- 1Department of Medical Oncology, Princess Margaret Cancer Centre, Toronto, ON, Canada
- 2Department of Medical Oncology, London Regional Cancer Centre, London, ON, Canada
Sensitizing mutations in the epidermal growth factor receptor (EGFR) predict response to EGFR tyrosine kinase inhibitors (TKIs) and both first- and second-generation TKIs are available as first-line treatment options in patients with advanced EGFR-mutant non-small cell lung cancer. Eventual resistance develops with multiple mechanisms identifiable both upon repeat biopsy and in plasma circulating tumor DNA. The T790M gatekeeper mutation is responsible for almost 60% of cases. A number of third-generation TKIs are in clinical development, and osimertinib has been approved by the US Food and Drug Administration for the treatment of patients with EGFR T790M mutant lung cancer after failure of initial EGFR kinase therapy. Resistance mechanisms are being identified to these novel agents, and the treatment landscape of EGFR-mutant lung cancer continues to evolve. The sequence of EGFR TKIs may change in the future and combination therapies targeting resistance appear highly promising.
Introduction
Non-small cell lung cancer (NSCLC) accounts for over 80% of lung cancer cases and is a leading cause of morbidity and mortality internationally (1, 2). When treated with platinum-based chemotherapy, the median survival in patients with metastatic disease is 8 months (3). Mutations in the epidermal growth factor receptor gene (EGFR) are found in 10–15% of lung cancers in Caucasians, and 30–40% of East Asian patients (4, 5). These patients most commonly have adenocarcinoma, are lifetime non- or light smokers and are more frequently female. The most commonly described mutations are deletions in exon 19 (del19) and the exon 21 L858R point mutation (from leucine to arginine). The discovery of EGFR mutations as a predictor of response to tyrosine kinase inhibitors (TKIs) heralded a paradigm shift in the treatment of NSCLC (6–8).
In the advanced setting, options for first-line treatment of EGFR-positive lung cancer include first-generation TKIs (erlotinib, gefitinib) and afatinib, a second-generation kinase inhibitor. These agents have an impressive body of evidence confirming better response, improved progression-free survival (PFS), and quality of life compared to chemotherapy (9–16). The pooled analysis of the LUX Lung 3 and 6 studies also suggested an overall survival advantage of afatinib relative to chemotherapy in the first-line setting for the subgroup of patients with exon 19 deletions (17). Recently, afatinib has been shown to have improved PFS compared to gefitinib, however, at the expense of greater toxicity (18). Resistance to both first- and second-generation TKIs is common and develops at a median time of 9–16 months (9, 11, 14, 19). This review summarizes known mechanisms of TKI resistance, clinical approaches to resistance with a focus on third-generation EGFR TKIs, their preclinical and clinical evidence for use, and future directions to improve the outcomes of patients with EGFR mutation-positive lung cancer.
Resistance to First- and Second-Generation Inhibitors
By performing biopsies in patients with progression on first-generation TKIs, Yu et al. elucidated the common mechanisms of resistance to first-generation TKIs (20). In approximately 60% of cases, a T790M point mutation in exon 20 was identified. Other mechanisms include downstream signaling pathway mutations in BRAF or PIK3CA, or parallel signaling pathway activation via changes in MET, HER2, FGFR, and AXL. A small group (3%) had histologic transformation: epithelial to mesenchymal transition or high-grade neuroendocrine transformation. A total of 18% did not have an identifiable cause. Multiple mechanisms of resistance were shown in 10% cases and have been postulated to be up to 15% of cases in other series (20–22).
More recently, the rate of T790M mutation have been reported to be much higher when analyzing circulating tumor DNA (ctDNA), highlighting the limitations of a single biopsy in the context of tumor heterogeneity (23). Tissue biopsies are associated with risks, delays, and an increased economic burden (24). Liquid biopsies are an attractive alternative to this and can accurately detect T790M mutations in ctDNA with a high positive predictive value. In the study by Oxnard et al., of 58 patients with a T790M negative tissue biopsy, one-third had T790M detected in plasma with similar response rates (RRs) to patients with the mutation identified in tumor biopsy samples (25). Recently, two studies have reported the detection of T790M several weeks to months prior to radiological progression, which emphasizes the potential use of serial plasma monitoring in this population (26, 27). However, plasma genotyping may still result in false negatives and it is unlikely that repeat tumor biopsies in clinic can be completely eliminated for all patients. But an approach whereby initial blood-based screening is used, followed by biopsy in only those without the mutation identified, may decrease the morbidity and delays involved in serial genomic testing.
Managing Resistance to Initial TKI Therapy
Platinum-based chemotherapy has been considered the standard treatment upon progression for patients on initial EGFR kinase therapy; however, few patients are well enough or agree to have cytotoxic chemotherapy (28). Intercalation or combination with chemotherapy has been minimally successful with added toxicity and no consistent survival benefit (29). The IMPRESS study showed that continuing TKI therapy with chemotherapy did not provide a PFS benefit and was associated with increased toxicity (30).
For oligo progressive disease, administering local therapy and continuing the original kinase inhibitor is a common approach (31). In a small single-arm phase II study (ASPIRATION), patients with minimally symptomatic or asymptomatic progression were randomized to continue erlotinib past progression or to stop, and those continuing remained on treatment for a median of an additional 3.7 months after the initial PFS of 11 months (32).
Despite in vitro T790M inhibition, the second-generation TKIs have not demonstrated significant single-agent activity in T790M mutation positive disease. Dual inhibition of EGFR signaling has generated interest, with a phase II study of afatinib and cetuximab in TKI-resistant patients, demonstrating a RR of 29% in T790M-positive and -negative subgroups. Thus, EGFR pathway signaling remains an important driver of disease, with trials ongoing (33).
The most significant development in treating resistance has been through third-generation kinase inhibitors that target T790M.
Third-Generation TKIs
Not only do these agents have activity in T790M mutant lung cancer but many have the advantage of limited wild-type inhibition, therefore, overcoming toxicities associated with first- and second-generation TKIs. WZ4002, a covalent pyrimidine EGFR TKI, was one of the first compounds to show in vitro and in vivo EGFR T790M inhibition with relative WT sparing (34). Several agents have now been tested in clinical trials, with osimertinib recently approved by the US Food and Drug Administration (FDA) and other regulatory agencies in patients with EGFR T790M mutant NSCLC post failure of first-/second-generation TKIs.
Osimertinib (AZD9291, Previously Merelitinib)
Osimertinib is an oral, irreversible TKI that forms a covalent bond with the cysteine residue in position 797 of EGFR within the ATP-binding site. Osimertinib and its active metabolites also interact with a number of other kinases harboring the cysteine residue. Osimertinib is a potent inhibitor of T790M with little wild-type activity and shows tumor regression in murine models (35).
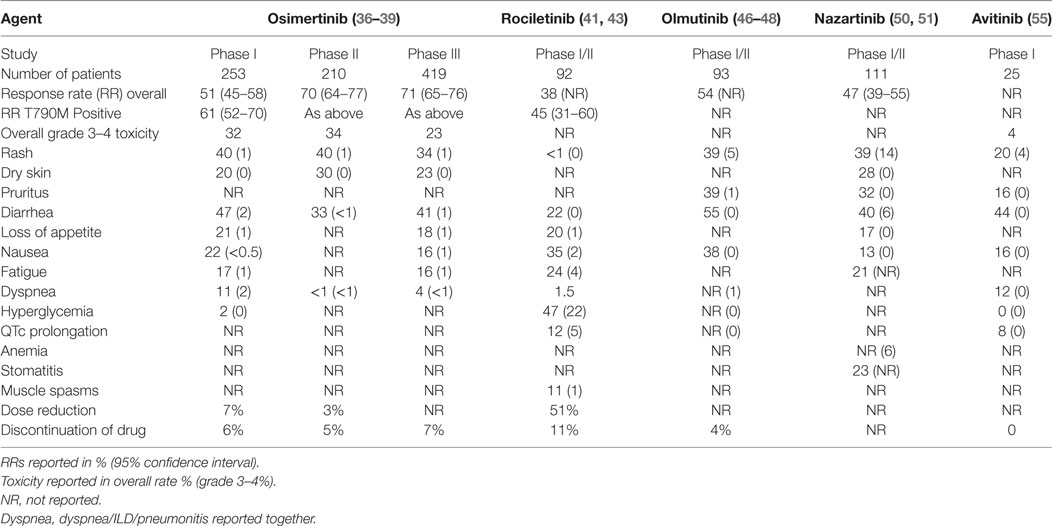
AURA (a phase I dose escalation study) (36) was performed in patients with EGFR mutation-positive advanced NSCLC with acquired resistance to TKI. No dose-limiting toxicity (DLT) was observed; the most common adverse events were diarrhea, rash, anorexia, and nausea (see Table 1). The overall RR was 51% [95% confidence interval (CI), 45–58]; higher in the T790M mutation-positive group than the T790M mutation-negative group (61 versus 21%). The median PFS was 9.6 months (95% CI, 8.3 to not reached) in EGFR T790M-positive patients.
Updated results of the 80 mg (RP2D) cohorts from three AURA studies were presented (37), and confirm a high RR (66% in phase I study and 71% in phase II studies). Encouraging duration of response (12.5 months in pooled phase II studies) and PFS (11.0 months in same pooled analysis) was seen (37).
AURA2 is a single-arm, open-label phase II study of osimertinib 80 mg daily in T790M mutation positive NSCLC after first-line TKI. A total of 140 (70%; 95% CI 64–77) of 199 patients (with measurable disease) achieved an objective response. There was a disease control rate (dCR) of 92%. Toxicity was manageable with low rates of grade 3 or higher toxicity (see Table 1) (38).
AURA3 was a phase III randomized trial that assessed the efficacy and safety of osimertinib (80 mg daily) versus platinum-doublet chemotherapy after initial TKI failure in 419 patients with T790M mutation-positive advanced NSCLC (39). The trial demonstrated superior PFS 10.1 versus 4.4 months (HR 0.30; 95% CI 0.23–0.41; p < 0.001) and higher RR with osimertinib, 71%, versus chemotherapy, 31% (39). Grade 3 or 4 adverse events occurred in 23% of patients on osimertinib, compared to 47% with chemotherapy (Table 1). Quality of life results are pending.
In November 2015, osimertinib received accelerated approval by the FDA, representing rapid progress through drug development—the first AURA patient was enrolled in March 2013 and FDA accelerated approval was granted in November 2015.
Rociletinib (CO1686)
Rociletinib is an irreversible orally delivered third-generation TKI that targets L858R, del19, and T790M mutations of EGFR with little WT activity. Rociletinib also modifies the C797 site through covalent binding. Tumor xenograft and transgenic models documented tumor regression in preclinical studies (40). In the phase I/dose expansion study, TIGERX, 130 patients with progression following EGFR TKI were enrolled but the maximum tolerated dose was not reached (41). The RR was 59% in T790M-positive patients; however, pooled data from this TIGER-X study and the phase II TIGER-2 was initially reported to be 30.2% (42) and later updated and published as 45% (43).
The most frequent grade 3/4 AEs, which occurred in more than 10%, included hyperglycemia and QTc prolongation (Table 1). The hyperglycemia is thought to be mediated by a metabolite of rociletinib that inhibits insulin-like growth factor-1 receptor and to a lesser extent, insulin receptor kinases. Clovis has suspended development of rociletinib and terminated enrollment in clinical trials in 2016, soon after the FDA rejected the request for accelerated approval (44).
Olmutinib (HM61713; Formerly BI148269)
Olmutinib is an oral selective inhibitor for EGFR including T790M mutant kinases and acts by binding to a cysteine residue close to the kinase domain. Potent inhibition of representative cell lines and in vivo activity have been reported (45). A phase I trial of 173 patients with EGFR-mutant lung cancer that had failed previous TKI therapy demonstrated a favorable safety profile and promising antitumor activity (46, 47). The MTD was established as 800 mg once daily. Treatment-related adverse events occurred in 87.3% of 165 patients, mainly diarrhea, rash, skin exfoliation, nausea, pruritus, decreased appetite, and dry skin. Grade 3 or greater toxicity was 2% in the initial study report (2/93), although not in the updated results (Table 1) (46–48).
In 34 patients with centrally confirmed T790M tumor mutations who received olmutinib at a dose greater than 650 mg daily, the RR was 59% (10 confirmed, 10 unconfirmed partial responses) and 13 achieved disease stabilization (dCR 97%). The phase II study has been suspended early with three cases of severe skin toxicity, including two reports of toxic epidermal necrolysis (one fatal), and one case of non-fatal Stevens–Johnson syndrome. The future of the drug’s development is uncertain.
Nazartinib (EGF816)
EGF816 is an oral, irreversible EGFR TKI that also forms a covalent bond with C797. Low IC50 values and in vivo activity against L858R–T790M and del19–T790M have been reported (49). Data from the phase I/II study of EGF816 in advanced T790M-positive lung cancer are now available (50, 51). Dose escalation began at 75 mg daily up to 350 mg daily for capsules and 100–225 mg daily for tablets. Diarrhea, stomatitis, rash, and pruritus were the most common toxicities (see Table 1), and the confirmed RR was 44% (56/127) and dCR was 91% (NCT02108964). Combination studies with immunotherapy are now recruiting (NCT02323126; NCT02335944; NCT02900664).
ASP8273
ASP8273 is an EGFR TKI that selectively and irreversibly inhibits mutant EGFR kinases including T790M by the formation of a covalent bond with C797. Both in vitro and in vivo studies confirm activity in T790M mutant lung cancer with relative WT sparing (52). In a phase I study, doses were escalated to 500 mg but the RP2D has been deemed 300 mg, although the details of DLT and maximum tolerated dose levels are not published (53). Of 60 patients treated with ASP8273 at the 300 mg dose, there was no DLT. All patients were EGFR positive with 90% having a T790M mutation. PR was demonstrated in 16 of 45 evaluable patients; dCR was 62% (n = 28/45). For the 40 T790M mutation-positive subjects with evaluable data, 38% (15/40) had PR and dCR was 65% (26/40) (NCT02113813). The Phase III SOLAR study is underway comparing initial ASP8273 with a first-generation TKI in patients with EGFR-mutant lung cancer (NCT02588261) (53).
PF06747775
PF0677775 is another oral inhibitor of EGFR T790M with 26-fold increased selectivity of mutant versus wild-type EGFR. It is currently under evaluation in a phase I/II study in patients with advanced EGFR mutation-positive lung cancer (del19 or L858R, T790M positive and negative) (NCT02349633) and early results have demonstrated activity and tolerability (54).
Avitinib (AC0010)
Avitinib is a new, irreversible, EGFR mutation selective TKI being evaluated in a phase I/II clinical trial (NCT02274337). In the reported dose escalation study (55), 25 patients were treated. The most common AEs were diarrhea, rash, and pruritus. Although diarrhea and rash increased in frequency in a dose-dependent manner, the majority of them were grade 1 (Table 1). There was no drug discontinuation in all treated patients. Outcomes for two patients with T790M-positive lung cancer showed partial responses. The clinical characteristics and efficacy outcomes of the remaining patients are not reported (55).
Special Populations
Uncommon Mutations
The “uncommon” EGFR mutations represent a heterogeneous group and can account for up to 10–18% of EGFR mutations (56, 57). The most frequent include exon 20 insertions (exon20ins), and point mutations G719X, L861Q, and S768I. The latter three mutations may have a superior response to afatinib (58). The majority of (exon20ins) are thought resistant to EGFR TKIs with the exception of A763_Y764insFQEA. In a preclinical study, osimertinib demonstrated potency with a wide therapeutic window in the exon20ins studied (Y764_V765insHH, A767V769dupASV, and D770_N771insNPG) (59). More recently, it has been revealed that EGFR amplification may occur in a subset of exon20ins. The dual EGFR blockade with osimertinib and cetuximab has demonstrated significant growth inhibition in in vivo models (60). EGF816 has shown both in vitro and in vivo efficacy in a number of exon20ins and in a patient-derived xenograft model, 100 mg/kg dosing resulted in tumor regression of 81% (49). AP32788 has also been shown to inhibit exon20ins in BA/F3 cell lines (61). The activity of third-generation TKIs in preclinical models has led to clinical trials for exon20 insertions including the phase I/II study of AP32788 (NCT02716116).
Brain Metastases
Approximately 30–50% of patients with NSCLC develop central nervous system (CNS) disease (62, 63). The association between EGFR mutation-positive NSCLC and the incidence of brain metastases is controversial with some studies suggesting an increased risk of CNS disease at diagnosis (64, 65). CNS disease can reduce survival and both brain metastases and loco regional therapies can impact neurological function and quality of life. First-generation EGFR TKIs have shown intracranial activity with erlotinib exhibiting higher CSF concentrations than gefitinib (66). Afatinib in patients pretreated with chemotherapy and a first-generation TKI has demonstrated a CNS dCR of 66% (67). A recent preclinical study has shown superior blood–brain barrier penetration of osimertinib compared to gefitinib, afatinib, and rociletinib (68). Sustained tumor regression in a murine brain metastases model has also been reported with osimertinib, doses of 80 mg in humans were predicted to target human brain metastases using an adaptive pharmacokinetic/pharmacodynamics model (69). AZD3759 is an innovative EGFR TKI developed to penetrate the blood–brain barrier but does not have T790M activity. BLOOM (NCT02228369) is a study testing the safety and efficacy of osimertinib 160 mg/day and AZD3759 in NSCLC patients with leptomeningeal disease; early data have reported disease control in three-quarters of patients and responses in 7 of 20 patients (70).
Osimertinib CNS activity was also confirmed in AURA and AURA2 (71). In the recently reported phase III study of osimertinib versus platinum-pemetrexed doublet, a significant improvement in PFS in patients with brain metastases was evident in the osimertinib group (8.5 versus 4.2 months; hazard ratio 0.32; 95% CI, 0.21–0.49) (39).
Resistance to Third-Generation TKIs and Combination Treatment
C797S and Other EGFR-Dependent Mechanisms
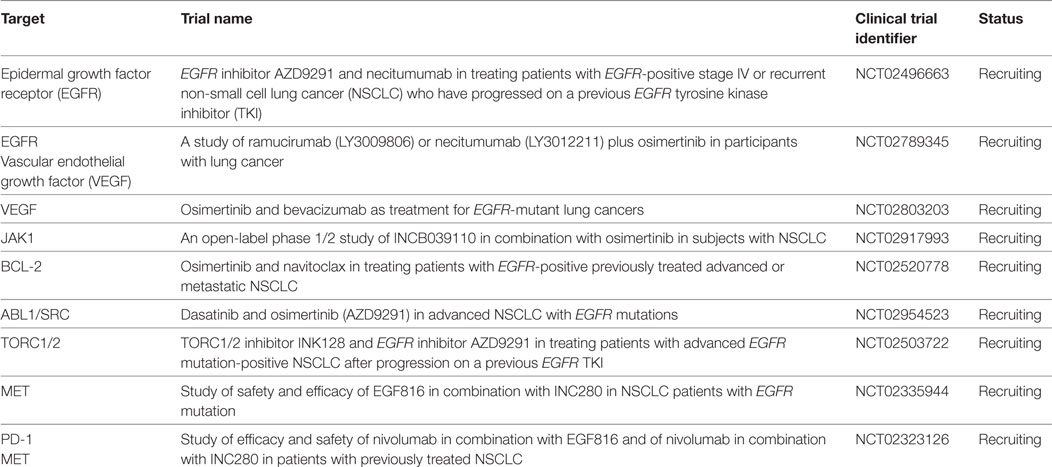
The point mutation C797S in exon 20 represents the most common resistance mechanism identified in third-generation EGFR TKIs. Most third-generation TKIs use the site of the cysteine amino acid located at position 797 for covalent binding, and the serine amino acid substitution reduces the capacity for TKI binding. Analysis of both plasma and tissue has confirmed this as a mechanism of resistance to osimertinib and olmutinib (72, 73). C797S as a cause of rociletinib resistance has been found to be much lower, but emergence of other uncommon EGFR mutations including L798I, L692V, and E709K have been implicated (23). Interestingly in preclinical models, if the activating mutation (del19 or L858R) is retained in the presence of C797S but without T790M, the tumor remains sensitive to gefitinib or afatinib. If T790M is present, in vitro analysis has demonstrated partial cetuximab sensitivity (74). Cetuximab with EAI045, a novel EGFR resistance mutation selective allosteric inhibitor was also effective in a mouse model of the triple-mutant EGFR L858R/T790M/C797S (75). Necitumumab is also being trialed in combination with osimertinib (Table 2) (NCT02496663, NCT02789345). Notably, brigatinib with or without the combination of an anti-EGFR antibody has demonstrated activity in preclinical models for the “triple-positive” tumors (76). First- and third-generation TKIs may also be combined effectively, but this is only likely if the C797S and T790M mutations occur in trans (77). Other acquired EGFR mutations have been reported by Chabon et al. including L798I, L762V, and E709K (23). EGFR amplification and copy number alterations are also important resistance mechanisms.
RAS/RAF/MEK
Cell line studies have identified the RAS pathway as important in emerging osimertinib resistance, including mutations in NRAS as well as copy number gains. The BRAF V600E mutation is also a known acquired resistance mutation (78). The addition of selumetinib (MEK inhibitor) delayed and prevented resistance in preclinical models and tumor regression has been documented in an osimertinib-resistant transgenic mouse model (79). The TATTON phase 1b study is a three-arm trial of TKI naive and pretreated patients that includes combinations of osimertinib with selumetinib (AZD6094), savolitinib, a MET inhibitor, and durvalumab (NCT02143466).
MET Amplification
MET amplification as a cause for EGFR TKI acquired resistance has been described in case reports and crizotinib led to a response in one of these (80–82). In a further study of rociletinib resistance, MET amplification accounted for 26% of patients. Patient-derived xenograft models in this study were again successfully targeted using crizotinib (83). Notable in the xenografts and in the case report by Planchard et al., selective pressure permitted the emergence of MET amplifications without detectable T790M suggesting that MET may induce resistance to third-generation TKIs. Chabon et al. also described the preexistence of co-occurring MET amplification with T790M, which correlated with inferior responses to rociletinib (23).
Immunotherapy Combinations
Although immune checkpoint inhibitors have made huge advances in shifting the treatment paradigm in NSCLC, their role in EGFR-mutant disease is unclear. The third arm of TATTON investigated the combination of osimertinib with durvalumab and has reported early data (84). Patients were treated with osimertinib 80 mg daily with varying dosing and scheduling of durvalumab. In EGFR TKI naïve patients, the RR was 70% and in pretreated T790M-positive patients and T790M-negative patients RR was 67 and 21%, respectively. The combined rate of interstitial lung disease was 38% and as such this arm is currently on hold. Similarly, the CAURAL (NCT02454933) study investigating the durvalumab combination versus single-agent osimertinib has been halted due to toxicity concerns.
Other Resistance Mechanisms
Other potentially targetable resistance mechanisms include HER2 amplification, FGFR1 amplification, and the PIK3CA E545K mutation. Epithelial to mesenchymal transition (EMT) and small cell transformation are also well recognized. Preclinical models have successfully targeted EMT with Akt inhibitors (40). Navitoclax, a BCL-2 inhibitor when combined with WZ4002, was shown to induce greater apoptosis than with the EGFR TKI alone (85). A phase 1b study is accruing (NCT02520778). Vascular endothelial growth factor (VEGF) and EGFR pathways are intimately related. The upregulation of VEGF receptors may be responsible for EGFR resistance and combination studies are ongoing (86) (Table 2). Early trials have already confirmed the benefit of dual inhibition with bevacizumab and erlotinib (87, 88). One small retrospective study has suggested the possibility of rechallenging with EGFR TKIs in addition to bevacizumab to gain further disease control (89).
Future Directions
It is not just the complexity of resistance mechanisms that poses challenges to physicians treating the EGFR mutation-positive population. It is also unclear as to whether third-generation TKIs should be used in the first-line setting or should remain the option for T790M resistance in the second line. The results of the phase III FLAURA study which compares osimertinib to either gefitinib or erlotinib are awaited. The ADAURA study will also investigate the potential role of adjuvant osimertinib in stage IB–IIIA resected NSCLC with and without chemotherapy. The roadmap of resistance continues to grow and it is very likely that ctDNA analysis will at least complement if not replace repeat tumor biopsies in building the knowledge of resistance mechanisms. Studies have already demonstrated the emergence of T790M prior to radiographic changes but whether this should mean a switch in TKI is uncertain at present.
Author Contributions
Conception and design: TB and GO. Manuscript writing; final approval of manuscript: all authors.
Conflict of Interest Statement
TB: no conflict of interest; GO: no conflict of interest; MV: honoraria: AstraZeneca, B-I, Roche BMS, Merck, and Novartis; NL: accredited CME honoraria: AstraZeneca, BMS, MSD, and Pfizer; Institutional research: Novartis.
Funding
There was no funding body for this work.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin (2011) 61(2):69–90. doi: 10.3322/caac.20107
2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin (2014) 64(1):9–29. doi:10.3322/caac.21208
3. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med (2002) 346(2):92–8. doi:10.1056/NEJMoa011954
4. Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol (2012) 9(2):154. doi:10.1097/JTO.0000000000000033
5. Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst (2005) 97(5):339–46. doi:10.1093/jnci/dji055
6. Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med (2004) 350(21):2129–39. doi:10.1056/NEJMoa040938
7. Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (2004) 304(5676):1497–500. doi:10.1126/science.1099314
8. Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A (2004) 101(36):13306–11. doi:10.1073/pnas.0405220101
9. Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med (2009) 361(10):947–57. doi:10.1056/NEJMoa0810699
10. Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol (2010) 11(2):121–8. doi:10.1016/S1470-2045(09)70364-X
11. Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med (2010) 362(25):2380–8. doi:10.1056/NEJMoa0909530
12. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol (2015) 26(9):1877–83. doi:10.1093/annonc/mdv276
13. Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol (2012) 30(10):1122–8. doi:10.1200/JCO.2011.36.8456
14. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13(3):239–46. doi:10.1016/S1470-2045(11)70393-X
15. Sequist LV, Yang JC-H, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol (2013) 31(27):3327–34. doi:10.1200/JCO.2012.44.2806
16. Wu Y-L, Zhou C, Hu C-P, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol (2014) 15(2):213–22. doi:10.1016/S1470-2045(13)70604-1
17. Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol (2015) 16(2):141–51. doi:10.1016/S1470-2045(14)71173-8
18. Park K, Tan EH, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol (2016) 17(5):577–89. doi:10.1016/S1470-2045(16)30033-X
19. Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol (2012) 13(5):528–38. doi:10.1016/S1470-2045(12)70087-6
20. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res (2013) 19(8):2240–7. doi:10.1158/1078-0432.CCR-12-2246
21. Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med (2011) 3(75):75ra26. doi:10.1126/scitranslmed.3002003
22. Ohashi K, Sequist LV, Arcila ME, Moran T, Chmielecki J, Lin YL, et al. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc Natl Acad Sci U S A (2012) 109(31):E2127–33. doi:10.1073/pnas.1203530109
23. Chabon JJ, Simmons AD, Lovejoy AF, Esfahani MS, Newman AM, Haringsma HJ, et al. Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun (2016) 7:11815. doi:10.1038/ncomms11815
24. Lim C, Sung M, Shepherd FA, Nouriany N, Sawczak M, Paul T, et al. Patients with advanced non-small cell lung cancer: are research biopsies a barrier to participation in clinical trials? J Thorac Oncol (2016) 11(1):79–84. doi:10.1016/j.jtho.2015.09.006
25. Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol (2016) 34(28):3375–82. doi:10.1200/JCO.2016.66.7162
26. Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O’Connell A, Messineo MM, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res (2014) 20(6):1698–705. doi:10.1158/1078-0432.CCR-13-2482
27. Zheng D, Ye X, Zhang MZ, Sun Y, Wang JY, Ni J, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep (2016) 6:20913. doi:10.1038/srep20913
28. Martin P, Shiau CJ, Pasic M, Tsao M, Kamel-Reid S, Lin S, et al. Clinical impact of mutation fraction in epidermal growth factor receptor mutation positive NSCLC patients. Br J Cancer (2016) 114(6):616–22. doi:10.1038/bjc.2016.22
29. Zhou C, Yao LD. Strategies to improve outcomes of patients with EGFR-mutant non-small-cell lung cancer: review of the literature. J Thorac Oncol (2016) 11(2):174–86. doi:10.1016/j.jtho.2015.10.002
30. Soria JC, Wu YL, Nakagawa K, Kim SW, Yang JJ, Ahn MJ, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol (2015) 16(8):990–8. doi:10.1016/S1470-2045(15)00121-7
31. Yu HA, Sima CS, Huang J, Solomon SB, Rimner A, Paik P, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol (2013) 8(3):346–51. doi:10.1097/JTO.0b013e31827e1f83
32. Park K, Ahn M, Yu C, Kim S, Lin M, Sriuranpong V, et al. ASPIRATION: first-line erlotinib (E) until and beyond RECIST progression (PD) in Asian patients with EGFR mutation positive (MUT+) NSCLC. Ann Oncol (2014) 25(Suppl 4: 12230):iv426–7. doi:10.1093/annonc/mdu349.2
33. Janjigian YY, Smit EF, Groen HJ, Horn L, Gettinger S, Camidge DR, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov (2014) 4(9):1036–45. doi:10.1158/2159-8290.CD-14-0326
34. Zhou W, Ercan D, Chen L, Yun CH, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature (2009) 462(7276):1070–4. doi:10.1038/nature08622
35. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov (2014) 4(9):1046–61. doi:10.1158/2159-8290.CD-14-0337
36. Janne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med (2015) 372(18):1689–99. doi:10.1056/NEJMoa1411817
37. Yang J, Ramalingam SS, Jänne PA, Cantarini M, Mitsudomi T. LBA2_PR: osimertinib (AZD9291) in pre-treated pts with T790M-positive advanced NSCLC: updated phase 1 (P1) and pooled phase 2 (P2) results. J Thorac Oncol (2016) 11(4):S152–3. doi:10.1016/S1556-0864(16)30325-2
38. Goss G, Tsai C-M, Shepherd FA, Bazhenova L, Lee JS, Chang G-C, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol (2016) 17(12):1643–52. doi:10.1016/S1470-2045(16)30508-3
39. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med (2017) 376(7):629–40. doi:10.1056/NEJMoa1612674
40. Walter AO, Sjin RT, Haringsma HJ, Ohashi K, Sun J, Lee K, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov (2013) 3(12):1404–15. doi:10.1158/2159-8290.CD-13-0314
41. Sequist LV, Soria J-C, Goldman JW, Wakelee HA, Gadgeel SM, Varga A, et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med (2015) 372(18):1700–9. doi:10.1056/NEJMoa1413654
42. Sussman A, Burkart B, Colorado B. Clovis Oncology Announces Regulatory Update for Rociletinib NDA Filing. Business Wire (2015).
43. Sequist LV, Soria J-C, Camidge DR. Update to rociletinib data with the RECIST confirmed response rate. N Engl J Med (2016) 374(23):2296–7. doi:10.1056/NEJMc1602688
44. Broderick J. Clovis Ends Development of Rociletinib in Lung Cancer. (2016). Available from: http://www.onclive.com/web-exclusives/clovis-ends-development-of-rociletinib-in-lung-cancer
45. Lee KO, Cha MY, Kim M, Song JY, Lee JH, Kim YH, et al. Abstract LB-100: discovery of HM61713 as an orally available and mutant EGFR selective inhibitor. Cancer Res (2014) 74(19 Suppl):LB–100.
46. Kim DW, Lee DH, Kang JH, Park K, Han JY, Lee JS, et al. Clinical activity and safety of HM61713, an EGFR-mutant selective inhibitor, in advanced non-small cell lung cancer (NSCLC) patients (pts) with EGFR mutations who had received EGFR tyrosine kinase inhibitors (TKIs). J Clin Oncol (2014) 32:5s.
47. Park K, Lee J-S, Lee KH, Kim J-H, Min YJ, Cho JY, et al. Updated safety and efficacy results from phase I/II study of HM61713 in patients (pts) with EGFR mutation positive non-small cell lung cancer (NSCLC) who failed previous EGFR-tyrosine kinase inhibitor (TKI). J Clin Oncol (2015) 33(15 suppl):8084.
48. Park K, Lee J-S, Lee K-H, Kim J-H, Cho B-C, Min Y-J, et al. BI1482694 (HM61713), an EGFR mutant-specific inhibitor, in T790M+ NSCLC: efficacy and safety at the RP2D. J Clin Oncol (2016) 34(Suppl):abstr 9055.
49. Jia Y, Juarez J, Li J, Manuia M, Niederst MJ, Tompkins C, et al. EGF816 exerts anticancer effects in non-small-cell lung cancer by irreversibly and selectively targeting primary and acquired activating mutations in the EGF receptor. Cancer Res (2016) 76(6):1591–602. doi:10.1158/0008-5472.CAN-15-2581
50. Tan DSW, Seto T, Leighl NB, Riely GJ, Sequist LV, Felip E, et al. First-in-human phase I study of EGF816, a third generation, mutant-selective EGFR tyrosine kinase inhibitor, in advanced non-small cell lung cancer (NSCLC) harboring T790M. J Clin Oncol (2015) 33(Suppl):abstr 8013.
51. Tan DSW, Yang J, Leighl NB, Riely GJ, Sequist LV, Felip E, et al. Updated results of a phase 1 study of EGF816, a third-generation, mutant-selective EGFR tyrosine kinase inhibitor (TKI), in advanced non-small cell lung cancer (NSCLC) harboring T790M. J Clin Oncol (2016) 34(Suppl):abstr 9044.
52. Sakagami H, Konagai S, Yamamoto H, Tanaka H, Matsuya T, Mori M, et al. ASP8273, a novel mutant-selective irreversible EGFR inhibitor, inhibits growth of non-small cell lung cancer (NSCLC) cells with EGFR activating and T790M resistance mutations. Cancer Res (2014) 74(19 Suppl):1728. doi:10.1158/1538-7445.AM2014-1728
53. Yu HA, Spira AI, Horn L, Weiss J, West HJ, Giaccone G, et al. Antitumor activity of ASP8273 300 mg in subjects with EGFR mutation-positive non-small cell lung cancer: interim results from an ongoing phase 1 study. J Clin Oncol (2016) 34(Suppl):abstr 9050.
54. Husain H, Martins R, Goldberg S, Senico P, Ma W, Masters J, et al. P3. 02b-001 phase 1 dose escalation of PF-06747775 (EGFR-T790M inhibitor) in patients with advanced EGFRm (Del 19 or L858R±T790M) NSCLC: topic: EGFR biomarkers. J Thorac Oncol (2017) 12(1):S1185. doi:10.1016/j.jtho.2016.11.1668
55. Xu X, Mao L, Xu W, Tang W, Zhang X, Xi B, et al. AC0010, an irreversible EGFR inhibitor selectively targeting mutated EGFR and overcoming T790M-induced resistance in animal models and lung cancer patients. Mol Cancer Ther (2016) 15(11):2586–97. doi:10.1158/1535-7163.MCT-16-0281
56. Beau-Faller M, Prim N, Ruppert AM, Nanni-Metellus I, Lacave R, Lacroix L, et al. Rare EGFR exon 18 and exon 20 mutations in non-small-cell lung cancer on 10 117 patients: a multicentre observational study by the French ERMETIC-IFCT network. Ann Oncol (2014) 25(1):126–31. doi:10.1093/annonc/mdt418
57. De Pas T, Toffalorio F, Manzotti M, Fumagalli C, Spitaleri G, Catania C, et al. Activity of epidermal growth factor receptor-tyrosine kinase inhibitors in patients with non-small cell lung cancer harboring rare epidermal growth factor receptor mutations. J Thorac Oncol (2011) 6(11):1895–901. doi:10.1097/JTO.0b013e318227e8c6
58. Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol (2015) 16(7):830–8. doi:10.1016/S1470-2045(15)00026-1
59. Hirano T, Yasuda H, Tani T, Hamamoto J, Oashi A, Ishioka K, et al. In vitro modeling to determine mutation specificity of EGFR tyrosine kinase inhibitors against clinically relevant EGFR mutants in non-small-cell lung cancer. Oncotarget (2015) 6(36):38789–803. doi:10.18632/oncotarget.5887
60. Riess JW, Gandara DR, Frampton GM, Cheng M, Lara P, Kelly K, et al. Comprehensive genomic profiling and PDX modeling of EGFR exon 20 insertions: evidence for osimertinib based dual EGFR blockade. In: Oral Presentation at WCLC. Vienna, Austria (2016). OA10 ID 382. Available from: http://library.iaslc.org/virtual-library-search?product_id=6&author=&category=&date=&session_type=&session=&presentation=&keyword=osimertinib
61. Gonzalvez F, Xhu X, Huang WS, Baker TE, Ning Y, Wardwell SD, et al. AP32788, a potent, selective inhibitor of EGFR and HER2 oncogenic mutants, including exon 20 insertions, in preclinical models. Cancer Res (2016) 76(14 Suppl):2644. doi:10.1158/1538-7445.AM2016-2644
62. Arrieta O, Villarreal-Garza C, Zamora J, Blake-Cerda M, de la Mata MD, Zavala DG, et al. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol (2011) 6:166. doi:10.1186/1748-717X-6-166
63. Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer (1954) 7(4):682–9. doi:10.1002/1097-0142(195407)7:4<682::AID-CNCR2820070409>3.0.CO;2-S
64. Stanic K, Zwitter M, Hitij NT, Kern I, Sadikov A, Cufer T. Brain metastases in lung adenocarcinoma: impact of EGFR mutation status on incidence and survival. Radiol Oncol (2014) 48(2):173–83. doi:10.2478/raon-2014-0016
65. Guerin A, Sasane M, Zhang J, Culver KW, Dea K, Nitulescu R, et al. Brain metastases in patients with ALK+ non-small-cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ (2015) 18(4):312–22. doi:10.3111/13696998.2014.1003644
66. Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small-cell lung cancer. Cancer Chemother Pharmacol (2012) 70(3):399–405. doi:10.1007/s00280-012-1929-4
67. Hoffknecht P, Tufman A, Wehler T, Pelzer T, Wiewrodt R, Schutz M, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol (2015) 10(1):156–63. doi:10.1097/JTO.0000000000000380
68. Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res (2016) 22(20):5130–40. doi:10.1158/1078-0432.CCR-16-0399
69. Kim DW, Yates J, Cross D, Billard P, Yang P, Yates J. Preclinical evidence and clinical cases of AZD9291 activity in EGFR-mutant non-small cell lung cancer (NSCLC) brain metastases (BM). European Society for Medical Oncology (ESMO) Annual Meeting. Madrid (2014). p. 26–30.
70. Yang JC-H, Kim D-W, Kim S-W, Cho BC, Lee JS, Ye X, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): updated results from BLOOM, a phase I study. J Clin Oncol (2016) 34(Suppl):9002.
71. Ahn MJ, Tsai CM, Yang JCH, Shepherd FA, Satouchi M, Kim DW, et al. 3083 AZD9291 activity in patients with EGFR-mutant advanced non-small cell lung cancer (NSCLC) and brain metastases: data from phase II studies. Eur J Cancer (2015) 51:S625–6. doi:10.1016/S0959-8049(16)31724-5
72. Song HN, Jung KS, Yoo KH, Cho J, Lee JY, Lim SH, et al. Acquired C797S mutation upon treatment with a T790M-specific third-generation EGFR inhibitor (HM61713) in non-small-cell lung cancer. J Thorac Oncol (2016) 11(4):e45–7. doi:10.1016/j.jtho.2015.12.093
73. Thress KS, Paweletz CP, Felip E, Cho BC, Stetson D, Dougherty B, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med (2015) 21(6):560–2. doi:10.1038/nm.3854
74. Ercan D, Choi HG, Yun CH, Capelletti M, Xie T, Eck MJ, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res (2015) 21(17):3913–23. doi:10.1158/1078-0432.CCR-14-2789
75. Jia Y, Yun CH, Park E, Ercan D, Manuia M, Juarez J, et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature (2016) 534(7605):129–32. doi:10.1038/nature17960
76. Uchibori K, Inase N, Araki M, Kamada M, Sato S, Okuno Y, et al. Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun (2017) 8:14768. doi:10.1038/ncomms14768
77. Niederst MJ, Hu H, Mulvey HE, Lockerman EL, Garcia AR, Piotrowska Z, et al. The allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategies. Clin Cancer Res (2015) 21(17):3924–33. doi:10.1158/1078-0432.CCR-15-0560
78. Ho CC, Liao WY, Lin CA, Shih JY, Yu CJ, Chih-Hsin Yang J. Acquired BRAF V600E mutation as resistant mechanism after treatment with osimertinib. J Thorac Oncol (2017) 12(3):567–72. doi:10.1016/j.jtho.2016.11.2231
79. Eberlein CA, Stetson D, Markovets AA, Al-Kadhimi KJ, Lai Z, Fisher PR, et al. Acquired resistance to the mutant-selective EGFR inhibitor AZD9291 is associated with increased dependence on RAS signaling in preclinical models. Cancer Res (2015) 75(12):2489–500. doi:10.1158/0008-5472.CAN-14-3167
80. Ou SH, Agarwal N, Ali SM. High MET amplification level as a resistance mechanism to osimertinib (AZD9291) in a patient that symptomatically responded to crizotinib treatment post-osimertinib progression. Lung Cancer (2016) 98:59–61. doi:10.1016/j.lungcan.2016.05.015
81. Planchard D, Loriot Y, Andre F, Gobert A, Auger N, Lacroix L, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol (2015) 26(10):2073–8. doi:10.1093/annonc/mdv319
82. Ortiz-Cuaran S, Scheffler M, Plenker D, Dahmen L, Scheel AH, Fernandez-Cuesta L, et al. Heterogeneous mechanisms of primary and acquired resistance to third-generation EGFR inhibitors. Clin Cancer Res (2016) 22(19):4837–47. doi:10.1158/1078-0432.CCR-15-1915
83. Chabon JJ, Simmons AD, Lovejoy AF, Esfahani MS, Newman AM, Haringsma HJ, et al. Corrigendum: circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun (2016) 7:13513. doi:10.1038/ncomms13513
84. Ahn MJ, Yang J, Yu H, Saka H, Ramalingam S, Goto K, et al. 136O: osimertinib combined with durvalumab in EGFR-mutant non-small cell lung cancer: results from the TATTON phase Ib trial. J Thorac Oncol (2016) 11(4 Suppl):S115. doi:10.1016/S1556-0864(16)30246-5
85. Hata AN, Niederst MJ, Archibald HL, Gomez-Caraballo M, Siddiqui FM, Mulvey HE, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med (2016) 22(3):262–9. doi:10.1038/nm.4040
86. Garon EB, Reck M, Paz-Ares L, Ponce S, Jaime JC, Juan O, et al. Treatment rationale and study design for the RELAY study: a multicenter, randomized, double-blind study of erlotinib with ramucirumab or placebo in patients with epidermal growth factor receptor mutation-positive metastatic non-small-cell lung cancer. Clin Lung Cancer (2017) 18(1):96–9. doi:10.1016/j.cllc.2016.05.023
87. Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol (2014) 15(11):1236–44; [Erratum appears in Lancet Oncol (2014) 15(11):e475]. doi:10.1016/S1470-2045(14)70381-X
88. Stahel RA, Dafni U, Gautschi O, Felip E, Curioni-Fontecedro A, Peters S, et al. 3BA A phase II trial of erlotinib (E) and bevacizumab (B) in patients with advanced non-small-cell lung cancer (NSCLC) with activating epidermal growth factor receptor (EGFR) mutations with and without T790M mutation. The Spanish Lung Cancer group (SLCG) and the European Thoracic Oncology Platform (ETOP) BELIEF trial. Eur J Cancer (2015) 51:S711–2. doi:10.1016/S0959-8049(15)30068-X
Keywords: lung cancer, lung cancer treatment, epidermal growth factor receptor, tyrosine kinase inhibitors, T790M
Citation: Barnes TA, O’Kane GM, Vincent MD and Leighl NB (2017) Third-Generation Tyrosine Kinase Inhibitors Targeting Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer. Front. Oncol. 7:113. doi: 10.3389/fonc.2017.00113
Received: 22 February 2017; Accepted: 15 May 2017;
Published: 31 May 2017
Edited by:
Stephen V. Liu, Georgetown University, United StatesReviewed by:
Weiqiang Zhao, The Ohio State University, Wexner Medical Center, United StatesIacopo Petrini, Sant’Anna School of Advanced Studies, Italy
Copyright: © 2017 Barnes, O’Kane, Vincent and Leighl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Natasha B. Leighl, bmF0YXNoYS5sZWlnaGxAdWhuLmNh
†These authors have contributed equally to this work.
 Tristan A. Barnes
Tristan A. Barnes Grainne M. O’Kane
Grainne M. O’Kane Mark David Vincent
Mark David Vincent Natasha B. Leighl
Natasha B. Leighl