.
Synthesized Findings
Clinical Characteristics
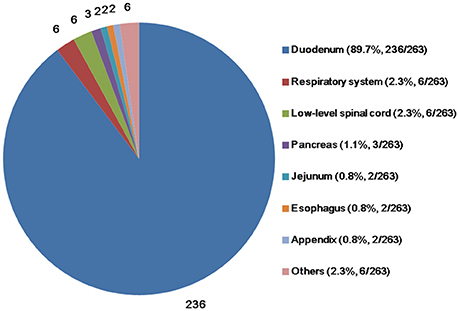
The mean age of the patients (n = 263) at the time of diagnosis was 53.5 (range, 15.0–84.0) years. The majority of the patients were male, with a male-to-female ratio of 157:104 (n = 261; two papers did not report patient sex). The mean tumor size (n = 203) was 25.7 (range, 5.5–100.0) mm. The predominant site of the primary tumor was the duodenum (n = 236; 89.7%), followed by the respiratory system (n = 6; 2.3%), low-level spinal cord (n = 6; 2.3%), pancreas (n = 3; 1.1%), jejunum (n = 2; 0.8%), esophagus (n = 2; 0.8%), appendix (n = 2; 0.8%), and other sites (Figure 2).
Clinical signs and/or symptoms were described in 217 patients. The most common clinical sign of GP was gastrointestinal bleeding (n = 104; 47.9%). Other signs and/or symptoms of GP included abdominal pain (n = 97; 44.7%), anemia (n = 44; 20.3%), incidental findings (n = 28; 12.9%), nausea (n = 15; 6.9%), weight loss (n = 12; 5.5%), general fatigue (n = 11; 5.1%), jaundice (n = 10; 4.6%), and incidental autopsy findings (n = 11; 5.1%).
In 263 GP cases, LNM and liver metastasis were observed in 11.4% (n = 30) and 1.1% (n = 3) of patients, respectively (2–4). GP recurrence was reported in only three patients (4, 35, 36). Unfortunately for one patient, GP was the cause of death (4). Twenty-seven patients underwent endoscopic treatment. Although one patient required additional surgery owing to the presence of residual tumor following the initial treatment (37), no additional treatments were conducted in the remaining 26 patients.
Pathological Characteristics
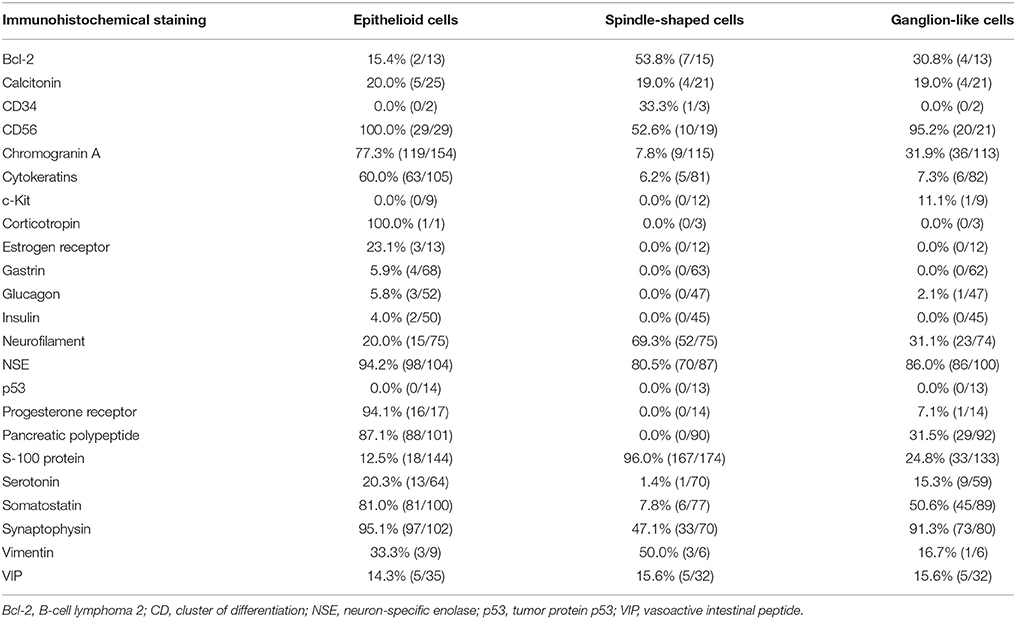
GPs contain three characteristic cell types: epithelioid, spindle-shaped, and ganglion-like. The distribution of these three cell types often varies considerably (Figures 3A,B) (9). Each of the three characteristic cell types have unique immunohistochemical profiles (the denominator varied for each immunohistochemical marker because only papers describing positive or negative immunohistochemical findings were evaluated). The positive immunoreactivity rates of markers in the epithelioid cells were as follows: CD56 (100.0% [29/29]), synaptophysin (95.1% [97/102]), neuron-specific enolase (94.2% [98/104]), progesterone receptor (94.1% [16/17]), pancreatic polypeptide (87.1% [88/101]), somatostatin (81.0% [81/100]), chromogranin A (77.3% [119/154]), and cytokeratins (60.0% [63/105]). In spindle-shaped cells, S-100 protein exhibited the highest positive immunoreactivity rate (96.0% [167/174]), followed by neuron-specific enolase (80.5% [70/87]), neurofilament (69.3% [52/75]), CD56 (52.6% [10/19]), vimentin (50.0% [3/6]), synaptophysin (47.1% [33/70]), and chromogranin A (7.8% [9/115]). In ganglion-like cells, CD56 exhibited the highest positive immunoreactivity rate (95.2% [20/21]), followed by synaptophysin (91.3% [73/80]), neuron-specific enolase (86.0% [86/100]), somatostatin (50.6% [45/89]), chromogranin A (31.9% [36/113]), neurofilament (31.8% [23/74]), pancreatic polypeptide (31.5% [29/92]), and S-100 protein (24.8% [33/133]; Table 1).
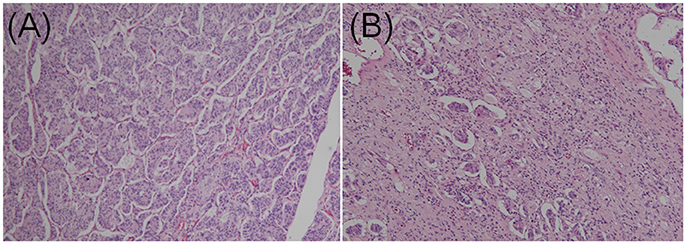
Figure 3. Contrasting histopathological features of gangliocytic paraganglioma. (A) Epithelioid cells accounted for the majority of the tumor. Dense proliferation was observed. Cells were arranged in cords in a nested Zellballen pattern. (B) Epithelioid cells exhibited sporadic proliferation. Spindle cells were predominant in the stroma and arranged in a chaotic pattern (hematoxylin and eosin staining; original magnification × 100).
In two previous multi-institutional retrospective studies (9, 38), we found that the majority of GP epithelioid cells exhibited positive immunoexpression for pancreatic polypeptide and progesterone receptor (Figures 4A,B), whereas NET G1 cells stained negative for pancreatic polypeptide and progesterone receptor. Histopathological findings of biopsy specimens obtained before surgery or endoscopic treatment were described in 63 cases. However, among these 63 cases, only 12 were successfully diagnosed with GP; 41 showed no tumor cells, nine were diagnosed with or suspected of having a different NET (NET G1 [n = 6], paraganglioma [n = 2], or ganglioneuroma [n = 1]), and one was diagnosed with atypical cells. Thus, only 19.0% of patients were preoperatively diagnosed with GP.
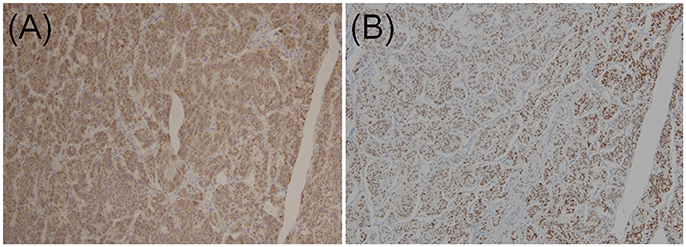
Figure 4. Immunohistochemical staining of (A) pancreatic polypeptides and (B) progesterone receptors in epithelioid cells of duodenal gangliocytic paraganglioma (original magnification × 100).
Risk Factors for LNM
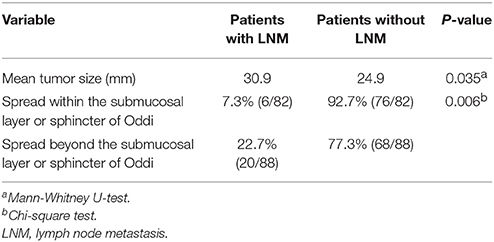
In the univariate analysis, mean tumor size and depth of tumor invasion were significant risk factors for LNM in patients with duodenal GP. The mean tumor size in patients with and without LNM was 30.6 and 24.9 mm, respectively. Thus, patients with LNM had significantly larger tumors than those without LNM (Mann-Whitney U-test, P = 0.035). With respect to depth of tumor invasion (n = 170 cases), the rate of LNM was higher in patients with GP penetrating beyond the submucosal layer or sphincter of Oddi (n = 88) compared to those with GP located within these layers (n = 82). In the latter, six patients (7.3%) had LNM and in the former 20 patients (22.7%) had LNM (Chi-square test, P = 0.006; Table 2). No significant differences were observed with respect to age or sex between patients with and without LNM.
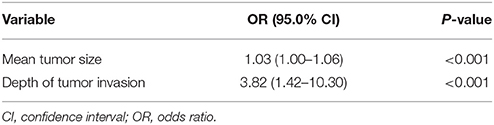
To elucidate the impact of these risk factors, we conducted multivariate logistic regression analysis. The results showed that both mean tumor size and depth of tumor invasion were significant risk factors for LNM. However, odds ratios differed. Mean tumor size had an odds ratio of 1.03 (95.0% confidence interval: 1.00–1.06; P < 0.01), while depth of tumor invasion had a higher odds ratio of 3.82 (95.0% confidence interval: 1.42–10.30; P < 0.01; Table 3). To further elucidate the cause of this result (given that depth of tumor invasion is an important risk factor for LNM), we conducted a statistical analysis of the relationship between mean tumor size and depth of tumor invasion. However, no significant relationship was observed. The mean tumor size in patients with tumor spread within or beyond the submucosal layer or sphincter of Oddi was 26.6 and 24.2 mm, respectively (Mann-Whitney U-test, P = 0.82).
Risk of Bias
Unfortunately, because this systematic review involved a cumulative case series, there is an inherent publication bias. This is a key limitation of the data.
Discussion
Summary of Main Findings
The predominant site of the primary GP was the duodenum. There was also a slight male preponderance. Through our literature survey, we found that gastrointestinal bleeding, a cause of severe anemia, was the most commonly reported clinical symptom of GP. One patient had recurrence due to the presence of residual tumor following the initial treatment (35). These findings suggest that surgery or endoscopic treatment of GP should be considered and follow-up alone should be avoided.
Twenty-seven patients underwent endoscopic treatment, while only one patient required additional surgery owing to the margin-positive results of the initial endoscopic procedure (37). Thus, endoscopic treatment can potentially produce favorable outcomes. Indeed, two patients also received irradiation (4, 39). However, it is possible that patients with negative surgical margins may not require irradiation because no recurrence or metastasis has been reported in such patients.
It should be noted that the distribution of the three characteristic tumor cells varied considerably. To accurately diagnose GP, pathologists should be aware that the histopathological features of GP are variable.
In the present study, approximately 10.0% of patients had LNM. Univariate analysis identified mean tumor size and depth of tumor invasion as significant risk factors for LNM in patients with GP. However, multivariate logistic regression analysis showed that depth of tumor invasion was a more significant risk factor than mean tumor size. Although mean tumor size is considered an important predictive factor of tumor progression, no significant relationship was observed between mean tumor size and depth of tumor invasion in duodenal GP. Moreover, the primary layer alone influences the probability of LNM in GP (GP arising from deep layers has a higher risk of LNM). This suggests, along with histological heterogeneity, that GP may have hamartomatous characteristics.
Further discussion is warranted regarding the proliferative activity of GP. Previous studies (4, 9) have shown that GP has no significant mitotic figures or Ki-67 protein, tumor protein p53, and B-cell lymphoma two immunoexpression, which are prognostic factors for several types of NETs (40–42), irrespective of the presence of LNM. Thus, general immunohistochemical prognostic factors in NETs are not useful for assessing the malignant potential of GP tumors.
Unfortunately, a typical biopsy has limited accuracy in diagnosing GP. However, one patient was successfully diagnosed with GP following multiple boring biopsies (43). These findings suggest that a boring biopsy may be a useful adjunctive procedure in selected cases. Schwannoma, gastrointestinal stromal tumor, leiomyoma, paraganglioma, and NET G1 are examples of differential diagnoses that can be derived from biopsy specimens (5). In the majority of cases, schwannoma, gastrointestinal stromal tumor, and leiomyoma can be easily distinguished using immunohistochemical staining (S-100 protein, c-Kit, CD34, synaptophysin, and chromogranin A). Paraganglioma has similar morphological, immunohistochemical, and genetic features to GP (hypoxia-inducible factor-2α gain-of-function mutations have been detected in both GP and paraganglioma) (44, 45). Thus, pathologists should consider the primary lesion and the presence of ganglion-like cells. For instance, ganglion-like cells are absent from paraganglioma, which rarely arises from the duodenum (46). Although NET G1 is the most important differential diagnosis, GP is often misdiagnosed as NET G1 (8). Nevertheless, as a benign course is more common in cases of GP than in cases of NET G1 (47), it is important to distinguish between GP and NET G1. In previous multi-institutional retrospective studies (9, 38), we showed that immunohistochemical examination of pancreatic polypeptide and progesterone receptor could be used to distinguish between GP and NET G1, even with small biopsy specimens. Finally, our previous multi-institutional retrospective studies suggest that GP accounts for a consistent proportion of duodenal NETs.
Limitations
Unfortunately, because this systematic review involved a cumulative case series, there is an inherent publication bias. This is a key limitation of the data.
Conclusions
The present review of 263 cases revealed more detailed clinicopathological characteristics of GP, including risk factors for LNM, differential diagnostic approaches, and improvements in the clinical management of this rare NET. In particular, GP may have hamartomatous characteristics. Occasionally GP is misdiagnosed as NET G1. To avoid such misdiagnoses, we emphasized the utility of immunohistochemical staining with progesterone receptor and pancreatic polypeptide to differentiate GP from NET G1, even with small biopsy specimens.
Author Contributions
YO collected the data, performed the statistical analysis, and wrote the manuscript. EY, MS, and KW performed the statistical analysis and revised the manuscript. KK and YK advised the first author on GPs as senior doctors and revised the manuscript. TY performed the statistical analysis and revised the manuscript as the senior author. All authors have read and approved the final version of the manuscript.
Funding
This work was supported by JSPS KAKENHI to YO (Grant Number: JP17K08713) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by Kanagawa Prefectural Hospitals Cancer Fund granted to YO (Grant Number: None).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank Sachie Osanai and Mitsuyo Yoshihara for their excellent technical support. We also wish to thank Editage (www.editage.jp) for English language editing.
Abbreviations
GP, gangliocytic paraganglioma; LNM, lymph node metastasis; NET, neuroendocrine tumor.
References
1. Nonaka K, Matsuda Y, Okaniwa A, Kasajima A, Sasano H, Arai T. Pancreatic gangliocytic paraganglioma harboring lymph node metastasis: a case report and literature review. Diagn Pathol. (2017) 12:57. doi: 10.1186/s13000-017-0648-x
2. Amin SM, Albrechtsen NW, Forster J, Damjanov I. Gangliocytic paraganglioma of duodenum metastatic to lymph nodes and liver and extending into the retropancreatic space. Pathologica (2013) 105:90–3.
3. Rowsell C, Coburn N, Chetty R. Gangliocytic paraganglioma: a rare case with metastases of all 3 elements to liver and lymph nodes. Ann Diagn Pathol. (2011) 15:467–71. doi: 10.1016/j.anndiagpath.2010.07.009
4. Li B, Li Y, Tian XY, Luo BN, Li Z. Malignant gangliocytic paraganglioma of the duodenum with distant metastases and a lethal course. World J Gastroenterol. (2014) 20:15454–61. doi: 10.3748/wjg.v20.i41.15454
5. Okubo Y, Yokose T, Motohashi O, Miyagi Y, Yoshioka E, Suzuki M, et al. Duodenal rare neuroendocrine tumor: clinicopathological characteristics of patients with gangliocytic paraganglioma. Gastroenterol Res Pract. (2016) 2016:5257312. doi: 10.1155/2016/5257312
6. Dahl EV, Waugh JM, Dahlin DC. Gastrointestinal ganglioneuromas; brief review with report of a duodenal ganglioneuroma. Am J Pathol. (1957) 33:953–65.
7. Okubo Y, Wakayama M, Nemoto T, Kitahara K, Nakayama H, Shibuya K, et al. Literature survey on epidemiology and pathology of gangliocytic paraganglioma. BMC Cancer (2011) 11:187. doi: 10.1186/1471-2407-11-187
8. Okubo Y. Gangliocytic paraganglioma is often misdiagnosed as neuroendocrine tumor G1. Arch Pathol Lab Med. (2017) 141:1309. doi: 10.5858/arpa.2017-0102-LE
9. Okubo Y, Nemoto T, Wakayama M, Tochigi N, Shinozaki M, Ishiwatari T, et al. Gangliocytic paraganglioma: a multi-institutional retrospective study in Japan. BMC Cancer (2015) 15:269. doi: 10.1186/s12885-015-1308-8
10. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
11. Henry C, Ghalel-Mechaoui H, Bottero N, Pradier T, Moindrot H. Gangliocytic paraganglioma of the pancreas with bone metastasis. Ann Chir. (2003) 128:336–8. doi: 10.1016/S0003-3944(03)00097-X
12. Eugene C, Bergue A, Quevauvilliers J, Pourcher J, Fingerhut A, Ronat R. Nonchromaffin paraganglyoma of the duodenum. Study of a case revealed by melena. Nouv Presse Med. (1977) 6:1551–2, 7.
13. Kermarec J, Duplay H, Lesbros F. Gangliocytic paraganglioma of the duodenum. One case with electron microscope study (author's transl). Arch Anat Cytol Pathol. (1976) 24:261–8.
14. Lauzon A, Cadotte M. Gangliocytic paraganglioma of the duodenum. Union Med Can. (1972) 101:1584–6.
15. Bergis D, Bojunga J, Lubomierski N, Frank M, Zeuzem S, Trojan J. Gangliocytic paraganglioma–a rare differential diagnosis of a submucosal duodenal tumor. Z Gastroenterol. (2011) 49:1412–6. doi: 10.1055/s-0029-1246103
16. Sandmann M, Fahndrich M, Lorenzen J, Heike M. Gangliocytic paraganglioma–a rare cause of an upper gastrointestinal bleeding. Z Gastroenterol. (2010) 48:1297–300. doi: 10.1055/s-0029-1245521
17. Huffer M, Riechmann M, Muller J, Burrig KF, Richter A. Gangliocytic paraganglioma of the ampulla of Vater. Zentralbl Chir. (2003) 128:232–5. doi: 10.1055/s-2003-38538
18. Hardt PD, Doppl WE, Klor HU, Hinrichs B. A rare combination of pheochromocytoma ans somatostatin-rich neuroendocrine tumor of Vater's papilla (carcinoid) in a patient with von Recklinghausen neurofibromatosis. Z Gastroenterol. (1998) 36:233–8.
19. Duff CA, von Segesser LK, Bino M, Schmid ER, Stamm B, Turina M. Duodenal paraganglioma. Case report and review of the literature. Chirurg (1991) 62:144–7.
20. Barten M, Kloppel G. Gangliocytic paraganglioma of the duodenum. Case report with immunocytochemical characterization. Zentralbl Allg Pathol. (1990) 136:181–7.
21. Buchler M, Beger HG, Malfertheiner P. Juxtapapillary gangliocytic paraganglioma of the duodenum. Dtsch Med Wochenschr. (1989) 114:1138.
22. Born P, Bauer M, Eimiller A, Busing CM, Paul F. Gangliocytic paraganglioma of the duodenum. Z Gastroenterol. (1989) 27:339–40.
23. Grouls V, Stein U, Vogel J. Juxtapapillary gangliocytic paraganglioma of the duodenum. Dtsch Med Wochenschr. (1989) 114:584–8. doi: 10.1055/s-2008-1066639
24. Grouls V, Pickartz H, Reis HE. Pedunculated polypoid gangliocytic paraganglioma of the afferent jejunal loop of a Billroth II stomach. Leber Magen Darm (1987) 17:327–33.
25. Cremer H, Addicks K. Polypoid gangliocytic paraganglioma of the duodenum. Pathologe (1985) 6:211–6.
26. Gavinelli M, Montagnolo G, Tiberio GA, Massari M, Bensi E, Tiberio G. Duodenal gangliocytic paraganglioma: intraoperative ultrasound evaluation. Chir Ital. (1998) 50:83–6.
27. Alvisi C, Valle C, Ursini G, Perego M. Gangliocytic paraganglioma. A case report and review of the literature. Minerva Gastroenterol Dietol. (1997) 43:41–5.
28. Zhang C, Wu J, Sun SA, Liu HY, Zhou WB, Li XF, et al. Duodenal gangliocytic paraganglioma: report of a case. Zhonghua Bing Li Xue Za Zhi (2012) 41:55–6.
29. Kim JH, Kim HM, Song SY, Kim YJ, Hahn CH, Park SW, et al. A case of gangliocytic paraganglioma in duodenum. Korean J Gastroenterol. (2004) 43:47–51.
30. Kepes JJ, Zacharias DL. Gangliocytic paragangliomas of the duodenum. A report of two cases with light and electron microscopic examination. Cancer (1971) 27:61–7. doi: 10.1002/1097-0142(197101)27:1<61::AID-CNCR2820270111>3.0.CO;2-I
31. Babaryka I. Polypoid nonchromaffin paraganglioma of the duodenum. Virchows Arch A Pathol Anat Histol. (1978) 377:181–7. doi: 10.1007/BF00427005
32. Weitzner S. Benign nonchromaffin paraganglioma of the duodenum. Report of a case and review of the literature. Am J Gastroenterol. (1970) 53:365–9.
33. Qizilbash AH. Benign paraganglioma of the duodenum. Case report with light and electron microscopic examination and brief review of literature. Arch Pathol. (1973) 96:276–80.
34. Buchler M, Malfertheiner P, Baczako K, Krautzberger W, Beger HG. A metastatic endocrine-neurogenic tumor of the ampulla of Vater with multiple endocrine immunoreaction–malignant paraganglioma? Digestion (1985) 31:54–9. doi: 10.1159/000199178
35. Dookhan DB, Miettinen M, Finkel G, Gibas Z. Recurrent duodenal gangliocytic paraganglioma with lymph node metastases. Histopathology (1993) 22:399–401. doi: 10.1111/j.1365-2559.1993.tb00145.x
36. Kee AR, Forrest CH, Brennan BA, Papadimitriou JM, Glancy RJ. Gangliocytic paraganglioma of the bronchus: a case report with follow-up and ultrastructural assessment. Am J Surg Pathol. (2003) 27:1380–5. doi: 10.1097/00000478-200310000-00011
37. Witkiewicz A, Galler A, Yeo CJ, Gross SD. Gangliocytic paraganglioma: case report and review of the literature. J Gastrointest Surg. (2007) 11:1351–4. doi: 10.1007/s11605-007-0217-9
38. Okubo Y, Kasajima R, Suzuki M, Miyagi Y, Motohashi O, Shiozawa M, et al. Risk factors associated with the progression and metastases of hindgut neuroendocrine tumors: a retrospective study. BMC Cancer (2017) 17:769. doi: 10.1186/s12885-017-3769-4
39. Wong A, Miller AR, Metter J, Thomas CR Jr. Locally advanced duodenal gangliocytic paraganglioma treated with adjuvant radiation therapy: case report and review of the literature. World J Surg Oncol. (2005) 3:15. doi: 10.1186/1477-7819-3-15
40. Okubo Y, Okubo T, Okubo Y, Ishiwatari T. Neuroendocrine differentiation in breast cancer: clinicopathological significance of Bcl-2 positive solid papillary carcinoma. Case Rep Med. (2016) 2016:9501410. doi: 10.1155/2016/9501410
41. Okubo Y, Yokose T, Tuchiya M, Mituda A, Wakayama M, Hasegawa C, et al. Duodenal gangliocytic paraganglioma showing lymph node metastasis: a rare case report. Diagn Pathol. (2010) 5:27. doi: 10.1186/1746-1596-5-27
42. Ishiwatari T, Okubo Y, Tochigi N, Wakayama M, Nemoto T, Kobayashi J, et al. Remodeling of the pulmonary artery induced by metastatic gastric carcinoma: a histopathological analysis of 51 autopsy cases. BMC Cancer (2014) 14:14. doi: 10.1186/1471-2407-14-14
43. Matsubayashi H, Ishiwatari H, Matsui T, Fujie S, Uesaka K, Sugiura T, et al. Gangliocytic Paraganglioma of the minor papilla of the Duodenum. Intern Med. (2017) 56:1029–35. doi: 10.2169/internalmedicine.56.7812
44. Zhuang Z, Yang C, Ryska A, Ji Y, Hou Y, Graybill SD, et al. HIF2A gain-of-function mutations detected in duodenal gangliocytic paraganglioma. Endocr Relat Cancer (2016) 23:L13–6. doi: 10.1530/ERC-16-0148
45. Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, et al. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med. (2012) 367:922–30. doi: 10.1056/NEJMoa1205119
46. Okubo Y. Gangliocytic paraganglioma: a diagnostic pitfall of rare neuroendocrine tumor. Endocr Pathol. (2017) 28:186. doi: 10.1007/s12022-017-9481-8
Keywords: gangliocytic paraganglioma, literature survey, metastasis, neuroendocrine tumor, pancreatic polypeptide, progesterone receptor
Citation: Okubo Y, Yoshioka E, Suzuki M, Washimi K, Kawachi K, Kameda Y and Yokose T (2018) Diagnosis, Pathological Findings, and Clinical Management of Gangliocytic Paraganglioma: A Systematic Review. Front. Oncol. 8:291. doi: 10.3389/fonc.2018.00291
Received: 24 May 2018; Accepted: 11 July 2018;
Published: 27 July 2018.
Edited by:
John James Tentler, University of Colorado, United StatesReviewed by:
Ali Coskun, Ízmir Bozyaka Eğitim ve Araştırma Hastanesi, TurkeyQingfeng Zhu, Johns Hopkins Medicine, United States
Copyright © 2018 Okubo, Yoshioka, Suzuki, Washimi, Kawachi, Kameda and Yokose. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoichiro Okubo, eW9pY2hpcm8wMjA3QGhvdG1haWwuY29t
 Yoichiro Okubo
Yoichiro Okubo Emi Yoshioka
Emi Yoshioka