- 1Division of Nuclear Medicine, Hospital of Neuchâtel, Neuchâtel, Switzerland
- 2Division of Nuclear Medicine and Molecular Imaging, Geneva University Hospital, Geneva, Switzerland
- 3Division of Radiology, Geneva University Hospital, Geneva, Switzerland
- 4Faculty of Medicine, Geneva University, Geneva, Switzerland
- 5Division of Radiation-Oncology, Geneva University Hospital, Geneva, Switzerland
- 6Department of Radiology and Nuclear Medicine, Stadtspital Triemli, Zurich, Switzerland
Salvage radiotherapy (SRT) represents the main treatment option for relapsing prostate cancer in patients after radical prostatectomy. Several open questions remain unanswered in terms of target volumes definition and delivered doses for SRT: the effective dose necessary to achieve biochemical control in the SRT setting may be different if the tumor recurrence is micro- or macroscopic. At the same time, irradiation of only the prostatic bed or of the whole pelvis will depend on the localization of the recurrence, local or locoregional. In the “theragnostic imaging” era, molecular imaging using positron emission tomography (PET) constitutes a useful tool for clinicians to define the site of the recurrence, the extent of disease, and individualize salvage treatments. The best option currently available in clinical routine is the combination of radiolabeled choline PET imaging and multiparametric magnetic resonance imaging (MRI), associating the nodal and distant metastases identification based on PET with the local assessment by MRI. A new generation of targeted tracers, namely, prostate-specific membrane antigen, show promising results, with a contrast superior to choline imaging and a higher detection rate even for low prostate-specific antigen levels; validation studies are ongoing. Finally, imaging targeting bone remodeling, using whole-body SPECT–CT, is a relevant complement to molecular/metabolic PET imaging when bone involvement is suspected.
Introduction
Although radical prostatectomy (RP) with or without lymphadenectomy remains one of the main curative options for prostate cancer (PCa), more than 30% of the patients will relapse during follow-up (1). Salvage radiotherapy (SRT) represents the main treatment option for relapsing patients after RP, and durable biochemical response rates have been reported (2). Despite gains in understanding how to select patients for salvage treatment, the variable clinical course of these patients still leaves uncertainties about how and when to appropriately manage these patients.
Early identification of relapsing disease by modern imaging techniques has been demonstrated to significantly influence final treatment decisions and drive SRT in locally or locoregionally relapsing patients in terms of target volume definition as well as planned doses. Indeed, the effective dose necessary to achieve biochemical control in the SRT setting may be different if the tumor recurrence is micro- or macroscopic (3). At the same time, irradiation of only the prostatic bed or of the whole pelvis will depend on the precise location of the recurrence, local or loco-regional.
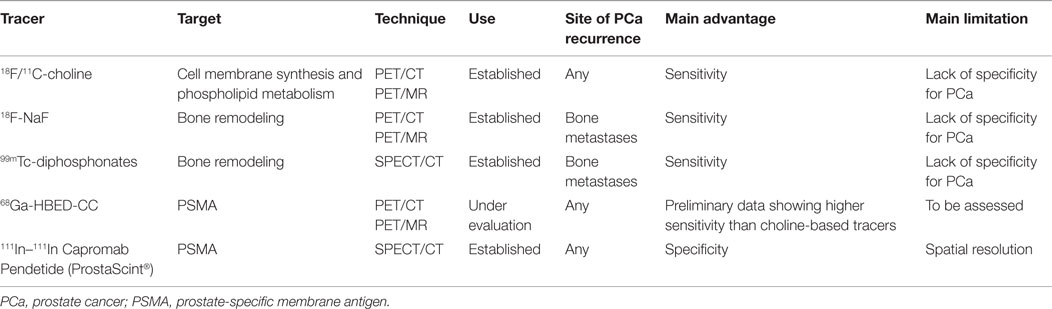
In the “theragnostic imaging” era, molecular imaging using positron emission tomography (PET) and single-photon emission computed tomography (SPECT) constitutes a useful tool for clinicians to define the site of the recurrence, the extent of disease, and allows, therefore, for individualizing salvage treatments. In the following review, we report on the evidence concerning the use of molecular imaging in the SRT setting in patients presenting with biochemical relapse after RP, with a special focus on new PCa-specific PET tracers. Table 1 provides a summary of the most relevant tracers available in the setting of post-prostatectomy relapsing PCa.
Evaluation of Local and Lymph Node Involvement Recurrence by Choline PET Tracers
18F-fluorodeoxyglucose (FDG) PET imaging is a well-established tool in radiation therapy planning, extensively used in many tumor types. The lack of FDG avidity in most PCa has motivated the search for alternative metabolic tracers, and among them, the most commonly used are choline tracers. Three main choline-based PET tracers exist, namely, 11C-choline, 18F-methylcholine, and 18F-ethylcholine: regardless of the slight chemical differences impacting overall distribution and the lack of formal comparative studies, available data suggest that their diagnostic performance is overall similar (4). 11C-acetate is another tracer, less commonly used in PCa, sharing with choline tracers a similar distribution, and being transformed to phosphatidylcholine after uptake (5). Studies have shown that performance is similar to 18F-choline (6).
The literature on the use of choline PET in recurrent PCa is vast but inhomogeneous, and for this reason, its use in recent guidelines is suggested but not established, yet. Two recent meta-analyses have tried to overcome this limitation, with encouraging and converging results when selecting studies with common inclusion criteria, protocols, and standard of reference (7, 8). Both analyses obtained pooled sensitivities and specificities above 85% in patients with biochemical recurrence. For local recurrence, in particular, the sensitivity was 61% and the specificity 97% (8).
Indeed, when assessing a biochemical recurrence of PCa after RP, it should be taken in account that the detection rates vary with prostate-specific antigen (PSA) levels when using choline-labeled tracers (9–11). Choline PET–CT has shown interesting results when assessing lymph node recurrences with PSA >1 ng/mL, with sensitivity of 90% and specificity of 100% in a per-patient analysis, and 67 and 96% in a per-region analysis, respectively (12). Below this level of PSA, the recurrence detection rate with choline-labeled tracers decreases, essentially because of the lack of ability for PET to detect small lesions (of a few millimeters), presenting with low metabolism due to the spatial resolution limit of the technique (9, 10, 13, 14). Nevertheless, the sensitivity of choline PET is still above 50% in patients with PSA <1 ng/mL when PSA doubling time is <6 months or PSA velocity is >1 ng/mL/year (10, 15, 16). When the 1 ng/mL threshold is not reached and other criteria, such as PSA doubling time and velocity, are not met, prostate-targeted magnetic resonance imaging (MRI) is considered the best choice to detect local recurrences. Conventional imaging, including CT and standard MRI, is, however, of limited value to identify metastatic lymph nodes since up to 80% of involved lymph nodes are smaller than 1 cm (17–19), and the evaluation of nodal involvement in prostate MRI studies is limited to the pelvic field of view. Integrated whole-body choline PET/MRI might thus be the modality of choice to overcome these limitations.
Choline PET–CT has been used to guide SRT planning, as recently reviewed (20). Despite the lack of large multicenter validation studies, single-center experiences consistently show that nodal and oligometastatic disease can be efficiently targeted (21–24). The limited spatial resolution remains the main obstacle for accurate targeting of the local relapse. Finally, more recent evidence has shown that choline PET also has a prognostic value among the candidates for curative radiation treatment (24, 25).
The Added Value of Combined PET–MRI
Magnetic resonance imaging is the most frequently used imaging modality to evaluate local PCa recurrence. T2-weighted imaging depicts recurrence with wide ranges of sensitivity and specificity with values of 48–100 and 50–100%, respectively, after RP and of 25–86 and 64–100%, respectively, after radiation therapy (26). Multiparametric imaging, such as spectroscopy, diffusion-weighted imaging, and dynamic contrast-enhanced MRI, have gained acceptance to complement T2-weighted MRI for primary and recurrent PCa detection (27–29). However, there is still an important need to further improve the accuracy of PCa imaging. The question arises whether associating metabolic PET data with MRI might potentially enhance PCa imaging. Preliminary reports using both modalities have provided contradictory results that could be explained in part by the difficulty to perform an accurate coregistration of the PET and MR images (30, 31). To solve this issue, hybrid PET–MRI systems have been designed to allow serial or simultaneous PET and MRI acquisitions during a single examination, with a common referential of the patient’s position. Acquiring fluorocholine PET and MRI in one single examination session showed a relevant improvement of the accuracy of PCa lesions’ detection (32–34) (Figure 1).
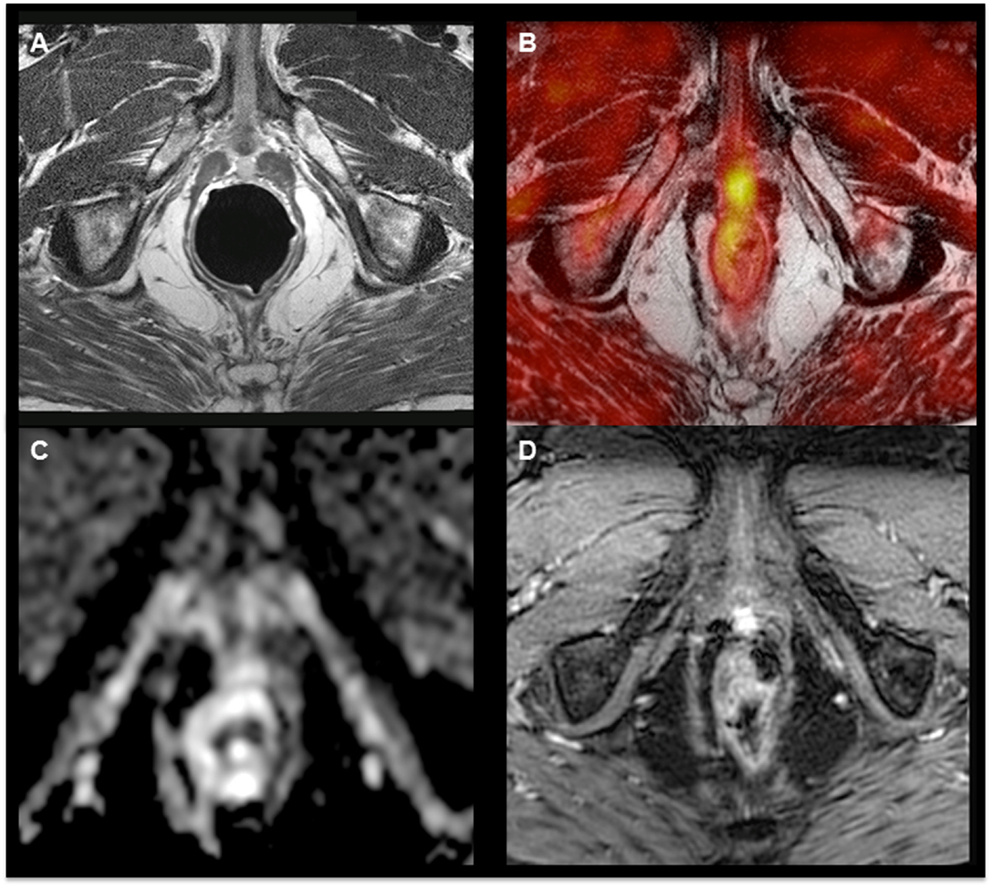
Figure 1. 18F-Fluorocholine hybrid PET–MRI images showing hyperintensity on the T2-weighted sequence (A) and focal hypermetabolism (B) in a nodule with limited diffusion restriction on ADC map (C) and hyperperfusion (D) in a patient with a biochemical relapse (PSA = 1.75 ng/mL, doubling time = 11 months) 9 years after radical prostatectomy.
The adjunction of the PET acquisition leads to an important gain of the specificity of cancer detection when compared to MRI alone, without significant reduction of sensitivity for primary PCa staging. The sensitivity and specificity for the multiparametric MRI alone were 84.4 and 68.6%, respectively, and 81.2 and 87.1%, respectively, for the use of integrated PET–MRI (33). Another study showed that PCa was correctly detected in 80% of patients using 18F-choline PET alone, in 83.3% of patients using multiparametric MRI, and in 93.3% using integrated PET–MRI (34). These data show the ability of the PET–MRI scanner to perform MRI examinations of high diagnostic quality without artifacts related to the presence of the PET gantry and demonstrate that the information obtained from MRI (T2 anatomical sequences, diffusion, and perfusion) and PET (SUVmax) are complementary. Hitherto, no study has been published concerning the specific use of hybrid PET–MRI systems for recurrence detection or radiation therapy planning. However, there are ongoing studies scoping the development of dedicated positioning devices and dosimetric approaches (35, 36).
Bone Metastases Assessment
Current guidelines recommend bone imaging only in selected high-risk cases. However, this definition is not homogenous in the literature (37, 38). In clinical practice, bone imaging is frequently performed in patients presenting with biochemical recurrence. Several choices exist, including bone scintigraphy, 18F-NaF PET–CT, or choline-labeled (18F or 11C) PET–CT (39).
Bone scintigraphy remains a widely used imaging modality in the metastatic workup of PCa patients. It allows for whole-body screening and is highly sensitive in the detection of metastases, but its specificity is limited due to benign conditions presenting also with altered tracer uptake (e.g., degenerative joint diseases, fractures, infections, or benign bone tumors) (40, 41). During the last decade, SPECT–CT has gained a wide acceptance for bone scanning. Many studies have shown that SPECT–CT reduces the rate of equivocal lesions compared to planar bone scan due to better anatomic localization of lesions and higher lesion-to-background contrast. By consequence, it increases diagnostic accuracy over SPECT alone or planar scintigraphy alone (42–46). Some authors use SPECT–CT only to clarify the origin of equivocal lesions based on planar scintigraphy, whereas others recommend to systematically acquire whole-body SPECT–CT from the cervical spine to the proximal femurs (43, 47). The proportion of indeterminate bone lesions can be reduced from a rate between 48 and 72% with planar whole-body scintigraphy and/or SPECT without CT, to a rate between 0 and 15% when adding SPECT with CT. Furthermore, SPECT–CT has been able to correctly convert a metastatic status into a non-metastatic status (downstaging) in 29.5% of the patients, with a sensitivity and specificity of 96.4 and 94.2%, respectively, on a per-patient analysis (47).
18F-NaF PET–CT is considered to have superior pharmacokinetic characteristics, such as high bone affinity, rapid clearance, and low protein binding, compared to 99mTc-diphosphonates. Its impact in PCa management has been recently evaluated by the National Oncologic PET Registry (NOPR) in the US, showing a 44% rate of change in management in recurrent PCa (48). The patient-based analysis showed that sensitivity and specificity of 18F-fluoride PET–CT and bone scan were 96 versus 88% and 91 versus 80%, respectively (49). Although 18F-NaF PET–CT has been reported to be more sensitive for detection of metastases than planar bone scan, the question arose to know whether 18F-NaF PET–CT outperforms whole-body SPECT–CT. Indeed, the comparative studies available hitherto only compare 18F-NaF PET–CT to standalone SPECT acquisitions, which are intrinsically limited by the lack of anatomical correlation (50).
Radiolabeled choline PET–CT is used in the assessment of PCa recurrence in the prostate bed or in lymph nodes but can also highlight bone metastases (9, 14, 51). It has been reported that 18F-choline PET–CT was more specific than 18F-NaF PET–CT (99 versus 93%) but that 18F-choline PET–CT suffered from slightly lower sensitivity (74 versus 81%) (49, 52). There is still an uncertainty whether these choline-negative lesions could be a result of androgen-deprivation therapy, since many patients enrolled in trials are under androgen deprivation. Based on this finding, it is recommended to systematically carry out imaging reflecting bone remodeling (18F-NaF PET–CT or whole-body SPECT–CT) in addition to choline PET imaging for bone assessment, both for diagnostic and for treatment planning purposes, whenever bone involvement is suspected clinically.
Future Tracers
While PET imaging currently validated for clinical practice is based on relatively unspecific tracers, such as FDG and choline, ongoing research focuses on the development of new tracers targeting tumor-specific antigens. The most promising tracers for prostate imaging are summarized below. No validation about their use in SRT is yet available, even if this has been tested for prostate-specific membrane antigen (PSMA) and anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (FACBC) tracers (53, 54).
Prostate-Specific Membrane Antigen Tracers
Prostate-specific membrane antigen is a transmembrane protein overexpressed in PCa and highly expressed in androgen-independent disease (55). Preclinical and in vitro studies suggest a good specificity of this target when compared to normal prostatic tissue or post-radiation therapy fibrotic changes (56). The high specificity of this target has also motivated the development of therapeutic or combined diagnostic/therapeutic (or “theragnostic”) agents, radiolabeled with 111In or 177Lu (57, 58). PSMA imaging is performed using 111In Capromab Pendetide (ProstaScint®), a monoclonal murine antibody. This tracer is FDA approved for staging high-risk PCa and for recurrent PCa post-prostatectomy. Prostascint imaging has, however, some disadvantages: a complex biodistribution, requiring imaging up to 6 days after administration, an intracellular epitope, not accessible in living cells, non-specific signal in the presence of inflammation, and the intrinsic lower resolution of SPECT imaging as compared with PET (59).
A comprehensive description of all tracers developed in preclinical studies for this target goes beyond the scope of this paper. Therefore, we will only briefly summarize the results of the clinical studies performed so far in recurrent PCa. Four tracers have been used in human studies, three of them using 18F as radioisotope and one using 68Ga.
18F-DCFBC
A dosimetry study in five metastatic patients showed the ability of the tracer to detect probable metastatic lesions in lymph nodes and the skeleton (60). The tracer has also been evaluated in primary PCa cancer characterization in 13 patients, showing a high specificity for tumor lesions over benign hypertrophy, even higher than MRI (61).
18F-BAY1075553
Only a single phase I study has been published, including 12 patients (9 at staging and 3 with recurrent PCa), and comparing the diagnostic performance of this tracer to 18F-choline, showing a similar performance of the two tracers for the characterization of prostatic lesions. However, 18F-choline has been shown to be superior for nodal and bone marrow lesions’ detection (62).
18F-DCFPyL
Only two studies used this tracer in patients, one of them performing whole-body dosimetry and the other providing a preliminary comparison with 68Ga-HBED-CC in 14 patients with recurrent PCa (63, 64).
68Ga-HBED-CC
This is the most extensively evaluated PSMA tracer so far, with already over 20 published studies. All of them showed high proportions of positive findings in recurrent disease, with detections rates ranging from 82.8 to 89.5%, in the two largest studies (65, 66). In patients with PSA values between 0.2 and 0.5 ng/mL, the detection rate was 57.9% (66). One study suggests superiority in comparison with 18F-choline, with higher contrast and more lesions identified by the PSMA marker (67). Discordant results were found with respect to the impact of PSA doubling time on PET positivity (66, 68). Only one recent study has evaluated the impact of this tracer on radiation therapy planning, showing a change in strategy in about 50% of the cases, which is in line with the range of the management changes rate reported for choline (54, 69, 70).
Amino Acids
Amino acid demand and transport are increased in malignant prostatic cells, reflecting protein synthesis. Some radiolabeled amino acids have been developed in order to explore this metabolic pathway. Anti-(18F)-FACBC (anti-1-amino-3-18F-FACBC or fluciclovine) appears to be a promising PET amino-acid radiotracer: it is a synthetic l-leucine analog, leucine being an essential nutrient for protein synthesis and cell growth, with high uptake in the majority of PCa lesions and metastasis. In a recent meta-analysis of six studies concerning the performances of 18F-FACBC PET–CT in patients with a suspicion of PCa recurrence, the pooled sensitivity and specificity for this radiotracer were 87 and 66%, respectively (71). Comparative studies with choline tracers showed a higher sensitivity and specificity, with an approximately 20% higher detection rate when using 18F-FACBC (72–75).
Gastrin-Releasing Peptide Receptors
Gastrin-releasing peptide receptors (GRPR) are overexpressed in a majority of PCa cells. Therefore, they represent a potential target for diagnostic imaging procedures. Bombesin, which can be labeled with positron-emitting radionuclides, is one of those tracers. Different radiolabeled bombesin analogs have been tested in primary and metastatic PCa (76, 77) as well as in cases of biological recurrence after surgery or hormonal therapy (76). Kähkönen et al., using 68Ga-labeledDOTA-4-amino-1-carboxymethyl-piperidine-d-Phe–Gln–Trp–Ala–Val–Gly–His–Sta–Leu-NH2 peptide (BAY 86-7548), found satisfying results in detection of recurrence in prostatic bed and nodal relapse but poor ability to detect bone metastases (76). Sah et al. published a first-in-man study concerning BAY 864367, a slightly different 18F-labeled bombesin tracer (78). They found that the tracer uptake was higher in primary PCa than in recurrent lesions. Mitsakis et al. compared 68Ga-NODAGA-MJ9 (MJ9) PET–CT with 18F-flurocholine in 33 patients with recurrent PCa and concluded that MJ9 missed 75% of the 24 bone lesions identified on 18F-choline PET. However, 18% of metastatic lymph nodes that were positive on 18-flurocholine were negative on MJ9, and inversely, 13% of lesions in lymph nodes were positive on MJ9 but negative on 18F-flurocholine PET/CT, with a greater signal-to-background ratio on MJ9 images (79).
Fluoro-5-Dihydrotestosterone
16β-(18F)-fluoro-5-dihydrotestosterone (FDHT) is a fluorinated testosterone analog that can detect the overexpression of androgen receptors in PCa lesions. The first study concerning the use of FDHT in patients with progressive metastatic PCa showed a high tumor-to-background ratio and a detection rate of 78% of the 59 lesions identified on conventional imaging methods in a group of seven patients (80). Tumor uptake of FDHT is receptor mediated (81), and thus, the results of the FDHT–PET may be able to predict which lesions will show a good response to androgen deprivation therapy and which ones will not, therefore, needing another type of treatment (82). Moreover, the intensity of FDHT uptake in bone metastases of castration-resistant PCa patients was a negative prognostic factor in terms of patient survival (83). No studies on the use of FDHT in recurrent PCa after RP have been published, yet.
Conclusion
The combination of radiolabeled-choline PET and MRI appears to be the modality of choice in clinical routine for the assessment of recurrence of PCa, associating the identification of nodal and distant disease based on PET and the local assessment by multiparametric MRI. While the availability of integrated PET–MRI systems will presumably remain confined to academic centers, at least in the near future, the use of software allowing automated fusion of PET and MRI sequences acquired at different times is already widely used in SRT planning. A new generation of targeted tracers, such as PSMA and FACBC, has shown promising results, with a lesion-to-background contrast superior to choline imaging and a higher detection rate of lesions even for very low PSA levels. Results of ongoing validation studies are warranted. Bone remodeling tracers, including standard bone scans with SPECT–CT, remain of great interest in assessment of bone extension and should be systematically associated with metabolic imaging.
Author Contributions
TZ, VG, ORager, and GA are responsible for the study design and contributed equally to the manuscript. TZ, VG, ORager, GA, and CT-V drafted the manuscript. RM, GG, ORatib, TP, and ES revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bianco FJ Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”). Urology (2005) 66:83–94. doi: 10.1016/j.urology.2005.06.116
2. Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol (2007) 25:2035–41. doi:10.1200/JCO.2006.08.9607
3. Zilli T, Jorcano S, Peguret N, Caparrotti F, Hidalgo A, Khan HG, et al. Results of dose-adapted salvage radiotherapy after radical prostatectomy based on an endorectal MRI target definition model. Am J Clin Oncol (2014). doi:10.1097/COC.0000000000000130
4. Calabria F, Gallo G, Schillaci O, Cascini GL. Bio-distribution, imaging protocols and diagnostic accuracy of PET with tracers of lipogenesis in imaging prostate cancer: a comparison between 11C-choline, 18Ffluoroethylcholine and 18F-methylcholine. Curr Pharm Des (2015) 21:4738–47. doi:10.2174/1381612821666150818110422
5. Yoshimoto M, Waki A, Yonekura Y, Sadato N, Murata T, Omata N, et al. Characterization of acetate metabolism in tumor cells in relation to cell proliferation: acetate metabolism in tumor cells. Nucl Med Biol (2001) 28:117–22. doi:10.1016/S0969-8051(00)00195-5
6. Buchegger F, Garibotto V, Zilli T, Allainmat L, Jorcano S, Vees H, et al. First imaging results of an intraindividual comparison of (11)C-acetate and (18)F-fluorocholine PET/CT in patients with prostate cancer at early biochemical first or second relapse after prostatectomy or radiotherapy. Eur J Nucl Med Mol Imaging (2014) 41:68–78. doi:10.1007/s00259-013-2540-6
7. Umbehr MH, Muntener M, Hany T, Sulser T, Bachmann LM. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol (2013) 64:106–17. doi:10.1016/j.eururo.2013.04.019
8. Fanti S, Minozzi S, Castellucci P, Balduzzi S, Herrmann K, Krause BJ, et al. PET/CT with C-choline for evaluation of prostate cancer patients with biochemical recurrence: meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging (2016) 43(1):55–69. doi:10.1007/s00259-015-3202-7
9. Picchio M, Briganti A, Fanti S, Heidenreich A, Krause BJ, Messa C, et al. The role of choline positron emission tomography/computed tomography in the management of patients with prostate-specific antigen progression after radical treatment of prostate cancer. Eur Urol (2011) 59:51–60. doi:10.1016/j.eururo.2010.09.004
10. Giovacchini G, Picchio M, Garcia-Parra R, Mapelli P, Briganti A, Montorsi F, et al. [11C]choline positron emission tomography/computerized tomography for early detection of prostate cancer recurrence in patients with low increasing prostate specific antigen. J Urol (2013) 189:105–10. doi:10.1016/j.juro.2012.09.001
11. Mapelli P, Panebianco V, Picchio M. Prostate cancer recurrence: can PSA guide imaging? Eur J Nucl Med Mol Imaging (2015) 42:1781–3. doi:10.1007/s00259-015-3091-9
12. Kitajima K, Murphy RC, Nathan MA, Froemming AT, Hagen CE, Takahashi N, et al. Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med (2014) 55:223–32. doi:10.2967/jnumed.113.123018
13. Castellucci P, Fuccio C, Rubello D, Schiavina R, Santi I, Nanni C, et al. Is there a role for (1)(1)C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase <1.5 ng/ml? Eur J Nucl Med Mol Imaging (2011) 38:55–63. doi:10.1007/s00259-010-1604-0
14. Mamede M, Ceci F, Castellucci P, Schiavina R, Fuccio C, Nanni C, et al. The role of 11C-choline PET imaging in the early detection of recurrence in surgically treated prostate cancer patients with very low PSA level <0.5 ng/mL. Clin Nucl Med (2013) 38:e342–5. doi:10.1097/RLU.0b013e31829af913
15. Schillaci O, Calabria F, Tavolozza M, Caracciolo CR, Finazzi Agro E, Miano R, et al. Influence of PSA, PSA velocity and PSA doubling time on contrast-enhanced 18F-choline PET/CT detection rate in patients with rising PSA after radical prostatectomy. Eur J Nucl Med Mol Imaging (2012) 39:589–96. doi:10.1007/s00259-011-2030-7
16. Castellucci P, Ceci F, Graziani T, Schiavina R, Brunocilla E, Mazzarotto R, et al. Early biochemical relapse after radical prostatectomy: which prostate cancer patients may benefit from a restaging 11C-choline PET/CT scan before salvage radiation therapy? J Nucl Med (2014) 55:1424–9. doi:10.2967/jnumed.114.138313
17. Heesakkers RA, Hovels AM, Jager GJ, van den Bosch HC, Witjes JA, Raat HP, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol (2008) 9:850–6. doi:10.1016/S1470-2045(08)70203-1
18. Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol (2008) 63:387–95. doi:10.1016/j.crad.2007.05.022
19. Mapelli P, Picchio M. Initial prostate cancer diagnosis and disease staging-the role of choline-PET-CT. Nat Rev Urol (2015) 12:510–8. doi:10.1038/nrurol.2015.191
20. De Bari B, Alongi F, Lestrade L, Giammarile F. Choline-PET in prostate cancer management: the point of view of the radiation oncologist. Crit Rev Oncol Hematol (2014) 91:234–47. doi:10.1016/j.critrevonc.2014.04.002
21. Vees H, Steiner C, Dipasquale G, Chouiter A, Zilli T, Velazquez M, et al. Target volume definition in high-risk prostate cancer patients using sentinel node SPECT/CT and 18 F-choline PET/CT. Radiat Oncol (2012) 7:134. doi:10.1186/1748-717X-7-134
22. Schick U, Jorcano S, Nouet P, Rouzaud M, Vees H, Zilli T, et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol (2013) 52:1622–8. doi:10.3109/0284186X.2013.764010
23. Miralbell R, Buchegger F. PET/CT imaging and the oligometastatic prostate cancer patient: an opportunity for a curative approach with high-dose radiotherapy? Eur J Nucl Med Mol Imaging (2014) 41:1267–9. doi:10.1007/s00259-014-2793-8
24. Picchio M, Berardi G, Fodor A, Busnardo E, Crivellaro C, Giovacchini G, et al. (11)C-Choline PET/CT as a guide to radiation treatment planning of lymph-node relapses in prostate cancer patients. Eur J Nucl Med Mol Imaging (2014) 41:1270–9. doi:10.1007/s00259-014-2734-6
25. Incerti E, Fodor A, Mapelli P, Fiorino C, Alongi P, Kirienko M, et al. Radiation treatment of lymph node recurrence from prostate cancer: is 11C-choline PET/CT predictive of survival outcomes? J Nucl Med (2015) 56(12):1836–42. doi:10.2967/jnumed.115.163741
26. Barchetti F, Panebianco V. Multiparametric MRI for recurrent prostate cancer post radical prostatectomy and postradiation therapy. Biomed Res Int (2014) 2014:316272. doi:10.1155/2014/316272
27. Kitajima K, Kaji Y, Fukabori Y, Yoshida K, Suganuma N, Sugimura K. Prostate cancer detection with 3 T MRI: comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. J Magn Reson Imaging (2010) 31:625–31. doi:10.1002/jmri.22075
28. Padhani AR. Integrating multiparametric prostate MRI into clinical practice. Cancer Imaging (2011) 11(Spec No A):S27–37. doi:10.1102/1470-7330.2011.9007
29. Schlemmer HP. Prostate cancer: localizing the cancer in patients with persistent negative biopsies. Cancer Imaging (2011) 11(Spec No A):S1. doi:10.1102/1470-7330.2011.9001
30. Jambor I, Borra R, Kemppainen J, Lepomaki V, Parkkola R, Dean K, et al. Improved detection of localized prostate cancer using co-registered MRI and (11)C-acetate PET/CT. Eur J Radiol (2012) 81(11):2966–72. doi:10.1016/j.ejrad.2011.12.043
31. Van den Bergh L, Koole M, Isebaert S, Joniau S, Deroose CM, Oyen R, et al. Is there an additional value of (11)C-Choline PET-CT to T2-weighted MRI images in the localization of intraprostatic tumor nodules? Int J Radiat Oncol Biol Phys (2012) 83(5):1486–92. doi:10.1016/j.ijrobp.2011.10.046
32. Souvatzoglou M, Eiber M, Takei T, Furst S, Maurer T, Gaertner F, et al. Comparison of integrated whole-body [11C]choline PET/MR with PET/CT in patients with prostate cancer. Eur J Nucl Med Mol Imaging (2013) 40:1486–99. doi:10.1007/s00259-013-2467-y
33. de Perrot T, Rager O, Scheffler M, Lord M, Pusztaszeri M, Iselin C, et al. Potential of hybrid (1)(8)F-fluorocholine PET/MRI for prostate cancer imaging. Eur J Nucl Med Mol Imaging (2014) 41:1744–55. doi:10.1007/s00259-014-2786-7
34. Kim YI, Cheon GJ, Paeng JC, Cho JY, Kwak C, Kang KW, et al. Usefulness of MRI-assisted metabolic volumetric parameters provided by simultaneous (18)F-fluorocholine PET/MRI for primary prostate cancer characterization. Eur J Nucl Med Mol Imaging (2015) 42:1247–56. doi:10.1007/s00259-015-3026-5
35. Paulus DH, Thorwath D, Schmidt H, Quick HH. Towards integration of PET/MR hybrid imaging into radiation therapy treatment planning. Med Phys (2014) 41:072505. doi:10.1118/1.4881317
36. Kim J, Garbarino K, Schultz L, Levin K, Movsas B, Siddiqui MS, et al. Dosimetric evaluation of synthetic CT relative to bulk density assignment-based magnetic resonance-only approaches for prostate radiotherapy. Radiat Oncol (2015) 10:239. doi:10.1186/s13014-015-0549-7
37. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol (2014) 65:467–79. doi:10.1016/j.eururo.2013.11.002
38. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Prostate Cancer (2016). Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
39. Picchio M, Mapelli P, Panebianco V, Castellucci P, Incerti E, Briganti A, et al. Imaging biomarkers in prostate cancer: role of PET/CT and MRI. Eur J Nucl Med Mol Imaging (2015) 42:644–55. doi:10.1007/s00259-014-2982-5
40. Love C, Din AS, Tomas MB, Kalapparambath TP, Palestro CJ. Radionuclide bone imaging: an illustrative review. Radiographics (2003) 23:341–58. doi:10.1148/rg.232025103
41. Damle NA, Bal C, Bandopadhyaya GP, Kumar L, Kumar P, Malhotra A, et al. The role of 18F-fluoride PET-CT in the detection of bone metastases in patients with breast, lung and prostate carcinoma: a comparison with FDG PET/CT and 99mTc-MDP bone scan. Jpn J Radiol (2013) 31:262–9. doi:10.1007/s11604-013-0179-7
42. Schillaci O, Danieli R, Manni C, Simonetti G. Is SPECT/CT with a hybrid camera useful to improve scintigraphic imaging interpretation? Nucl Med Commun (2004) 25:705–10. doi:10.1097/01.mnm.0000130240.83949.54
43. Romer W, Nomayr A, Uder M, Bautz W, Kuwert T. SPECT-guided CT for evaluating foci of increased bone metabolism classified as indeterminate on SPECT in cancer patients. J Nucl Med (2006) 47:1102–6.
44. Utsunomiya D, Shiraishi S, Imuta M, Tomiguchi S, Kawanaka K, Morishita S, et al. Added value of SPECT/CT fusion in assessing suspected bone metastasis: comparison with scintigraphy alone and nonfused scintigraphy and CT. Radiology (2006) 238:264–71. doi:10.1148/radiol.2373041358
45. Helyar V, Mohan HK, Barwick T, Livieratos L, Gnanasegaran G, Clarke SE, et al. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur J Nucl Med Mol Imaging (2010) 37:706–13. doi:10.1007/s00259-009-1334-3
46. Zhang Y, Shi H, Gu Y, Xiu Y, Li B, Zhu W, et al. Differential diagnostic value of single-photon emission computed tomography/spiral computed tomography with Tc-99m-methylene diphosphonate in patients with spinal lesions. Nucl Med Commun (2011) 32:1194–200. doi:10.1097/MNM.0b013e32834bd82e
47. Palmedo H, Marx C, Ebert A, Kreft B, Ko Y, Turler A, et al. Whole-body SPECT/CT for bone scintigraphy: diagnostic value and effect on patient management in oncological patients. Eur J Nucl Med Mol Imaging (2014) 41:59–67. doi:10.1007/s00259-013-2532-6
48. Hillner BE, Siegel BA, Hanna L, Duan F, Shields AF, Coleman RE. Impact of 18F-fluoride PET in patients with known prostate cancer: initial results from the National Oncologic PET Registry. J Nucl Med (2014) 55:574–81. doi:10.2967/jnumed.113.130005
49. Shen CT, Qiu ZL, Han TT, Luo QY. Performance of 18F-fluoride PET or PET/CT for the detection of bone metastases: a meta-analysis. Clin Nucl Med (2015) 40:103–10. doi:10.1097/RLU.0000000000000592
50. Even-Sapir E, Metser U, Mishani E, Lievshitz G, Lerman H, Leibovitch I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single – and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med (2006) 47:287–97.
51. Chondrogiannis S, Marzola MC, Ferretti A, Maffione AM, Rampin L, Grassetto G, et al. Role of (1)(8)F-choline PET/CT in suspicion of relapse following definitive radiotherapy for prostate cancer. Eur J Nucl Med Mol Imaging (2013) 40:1356–64. doi:10.1007/s00259-013-2433-8
52. Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Loidl W, et al. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging (2008) 35:1766–74. doi:10.1007/s00259-008-0788-z
53. Brunocilla E, Schiavina R, Nanni C, Borghesi M, Cevenini M, Molinaroli E, et al. First case of 18F-FACBC PET/CT-guided salvage radiotherapy for local relapse after radical prostatectomy with negative 11C-Choline PET/CT and multiparametric MRI: new imaging techniques may improve patient selection. Arch Ital Urol Androl (2014) 86:239–40. doi:10.4081/aiua.2014.3.239
54. Sterzing F, Kratochwil C, Fiedler H, Katayama S, Habl G, Kopka K, et al. Ga-PSMA-11 PET/CT: a new technique with high potential for the radiotherapeutic management of prostate cancer patients. Eur J Nucl Med Mol Imaging (2016) 43:34–41. doi:10.1007/s00259-015-3188-1
55. Mease RC, Foss CA, Pomper MG. PET imaging in prostate cancer: focus on prostate-specific membrane antigen. Curr Top Med Chem (2013) 13:951–62. doi:10.2174/1568026611313080008
56. Rybalov M, Ananias HJ, Hoving HD, van der Poel HG, Rosati S, de Jong IJ. PSMA, EpCAM, VEGF and GRPR as imaging targets in locally recurrent prostate cancer after radiotherapy. Int J Mol Sci (2014) 15:6046–61. doi:10.3390/ijms15046046
57. Ahmadzadehfar H, Rahbar K, Kurpig S, Bogemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res (2015) 5:114. doi:10.1186/s13550-015-0114-2
58. Maurer T, Weirich G, Schottelius M, Weineisen M, Frisch B, Okur A, et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur Urol (2015) 68:530–4. doi:10.1016/j.eururo.2015.04.034
59. Feneley MR, Jan H, Granowska M, Mather SJ, Ellison D, Glass J, et al. Imaging with prostate-specific membrane antigen (PSMA) in prostate cancer. Prostate Cancer Prostatic Dis (2000) 3:47–52. doi:10.1038/sj.pcan.4500390
60. Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med (2012) 53:1883–91. doi:10.2967/jnumed.112.104661
61. Rowe SP, Gage KL, Faraj SF, Macura KJ, Cornish TC, Gonzalez-Roibon N, et al. (1)(8)F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med (2015) 56:1003–10. doi:10.2967/jnumed.115.154336
62. Beheshti M, Kunit T, Haim S, Zakavi R, Schiller C, Stephens A, et al. BAY 1075553 PET-CT for staging and restaging prostate cancer patients: comparison with [18F] Fluorocholine PET-CT (Phase I Study). Mol Imaging Biol (2015) 17:424–33. doi:10.1007/s11307-014-0800-x
63. Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomacker K, et al. Comparison of [F]DCFPyL and [Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol (2015) 17:575–84. doi:10.1007/s11307-015-0866-0
64. Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, et al. Initial evaluation of [F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol (2015) 17:565–74. doi:10.1007/s11307-015-0850-8
65. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging (2015) 42:197–209. doi:10.1007/s00259-014-2949-6
66. Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid (6)(8)Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med (2015) 56:668–74. doi:10.2967/jnumed.115.154153
67. Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging (2014) 41:11–20. doi:10.1007/s00259-013-2525-5
68. Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging (2015) 42:1284–94. doi:10.1007/s00259-015-3078-6
69. Ceci F, Herrmann K, Castellucci P, Graziani T, Bluemel C, Schiavina R, et al. Impact of 11C-choline PET/CT on clinical decision making in recurrent prostate cancer: results from a retrospective two-centre trial. Eur J Nucl Med Mol Imaging (2014) 41:2222–31. doi:10.1007/s00259-014-2872-x
70. Goldstein J, Even-Sapir E, Ben-Haim S, Saad A, Spieler B, Davidson T, et al. Does choline PET/CT change the management of prostate cancer patients with biochemical failure? Am J Clin Oncol (2014). doi:10.1097/COC.0000000000000139
71. Ren J, Yuan L, Wen G, Yang J. The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: a meta-analysis. Acta Radiol (2016) 57:487–93. doi:10.1177/0284185115581541
72. Schuster DM, Savir-Baruch B, Nieh PT, Master VA, Halkar RK, Rossi PJ, et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In-capromab pendetide SPECT/CT. Radiology (2011) 259:852–61. doi:10.1148/radiol.11102023
73. Nanni C, Schiavina R, Boschi S, Ambrosini V, Pettinato C, Brunocilla E, et al. Comparison of 18F-FACBC and 11C-choline PET/CT in patients with radically treated prostate cancer and biochemical relapse: preliminary results. Eur J Nucl Med Mol Imaging (2013) 40(Suppl 1):S11–7. doi:10.1007/s00259-013-2373-3
74. Nanni C, Schiavina R, Brunocilla E, Borghesi M, Ambrosini V, Zanoni L, et al. 18F-FACBC compared with 11C-choline PET/CT in patients with biochemical relapse after radical prostatectomy: a prospective study in 28 patients. Clin Genitourin Cancer (2014) 12:106–10. doi:10.1016/j.clgc.2013.08.002
75. Nanni C, Schiavina R, Brunocilla E, Boschi S, Borghesi M, Zanoni L, et al. 18F-fluciclovine PET/CT for the detection of prostate cancer relapse: a comparison to 11C-choline PET/CT. Clin Nucl Med (2015) 40:e386–91. doi:10.1097/RLU.0000000000000849
76. Kähkönen E, Jambor I, Kemppainen J, Lehtio K, Gronroos TJ, Kuisma A, et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin Cancer Res (2013) 19:5434–43. doi:10.1158/1078-0432.CCR-12-3490
77. Wieser G, Mansi R, Grosu AL, Schultze-Seemann W, Dumont-Walter RA, Meyer PT, et al. Positron emission tomography (PET) imaging of prostate cancer with a gastrin releasing peptide receptor antagonist – from mice to men. Theranostics (2014) 4:412–9. doi:10.7150/thno.7324
78. Sah BR, Burger IA, Schibli R, Friebe M, Dinkelborg L, Graham K, et al. Dosimetry and first clinical evaluation of the new 18F-radiolabeled bombesin analogue BAY 864367 in patients with prostate cancer. J Nucl Med (2015) 56:372–8. doi:10.2967/jnumed.114.147116
79. Mitsakis P, Zilli T, Kosinski M, Delage J, Maecke H, Mansi R, et al. A direct comparison study of Ga-68-NODAGA-MJ9 (MJ9 bombesin) to F-18-FCH in recurrent prostate cancer. Eur J Nucl Med Mol Imaging (2015) 42(Suppl 1):S124–5. doi:10.1007/s00259-015-3198-z
80. Larson SM, Morris M, Gunther I, Beattie B, Humm JL, Akhurst TA, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med (2004) 45:366–73.
81. Dehdashti F, Picus J, Michalski JM, Dence CS, Siegel BA, Katzenellenbogen JA, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging (2005) 32:344–50. doi:10.1007/s00259-005-1764-5
82. Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet (2010) 375:1437–46. doi:10.1016/S0140-6736(10)60172-9
83. Vargas HA, Wassberg C, Fox JJ, Wibmer A, Goldman DA, Kuk D, et al. Bone metastases in castration-resistant prostate cancer: associations between morphologic CT patterns, glycolytic activity, and androgen receptor expression on PET and overall survival. Radiology (2014) 271:220–9. doi:10.1148/radiol.13130625
Keywords: prostate cancer, PET, MRI, salvage radiotherapy, choline, PSMA
Citation: Amzalag G, Rager O, Tabouret-Viaud C, Wissmeyer M, Sfakianaki E, de Perrot T, Ratib O, Miralbell R, Giovacchini G, Garibotto V and Zilli T (2016) Target Definition in Salvage Radiotherapy for Recurrent Prostate Cancer: The Role of Advanced Molecular Imaging. Front. Oncol. 6:73. doi: 10.3389/fonc.2016.00073
Received: 20 December 2015; Accepted: 14 March 2016;
Published: 31 March 2016
Edited by:
Scott T. Tagawa, Weill Cornell Medical College, USAReviewed by:
Jaspreet Singh Batra, Weill Cornell Medical College, USAStefano Vagge, A.O.U. IRCCS San Martino IST National Cancer Research Institute and University, Italy
Copyright: © 2016 Amzalag, Rager, Tabouret-Viaud, Wissmeyer, Sfakianaki, de Perrot, Ratib, Miralbell, Giovacchini, Garibotto and Zilli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Zilli, dGhvbWFzLnppbGxpQGhjdWdlLmNo
†Gaël Amzalag, Olivier Rager, Valentina Garibotto, and Thomas Zilli contributed equally to this work.
 Gaël Amzalag
Gaël Amzalag Olivier Rager
Olivier Rager Claire Tabouret-Viaud2
Claire Tabouret-Viaud2 Thomas Zilli
Thomas Zilli