- 1Hermann Buhl Institute for Hypoxia and Sleep Medicine Research, Bad Aibling, Germany
- 2Department of Sports Science, University Innsbruck, Innsbruck, Austria
- 3Division of Sports Medicine and Rehabilitation, Department of Medicine, University Ulm, Ulm, Germany
- 4Institute of Human Genetics, University of Ulm, Ulm, Germany
Objective: The development of breast cancer cells is linked to hypoxia. The hypoxia-induced factor HIF-1α influences metastasis through neovascularization. Hypoxia seems to decrease the responsiveness to hormonal treatment due to loss of estrogen receptors (ERs). Obesity is discussed to increase hypoxia in adipocytes, which promotes a favorable environment for tumor cells in mammary fat tissue, whereas, tumor cells profit from good oxygen supply and are influenced by its deprivation as target regions within tumors show. This review gives an overview of the current state on research of hypoxia and breast cancer in human adipose tissue.
Methods: A systematic literature search was conducted on PubMed (2000–2016) by applying hypoxia and/or adipocytes and breast cancer as keywords. Review articles were excluded as well as languages other than English or German. There was no restriction regarding the study design or type of breast cancer. A total of 35 papers were found. Eight studies were excluded due to missing at least two of the three keywords. One paper was removed due to Russian language, and one was dismissed due to lack of adherence. Seven papers were identified as reviews. After applying exclusion criteria, 18 articles were eligible for inclusion.
Results: Two articles describe the impairment of mammary epithelial cell polarization through hypoxic preconditioning. A high amount of adipocytes enhances cancer progression due to the increased expression of HIF-1α which causes the loss of ER α protein as stated in four articles. Four articles analyzed that increased activation of HIF’s induces a series of transcriptions resulting in tumor angiogenesis. HIF inhibition, especially when combined with cytotoxic chemotherapy, holds strong potential for tumor suppression as stated in further four articles. In two articles there is evidence of a strong connection between hypoxia, oxidative stress and a poor prognosis for breast cancer via HIF regulated pathways. Acute hypoxia seems to normalize the microenvironment in breast cancer tissue and has proven to affect tumor growth positively as covered in two articles.
Conclusion: This review indicates that the development of breast cancer is influenced by hypoxia. A high amount of adipocytes enhances cancer progression due to the increased expression of HIF-1α.
Introduction
Breast cancer is the most commonly diagnosed cancer in women (1). In 2012, over a million new cases were identified and figures are rising due to late diagnosis at already quite advanced cancer stages (World Cancer Research Fund International, 2012) (2). Breast cancer represents 25% of all cancer types in women and is the fifth most common cause of death. It is classified into three main groups (3). The hormone receptor (HR) positive group, which expresses estrogen receptor (ER) or progesterone receptor (PR); the epidermal growth factor receptor 2 (HER2) positive group and the triple-negative breast cancer (TNBC) group without expression of ER, PR, and HER2. 90% of breast cancer patients die in consequence of metastasis most commonly found in bone tissue (4).
Several prospective, epidemiological studies show that there is a direct relationship between obesity and cancer (5–9). Especially, the manifestation of breast cancer seems to be linked to obesity (10). Notably, female obese breast cancer patients show a less sufficient response to the same dosage of chemotherapy compared to female lean breast cancer patients (11). In premenopausal women, the risk for breast cancer is reduced with increasing body mass index (BMI). Thus, postmenopausal women are at higher risk for breast cancer development if BMI is increased (10). There is a strong association between BMI and breast cancer in ER−/PR+ receptor positive breast cancer types as found in a dose–response meta-analysis (12). This could be due to an increase in sex-hormones triggered by an increase in estradiol production of adipose tissue, caused by a higher activity of aromatase enzymes (13). Adipose tissue is divided into brown adipose tissue (BAT) and white adipose tissue (WAT). BAT is only 50 g compared to kilograms of WAT, which is an endocrine organ producing a large number of adipokines and cytokines (14). In the presence of hypertrophy, the protein synthesis of white adipocytes is changed toward producing pro-inflammatory adipokines, such as tumor necrosis factor-alpha. On the contrary, adiponectin is an anti-inflammatory adipokine with cardio-protective and anti-tumor actions. Dysfunctional adipose tissue in obesity causes defective adipokines with increased levels of pro-inflammatory factors (14). The currently available therapies for advanced breast cancer stages in obese women seem to achieve a rather poor clinical outcome. Conclusively, a long-lasting reduced-calorie diet seems to lower the risk for breast cancer (15).
It remains difficult to identify single impact factors as dietary changes, energy balance, amount of physical activity, and obesity on cancer development and progression (16, 17). It also remains unclear if the higher amount of adipose tissue and the resulting tissue hypoxia in obesity contributes to the development of cancer. Especially, the elevated activation of HIF’s seems to increase metastasis and worsen the prognosis of patient survival (18). Intra-tumoral partial pressure of oxygen (PO2) is decreased by 20% compared to healthy tissue (19). PO2 values below 10 mmHg have shown to drive cancer growth, metastasis, and mortality. In cancer tissue, oxygen supply can be restrained due to the proliferation of vessels. Therefore, HIF’s, as the key factors of hypoxic cancer cells, seem to stimulate inflammation and angiogenesis (18).
The concurrence of adipose tissue hypoxia to cancer development is not fully explained, but tumors are most likely surrounded by adipose tissue (20–22). Hence, it is likely that such a malignant environment may promote tumor development (22).
Methods
A literature search was conducted according to preferred reporting items for review and meta-analysis protocols (PRISMA-2015) statement. Via PubMed (2000–2016) search and manual searches of reference lists, studies examining the relationship between hypoxia, adipocytes, and breast cancer were identified. The keywords for the search were (hypoxia and/or adipocyte) and breast cancer. Articles had to be in English or German language. Review articles were excluded. There was no restriction regarding study design or certain breast cancer types. After this search, a total of 35 papers were identified. After title and abstract evaluation, eight studies were excluded due to lack of coherences with the topic. Out of four papers not offering open access, one paper was excluded due to Russian language, and another paper was also excluded due to lack of coherence. After assessing full-text articles for eligibility, seven papers were identified as reviews. Finally, 18 articles were eligible for inclusion in this review and selected for analysis. Figure 1 shows a flow diagram according to PRISMA-2015 protocols displaying the process of literature identification, screening, eligibility, and inclusion.
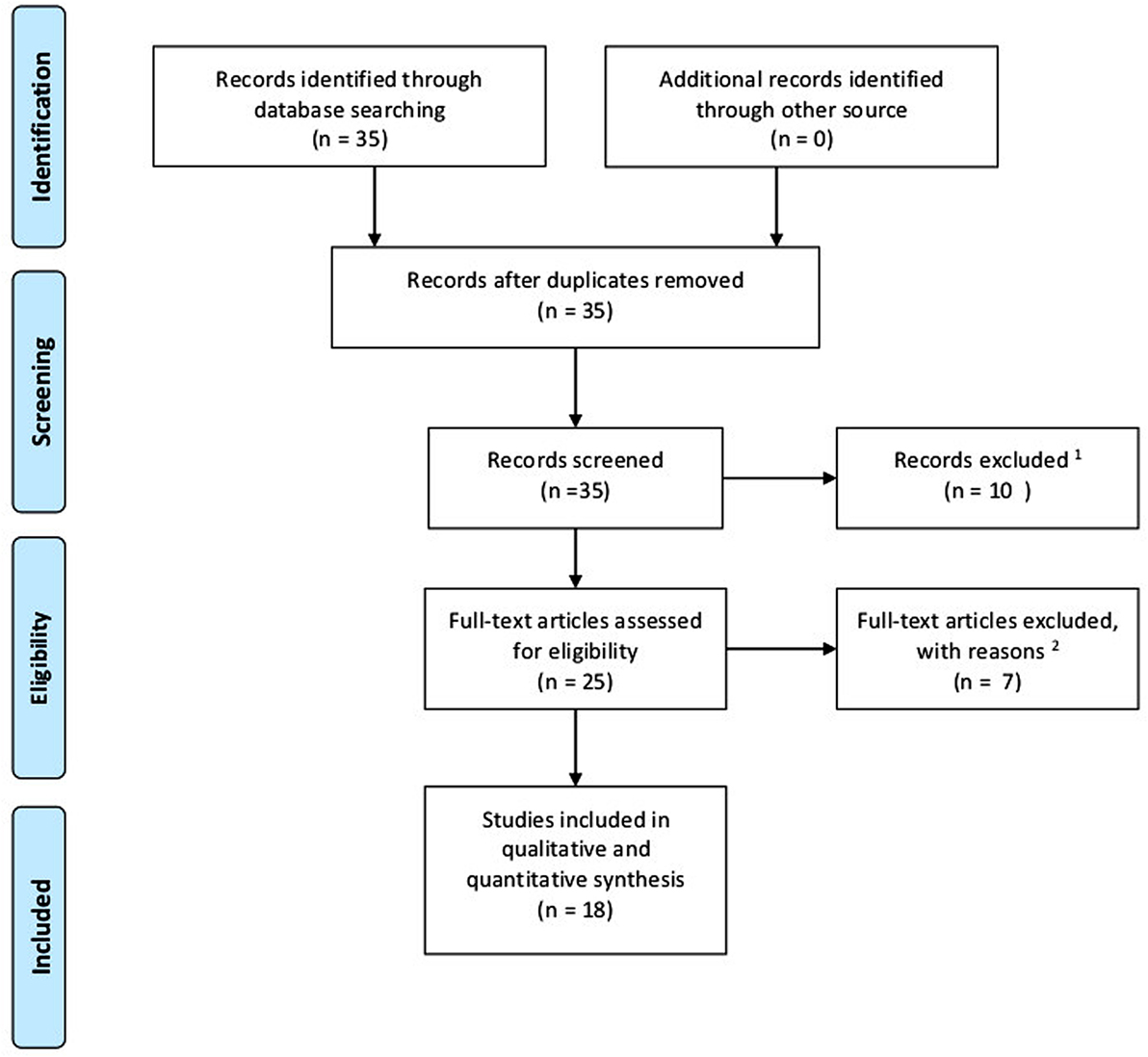
Figure 1. Flow diagram according to PRISMA-2015 protocols displaying process of literature identification, screening, eligibility, and inclusion. 1Language other than English or German; at least two of the three keywords missing. 2Fulltexts identified as reviews.
Results
After the final evaluation of the 18 included articles, six on-topic categories were identified. Two studies identify the impact of hypoxic conditioning on malignant and non-malignant mammary epithelial cells. Two studies examine the role of hypoxic adipocytes in the development of breast cancer cells. Five studies approach the activation of HIF’s occurring in hypoxic adipocytes, which promotes breast cancer cell growth. Two studies identify the distinct biochemical responses of the body responsible for HIF inhibition. Three studies investigate medical interventions for HIF inhibition and limitation of breast cancer cell growth. Two studies express alternatives to drug cure of breast cancer inhibiting breast cancer via HIF pathways. Table 1 displays a study summary of HIF-related effects through different physiological and biochemical pathways on breast cancer progression.
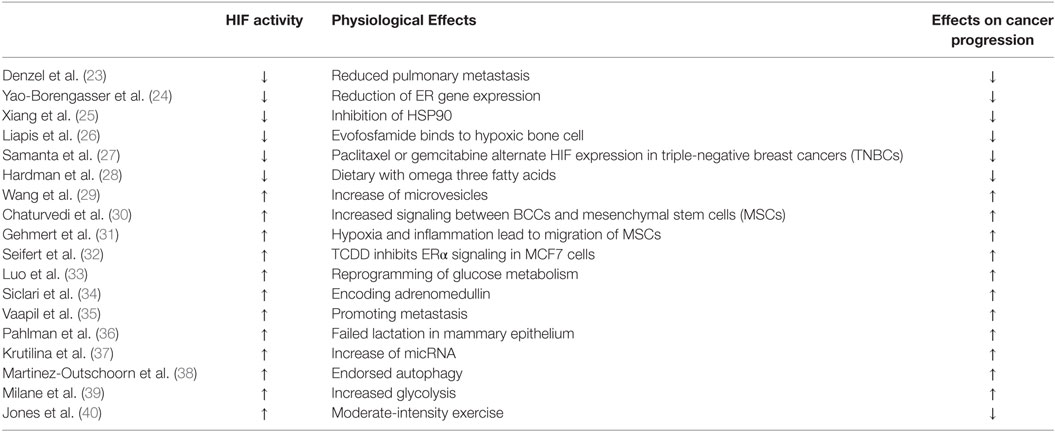
Table 1. Study summary of HIF-related effects through different physiological and biochemical pathways on breast cancer progression.
Hypoxic Preconditioning
The most important element for tumor growth is the development of tumor vasculature (41–43). This vasculature is highly disorganized and constantly changing due to blood vessel gain and loss. A consequence of this alteration is the fluctuation of oxygen- and glucose levels, which result in heterogeneous states of hypoxia, anaerobic, and aerobic glycolysis (42). If a cell happens to be above its diffusion limit of oxygen, chronic hypoxia occurs (44). Transient hypoxia occurs due to local oxygen depletion (44). As a result of the fluctuant oxygenation within a tumor, it is possible that the hypoxia-induced glycolysis pre-conditions cancer cells for aerobic glycolysis (45). Increased glycolysis with and without the presence of oxygen is an important indicator for cancer and the connecting link between multidrug-resistant breast cancer cells and hypoxia (46–49). Milane et al. (39) extracted proteins of TNBC and ovarian cancer cell lines pre-exposed to either normoxic or hypoxic conditions. The TNBC cell line MDA-MB-231 experienced the most significant hypoxic transformation with an increase in all glycolytic proteins glucose transporters (GLUT-1 and GLUT-3), hexokinase 1 and 2, phosphofructokinase (PFK), aldolase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerate kinase (PGK), enolase, pyruvate kinase, and lactate dehydrogenase (LDH). That indicates that each cell line has a time-specific threshold for hypoxic transformation inducing glycolysis (39). This finding is based on malignant breast cancer cells, but little is known about the effects of hypoxia on non-malignant cells. Vaapil et al. (35) cultivated normal human primary breast epithelial cells and non-malignant mammary epithelial MCF-10A cells under hypoxia and normoxia. The breast epithelial cells with high HIF-levels were found to be immature compared to the well-oxygenated cells. Due to the fact that constant cell proliferation is followed by high HIF-levels in certain compartments of the tumor, cellular differentiation of non-malignant human mammary epithelial cells is restrained (35).
Hypoxic preconditioning impairs polarization and organization of mammary epithelial cells and enhances cancer manifestation and progression.
The Role of Hypoxic Adipocytes in the Development of Breast Cancer Cells
Obesity is accompanied with the development of hypoxic fat tissue and an increase of oxidative stress (50, 51). Conditioned by rising cell size, oxygen (O2) diffusion is decreased and vascular growth impaired in the hypoxic fat tissue (52). The mitochondrial production of excessive free fatty acids leads to increased procreation of reactive oxygen species (ROS), which causes oxidative stress (53, 54). As a consequence, the production of adipokines, cell signaling proteins secreted by adipose tissue, is defective and leads to angiogenesis and inflammation (50). This reaction chain creates a pro-malignancy setting in epithelial tissue for the development of breast cancer cells. Gehmert et al. (31) isolated mesenchymal stem cells (MSCs) from subcutaneous fat tissue. Breast cancer cells were injected into mammary fat pad and it showed that MSCs migrated primarily toward an inflammatory milieu in tumor stroma and vasculature independent of biological processes causing inflammation. It is suggested that the migration of MSCs depends on cancer-secreted cytokines due to the lack of inflammatory response by the immune system (31). Furthermore, Yao-Borengasser et al. (24) co-cultured the progressive breast cancer cell line MCF7 with human adipocytes. The MCF7 cell line is the most investigated cell line to analyze the cross talk of estrogen and ERα protein (estrogen receptor alpha protein) (32). They found a decreased level of ERα protein caused by deregulated adipocytes under hypoxic cell conditions. In human adipocyte cells, HIF-1α gene expression was increased and accompanied by a reduction of ER gene expression. With the loss of ERα protein, the tumor progresses and hormone therapy is less efficient (24). Seifert et al. also analyzed MCF7 cell lines cultivated under mild hypoxic conditions (5% of O2 for a duration of 6 h) (32). These cell lines were exposed to TCDD (2,3,7,8-tetrachlorodibenzo-para-dioxin), a pollutant causing a variety of biochemical and toxic effects, accumulating in adipose tissue. The prevalence of breast cancer cells was significantly higher due to the positive correlation with increased TCDD serum levels. TCDD reduces the hypoxia-induced stabilization and activation of HIF-1α (32).
Denzel et al. states that drug inhibition of the pro-angiogenic HIF-1α pathway only leads to temporary improvement and breast cancer resists treatment after a limited time frame (23). The effect of HIF on changes in human adipocytes inclines with extended exposure time (55, 56). They investigated cellular functions of adiponectin in breast cancer cells creating an adiponectin null mouse model of mammary cancer. The treatment of adiponectin leads to a reduction of human breast cancer cells due to adiponectins’ cancer-protective functions. Vessel density is restrained through tumor vasculature because of adiponectin deficit. This limits the supply of oxygen and nutrients (23). Therefore, high adiponectin levels in women are associated with a lower risk of breast cancer and tumor metastasis (57, 58).
A high amount of adipocytes enhances cancer progression due to the increased expression of HIF-1α which causes the loss of ERα protein. Thus, a high amount of the peptide hormone adiponectin appears to be cancer protective.
Activation of HIF’s and the Impact on Breast Cancer Cell Growth
HIF-1α and HIF-2α are linked to breast cancer metastasis and poor patients’ survival (21, 59). The expression of HIF-1α and HIF-2α occurs differently during separate phases of mammary gland development and function (36). Selective inhibition of HIF-1α expression in mammary epithelium leads to lactation failure and in breast cancer models to increased tumor growth (60–62). Pahlman et al. investigated the separate phases using different mouse models with MCF-7 breast cancer cells. They found that the regulation and expression of the two factors and its subunits is not merely dependent on the availability of oxygen (36). Under hypoxic condition, HIFs are stabilized. In a malignant setting, the activation of HIF-induced transcriptions is implemented in extracellular proteolytic activity, invasion, and angiogenesis (36). Wang et al. cultivated TNBC cell lines that were exposed to hypoxia (29). These cells increased their production of microvesicles due to HIF expression. Microvesicles contain proteins that stimulate the invasion and metastasis of breast cancer cells (63). Chaturvedi et al. found that tumor growth, which promotes signals between TBNC’s and MSCs is stimulated by HIF activity (30). HIF activates transcription genes, which encode proteins that play a role in proliferation of breast cancer cells. As stated by Luo et al., some of these proteins only interact with HIF-1α, but not with HIF-2α. The consequence is the reprogramming of the glucose metabolism of breast cancer cells which generates macromolecular blocks, such as amino acids and acetyl CoA, that release more breast cancer cells (33). Furthermore, Siclari et al. (34) identify adrenomedullin as a 52-amino acid peptide for which gene transcription is increased by the HIF-1α pathway. This peptide stimulates angiogenesis and proliferation. Many cancer types release adrenomedullin and its receptors which is indirectly connected to poor survival probability (64).
Taken together, increased activation of HIF’s induces a series of transcriptions resulting in tumor invasion and angiogenesis. Adrenomedullin is one of them which plays a major role.
Inhibition of HIF’s and the Impact on Breast Cancer Cell Growth
Tumor hypoxia contributes to a great degree to treatment failure and increased patients’ mortality for a broad range of malignancies (65). Hypoxic regions within a solid tumor contain cancer cells that resist conventional chemotherapy or radiotherapy (66). This leads to cancer recurrence and metastasis (67). HIF’s activate two main transcription processes. First, the gene expression of vascular endothelial growth factors (VEGFs) which contributes to vascularization (68, 69) and, second, the expression of proteins regulating the change from mainly oxidative to glycolytic metabolism (70). The identification of chemical HIF inhibitors and their mechanisms has been a relevant target in anti-cancer research (71). The difficulty in analyzing the development of HIF inhibitors is the lack of specificity. In the ER−/PR+ cancer group, there are already appropriate receptor-blocking inhibitors in use while we still lack comparable methods for TNBCs (72). This type of cancer is associated with increased mortality compared to other types. Inhibition of HIF’s and its target genes in consequence could provide a feasible method for tumor suppression.
Xiang et al. showed that in human breast cancer cell cultures the drug Ganetesip inhibits, among others, the expression of the heat shock protein 90 (HSP90). The lack of HSP90 leads to a degeneration of HIF-1α (25). The distribution of HIF-1α was decreased by 35% in breast cancer cells and the expression of VEGFs was reduced as well. The inhibition of HSP90 resulted in a reduction of tumor weight and -growth (25). Another prodrug exhibiting hypoxia-selective cytotoxicity on breast cancer cells is Evofosfamide (TH-302). Liapis et al. show that by binding to hypoxic bone cells, the drug is able to destroy 50–90% of hypoxic cancer cells in bone tissue (26). The advantage of Evofosfamide therapy seems to be greatest when combined with cytotoxic chemotherapy. The combination of chemotherapy and HIF inhibiting drugs is also suggested by Samanta et al. This is based on the finding that paclitaxel as well as gemcitabine change the activity of HIF expression and transcription in human TNBC cell lines (27).
HIF inhibition, especially when combined with cytotoxic chemotherapy, holds strong potential for tumor suppression as well as for the reduction of metastasis.
Self-Regulating Mechanisms of the Human Body and Its Impact on HIF Expression
The human body offers several regulating mechanisms affecting HIF expression and in consequence tumor progression. One important regulating mechanism seems to be the distinct expression profiles of microRNAs that are associated with molecular subgroups and pathological characteristics in breast cancer (73). Krutilina et al. (37) link the expression of microRNAs to a HIF-1α dependent hypoxic response. A growing number of microRNAs have been described as oncogenes and tumor suppressors. Within solid tumors, microRNAs have proven to be downregulated which causes a higher expression of HIF-1α. In consequence, the downregulation of microRNAs withholds a higher probability for metastasis (74–76). One of the most frequently deregulated microRNAs-encoding genes in human cancer is the polycistronic MIR17HG gene, which encodes six microRNAs including miR-18a. Increased expression of miR-18a in MDA-MB-231 breast cancer cell lines has shown to reduce primary tumor growth and lung metastasis and miR-18a inhibition promotes tumor growth and lung metastasis (37). Besides other self-regulating mechanisms of the human body, the regulation of autophagy heavily affects the development and growth of breast cancer. Autophagy is a catabolic process responsible for the systematic degradation and recycling of cellular components (38). Yao-Borengasser et al. show that hypoxia and oxidative stress promote autophagy and support a pro-malignancy setting in epithelial tissue for the development of breast cancer cells (24, 77). Furthermore, as stated by Martinez-Outschoorn et al. (38) in some cases, autophagy promotes tumor progression while in other cases autophagy has shown to have tumor-suppressive effects. Increased HIF expression promotes autophagy and stromal caveolin-1 is degraded. Caveolin-1 appears to be tumor suppressive and low levels carry a poor prognosis for tumor development for the patient (78–80).
The human body offers a range of tumor affecting mechanisms that are not fully understood. Nevertheless, there seems to be a strong connection between hypoxia, oxidative stress, and a poor prognosis for breast cancer via HIF-regulated pathways.
Alternatives to Drug Cure of Breast Cancer Inhibiting Breast Cancer Cell Growth
Physical activity is discussed as a supportive factor for breast cancer therapy and has proven to be quite effective (81, 82). Jones et al. (40) investigated effects of moderate aerobic exercise on tumor characteristics, such as vascularization, angiogenesis, and metabolism. MDA-MB-231 breast cancer cell line implanted mice were randomly assigned to voluntary wheel running. Moderate aerobic exercise has shown to increase intra tumor vascularization, which leads to normalization of tissue environment. This is one of the first studies to evaluate the impact of an exercise intervention on the microenvironment in cancer tissue (40). In contrast to other studies exercise-induced high concentration of HIF is associated with a normalization of cancer microenvironment. This is thought to improve oxygenation and removal of by-products in the long run. In several other studies, regular moderate-intensity exercise is associated with a 30–50% reduction in the risk of mortality in cancer, a fact which supports this finding (83, 84). Physical exercise as well as dietary interventions has shown to affect tumor growth and progression. Hardman et al. investigated the effect of an omega-3 fatty acids enriched diet on mice bearing MDA-MB-231 breast cancer cells. This dietary delays tumor growth and vascularization which could be ought to the reduction of oxygen radicals and HIF expression in consequence (28).
Acute hypoxia seems to normalize the microenvironment in breast cancer tissue and has proven to affect tumor growth and progression positively.
Conclusion
There seems to be a strong linkage between adipose tissue hypoxia and the development, growth, and progression of breast cancer. HIF-1α and its target genes play a strong role in driving breast cancer cell proliferation. A high amount of adipocytes enhances cancer progression due to the increased expression of HIF-1α which causes the loss of ERα protein. Thus, a high amount of the peptide hormone adiponectin appears to be cancer protective. On the other hand, tissue hypoxia seems to provide a feasible pathway for the identification of cancer cells and their degeneration. Physical activity shows to improve tissue hypoxia and to reduce adipose tissue and is very likely to improve prognosis as well as therapy outcome in breast cancer (85).
Author Contributions
LR and SP: literature search and analysis and writing manuscript; JH: writing manuscript; and NN: designing research strategy, optimization of keywords, and revising manuscript.
Conflict of Interest Statement
We declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. James FR, Wootton S, Jackson A, Wiseman M, Copson ER, Cutress RI. Obesity in breast cancer – what is the risk factor? Eur J Cancer (2015) 51(6):705–20. doi:10.1016/j.ejca.2015.01.057
3. Smid M, Wang Y, Zhang Y, Sieuwerts AM, Yu J, Klijn JG, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res (2008) 68(9):3108–14. doi:10.1158/0008-5472.CAN-07-5644
4. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res (2006) 12(20 Pt 2):6243s–9s. doi:10.1158/1078-0432.CCR-06-0931
5. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med (2003) 348(17):1625–38. doi:10.1056/NEJMoa021423
6. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer (2004) 4(8):579–91. doi:10.1038/nrc1408
7. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (2008) 371(9612):569–78. doi:10.1016/S0140-6736(08)60269-X
8. Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc (2008) 67(3):253–6. doi:10.1017/S002966510800712X
9. Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev (2002) 11(Suppl 2):S94–100.
10. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ (2007) 335(7630):1134. doi:10.1136/bmj.39367.495995.AE
11. Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist (2010) 15(6):556–65. doi:10.1634/theoncologist.2009-0285
12. Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol (2008) 9(11):1039–47. doi:10.1016/S1470-2045(08)70235-3
13. de Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes (2013) 2013:291546. doi:10.1155/2013/291546
14. Divella R, de Luca R, Abbate I, Naglieri E, Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer (2016) 7(15):2346–59. doi:10.7150/jca.16884
15. Spindler SR. Rapid and reversible induction of the longevity, anticancer and genomic effects of caloric restriction. Mech Ageing Dev (2005) 126(9):960–6. doi:10.1016/j.mad.2005.03.016
16. Pallavi R, Giorgio M, Pelicci PG. Insights into the beneficial effect of caloric/dietary restriction for a healthy and prolonged life. Front Physiol (2012) 3:318. doi:10.3389/fphys.2012.00318
17. Imayama I, Ulrich CM, Alfano CM, Wang C, Xiao L, Wener MH, et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res (2012) 72(9):2314–26. doi:10.1158/0008-5472.CAN-11-3092
18. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell (2012) 148(3):399–408. doi:10.1016/j.cell.2012.01.021
19. Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal (2007) 9(8):1221–35. doi:10.1089/ars.2007.1628
20. Semenza GL. Advances in cancer biology and therapy. J Mol Med (Berl) (2013) 91(4):409. doi:10.1007/s00109-013-1024-2
21. Bos R, Zhong H, Hanrahan CF, Mommers EC, Semenza GL, Pinedo HM, et al. Levels of hypoxia-inducible factor-1 alpha during breast carcinogenesis. J Natl Cancer Inst (2001) 93(4):309–14. doi:10.1093/jnci/93.4.309
22. Schindl M, Schoppmann SF, Samonigg H, Hausmaninger H, Kwasny W, Gnant M, et al. Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res (2002) 8(6):1831–7.
23. Denzel MS, Hebbard LW, Shostak G, Shapiro L, Cardiff RD, Ranscht B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin Cancer Res (2009) 15(10):3256–64. doi:10.1158/1078-0432.CCR-08-2661
24. Yao-Borengasser A, Monzavi-Karbassi B, Hedges RA, Rogers LJ, Kadlubar SA, Kieber-Emmons T. Adipocyte hypoxia promotes epithelial-mesenchymal transition-related gene expression and estrogen receptor-negative phenotype in breast cancer cells. Oncol Rep (2015) 33(6):2689–94. doi:10.3892/or.2015.3880
25. Xiang L, Gilkes DM, Chaturvedi P, Luo W, Hu H, Takano N, et al. Ganetespib blocks HIF-1 activity and inhibits tumor growth, vascularization, stem cell maintenance, invasion, and metastasis in orthotopic mouse models of triple-negative breast cancer. J Mol Med (Berl) (2014) 92(2):151–64. doi:10.1007/s00109-013-1102-5
26. Liapis V, Zinonos I, Labrinidis A, Hay S, Ponomarev V, Panagopoulos V, et al. Anticancer efficacy of the hypoxia-activated prodrug evofosfamide (TH-302) in osteolytic breast cancer murine models. Cancer Med (2016) 5(3):534–45. doi:10.1002/cam4.599
27. Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A (2014) 111(50):E5429–38. doi:10.1073/pnas.1421438111
28. Hardman WE, Sun L, Short N, Cameron IL. Dietary omega-3 fatty acids and ionizing irradiation on human breast cancer xenograft growth and angiogenesis. Cancer Cell Int (2005) 5(1):12. doi:10.1186/1475-2867-5-12
30. Chaturvedi P, Gilkes DM, Takano N, Semenza GL. Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci U S A (2014) 111(20):E2120–9. doi:10.1073/pnas.1406655111
31. Gehmert S, Gehmert S, Bai X, Klein S, Ortmann O, Prantl L. Limitation of in vivo models investigating angiogenesis in breast cancer. Clin Hemorheol Microcirc (2011) 49(1–4):519–26. doi:10.3233/CH-2011-1502
32. Seifert A, Taubert H, Hombach-Klonisch S, Fischer B, Navarrete Santos A. TCDD mediates inhibition of p53 and activation of ERalpha signaling in MCF-7 cells at moderate hypoxic conditions. Int J Oncol (2009) 35(2):417–24.
33. Luo W, Chang R, Zhong J, Pandey A, Semenza GL. Histone demethylase JMJD2C is a coactivator for hypoxia-inducible factor 1 that is required for breast cancer progression. Proc Natl Acad Sci U S A (2012) 109(49):E3367–76. doi:10.1073/pnas.1217394109
35. Vaapil M, Helczynska K, Villadsen R, Petersen OW, Johansson E, Beckman S, et al. Hypoxic conditions induce a cancer-like phenotype in human breast epithelial cells. PLoS One (2012) 7(9):e46543. doi:10.1371/journal.pone.0046543
36. Pahlman S, Lund LR, Jogi A. Differential HIF-1alpha and HIF-2alpha expression in mammary epithelial cells during fat pad invasion, lactation, and involution. PLoS One (2015) 10(5):e0125771. doi:10.1371/journal.pone.0125771
37. Krutilina R, Sun W, Sethuraman A, Brown M, Seagroves TN, Pfeffer LM, et al. MicroRNA-18a inhibits hypoxia-inducible factor 1alpha activity and lung metastasis in basal breast cancers. Breast Cancer Res (2014) 16(4):R78. doi:10.1186/bcr3693
38. Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFkappaB activation in the tumor stromal microenvironment. Cell Cycle (2010) 9(17):3515–33. doi:10.4161/cc.9.17.12928
39. Milane L, Duan Z, Amiji M. Role of hypoxia and glycolysis in the development of multi-drug resistance in human tumor cells and the establishment of an orthotopic multi-drug resistant tumor model in nude mice using hypoxic pre-conditioning. Cancer Cell Int (2011) 11:3. doi:10.1186/1475-2867-11-3
40. Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol (1985) (2010) 108(2):343–8. doi:10.1152/japplphysiol.00424.2009
41. Cairns R, Papandreou I, Denko N. Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment. Mol Cancer Res (2006) 4(2):61–70. doi:10.1158/1541-7786.MCR-06-0002
42. Guppy M. The hypoxic core: a possible answer to the cancer paradox. Biochem Biophys Res Commun (2002) 299(4):676–80. doi:10.1016/S0006-291X(02)02710-9
43. Vaupel P. Tumor microenvironmental physiology and its implications for radiation oncology. Semin Radiat Oncol (2004) 14(3):198–206. doi:10.1016/j.semradonc.2004.04.008
44. Cosse J-P, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anticancer Agents Med Chem (2008) 8(7):790–7. doi:10.2174/187152008785914798
45. Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem (2002) 277(26):23111–5. doi:10.1074/jbc.M202487200
46. Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev (2007) 21(9):1037–49. doi:10.1101/gad.1529107
47. Lopez-Lazaro M. The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anticancer Agents Med Chem (2008) 8(3):305–12. doi:10.2174/187152008783961932
48. Seagroves TN, Ryan HE, Lu H, Wouters BG, Knapp M, Thibault P, et al. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol Cell Biol (2001) 21(10):3436–44. doi:10.1128/MCB.21.10.3436-3444.2001
49. Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr (2007) 39(3):231–4. doi:10.1007/s10863-007-9081-2
50. Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes (2007) 56(4):901–11. doi:10.2337/db06-0911
51. Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) (2008) 32(3):451–63. doi:10.1038/sj.ijo.0803744
52. Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab (2007) 293(4):E1118–28. doi:10.1152/ajpendo.00435.2007
53. Wojtczak L, Schonfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta (1993) 1183(1):41–57. doi:10.1016/0005-2728(93)90004-Y
54. Fridlyand LE, Philipson LH. Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes Metab (2006) 8(2):136–45. doi:10.1111/j.1463-1326.2005.00496.x
55. Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch (2007) 455(3):479–92. doi:10.1007/s00424-007-0301-8
56. Gatterer H, Haacke S, Burtscher M, Faulhaber M, Melmer A, Ebenbichler C, et al. Normobaric intermittent hypoxia over 8 months does not reduce body weight and metabolic risk factors – a randomized, single blind, placebo-controlled study in normobaric hypoxia and normobaric sham hypoxia. Obes Facts (2015) 8(3):200–9. doi:10.1159/000431157
57. Mantzoros C, Petridou E, Dessypris N, Chavelas C, Dalamaga M, Alexe DM, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab (2004) 89(3):1102–7. doi:10.1210/jc.2003-031804
58. Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY, et al. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Lett (2006) 237(1):109–14. doi:10.1016/j.canlet.2005.05.047
59. Helczynska K, Larsson AM, Holmquist Mengelbier L, Bridges E, Fredlund E, Borgquist S, et al. Hypoxia-inducible factor-2alpha correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res (2008) 68(22):9212–20. doi:10.1158/0008-5472.CAN-08-1135
60. Seagroves TN, Hadsell D, McManaman J, Palmer C, Liao D, McNulty W, et al. HIF1alpha is a critical regulator of secretory differentiation and activation, but not vascular expansion, in the mouse mammary gland. Development (2003) 130(8):1713–24. doi:10.1242/dev.00403
61. Schwab LP, Peacock DL, Majumdar D, Ingels JF, Jensen LC, Smith KD, et al. Hypoxia-inducible factor 1alpha promotes primary tumor growth and tumor-initiating cell activity in breast cancer. Breast Cancer Res (2012) 14(1):R6. doi:10.1186/bcr3087
62. Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res (2007) 67(2):563–72. doi:10.1158/0008-5472.CAN-06-2701
63. Galindo-Hernandez O, Villegas-Comonfort S, Candanedo F, González-Vázquez MC, Chavez-Ocaña S, Jimenez-Villanueva X, et al. Elevated concentration of microvesicles isolated from peripheral blood in breast cancer patients. Arch Med Res (2013) 44(3):208–14. doi:10.1016/j.arcmed.2013.03.002
64. Garayoa M, Martínez A, Lee S, Pío R, An WG, Neckers L, et al. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: a possible promotion mechanism of carcinogenesis. Mol Endocrinol (2000) 14(6):848–62. doi:10.1210/mend.14.6.0473
65. Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer (2004) 4(6):437–47. doi:10.1038/nrc1367
66. Chang J, Erler J. Hypoxia-mediated metastasis. Adv Exp Med Biol (2014) 772:55–81. doi:10.1007/978-1-4614-5915-6_3
67. Saggar JK, Yu M, Tan Q, Tannock IF. The tumor microenvironment and strategies to improve drug distribution. Front Oncol (2013) 3:154. doi:10.3389/fonc.2013.00154
68. Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol (1996) 16(9):4604–13. doi:10.1128/MCB.16.9.4604
69. Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med (2004) 10(8):858–64. doi:10.1038/nm1075
70. Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest (2013) 123(9):3664–71. doi:10.1172/JCI67230
71. Hu Y, Liu J, Huang H. Recent agents targeting HIF-1alpha for cancer therapy. J Cell Biochem (2013) 114(3):498–509. doi:10.1002/jcb.24390
72. Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol (2007) 8(3):235–44. doi:10.1016/S1470-2045(07)70074-8
73. Lujambio A, Lowe SW. The microcosmos of cancer. Nature (2012) 482(7385):347–55. doi:10.1038/nature10888
74. Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res (2005) 65(16):7065–70. doi:10.1158/0008-5472.CAN-05-1783
75. Persson H, Kvist A, Rego N, Staaf J, Vallon-Christersson J, Luts L, et al. Identification of new microRNAs in paired normal and tumor breast tissue suggests a dual role for the ERBB2/Her2 gene. Cancer Res (2011) 71(1):78–86. doi:10.1158/0008-5472.CAN-10-1869
76. Foekens JA, Sieuwerts AM, Smid M, Look MP, de Weerd V, Boersma AW, et al. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proc Natl Acad Sci U S A (2008) 105(35):13021–6. doi:10.1073/pnas.0803304105
77. Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem (2008) 283(16):10892–903. doi:10.1074/jbc.M800102200
78. Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene (1998) 16(11):1391–7. doi:10.1038/sj.onc.1201661
79. Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, et al. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol (2009) 174(6):2023–34. doi:10.2353/ajpath.2009.080873
80. Sloan EK, Ciocca DR, Pouliot N, Natoli A, Restall C, Henderson MA, et al. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol (2009) 174(6):2035–43. doi:10.2353/ajpath.2009.080924
81. Cummings SR, Tice JA, Bauer S, Browner WS, Cuzick J, Ziv E, et al. Prevention of breast cancer in postmenopausal women: approaches to estimating and reducing risk. J Natl Cancer Inst (2009) 101(6):384–98. doi:10.1093/jnci/djp018
82. Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr (2002) 132(11 Suppl):3456S–64S. doi:10.1503/cmaj.051073
83. Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer Epidemiol Biomarkers Prev (2008) 17(2):379–86. doi:10.1158/1055-9965.EPI-07-0771
84. Irwin ML, Smith AW, McTiernan A, Ballard-Barbash R, Cronin K, Gilliland FD, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the health, eating, activity, and lifestyle study. J Clin Oncol (2008) 26(24):3958–64. doi:10.1200/JCO.2007.15.9822
Keywords: hypoxia, adipocytes, breast cancer, HIF-1α, HIF-2α
Citation: Rausch LK, Netzer NC, Hoegel J and Pramsohler S (2017) The Linkage between Breast Cancer, Hypoxia, and Adipose Tissue. Front. Oncol. 7:211. doi: 10.3389/fonc.2017.00211
Received: 14 June 2017; Accepted: 28 August 2017;
Published: 25 September 2017
Edited by:
Michael Breitenbach, University of Salzburg, AustriaReviewed by:
Maja Cemazar, Institute of Oncology Ljubljana, SloveniaLuisa Lanfrancone, Istituto Europeo di Oncologia, Italy
Copyright: © 2017 Rausch, Netzer, Hoegel and Pramsohler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda K. Rausch, bC5yYXVzY2hAaGVybWFubi1idWhsLWluc3RpdHV0LmRl
 Linda K. Rausch
Linda K. Rausch Nikolaus C. Netzer
Nikolaus C. Netzer Josef Hoegel4
Josef Hoegel4 Stephan Pramsohler
Stephan Pramsohler