
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 13 June 2018
Sec. Genitourinary Oncology
Volume 8 - 2018 | https://doi.org/10.3389/fonc.2018.00221
This article is part of the Research Topic Updates, Emerging Methodologies, and Tools for Prostate Cancer Precision Medicine View all 7 articles
 Matteo Giulietti1†
Matteo Giulietti1† Matteo Santoni2†
Matteo Santoni2† Alessia Cimadamore3†
Alessia Cimadamore3† Francesco Carrozza4
Francesco Carrozza4 Francesco Piva1
Francesco Piva1 Liang Cheng5
Liang Cheng5 Antonio Lopez-Beltran6
Antonio Lopez-Beltran6 Marina Scarpelli3
Marina Scarpelli3 Nicola Battelli2
Nicola Battelli2 Rodolfo Montironi3*
Rodolfo Montironi3*
Tumor microenvironment constitutes a complex network in which tumor cells communicate among them and with stromal and immune cells. It has been shown that cancer cells are able to exchange genetic materials through small extracellular vesicles (EVs), a heterogeneous group of vesicles with different size and shape, cargo content, and function. The importance to investigate populations of circulating EVs would be of great importance as prostate cancer (PCa) biomarkers. In several neoplasms as well as in PCa, nanometer-sized EVs of endosomal origin are implicated in supporting tumor growth and metastatic spread by both altering local stroma cells and creating a protumor environment that favors the formation of pre-metastatic niches. Several techniques are applicable for the isolation and analysis of PCa-derived small EVs and are illustrated in this article. Due to the high sensitivity and specificity of these techniques, small EVs have become ideal candidates for early diagnosis. Moreover, we discuss the role of small EVs during PCa carcinogenesis, as well as in modulating the development of drug resistance to hormonal therapy and chemotherapy, thus underlining the potential of EV-tailored strategies in PCa patients.
Tumor microenvironment components represent promising therapeutic targets in cancer. Indeed, it is implicated in tumor carcinogenesis and progression, as well as in the suppression of antitumor immune response and in the development of drug resistance (1). These activities are the result of a complex network between normal and cancer cells, which supports tumor proliferation and metastatic spread. Recently, it has been shown that cancer cells are able to exchange genetic materials through extracellular vesicles (EVs), a heterogeneous group of vesicles with different size and shape, cargo content, and function (2).
Among them, nanometer-sized EVs of endosomal origin are involved in promoting tumor growth and metastasis (3) through the alteration of local stroma cells (4) and the creation of a protumor environment that sustains the formation of pre-metastatic niches (5, 6). These small size vesicles of ~30–150 nm in diameter are released into the extracellular space after fusion of multivesicular bodies with the cell membrane and could be found in blood, urine, and other biological fluids. Urine EVs have been shown to contain RNA, DNA, and miRNA, lipid and proteins/transporters specific to cells of the kidney and urogenital tract. Thus, urine represents a potentially useful non-invasive material for diagnosis and prognosis of genitourinary tract neoplasms (7, 8). Due to their involvement in the early phases of tumor development and dissemination, future diagnostic and therapeutic EV-tailored strategies are emerging in the armamentarium of cancer patients.
In genitourinary tumors, EVs represent potential novel biomarkers. Indeed, small EVs have been involved in kidney diseases and cancer, as well as in bladder cancer growth and progression (9). In prostate cancer (PCa), small EVs have been reported to contain high levels of molecules that stimulate cancer cell invasion, such as integrins β4 (ITGB4) and αvβ6, vinculin (VCL), transmembrane glycoprotein Trop-2, vimentin, and N-cadherin, well-known epithelial–mesenchymal transition markers (6, 10).
Furthermore, the efficacy of urine assays in PCa diagnostic could be improved by simple procedures like prostate massage to increase the amount of tumor-specific nucleic acids released (11). This review illustrates the main techniques for isolation and analysis of small EVs and emphasizes their role in the carcinogenesis, progression, and drug response in patients with prostate tumor, thus underlying the potential of emerging EV-tailored approaches in this setting.
The currently adopted methods for small EVs isolation include differential and density gradient ultracentrifugation, size-based methods (ultrafiltration and size-exclusion chromatography), polymer-based precipitation, and immunoaffinity capture. The choice of the isolation technique depends on the sample source, the sample amount, the type of the downstream molecular analyses, the desired purity level, and final concentration. Each method shows both advantages and disadvantages, summarized in Table 1. It should be noted that some isolation methods can be more easily integrated with analysis of vesicular RNAs and proteins, so fewer steps are needed. This can therefore improve the reliability and efficiency of small EVs analysis.
Ultracentrifugation is the most frequently used approach for small EVs isolation, mainly since it does not require high level of technical experience. This technique is based on the different sedimentation rates (depending on size and density) of small EVs from the other sample components (12). Generally, before ultracentrifugation, some steps of low-speed centrifugation (3,000–20,000 × g) are carried out to clean the sample from large particles, including cells, platelets, apoptotic bodies, and microvesicles. Typically, for ultracentrifugation, the used force ranges from 100,000 to 200,000 × g. Due to the high-speed centrifugation, the small EVs rupture represents a high risk and leads to the yield reduction of intact particles suitable for downstream analyses. It also requires high volumes of samples, it is time-consuming and not suitable for large number of samples (13).
Moreover, pellet obtained by this method may contain also EV aggregates and protein complexes similar to small EVs in size (14). A particular case regards the uromodulin (THP), which is the most abundant protein in urine and can form a polymeric network that facilitates small EVs’ aggregation. Uromodulin removal and the consequent increase of small EVs’ yield can be achieved by using the detergent CHAPS or the reducing agent dithiothreitol in urine samples (15). Finally, unlike free RNA that is degraded by urinary RNAses, DNA contamination is frequent, and a DNAse should be used if small EVs’ DNA is the target of downstream analyses (16).
The density gradient centrifugation method allows small EVs separation from other sample components by exploiting their typical density, which ranges between 1.13 and 1.19 g/ml (17). By using pre-constructed columns composed of solutions with different densities, after ultracentrifugation small EVs will be at a specific column layer.
It allows purer small EVs isolation than simple ultracentrifugation; in fact, it is generally used as a second step after other isolation methods to purify the extracts (18, 19). However, high-density lipoproteins represent a contaminant frequently co-isolated with small EVs; therefore, plasma and serum are not suitable sample types for density gradient centrifugation. In addition, it cannot distinguish small EVs from other EVs with similar density (20).
Ultrafiltration represents an efficient alternative to ultracentrifugation. It involves the use of nanomembrane filters of polyethersulfone or polyvinylidene difluoride with an approximately 50–100 kDa molecular mass cutoff (12). Generally, some steps of filtration with membrane filters having larger pores can be performed for the depletion of floating cells and large cell debris (21). Membranes allow the concentration of very diluted samples and therefore they are especially suited for cell culture media and urine samples.
Extracellular vesicles size can be also exploited by the size-exclusion chromatography that, using sepharose packed columns, allows the separation of the small EVs fraction from biofluids, with high purity and reproducibility (22). However, both ultrafiltration and size-exclusion chromatography have long run times that limit their scalability for clinical routine laboratory applications.
Some isolation kits for small EVs precipitation are commercially available (23, 24). They are based on water-excluding polymer mixtures that force insoluble components out. When these polymers are added to the sample and after a low-speed ultracentrifugation (<20,000 × g), they allow the precipitation of small EVs. Since precipitation method is very fast, easy to use, and no equipment is necessary, it is a method scalable for large sample sizes and therefore is suited for the clinical use.
Recently, performances of ultracentrifugation and four different precipitation methods have been assessed in urine samples. In this comparison, a precipitation method outperforms the others in detecting alterations of PCa small EVs’ markers (25). However, other EVs are frequently co-isolated, making this procedure not so specific for small EVs. Moreover, abundant proteins, including uromodulin, can also be recovered.
Finally, immunoaffinity isolation methods exploit the presence of specific proteins in the small EVs’ surface (26). Antibodies conjugated with magnetic beads or other materials can recognize the vesicular antigens and facilitate the precipitation by low-speed centrifugation or the isolation by magnetic techniques. This method is highly specific, allowing the isolation of only small EVs by using general small EV surface markers (e.g., CD81, CD9, and CD63) and even only small EV subpopulations, for example, tumor small EVs by using a tumor-specific marker.
Recently, specific methods for immuno-based small EVs isolation and detection for PCa have been proposed, both measuring total small EVs (27) and exploiting the anti-prostate-specific membrane antigen (PSMA) antibody (28). Moreover, it has been observed that immunoaffinity-based methods have better performance than purification methods in isolating PCa small EVs from patient plasma samples (29). However, this generally leads to low yields. In addition, immunoaffinity isolation requires several elaboration steps making it subjected to potential errors by operators.
After isolation, small EVs quantity, size, purity, and other properties can be assessed. A popular method to quantify total small EVs is the nanoparticle tracking analysis (NTA) (12, 30–32). It also allows the determination of a size distribution profile of particles in solution and, by verifying that the observed distribution ranges within typical small EV size, also the purity degree can be estimated. In particular, a laser beam makes particles visible and therefore their concentration can be assessed. The size distribution can be evaluated by measuring the velocity of the Brownian motion of each particle and, according to the Stokes–Einstein equation, speed of particles can be correlated to the particle size. However, protein complexes, lipoprotein particles, and vesicle aggregates similar to small EVs in size can be revealed by NTA and therefore the small EVs concentration could be overestimated. Flow cytometry is another popular method for both small EVs quantification and characterization (33). In particular, particles are illuminated by a laser beam, and the scattered light is detected. However, currently, there is a detection limit of 150 nm; therefore, the use of latex or magnetic beads coupling with small EVs is suggested to make these aggregates visible by flow cytometry (34). Flow cytometry has been used to perform “liquid biopsy” in PCa patient plasma samples. Biggs et al. measured circulating prostate microparticles (PMPs), a type of EVs, with a size ranging from 100 to 1,000 nm, immunoreactive to anti-PSMA mAb. PMP enumeration levels in plasma permit to identify patients with Gleason score ≥8 PCa independently from PSA value (35).
Regarding small EVs quantification, researchers frequently adopt the enzyme-linked immunosorbent assay (ELISA). Similar to immunoaffinity isolation methods, this approach exploits small EV surface antigens that can be detected by specific antibodies. To quantify total small EVs, CD63, CD9, and CD81 vesicular-specific proteins are generally measured by ELISA (34). The evaluation of the enzymatic activity of the acetyl-CoA acetylcholinesterase, which is enriched in small EVs, is another method used for quantification and purity assessments. Alternatively, to evaluate the purity degree of a small EVs preparation, Western blotting can be used to assess the presence of typical small vesicular proteins (CD63, CD9, CD81, Alix, and Tsg101) (12). However, this assay is not quantitative and gives no information on the particle size.
Upon small EVs isolation and quantification, their RNA, DNA, protein, and lipid content can be analyzed. RNA profiling can include mRNAs and miRNAs and can be achieved both by PCR-based assays for single RNA molecule profiling and by next-generation sequencing (RNA-seq) for large-scale analysis. For example, after isolation of small EVs in urine specimens, the levels of PCa-associated mRNA transcripts in these vesicles have been assessed by quantitative real-time PCR analysis (36, 37). In addition, quantification of vesicular miRNAs can be assessed by RNA-seq, as carried out for urinary small EVs from PCa patients (38). However, vesicular miRNA quantification can be affected by inadequate procedural choices, including the choice of optimal endogenous miRNA for expression normalization (39). Differential expression analysis of vesicular mRNAs or miRNAs between samples from primary PCa, metastatic cancers, and healthy subjects can lead to the identification of candidate biomarkers (40). In addition, other gene expression analysis methods, including co-expression network analysis, can suggest potential diagnostic and prognostic biomarkers (41–43). Analysis of urinary vesicular RNA and DNA has also been performed in prostate tumors to identify the presence of mutations (44). It has been observed that small EVs released by different PCa cell lines harbor specific DNA mutations (45). These results may allow the development of non-invasive tests for mutational status assessment in PCa. Interestingly, the specific molecular effects due to the identified mutations on transcription, splicing, miRNA binding at 3′UTR, and nucleocytoplasmic export of mRNA can be effectively predicted (46–49).
Furthermore, methods for single protein and multiple protein profiling exist. Western blot, cytofluorimetry, and ELISA represent popular methods for the assessment of specific small vesicular proteins and for validation of results obtained by other proteomic methods, also regarding urinary small EVs of PCa patients (50). Recently, a new aptamer-based method for the simultaneous analysis of around 1,000 proteins from small EVs of PCa patients has been developed (51). Moreover, mass spectrometry has been frequently used to identify all small EVs’ proteins from many sample types and, recently, it has also been exploited for the analysis of small EVs’ proteins in plasma samples of PCa patients (52). Interestingly, mass spectrometry can be also used to assess the lipid content of small EVs. In particular, lipid species in small EVs released by PCa cell lines and present in urine of PCa patients have been evaluated (53, 54). It should be also taken into account the heterogeneous nature of cancer EVs; different types of PCa cells are more prone to shed heterogeneous populations of EVs compared with other PCa cells. Indeed, El-Sayed and her group have recently showed mesenchymal-like prostate carcinoma cells have a propensity to release EVs of varied sizes with diameters ranging from 100 to 300 nm approximately, while epithelial-like PCa cells principally generate small vesicles (50–150 nm). The isolation method could be a technical limitation; further studies are needed to properly characterize and classify these vesicles and to investigate the potential effects of EVs as a whole, including small EVs’ fraction and others EVs’ subgroups (10).
In the past 10 years, small EVs have emerged as a novel effective and non-invasive clinical tool for the screening and early diagnosis of PCa. This evidence is supported by the specificity and sensitivity showed by small EVs analysis in this disease, together with the possibility of a non-invasive assessment of gene expression and mutations (36), thus leading to a year-by-year growing number of studies in this context. Indeed, it has been shown that only PCa patients are characterized by high levels of nanovesicles (125–180 nm, i.e., small EVs) expressing both CD81 and PSA (55). Such results were obtained analyzing 1 ml of plasma samples of 15 healthy donors, 15 benign prostatic hyperplasia, and 15 PCa patients by nanoscale flow cytometry and ELISA assay. Moreover, McKiernan and his group revealed that gene expression assay in urine small EVs was able to discriminate high-grade (Gleason score ≥7) from low-grade (GS6) cancer and benign disease. Small EVs’ RNA cycle threshold values of ERG, PCA3, and SPDEF were used to derive urine small EVs gene expression assay score that was tested in 255 men and then was prospectively validated in an independent cohort of 519 men. In this way, they identified patients with higher-grade PCa among men with elevated PSA levels, thus potentially reducing the number of unnecessary biopsies (56). In the same view, high Claudin 3 levels from isolated small EVs were able to predict GS ≥8 (57), while small EVs’ levels of gamma-glutamyltransferase 1, a cell-surface enzyme that regulates the catabolism of extracellular glutathione, were significantly higher in PCa patients compared with benign prostatic hyperplasia patients (58).
Interestingly, several miRNAs from isolated urinary small EVs, such as miR-2909 (59), miR-19b (60), miRNA-21, and miR-375 (61), have demonstrated to be effective as diagnostic biomarkers for prostate tumors. The isolation method used in these studies included precipitation of small EVs in fluids with low-speed centrifugation step (59) or differential centrifugation (60, 61) with selection of vesicles of 30–100 nm obtained by 0.1-µm filtration (60). The authenticity of these isolated small EVs was confirmed by subjecting them to morphological analysis by electronic microscopy (59, 61), immunostaining with antibodies against CD63, CD9, and CD24 (60), or Western blot analysis using CD63 antibody coupled with standard immunodetection procedure (59).
More recently, the analysis of the lipidome of urinary small EVs showed statistically significant difference between PCa patients (n = 15) and healthy volunteers (n = 13), in particular for the high presence of phosphatidylserine and lactosylceramide associated with PCa diagnosis (53). These pioneering studies, even if limited to a small number of samples, are promising and should be validated in larger independent cohorts.
Prostate cancer-derived small EVs are directly involved in PCa carcinogenesis and metastasis. Indeed, small EVs are able to significantly reduce apoptosis, increase cancer cell proliferation, and induce cell migration in LNCaP and RWPE-1 cells (62). Moreover, they can modulate bone cell formation by affecting the fusion and differentiation of osteoclasts in the metastatic sites (63), thus favoring the formation of pre-metastatic niches.
Hypoxia plays a crucial role in regulating small EVs activity. In fact, it has been shown that small EVs secreted by PCa cells under hypoxic conditions showed higher metalloproteinases (MMPs) activity and increased levels of proteins primarily implicated in the remodeling of epithelial adherens junction pathway compared with small EVs released from normoxic cells. Therefore, this enhanced the invasiveness and stemness of naïve PCa cells (64).
Beyond their potential use for PCa diagnosis, miRNAs are directly implicated in PCa development and progression. It has been shown that normal prostate fibroblasts (WPMY-1) transfected with miR-100-5p, miR-21-5p, and miR-139-5p augmented their migration and metastatic invasion by increasing the expression of MMP-2, -9, and -13 and RANKL (65). Furthermore, adipocyte differentiation-related protein can be detected in small EVs released by PCa cells and is able to induce neuroendocrine differentiation of these cells in a paracrine fashion (66). Interestingly, small EVs are also involved in modulating PCa-induced immunosuppression of dendritic cell functions (67) and promote immune evasion by downregulating NKG2D expression on natural killer cells and CD8+ T cells (68).
Even if most of the studies on biological effects of small EVs are conducted in vitro, they lay the foundations for following clinical investigations.
Recently, it has been shown that the expression of CD9 is increased in PCa of patients who suffer from disease recurrence in 5 years, indicating the role of CD9 in the progression of recurrent advanced PCa (69).
Using the established immunocapture and immunodetection method, Soekmadji and colleagues reported that the level of CD9+ EVs in plasma is increased in PCa patients compared to those with benign prostate hyperplasia and, on the contrary, CD63+ EVs level does not show a significant difference between the two groups. Moreover, they showed that in plasma obtained from a metastatic PCa patient cohort the level of CD9+ EVs were higher in circulating tumor cell (CTC)-positive PCa patients compared with CTC-negative patients. Instead, the CD63+ EVs level did not show significant differences between the cohorts (70, 71).
Such findings underline the importance to investigate particularly subpopulations of circulating EVs that would be more informative as PCa biomarkers.
The stability and low immunogenicity of EVs support their use as therapeutic delivery agents for cancer drugs and small molecules. The methodology for loading EVs with a therapeutic cargo consists into two different approaches: the first one is based on the indirect modification of EV membranes via the genetic engineering of their parental cell (72). The second one needs the direct encapsulation of a cargo into purified exosomes through active (i.e., sonification and electroporation) or passive (i.e., the introduction of hydrophobic drugs into EVs, the utilization of multivalent electrostatic interactions, permeabilization with saponin) loading methods (72). These technologies will represent a major step forward in the era of precision medicine for PCa patients and should be tested into future clinical trials.
Small EVs have been shown to be crucial for the development of drug resistance in patients with prostate tumor. In 2017, Del Re et al. assessed AR-V7 as a predictor of resistance to hormonal therapy by highly sensitive digital droplet polymerase chain reaction in plasma-derived small EVs’ RNA. They found that both median progression-free survival (20 vs. 3 months; p < 0.001) and overall survival (8 months vs. not reached; p < 0.001) were significantly longer in AR-V7-negative vs. AR-V7-positive patients (73).
Small EV-derived microRNAs contribute also to PCa chemoresistance (74) and can act as surrogate biomarker of tumor response to taxanes (75). It has been observed that the transfer of small EVs (in particular, small EVs’ MDR-1/P-gp) from docetaxel-resistant cell lines to DU145, 22Rv1, and LNCap PCa cell lines induces acquired resistance to this drug (76). In the same view, Kawakami et al. reported that β4 (ITGB4) and VCL in small EVs could be useful markers of PCa progression correlated with taxane resistance (77).
Interestingly, serum small EVs’ P-glycoprotein high levels are associated with resistance to docetaxel but not to cabazitaxel (78), thus representing a potential biomarker to guide the decision-making process in PCa patients.
Another important therapeutic use of EVs is vaccination to treat cancer. It has already been studied on mice injected with autologous tumor-derived nanovesicles, enriched with tumor-specific antigens. The high-level immunogenicity of these nanovesicles induced an antitumor immune responses in both primary and metastatic melanoma mouse models (79).
Some questions have to be answered before these preclinical evidences on EV-based therapy can be applied in a clinical scenario. Major problems are the EV’s production, purification, and concentration, the biodistribution of EVs, the targeting of recipient cells, the up-taking by the recipient cells, and the effects on the recipient immune system (80).
For years, Gleason score has represented the most relevant prognostic factor in patients with PCa, along with PSA levels measured at the time of diagnosis and the TNM score. Plasma quantification of PSA has markedly improved the early detection of PCa, but still lacks the required specificity. More recently, a new generation of potentially predictive and prognostic parameters has grown, opening the way to molecularly tailored approaches in PCa patients. Small EVs have been reported to act as mediators of cell-to-cell communication due to the regulatory functions of their content. The high sensitivity and specificity of data obtained from small EVs analysis, their presence in almost all human fluids, and the variety of their functions exerted during tumor carcinogenesis, progression, and response to treatments, support the notion that small EVs exploration will represent a cornerstone of future approaches in cancer patients. Further investigations are needed, particularly regarding the content of the different EVs and their possible use in clinical and therapeutic settings.
The current application of EVs engineering is limited by a series of key factors that should be overcrossed to introduce these methodologies into daily clinical practice. Among them, a standardization of present methods of isolation and analysis results fundamental. Interestingly, EVs is a wide term that includes different vesicle types such as exosomes, microvesicles, and apoptotic bodies. This nomenclature, based on size, biogenesis, and cellular release mechanisms, is currently not consistent throughout the literature (17, 81, 82). Although the International Society of EVs (ISEV) is making an effort to unify this controversial nomenclature, favoring the term EVs instead of exosomes and of other terms, a consensus criterion does not still exist (82). ISEV is involved also in the standardization of the methodologies of EVs isolation and characterization (81). Also in this case, there are not commonly accepted procedures. The lack of a consensus regarding EVs isolation and even storing conditions (83) can result in impure exosome preparations, raising a possible explanation regarding some inconsistent results in literature.
In conclusion, the search for effective predictive and prognostic factors in prostate neoplasms is still ongoing. The growing knowledge about molecular biology of this tumor is bringing us very rapidly to a new age of therapeutic possibilities. Nevertheless, the cost–benefit ratio of the massive application of these new potential prognostic factors represents a crucial point in the choice of the “best one.” Based on this scenario, small EVs may represent a cornerstone in the future diagnostic and treatment-decision processes of PCa, leading to a more tailored and personalized approach for patients with advanced disease (Figure 1) (84).
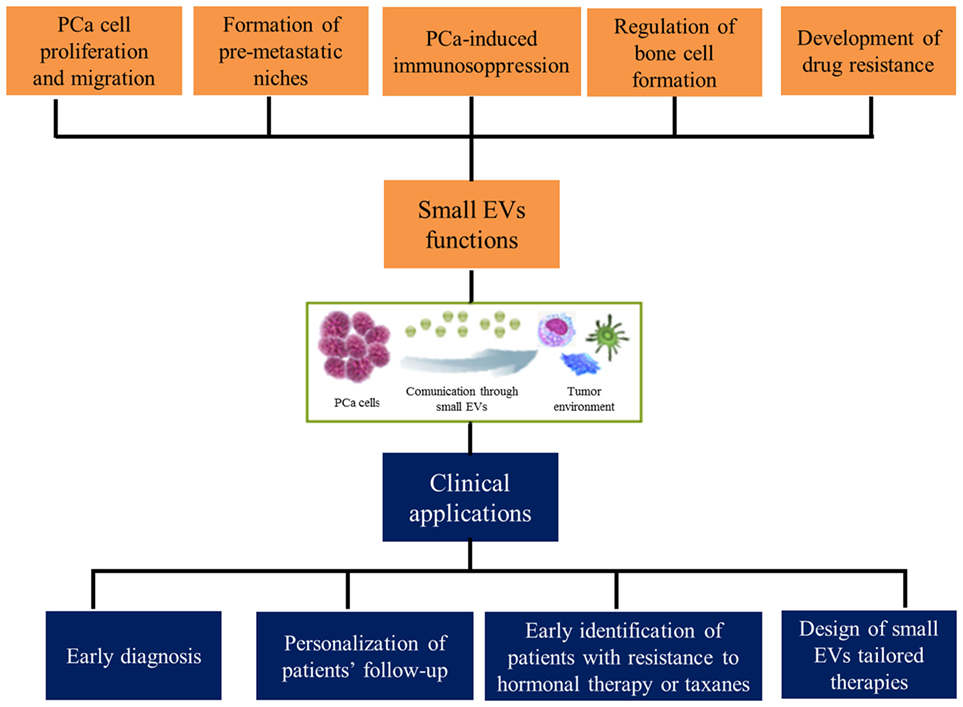
Figure 1. Functions exerted by small extracellular vesicles (EVs) in prostate cancer (PCa) microenvironment and their clinical implications.
RM and MS (Marina Scarpelli): conception and design. MG and MS (Matteo Santoni): drafting the manuscript. FC, FP, and AC: review of the literature. LC, NB, and AL-B: critical revision of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This paper was not funded. Declaration of interest: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
1. Langley RR, Fidler IJ. The seed and soil hypothesis revisited – the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer (2011) 128(11):2527–35. doi:10.1002/ijc.26031
2. El Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov (2013) 12(5):347–57. doi:10.1038/nrd3978
3. Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev (2013) 32:623–42. doi:10.1007/s10555-013-9441-9
4. Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signalling in breast cancer cell migration. Cell (2012) 151:1542–56. doi:10.1016/j.cell.2012.11.024
5. Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med (2012) 18:883–91. doi:10.1038/nm.2753
6. Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget (2015) 6:715–31. doi:10.18632/oncotarget.2711
7. Pisitkun T, Shen R-F, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A (2004) 101:13368–73. doi:10.1073/pnas.0403453101
8. Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol (2009) 20:363–79. doi:10.1681/ASN.2008040406
9. Nawaz M, Camussi G, Valadi H, Nazarenko I, Ekström K, Wang X, et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat Rev Urol (2014) 11:688–701. doi:10.1038/nrurol.2014.301
10. El-Sayed IY, Daher A, Destouches D, Firlej V, Kostallari E, Maillé P, et al. Extracellular vesicles released by mesenchymal-like prostate carcinoma cells modulate EMT state of recipient epithelial-like carcinoma cells through regulation of AR signaling. Cancer Lett (2017) 410:100–11. doi:10.1016/j.canlet.2017.09.010
11. Downes MR, Byrne JC, Pennington SR, Dunn MJ, Fitzpatrick JM, Watson RWG. Urinary markers for prostate cancer. BJU Int (2007) 99:263–8. doi:10.1111/j.1464-410X.2006.06610.x
12. Koh YQ, Almughlliq FB, Vaswani K, Peiris HN, Mitchell MD. Exosome enrichment by ultracentrifugation and size exclusion chromatography. Front Biosci (Landmark Ed) (2018) 23:865–74. doi:10.2741/4621
13. Jeppesen DK, Hvam ML, Primdahl-Bengtson B, Boysen AT, Whitehead B, Dyrskjøt L, et al. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J Extracell Vesicles (2014) 3:25011. doi:10.3402/jev.v3.25011
14. Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles (2012) 1:1. doi:10.3402/jev.v1i0.18397
15. Musante L, Saraswat M, Duriez E, Byrne B, Ravidà A, Domon B, et al. Biochemical and physical characterisation of urinary nanovesicles following CHAPS treatment. PLoS One (2012) 7(7):e37279. doi:10.1371/journal.pone.0037279
16. Miranda KC, Bond DT, McKee M, Skog J, Păunescu TG, Da Silva N, et al. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int (2010) 78(2):191–9. doi:10.1038/ki.2010.106
17. van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev (2012) 64(3):676–705. doi:10.1124/pr.112.005983
18. Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, et al. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles (2014) 3. doi:10.3402/jev.v3.24858
19. Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, et al. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics (2013) 13(22):3354–64. doi:10.1002/pmic.201300282
20. Böing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles (2014) 3. doi:10.3402/jev.v3.23430
21. Heinemann ML, Vykoukal J. Sequential filtration: a gentle method for the isolation of functional extracellular vesicles. Methods Mol Biol (2017) 1660:33–41. doi:10.1007/978-1-4939-7253-1_4
22. Lozano-Ramos I, Bancu I, Oliveira-Tercero A, Armengol MP, Menezes-Neto A, Del Portillo HA, et al. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J Extracell Vesicles (2015) 4:27369. doi:10.3402/jev.v4.27369
23. Kanchi Ravi R, Khosroheidari M, DiStefano JK. A modified precipitation method to isolate urinary exosomes. J Vis Exp (2015) 95:51158. doi:10.3791/51158
24. Alvarez ML. Isolation of urinary exosomes for RNA biomarker discovery using a simple, fast, and highly scalable method. Methods Mol Biol (2014) 1182:145–70. doi:10.1007/978-1-4939-1062-5_13
25. Royo F, Zuniga-Garcia P, Sanchez-Mosquera P, Egia A, Perez A, Loizaga A, et al. Different EV enrichment methods suitable for clinical settings yield different subpopulations of urinary extracellular vesicles from human samples. J Extracell Vesicles (2016) 5:29497. doi:10.3402/jev.v5.29497
26. Salih M, Fenton RA, Knipscheer J, Janssen JW, Vredenbregt-van den Berg MS, Jenster G, et al. An immunoassay for urinary extracellular vesicles. Am J Physiol Renal Physiol (2016) 310:F796–801. doi:10.1152/ajprenal.00463.2015
27. Duijvesz D, Versluis CY, van der Fels CA, Vredenbregt-van den Berg MS, Leivo J, Peltola MT, et al. Immuno-based detection of extracellular vesicles in urine as diagnostic marker for prostate cancer. Int J Cancer (2015) 137:2869–78. doi:10.1002/ijc.29664
28. Mizutani K, Terazawa R, Kameyama K, Kato T, Horie K, Tsuchiya T, et al. Isolation of prostate cancer-related exosomes. Anticancer Res (2014) 34:3419–23.
29. Brett SI, Lucien F, Guo C, Williams KC, Kim Y, Durfee PN, et al. Immunoaffinity based methods are superior to kits for purification of prostate derived extracellular vesicles from plasma samples. Prostate (2017) 77:1335–43. doi:10.1002/pros.23393
30. Oosthuyzen W, Sime NE, Ivy JR, Turtle EJ, Street JM, Pound J, et al. Quantification of human urinary exosomes by nanoparticle tracking analysis. J Physiol (2013) 591:5833–42. doi:10.1113/jphysiol.2013.264069
31. Perrini C, Strillacci MG, Bagnato A, Esposti P, Marini MG, Corradetti B, et al. Microvesicles secreted from equine amniotic-derived cells and their potential role in reducing inflammation in endometrial cells in an in-vitro model. Stem Cell Res Ther (2016) 7:169. doi:10.1186/s13287-016-0429-6
32. Lange-Consiglio A, Perrini C, Tasquier R, Deregibus MC, Camussi G, Pascucci L, et al. Equine amniotic microvesicles and their anti-inflammatory potential in a tenocyte model in vitro. Stem Cells Dev (2016) 25:610–21. doi:10.1089/scd.2015.0348
33. Nolan JP, Duggan E. Analysis of individual extracellular vesicles by flow cytometry. Methods Mol Biol (2018) 1678:79–92. doi:10.1007/978-1-4939-7346-0_5
34. Pedersen KW, Kierulf B, Neurauter A. Specific and generic isolation of extracellular vesicles with magnetic beads. Methods Mol Biol (2017) 1660:65–87. doi:10.1007/978-1-4939-7253-1_7
35. Biggs CN, Siddiqui KM, Al-Zahrani AA, Pardhan S, Brett SI, Guo QQ, et al. Prostate extracellular vesicles in patient plasma as a liquid biopsy platform for prostate cancer using nanoscale flow cytometry. Oncotarget (2016) 7:8839–49. doi:10.18632/oncotarget.6983
36. Pellegrini KL, Patil D, Douglas KJS, Lee G, Wehrmeyer K, Torlak M, et al. Detection of prostate cancer-specific transcripts in extracellular vesicles isolated from post-DRE urine. Prostate (2017) 77:990–9. doi:10.1002/pros.23355
37. Royo F, Zuniga-Garcia P, Torrano V, Loizaga A, Sanchez-Mosquera P, Ugalde-Olano A, et al. Transcriptomic profiling of urine extracellular vesicles reveals alterations of CDH3 in prostate cancer. Oncotarget (2016) 7:6835–46. doi:10.18632/oncotarget.6899
38. Rodriguez M, Bajo-Santos C, Hessvik NP, Lorenz S, Fromm B, Berge V, et al. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol Cancer (2017) 16:156. doi:10.1186/s12943-017-0726-4
39. Occhipinti G, Giulietti M, Principato G, Piva F. The choice of endogenous controls in exosomal microRNA assessments from biofluids. Tumour Biol (2016) 37:11657–65. doi:10.1007/s13277-016-5164-1
40. Guo K, Liang Z, Li F, Wang H. Comparison of miRNA and gene expression profiles between metastatic and primary prostate cancer. Oncol Lett (2017) 14:6085–90. doi:10.3892/ol.2017.6969
41. Giulietti M, Occhipinti G, Principato G, Piva F. Weighted gene co-expression network analysis reveals key genes involved in pancreatic ductal adenocarcinoma development. Cell Oncol (Dordr) (2016) 39:379–88. doi:10.1007/s13402-016-0283-7
42. Giulietti M, Occhipinti G, Principato G, Piva F. Identification of candidate miRNA biomarkers for pancreatic ductal adenocarcinoma by weighted gene co-expression network analysis. Cell Oncol (Dordr) (2017) 40:181–92. doi:10.1007/s13402-017-0315-y
43. Huang H, Zhang Q, Ye C, Lv JM, Liu X, Chen L, et al. Identification of prognostic markers of high grade prostate cancer through an integrated bioinformatics approach. J Cancer Res Clin Oncol (2017) 143(12):2571–9. doi:10.1007/s00432-017-2497-0
44. Motamedinia P, Scott AN, Bate KL, Sadeghi N, Salazar G, Shapiro E, et al. Urine exosomes for non-invasive assessment of gene expression and mutations of prostate cancer. PLoS One (2016) 11:e0154507. doi:10.1371/journal.pone.0154507
45. Lazaro-Ibanez E, Sanz-Garcia A, Visakorpi T, Escobedo-Lucea C, Siljander P, Ayuso-Sacido A, et al. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. Prostate (2014) 74:1379–90. doi:10.1002/pros.22853
46. Giulietti M, Piva F, D’Antonio M, D’Onorio De Meo P, Paoletti D, Castrignano T, et al. SpliceAid-F: a database of human splicing factors and their RNA-binding sites. Nucleic Acids Res (2013) 41:D125–31. doi:10.1093/nar/gks997
47. Piva F, Giulietti M, Nardi B, Bellantuono C, Principato G. An improved in silico selection of phenotype affecting polymorphisms in SLC6A4, HTR1A and HTR2A genes. Hum Psychopharmacol (2010) 25:153–61. doi:10.1002/hup.1100
48. Piva F, Giulietti M, Occhipinti G, Santoni M, Massari F, Sotte V, et al. Computational analysis of the mutations in BAP1, PBRM1 and SETD2 genes reveals the impaired molecular processes in renal cell carcinoma. Oncotarget (2015) 6:32161–8. doi:10.18632/oncotarget.5147
49. Giulietti M, Milantoni SA, Armeni T, Principato G, Piva F. ExportAid: database of RNA elements regulating nuclear RNA export in mammals. Bioinformatics (2015) 31:246–51. doi:10.1093/bioinformatics/btu620
50. Wang L, Skotland T, Berge V, Sandvig K, Llorente A. Exosomal proteins as prostate cancer biomarkers in urine: from mass spectrometry discovery to immunoassay-based validation. Eur J Pharm Sci (2017) 98:80–5. doi:10.1016/j.ejps.2016.09.023
51. Welton JL, Brennan P, Gurney M, Webber JP, Spary LK, Carton DG, et al. Proteomics analysis of vesicles isolated from plasma and urine of prostate cancer patients using a multiplex, aptamer-based protein array. J Extracell Vesicles (2016) 5:31209. doi:10.3402/jev.v5.31209
52. Turay D, Khan S, Diaz Osterman CJ, Curtis MP, Khaira B, Neidigh JW, et al. Proteomic profiling of serum-derived exosomes from ethnically diverse prostate cancer patients. Cancer Invest (2016) 34:1–11. doi:10.3109/07357907.2015.1081921
53. Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, et al. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer (2017) 70:122–32. doi:10.1016/j.ejca.2016.10.011
54. Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski A, et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta (2013) 1831:1302–9. doi:10.1016/j.bbalip.2013.04.011
55. Logozzi M, Angelini DF, Iessi E, Mizzoni D, Di Raimo R, Federici C, et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett (2017) 403:318–29. doi:10.1016/j.canlet.2017.06.036
56. McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, et al. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol (2016) 2(7):882–9. doi:10.1001/jamaoncol.2016.0097
57. Worst TS, von Hardenberg J, Gross JC, Erben P, Schnölzer M, Hausser I, et al. Database-augmented mass spectrometry analysis of exosomes identifies Claudin 3 as a putative prostate cancer biomarker. Mol Cell Proteomics (2017) 16(6):998–1008. doi:10.1074/mcp.M117.068577
58. Kawakami K, Fujita Y, Matsuda Y, Arai T, Horie K, Kameyama K, et al. Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer (2017) 17(1):316. doi:10.1186/s12885-017-3301-x
59. Wani S, Kaul D, Mavuduru RS, Kakkar N, Bhatia A. Urinary-exosomal miR-2909: a novel pathognomonic trait of prostate cancer severity. J Biotechnol (2017) 259:135–9. doi:10.1016/j.jbiotec.2017.07.029
60. Bryzgunova OE, Zaripov MM, Skvortsova TE, Lekchnov EA, Grigor’eva AE, Zaporozhchenko IA, et al. Comparative study of extracellular vesicles from the urine of healthy individuals and prostate cancer patients. PLoS One (2016) 11(6):e0157566. doi:10.1371/journal.pone.0157566
61. Foj L, Ferrer F, Serra M, Arévalo A, Gavagnach M, Giménez N, et al. Exosomal and non-exosomal urinary miRNAs in prostate cancer detection and prognosis. Prostate (2017) 77(6):573–83. doi:10.1002/pros.23295
62. Hosseini-Beheshti E, Choi W, Weiswald LB, Kharmate G, Ghaffari M, Roshan-Moniri M, et al. Exosomes confer pro-survival signals to alter the phenotype of prostate cells in their surrounding environment. Oncotarget (2016) 7(12):14639–58. doi:10.18632/oncotarget.7052
63. Karlsson T, Lundholm M, Widmark A, Persson E. Tumor cell-derived exosomes from the prostate cancer cell line TRAMP-C1 impair osteoclast formation and differentiation. PLoS One (2016) 11(11):e0166284. doi:10.1371/journal.pone.0166284
64. Ramteke A, Ting H, Agarwal C, Mateen S, Somasagara R, Hussain A, et al. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog (2015) 54(7):554–65. doi:10.1002/mc.22124
65. Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG, et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget (2016) 7(4):3993–4008. doi:10.18632/oncotarget.6540
66. Lin LC, Gao AC, Lai CH, Hsieh JT, Lin H. Induction of neuroendocrine differentiation in castration resistant prostate cancer cells by adipocyte differentiation-related protein (ADRP) delivered by exosomes. Cancer Lett (2017) 391:74–82. doi:10.1016/j.canlet.2017.01.018
67. Salimu J, Webber J, Gurney M, Al-Taei S, Clayton A, Tabi Z. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J Extracell Vesicles (2017) 6(1):1368823. doi:10.1080/20013078.2017.1368823
68. Lundholm M, Schröder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One (2014) 9(9):e108925. doi:10.1371/journal.pone.0108925
69. Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol (2004) 164:217–27. doi:10.1016/S0002-9440(10)63112-4
70. Soekmadji C, Riches JD, Russell PJ, Ruelcke JE, McPherson S, Wang C, et al. Modulation of paracrine signaling by CD9 positive small extracellular vesicles mediates cellular growth of androgen deprived prostate cancer. Oncotarget (2016) 8:52237–55. doi:10.18632/oncotarget.11111
71. Soekmadji C, Corcoran NM, Oleinikova I, Jovanovic L; Australian Prostate Cancer Collaboration BioResource, Ramm GA, et al. Extracellular vesicles for personalized therapy decision support in advanced metastatic cancers and its potential impact for prostate cancer. Prostate (2017) 77:1416–23. doi:10.1002/pros.23403
72. Mentkowski KI, Snitzer JD, Rusnak S, Lang JK. Therapeutic potential of engineered extracellular vesicles. AAPS J (2018) 20(3):50. doi:10.1208/s12248-018-0211-z
73. Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, et al. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. Eur Uro (2017) 71(4):680–7. doi:10.1016/j.eururo.2016.08.012
74. Li J, Yang X, Guan H, Mizokami A, Keller ET, Xu X, et al. Exosome-derived microRNAs contribute to prostate cancer chemoresistance. Int J Oncol (2016) 49(2):838–46. doi:10.3892/ijo.2016.3560
75. Kharaziha P, Chioureas D, Rutishauser D, Baltatzis G, Lennartsson L, Fonseca P, et al. Molecular profiling of prostate cancer derived exosomes may reveal a predictive signature for response to docetaxel. Oncotarget (2015) 6(25):21740–54. doi:10.18632/oncotarget.3226
76. Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One (2012) 7(12):e50999. doi:10.1371/journal.pone.0050999
77. Kawakami K, Fujita Y, Kato T, Mizutani K, Kameyama K, Tsumoto H, et al. Integrin β4 and vinculin contained in exosomes are potential markers for progression of prostate cancer associated with taxane-resistance. Int J Oncol (2015) 47(1):384–90. doi:10.3892/ijo.2015.3011
78. Kato T, Mizutani K, Kameyama K, Kawakami K, Fujita Y, Nakane K, et al. Serum exosomal P-glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. Urol Oncol (2015) 33(9):.e15–20. doi:10.1016/j.urolonc.2015.04.019
79. Lee EY, Park KS, Yoon YJ, Lee J, Moon HG, Jang SC, et al. Therapeutic effects of autologous tumor-derived nanovesicles on melanoma growth and metastasis. PLoS One (2012) 7:e33330. doi:10.1371/journal.pone.0033330
80. György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol (2015) 55:439–64. doi:10.1146/annurev-pharmtox-010814-124630
81. Simpson RJ, Mathivanan S. Extracellular microvesicles: the need for internationally recognised nomenclature and stringent purification criteria. J Proteomics Bioinform (2012) 5:ii–ii. doi:10.4172/jpb.10000e10
82. Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of EVs and their physiological functions. J Extracell Vesicles (2015) 4:27066. doi:10.3402/jev.v4.27066
83. Wu Y, Deng W, Klinke DJ. Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst (2015) 140(19):6631–42. doi:10.1039/c5an00688k
Keywords: prostate cancer, drug resistance, tumor microenvironment, tumor biomarkers, small extracellular vesicles
Citation: Giulietti M, Santoni M, Cimadamore A, Carrozza F, Piva F, Cheng L, Lopez-Beltran A, Scarpelli M, Battelli N and Montironi R (2018) Exploring Small Extracellular Vesicles for Precision Medicine in Prostate Cancer. Front. Oncol. 8:221. doi: 10.3389/fonc.2018.00221
Received: 05 December 2017; Accepted: 29 May 2018;
Published: 13 June 2018
Edited by:
Stéphane Terry, Institut Gustave Roussy, FranceReviewed by:
Ihsan Y. El-Sayed, Université de Lille, FranceCopyright: © 2018 Giulietti, Santoni, Cimadamore, Carrozza, Piva, Cheng, Lopez-Beltran, Scarpelli, Battelli and Montironi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodolfo Montironi, ci5tb250aXJvbmlAdW5pdnBtLml0
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.